Abstract
Purpose
To investigate long-term disease and toxicity outcomes for pediatric retinoblastoma patients treated with proton radiotherapy (PRT).
Methods and Materials
This is a retrospective analysis of 49 retinoblastoma patients (60 eyes) treated with PRT between 1986 and 2012.
Results
The majority (84%) of patients had bilateral disease, and nearly half (45%) had received prior chemotherapy. At a median follow-up of 8 years (range, 1 – 24 years), no patients died of retinoblastoma or developed metastatic disease. The post-PRT enucleation rate was low (18%), especially in patients with early-stage disease (11% for patients with International Classification for Intraocular Retinoblastoma [ICIR] Stage A-B disease vs 23% for patients with ICIR Stage C-D disease). Post-PRT ophthalmologic follow-up was available for 61% of the preserved eyes (30/49): 14/30 eyes (47%) had 20/40 visual acuity or better, 7/30 (23%) had moderate visual acuity (20/40 - 20/600), and 9/30 (30%) had little or no useful vision (worse than 20/600). Twelve of 60 treated eyes (20%) experienced a post-PRT event requiring intervention, with cataracts the most common (4 eyes). No patients developed an in-field second malignancy.
Conclusions
Long-term follow-up of retinoblastoma patients treated with PRT demonstrates that PRT can achieve high local control rates, even in advanced cases, and many patients retain useful vision in the treated eye. Treatment-related ocular side effects were uncommon and no radiation-associated malignancies were observed.
INTRODUCTION
Retinoblastoma is the most common intraocular malignancy of childhood, and nearly 300 new cases are diagnosed each year in the US. [1, 2] Approximately 60% of tumors are sporadic, while 40% are hereditary owing to germline loss or mutation of the RB1 tumor suppressor gene.[2, 3] Sporadic cases of retinoblastoma are typically unilateral, whereas most patients with hereditary disease present with bilateral tumors. Although not uncommonly seen in developing countries, metastases are rare at diagnosis in North America; therefore, definitive management relies on effective treatment of the ocular tumor(s).
External beam radiation therapy (EBRT) has been used in the treatment of retinoblastoma for over a century and was the preferred vision-sparing treatment approach for many years.[4-6] Long-term eye preservation rates range from 50-100% in several large series, with outcomes better for early-stage versus advanced tumors.[7-9] However, use of EBRT has decreased sharply over the past two decades owing to the advent of effective chemotherapeutic regimens as well as concern for radiation-induced side effects including growth abnormalities and second malignancies.[10-13] Currently, EBRT is typically reserved for cases of progressive or persistent disease following chemotherapy and focal treatment (cryotherapy or laser photocoagulation/thermotherapy), or in advanced cases when vitreous or subretinal seeding is present.[14, 15] Even in these advanced or refractory cases, ocular salvage and vision preservation can often be achieved.[5, 7]
EBRT techniques have evolved significantly over time and conformal photon techniques and proton RT (PRT) are now favored for treatment of retinoblastoma.[16, 17] Due to its unique physical properties and lack of exit dose, PRT minimizes normal tissue exposure and may be associated with lower rates of radiation-induced normal tissue injury and malignancies compared to contemporary photon-based techniques.[18] PRT has been used in the treatment of retinoblastoma at our institution since 1986, and here we report the long-term outcomes of patients treated with this approach.
METHODS
Patient Population
We identified all patients who received PRT for retinoblastoma at our institution between 1986 and 2010 and who had at least one year of follow-up data available (4 patients excluded due to inadequate follow-up). Patients with extraocular tumors or who had received previous radiation (EBRT or brachytherapy) were excluded from the analysis (N = 5). Patients receiving PRT following enucleation were also excluded (N = 3). For eligible patients, all available medical records were analyzed. This study was approved by our institutional review board.
