Ocriplasmin (Jetrea, Thrombogenics, Leuvin, Belgium) was recently FDA-approved for the treatment of symptomatic vitreomacular traction (VMT). The MIVI-TRUST phase 3 randomized control trials revealed that intravitreal ocriplasmin had superior efficacy for VMT resolution compared to placebo vehicle injection.1 Qualitative identification of outer retinal changes following ocriplasmin injection that were not previously recognized in the MIVI-TRUST trials have recently been described.2, 3, 4 In those reports, the outer segment ellipsoid zone (EZ), also referred to as inner segment/outer segment (IS/OS) junction, appeared to be most impacted. Another retinal alteration that has been described following ocriplasmin injection is increased subretinal fluid (SRF).2 The purpose of this study was to quantitatively evaluate retinal structural changes with spectral domain OCT (SD-OCT) following ocriplasmin therapy.
This was an IRB-approved retrospective consecutive case series and adhered to the tenets of the Declaration of Helsinki. Inclusion criteria included eyes that received intravitreal ocriplasmin and SD-OCT imaging at baseline and at least 1 week following injection. Exclusion criteria included poor quality SD-OCT and concurrent macular disease that could affect visual acuity (VA) and/or retinal architecture. All eyes received intravitreal ocriplasmin (125 µg). The SD-OCT examinations were performed with a Cirrus HD-OCT (Cirrus V.6.1 software, Carl Zeiss Meditec, Dublin, CA) at baseline and at post-injection time points.
Inner, middle and outer retinal thicknesses 1.2 mm nasal, temporal, superior, and inferior to the fovea were calculated as previously described.5 Additionally, the distance between the EZ to retinal pigmentary epithelium (EZ-RPE height) was quantified. All measurements were performed in a masked fashion. For SRF volumetric analysis, a custom OCT software analysis program was utilized to manually segment the SRF volume. To compare two groups, Mann-Whitney U tests were used with nonparametric distribution data. Multivariate analysis was used to investigate the relationships between retinal architectural alterations and VA. Spearman’s rank correlation was also performed, as appropriate. A p-value of < 0.05 was considered to be significant.
Nineteen eyes of 19 patients were identified that met the inclusion/exclusion criteria for this study. Mean age was 69.6 years (59–85) with 5 men (26%) and 14 women (74%). Twelve (63%) eyes were phakic and 7 (37%) eyes were pseudophakic. The mean visual acuity was 20/40 at baseline, 20/43 at 1 week post-injection, 20/38 at 1 month, and 20/32 at 3 months. Three months after injection, VMT release following ocriplasmin injection was observed in 9 of 19 (47%) eyes.
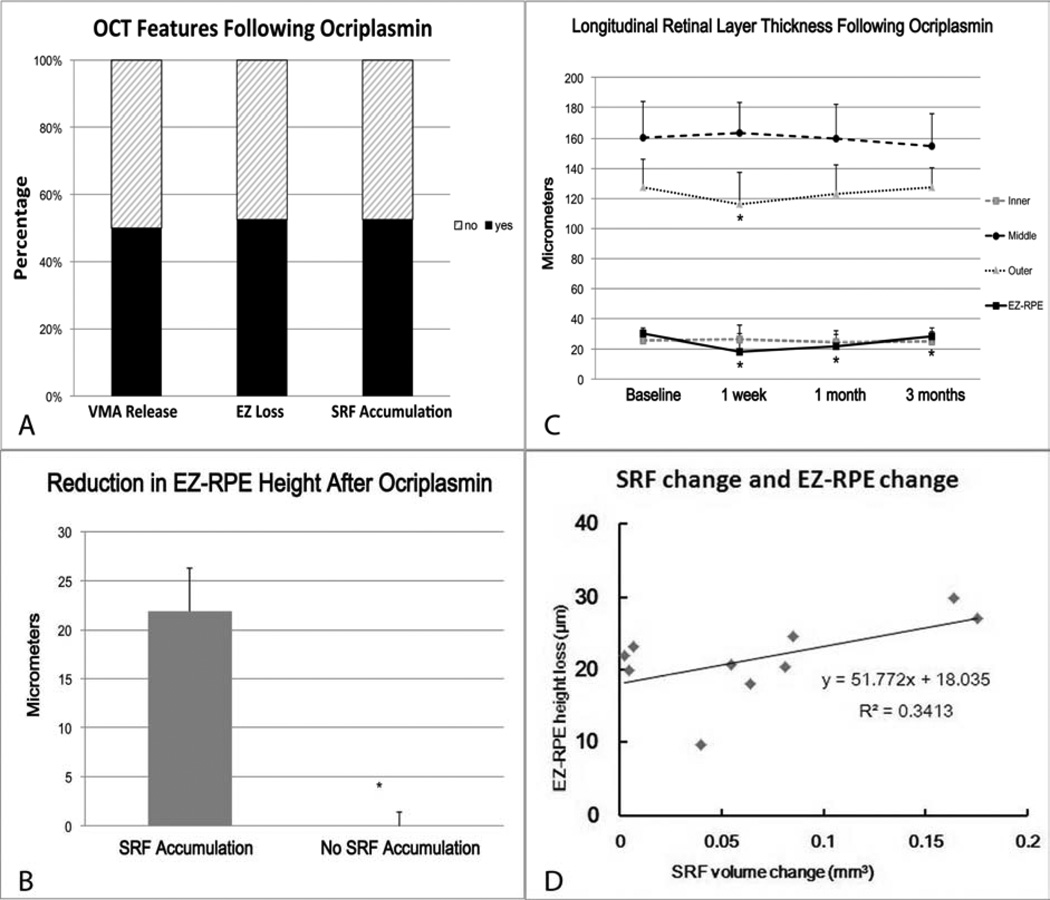
On SD-OCT analysis, 10 of 19 (53 %) eyes exhibited transient EZ loss [Figures 1 and 2 (available at http://aaojournal.org)]. Inner and middle retinal thicknesses were unchanged following ocriplasmin therapy. Mean outer retinal thickness significantly decreased at 1 week (p = 0.00029), but gradually recovered at 1 month (p = 0.09) and 3 months (p = 0.91) following injection. The mean EZ-RPE height at baseline was significantly reduced at 1 week, at 1 month and at 3 months (p = 0.0001, < 0.0001, 0.00099, respectively) following injection (Figure 1).