Treatment Details
All patients underwent exam under anesthesia (EUA) at the time of initial evaluation. Tumor stage was assigned based on description of EUA findings using the International Classification for Intraocular Retinoblastoma (ICIR) staging system.[19] Computed tomography (CT)-based radiation planning was performed for all patients, and patients were treated at XXXX. The target volume was defined jointly by the treating radiation oncologist and the ophthalmologist and contained the tumor(s) plus a margin of 5-8 mm depending upon treating physician preference. For large retinal tumors or tumors with vitreous seeding, the entire vitreous and retina were targeted with the anterior field edge placed just posterior to the lens and typically correlating with the ora serrata. For smaller tumors of the posterior retina, a single anterior oblique beam was used to minimize exposure of the bony orbit (Figure 1). Patients were treated under general anesthesia using a custom head immobilization device as well as a radio-opaque silicon suction cup to maintain a neutral forward gaze position during treatment. A custom brass aperture and range compensator were fashioned to control the shape and depth of the beam. A 3.5% CT density correction plus 1 mm were applied for range uncertainty, and 1 mm lateral margin was added to aperture edge for set up uncertainty.
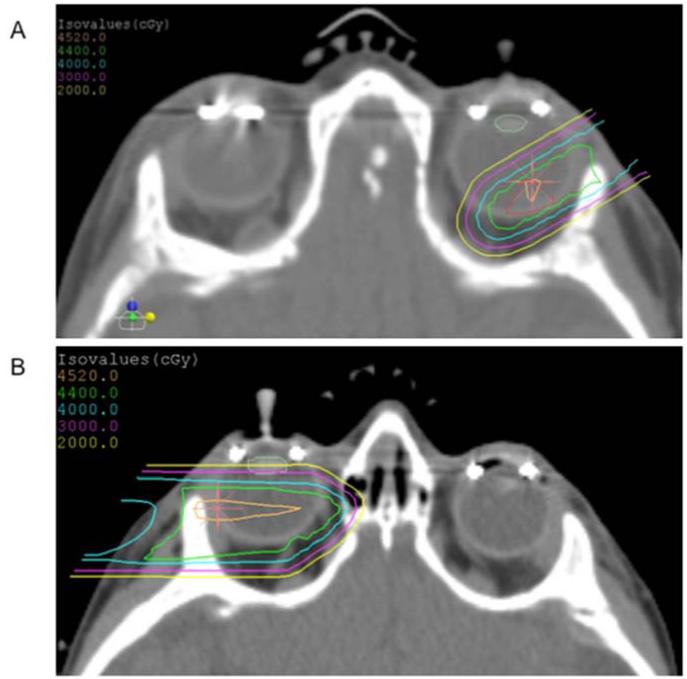
Figure 1.
Representative axial CT slice from two PRT plans. A. For small tumors without seeding, a single anterior oblique beam was used to minimize dose to the bony orbit (prescription dose 44 Gy[RBE]). The tumor is outlined in red, and the lens is outlined in pale green. B. For larger tumors, or if seeding was present, the posterior chamber was targeted with a single lateral beam (prescription dose 45 Gy[RBE]).
Follow-Up
Patients underwent EUA at the completion of RT to establish a post-treatment baseline, and then at lengthening intervals if they remained disease-free. Aside from the CT performed for radiation planning, no radiographic studies were routinely performed during treatment or follow-up. Length of follow-up was defined as the time from start of RT to the most recent recorded status of the treated eye(s).
Statistical Analysis
Fisher’s exact test was used to compare clinical and demographic characteristics between patient groups for binary outcomes. Wilcoxon-rank sum test was used for group comparison when the data were continuous or ordinal, including the three-category visual outcomes. Enucleation-free survival was defined from the first day of PRT until the date of enucleation or was censored at the date of last follow-up for non-enucleated eyes. Enucleation-free survival was estimated by the Kaplan-Meier method, with the differences between groups assessed by the log-rank test. All p-values are based on a two-sided hypothesis. Data analysis was performed using SAS 9.3 (SAS Institute, Cary, NC), while StatXact 6.0 (Cytel Software, Cambridge, MA) was used to compute the exact p-values in the analysis of enucleation-free survival and visual outcomes.
RESULTS
Patient and Treatment Characteristics
A total of 49 patients and 60 irradiated eyes were included in the analysis (Table 1). Approximately half of the patients (26/49, 53%) were female. The median age at diagnosis was six months and ranged from six days to 30 months.
Table 1. Patient and Treatment Characteristics.
| No. patients | 49 |
|
| |
| Female | 26 (53%) |
| Male | 23 (47%) |
|
| |
| Mechanism of Diagnosis (N=49) | |
|
| |
| Screening | 12 (25%) |
| Clinical Sign(s) | 29 (59%) |
| Leukocoria/abnormal light reflex | 18/29 |
| Strabismus or head tilt | 10/29 |
| Eye swelling | 1/29 |
| Unknown | 8 (16%) |
|
| |
| No. patients with positive family history for retinoblastoma | 14/49 (29%) |
|
| |
| Positive family history and bilateral disease | 10/14 |
| Positive family history and diagnosis by screening | 11/14 |
|
| |
| Median Age at Diagnosis (range) | 6 mo (6 d - 30 mo) |
|
| |
| Patients identified by screening (range) | 70 days (6 d - 8 mo) |
| Patients identified by clinical sign(s) (range) | 9 months (1 - 30 mo) |
| Patients with positive family history (range) | 55 days (6 d - 8 mo) |
| Patients without positive family history (range) | 11 mo (45 d - 30 mo) |
|
| |
| No. patients with bilateral disease at diagnosis | 41/49 (84%) |
|
| |
| Received bilateral PRT | 11/41 |
| Contralateral enucleation prior to PRT | 23/41 |
| Local therapy only to contralateral eye | 7/41 |
|
| |
| ICIR Stage at Diagnosis (N=60) | |
|
| |
| Stage A-B without disc/fovea involvement | 12 (20%) |
| Stage B due to disc/fovea involvement | 15 (25%) |
| Stage C-D | 31 (52%) |
| Stage unrecorded | 2 (3%) |
|
| |
| No. patients who received chemotherapy | 25/49 (51%) |
|
| |
| Prior to PRT | 22/25 |
| After PRT | 1/25 |
| Before and after PRT | 2/25 |
|
| |
| No. eyes treated with cryotherapy/laser | 43/60 (72%) |
|
| |
| Before PRT | 25/43 |
| After PRT | 12/43 |
| Before and after PRT | 6/43 |
|
| |
| Median age at PRT start (range) | 14 mo (1 - 82 mo) |
|
| |
| Median time elapsed between diagnosis and RT start (range) | 83 days (2 d - 62 mo) |
| Median time elapsed for patients not receiving chemo | 21 days |
| Median time elapsed for patients receiving chemo | 7 mo |
| Median PRT dose, Gy(RBE) (range) | 44.0 (40-46.8) |
ICIR: International Classification for Intraocular Retinoblastoma
Twenty-nine of the 49 patients (59%) presented with clinical signs of retinoblastoma. Leukocoria was the most common sign (18/29), followed by strabismus or head tilt (10/29). Approximately one quarter of patients were diagnosed by screening (12/49), and these patients were diagnosed at a significantly younger age than patients presenting with clinical signs (2.3 months vs 9 months; p=0.0003). The events preceding diagnosis were unknown for eight patients (16%).
Fourteen patients (29%) had a family history of retinoblastoma (defined as retinoblastoma in a first-degree relative), and these patients were diagnosed at a significantly younger age (1.8 months vs 11 months; p<0.0001) and were significantly more likely to be diagnosed by screening (92% vs 3%; p<0.0001) than patients without a family history. Ten of the 14 patients (71%) with a positive family history had bilateral tumors at diagnosis.
A total of 60 tumors were treated with PRT, and these were approximately equally split between early-stage (27/60 [45%] ICIR Stage A or B) and advanced-stage (31/60 [52%] ICIR Stage C-D) cases (Table 1). Of the 27 patients with early-stage disease, 15 (56%) had Stage B disease due to documented involvement of the optic disc and/or fovea. Two tumors (3%) lacked adequate information to determine the stage. No Stage E tumors were treated with PRT.
Bilateral tumors were present in 41 of the 49 patients (84%). Eleven of 41 patients with bilateral disease underwent bilateral PRT. Enucleation of one eye prior to irradiation of the other eye was performed in 23 patients, and seven patients received focal therapy (laser photocoagulation or cryotherapy) alone to the non-irradiated eye.
Approximately half of patients (25/49, 51%) received chemotherapy in addition to PRT. Nearly all of these patients received upfront chemotherapy (22/25), and subsequently required PRT for progressive or recurrent disease. One patient received chemotherapy after PRT and two received chemotherapy both before and after PRT. A variety of chemotherapy regimens were used, with carboplatin, vincristine, and etoposide being the most common agents. Patients with Stage C-D tumors were significantly more likely to be treated with chemotherapy prior to PRT than patients with Stage A-B tumors (68% vs 30%; p=0.008).
A majority of the irradiated eyes (43/60, 72%) were also treated using focal techniques (laser photocoagulation and/or cryotherapy). Twenty-five of the 43 eyes underwent focal treatment prior to PRT (typically at the time of diagnosis or during chemotherapy), 12 were treated after PRT (due to concern for non-regressing tumor), and six were treated both before and after PRT.
The median prescribed radiation dose was 44 Gy(RBE), and the range was 40 to 46.8 Gy(RBE). Radiation was given in 1.8 to 2.0 Gy(RBE) daily fractions delivered four or five times per week. The median age at the start of radiation was 14 months (range, 27 days – 82 months), and the median time from diagnosis to the start of radiation was 83 days (range, 2 days – 62 months). In patients not receiving chemotherapy prior to radiation, the median time between diagnosis and the start of radiation was 21 days, compared to 7 months in patients undergoing chemotherapy prior to radiation (p<0.0001).
Tumor Control & Enucleation Free Survival
The median length of follow-up in this cohort was 8 years (range, 1 – 24 years) and the median patient age at the time of last follow-up was 9 years (range, 2 – 24 years) (Table 2). No patients died of retinoblastoma and none developed metastatic disease during follow-up. No patients developed an in-field second malignancy; however, one patient with hereditary disease developed an osteosarcoma of the femur 10 years after completion of radiation for retinoblastoma.[18]
Table 2.
Follow-Up Details
| Median length of follow-up (range) | 8 yrs (1 -24 yrs) |
| Median age at last follow-up (range) | 9 yrs (2 - 24 yrs) |
|
| |
| No. irradiated eyes enucleated | 11/60 (18%) |
|
| |
| Stage A-B | 3/11 |
| Stage C-D | 7/11 |
| Stage unknown | 1/11 |
|
| |
| Enucleation location | |
|
| |
| Our institution | 6 (55%) |
| Outside institution | 5 (45%) |
|
| |
| Median time elapsed between PRT and enucleation (range) | 10 mo (5 - 44 mo) |
|
| |
| Median time when enucleation at our institution | 20 mo |
| Median time elapsed when enucleation at outside institution | 7 mo |
|
| |
| Indication for Enucleation | |
|
| |
| Progressive disease | 8/11 |
| Ocular complications | 2/11 |
| Unknown | 1/11 |
|
| |
| Non-enucleative ocular complications requiring procedure | |
|
| |
| Cataracts | 4 |
| Radiation retinopathy | 3 |
| Glaucoma | 1 |
| Neovascularization/Hemorrhage | 1 |
| Other | 2 |
| Multiple |
1 |
| No. patients with metastatic disease | 0 |
| No. patients with second malignancy | 1 |
| No. patients with in-field second malignancy | 0 |
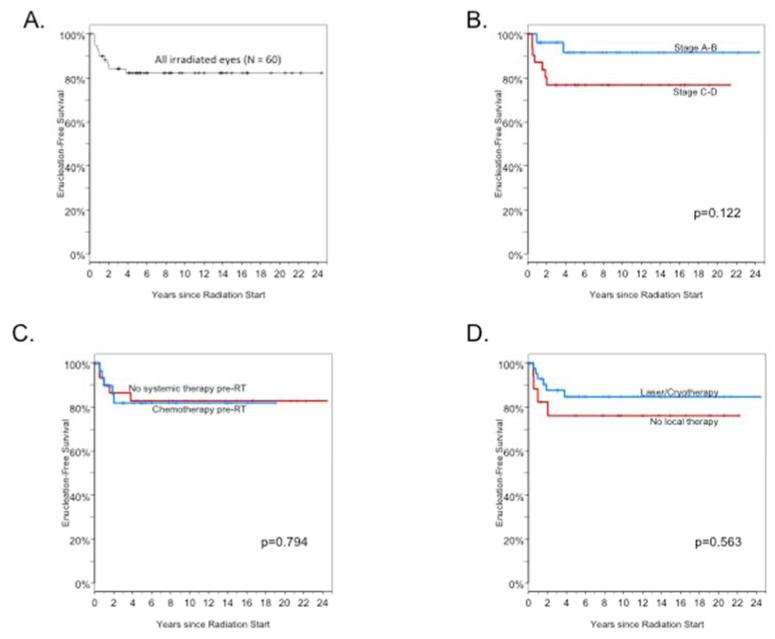
Eleven of 60 irradiated tumors (18%) ultimately required enucleation. Three of 27 Stage A-B tumors (11%) required enucleation following PRT, as did seven of 31 Stage C-D tumors (23%) (Table 2 and Figure 2A-B). All but one of enucleations took place within two years of completion of PRT, and no enucleations took place beyond four years. Five enucleations occurred among the 24 patients who did not receive chemotherapy, five occurred among 24 patients who received chemotherapy before (or before and after) PRT, and one enucleation occurred in the single patient who received chemotherapy after (but not before) PRT. There was no difference in enucleation-free survival among patients who received chemotherapy or focal therapy and those who did not (Figure 2C-D).
Figure 2.
Enucleation-free survival (EFS). A. EFS for all PRT-treated eyes. B. EFS stratified by ICIR tumor stage. C. EFS stratified by use of pre-PRT chemotherapy. D. EFS stratified by use of focal therapy.
The most common indication for enucleation was tumor progression (8/11), followed by non-tumor ocular complication (2/11). The indication and date for one enucleation was unknown. There was no difference in the median time interval from completion of PRT to enucleation for enucleations performed for progressive disease versus enucleations performed for non-tumor ocular complications (14 months vs 8 months, p=0.533). Radiation dose and overall treatment time were similar between enucleated and non-enucleated eyes.
Approximately half (6/11) of the enucleations were performed at our institution, and half (5/11) were performed at an outside institution. The median time from completion of PRT to enucleation was 10 months (range, 5 – 44 months), and there was a trend towards a longer median time interval between completion of PRT and enucleation when the enucleation was performed at our institution versus another institution (20 months vs 7 months; p=0.067).
Ocular Complications
Twelve of 60 irradiated eyes (20%) in 11 of the 49 patients (22%) developed a post-treatment ocular complication requiring procedural correction. All but one of the eyes requiring an intervention had a Stage C-D tumor at diagnosis. Two of the twelve eyes eventually required enucleation for persistent ocular complication. Cataracts were the most common complication (4/12), followed by radiation retinopathy (3/12), glaucoma (1/12), neovascularization (1/12), other complications (2/12), and one eye with multiple complications (1/12). The complications eventually requiring enucleation were multiple (1 case) and neovascularization with hemorrhage (1 case).
Visual Outcomes
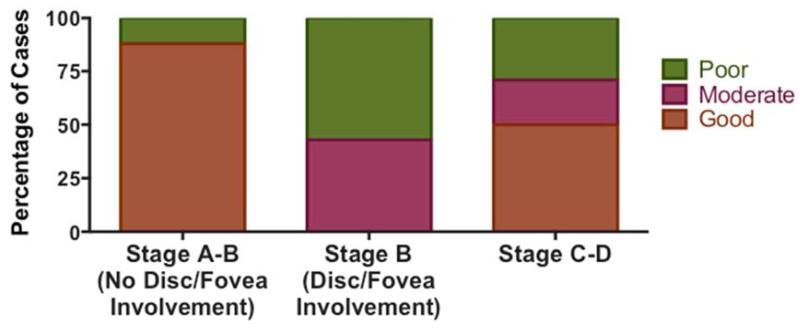
Formal ophthalmologic follow-up information was available for 61% (30/49) of non-enucleated irradiated eyes. Visual outcomes were characterized as good (20/40 visual acuity or better in treated eye), moderate (worse than 20/40 but better than 20/600), or poor (worse than 20/600) based on the most recent available ophthalmologic evaluation (Figure 3). Fourteen of 30 evaluated eyes (47%) had 20/40 visual acuity or better following PRT, including 7 of 15 eyes (47%) with Stage A-B disease and 7 of 14 eyes (50%) with Stage C-D disease. Seven of 30 treated eyes (23%) had moderate visual acuity (3 of 15 Stage A-B eyes [20%]; 3 of 14 Stage C-D eyes [21%]; one eye with unknown stage), and 9 of 30 treated eyes (30%) had poor visual acuity (5 of 15 Stage A-B eyes [33%] and 4 of 14 Stage C-D eyes [29%]). The subset of eyes with Stage B disease due to involvement of the optic disc or fovea had worse outcomes than other Stage A-B tumors (p=0.005) (Figure 3). Of seven eyes with optic disc or fovea involvement at diagnosis, there were three moderate and four poor visual acuity outcomes, suggesting that tumor location at diagnosis impacts visual outcomes following PRT. There did not appear to be differences in visual outcomes between patients who received chemotherapy and those who did not (p=0.289), or between eyes that received pre-PRT focal therapy and those that did not (p=0.287).
Figure 3.
Visual Outcomes. PRT-treated eyes with formal ophthalmologic evaluation were grouped by visual acuity into good (20/40 or better), moderate (20/40 to 20/600), or poor (worse than 20/600) outcome categories. The percentage of eyes in each category are shown for Stage A-B tumors without optic disc or fovea involvement, Stage B tumors with optic disc and/or fovea involvement, and Stage C-D tumors.
Cosmetic outcomes were often reported by providers at follow-up, and one third of patients (16 of 49, 33%) (including 3 patients with 2 outcomes each) had documented physical exam findings likely attributable to PRT, including orbital hypoplasia (15/49), hyperpigmentation (3/49), or soft tissue fibrosis over the treatment portal (1/49). Due to lack of standardized reporting, actual rates of cosmetic side effects of PRT may be higher; however, no procedures to address PRT-related cosmetic issues were recorded in this cohort.
DISCUSSION
Here, we report long-term outcomes of retinoblastoma patients treated with PRT. To our knowledge, this represents the largest and most mature experience available for this patient population.
For nearly a century, photon-based radiotherapy was the standard of care for retinoblastoma patients and was associated with high rates of disease control and vision preservation.[4, 5, 7-9, 20] However, patients remained at significant risk for toxicities including growth abnormalities and radiation-induced malignancies resulting from collateral irradiation of adjacent normal tissues.[7, 18, 21] In the past two decades, chemotherapy and focal treatments such as laser photocoagulation, cryotherapy, and episcleral brachytherapy have largely replaced EBRT in upfront treatment.[15] The cohort studied here represents patients from both eras: approximately one half of patients received PRT as primary treatment, whereas half (typically with more advanced disease) often received chemotherapy and/or focal treatments prior to PRT.
Among patients with advanced tumors who received PRT, disease control and eye preservation rates were quite high. Importantly, no patients in this cohort died of retinoblastoma or developed metastatic disease. Despite many of these patients receiving multiple treatments prior to PRT, approximately 75% of Stage C-D patients were able to preserve the treated eye. In approximately half of the preserved eyes, good visual outcomes (20/40 visual acuity or better) were achieved following PRT, and another 21% of patients had moderate visual acuity (20/40 to 20/600) following treatment. Patients with more advanced tumors did have higher rates of ocular complications than early-stage patients, with all but one of the noted complications occurring in Stage C-D patients. However, given that the majority of these patients had exhausted other treatment options, PRT appears to represent a safe alternative to enucleation and allows the majority of appropriately selected patients to be cured of their disease and preserve useful vision.
Approximately half of the tumors in this cohort (27/60, 45%) were early-stage (ICIR Stage A-B), and almost half of these patients (11/27, 41%) received PRT in the upfront setting. Eye preservation rates were very high and complication rates very low in this setting. Only 11% of Stage A-B tumors were not controlled with PRT and eventually required enucleation of the affected eye. Similarly, complications were uncommon, with only one preserved eye requiring a procedure following PRT. Given the favorable outcomes and minimal observed toxicity, PRT may be an appropriate upfront treatment for carefully selected early-stage tumors. However, because patients incur a lifetime elevated risk of radiation-induced malignancies, long term follow-up is necessary.
Interesting, there appeared to be two distinct subgroups with the early-stage patients with respect to visual acuity outcomes. Most patients with Stage A-B tumors that did not involve the optic disc or fovea had good visual acuity outcomes, whereas patients who had Stage B tumors with EUA-documented optic disc or fovea involvement had significantly worse outcomes (Figure 3). Due to the young age at presentation, none of the patients in this cohort had pre-treatment visual acuity testing. Therefore, it is not possible to determine the relative contribution of the tumor versus the treatment towards long-term visual outcomes. However, the relatively poor outcomes among patients with Stage B disease with optic disc/fovea involvement, even relative to patients with Stage C-D disease (which includes large tumors and presence of vitreous seeding), suggest that tumor location may play a significant role in determining post-treatment visual acuity. Achieving good visual acuity outcomes has proven to be difficult for tumors involving the optic disc or fovea whenever local therapy (cryotherapy, laser photocoagulation, or photon- or proton-based RT) is necessary; therefore, continued focus on developing new therapies remains an important goal for the field.
Given the relative rarity of retinoblastoma, newly-diagnosed patients are often referred to specialized centers for work-up and treatment. With the high success rates of modern therapies, the majority of patients can be cured of their disease. However, patients are often not able to return for routine follow-up at the treating center, and are instead followed locally. Practitioners unfamiliar with either the disease or expectant post-PRT treatment response of tumor stabilization with slow regression may find it difficult to distinguish PRT treatment effect versus tumor progression. Careful monitoring with serial EUAs is often required to discern between active and regressing tumor. We note a trend towards shortened time from end of PRT to enucleation for enucleations performed at outside institutions compared to enucleations performed at our institution and speculate that some of the enucleated cases at outside institutions may represent slowly responding tumors rather than true failures. Pathology was not available for these cases; however, even if available, responding tumor may not appear histologically different from viable disease despite its incapacity to proliferate.
Due to the unique physical properties of the proton beam, PRT represents the most conformal external beam radiation treatment option currently available for retinoblastoma. For the majority of early-stage cases in this cohort, the target was limited to the tumor(s) plus a small margin of 5-8 mm, thus sparing dose to uninvolved portions of the anterior retina, cornea, and lens. For more advanced tumors with seeding, the entire vitreous was treated in order to ensure full coverage of the involved areas. A single field was adequate to cover either of these treatment volumes, thus limiting normal tissue exposure to a small region of low-intermediate dose between the skin surface and the globe (Figure 1). Unlike photon-based RT, radiation to other facial structures and the CNS can be eliminated or markedly reduced with PRT. However, due to the entrance dose of the proton beam through the lateral or anterior oblique treatment portal, orbital hypoplasia and skin changes can occur, and were noted in a significant percentage of this cohort. Prospective studies are warranted to systematically characterize the long-terms effect of PRT on toxicity and quality-of-life.
In summary, long-term disease control can often be achieved with PRT in both early-stage and advanced tumors. Useful vision was preserved in the majority of cases; however, visual outcomes appear to depend upon the extent of tumor involvement of the optic disc and fovea at diagnosis. Ocular toxicities were more common among advanced tumors, but overall rates were low. Importantly, no radiation-associated malignancies were noted in this cohort.
SUMMARY.
Radiotherapy (RT) can be used in the primary or salvage treatment of retinoblastoma and has been associated with high cure rates but also with risk of radiation-related complications. Here, we present long-term follow-up for a large cohort of retinoblastoma patients treated with proton RT. Despite many patients having advanced disease, tumor control rates are high and functional vision is preserved in the majority of patients. Ocular toxicity was limited, and no radiation-associated malignancies were observed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this work were presented at the 55th Annual Meeting of the American Society for Radiation Oncology (ASTRO), Atlanta, GA, Sept. 22-25th, 2013.
Conflicts of interest:
H Shih is a senior editor of the IJROBP.
REFERENCES
- 1.U.S. Cancer Statistics Working Group United States Cancer Statistics: 1999-2009 Incidence and Mortality Web-based Report. 2013 Available from: http://www.cdc.gov/uscs.
- 2.Ries L, Smith M, Gurney J, et al., editors. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975-1995. National Institute of Health; Bethesda (MD): 1999. [Google Scholar]
- 3.Sachdeva UM, O’Brien JM. Understanding pRb: toward the necessary development of targeted treatments for retinoblastoma. J Clin Invest. 2012;122:425–34. doi: 10.1172/JCI57114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassady JR, Sagerman RH, Tretter P, et al. Radiation therapy in retinoblastoma. An analysis of 230 cases. Radiology. 1969;93:405–9. doi: 10.1148/93.2.405. [DOI] [PubMed] [Google Scholar]
- 5.Blach LE, McCormick B, Abramson DH. External beam radiation therapy and retinoblastoma: long-term results in the comparison of two techniques. Int J Radiat Oncol Biol Phys. 1996;35:45–51. doi: 10.1016/s0360-3016(96)85010-3. [DOI] [PubMed] [Google Scholar]
- 6.Hilgartner H. Report of a case of double glioma treated by x-rays. Tex Med J. 1903;18 [PMC free article] [PubMed] [Google Scholar]
- 7.Abramson DH, Beaverson KL, Chang ST, et al. Outcome following initial external beam radiotherapy in patients with Reese-Ellsworth group Vb retinoblastoma. Arch Ophthalmol. 2004;122:1316–23. doi: 10.1001/archopht.122.9.1316. [DOI] [PubMed] [Google Scholar]
- 8.Merchant TE, Gould CJ, Hilton NE, et al. Ocular preservation after 36 Gy external beam radiation therapy for retinoblastoma. J Pediatr Hematol Oncol. 2002;24:246–9. doi: 10.1097/00043426-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Schipper J, Tan KE, van Peperzeel HA. Treatment of retinoblastoma by precision megavoltage radiation therapy. Radiother Oncol. 1985;3:117–32. doi: 10.1016/s0167-8140(85)80016-5. [DOI] [PubMed] [Google Scholar]
- 10.Jairam V, Roberts KB, Yu JB. Historical trends in the use of radiation therapy for pediatric cancers: 1973-2008. Int J Radiat Oncol Biol Phys. 2013;85:e151–5. doi: 10.1016/j.ijrobp.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marees T, van Leeuwen FE, de Boer MR, et al. Cancer mortality in long-term survivors of retinoblastoma. Eur J Cancer. 2009;45:3245–53. doi: 10.1016/j.ejca.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Yu CL, Tucker MA, Abramson DH, et al. Cause-specific mortality in long-term survivors of retinoblastoma. J Natl Cancer Inst. 2009;101:581–91. doi: 10.1093/jnci/djp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong FL, Boice JD, Jr., Abramson DH, et al. Cancer incidence after retinoblastoma. Radiation dose and sarcoma risk. JAMA. 1997;278:1262–7. doi: 10.1001/jama.278.15.1262. [DOI] [PubMed] [Google Scholar]
- 14.Shields CL, Honavar SG, Meadows AT, et al. Chemoreduction plus focal therapy for retinoblastoma: factors predictive of need for treatment with external beam radiotherapy or enucleation. Am J Ophthalmol. 2002;133:657–64. doi: 10.1016/s0002-9394(02)01348-x. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez-Galindo C, Chantada GL, Haik BG, et al. Treatment of retinoblastoma: current status and future perspectives. Curr Treat Options Neurol. 2007;9:294–307. doi: 10.1007/s11940-007-0015-4. [DOI] [PubMed] [Google Scholar]
- 16.Krengli M, Hug EB, Adams JA, et al. Proton radiation therapy for retinoblastoma: comparison of various intraocular tumor locations and beam arrangements. Int J Radiat Oncol Biol Phys. 2005;61:583–93. doi: 10.1016/j.ijrobp.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Lee CT, Bilton SD, Famiglietti RM, et al. Treatment planning with protons for pediatric retinoblastoma, medulloblastoma, and pelvic sarcoma: how do protons compare with other conformal techniques? Int J Radiat Oncol Biol Phys. 2005;63:362–72. doi: 10.1016/j.ijrobp.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 18.Sethi RV, Shih HA, Yeap BY, et al. Second nonocular tumors among survivors of retinoblastoma treated with contemporary photon and proton radiotherapy. Cancer. 2014;120:126–33. doi: 10.1002/cncr.28387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linn Murphree A. Intraocular retinoblastoma: the case for a new group classification. Ophthalmol Clin North Am. 2005;18:41–53. doi: 10.1016/j.ohc.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Pradhan DG, Sandridge AL, Mullaney P, et al. Radiation therapy for retinoblastoma: a retrospective review of 120 patients. Int J Radiat Oncol Biol Phys. 1997;39:3–13. doi: 10.1016/s0360-3016(97)00156-9. [DOI] [PubMed] [Google Scholar]
- 21.Kleinerman RA, Yu CL, Little MP, et al. Variation of second cancer risk by family history of retinoblastoma among long-term survivors. J Clin Oncol. 2012;30:950–7. doi: 10.1200/JCO.2011.37.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]