Figure 1.
(A) Features identified on OCT 1 week following intravitreal ocriplasmin, including vitreomacular traction release, ellipsoid zone (EZ) loss and subretinal fluid (SRF) accumulation. (B) Bar graph comparing value of EZ-retinal pigment epithelium (RPE) height reduction and subretinal fluid (SRF) accumulation revealing that the EZ-RPE reduction was significantly larger when compared to the cases without SRF accumulation (P = 0.00024). (C) Longitudinal retinal layer thickness prior to and following intravitreal ocriplasmin. A significant reduction is seen in outer retinal thickness at 1 week and in the EZ-RPE height at all time points [* P < 0.01 versus baseline (Mann-Whitney U test)]. Error bars indicate the standard deviation. (D) Scatter plot shows the relationship between the EZ-RPE height loss and SRF volume.
Retinal thickness assessments based on VMT release and SRF accumulation were also performed. One week after injection, EZ-RPE height was reduced in eyes with VMT release (14.9 ± 9.1µm compared to baseline) and without VMT release (8.9 ± 13.2 µm). One week following injection, the EZ-RPE height was reduced in eyes with increased SRF (21.9 ± 4.5 µm) but not in eyes without increased SRF (−0.52 ± 2.0 µm) and this was significantly different (p = 0.00024), Figure 1 and Table 1 (available at http://aaojournal.org). The amount of decreased EZ-RPE height was strongly correlated to accumulation of SRF accumulation (p = 0.00021, correlation coefficient = 0.88), Figure 1.
As with any retrospective analysis, there are limitations to this study. The follow-up period is relatively short and the sample size small. Due to the retrospective nature, standardized follow-up could not be achieved. This study was also not controlled and did not have a comparison group, such as surgery, placebo injection, or pneumatic vitreolysis. Functional analysis within this study did not include potential important diagnostic testing, including ERG and microperimetry.
Recent case reports following ocriplasmin suggests similar acute panretinal dysfunction in rare cases. Outer retinal changes on SD-OCT and significant reduction in ERG amplitudes were present.3, 4 One report suggests that these changes may reflect degradation of laminin resulting in panretinal dysfunction secondary to ocriplasmin.4 In our study, 10 of 19 eyes showed decreased length between the EZ and RPE (EZ-RPE height) one week after ocriplasmin injection. All of these cases also exhibited increased SRF. This EZ-RPE height loss gradually recovered with time. Three months following injection, outer retinal thickness recovered to baseline.
The origin of the increased SRF remains unclear. Potential hypotheses include alterations from vitreoretinal traction, transient breakdown of the outer blood-retinal barrier, inherent RPE dysfunction, and accumulation of photoreceptor outer segments. All cases that showed reduction in EZ-RPE height also showed an increase in volume of the SRF compared to baseline. There was a strong correlation in the change in EZ-RPE height and increase in SRF. This may support that the SRF origin is related to the degradation of photoreceptor outer segments. Although the hyporeflective nature of the SRF seen in our cases does not correspond with the typical OCT reflectivity of outer segments, in the presence of potential enzymatic changes it is unclear what characteristics outer segments would have on OCT. In addition, the the loss of standard directional orientation of the outer segments would change the OCT appearance. It is important to recognize that although the EZ band appears to rapidly reappear on the OCT qualitatively within a few weeks, it takes significantly longer for the baseline EZ-RPE height to be restored to preinjection levels. This may reflect the slow reaccumulation of the outer segments.
Based on our review of the literature, we believe this represents the first quantitative assessment of the retinal alterations associated with ocriplasmin therapy. Our study suggests that the outer retinal changes and subretinal fluid accumulation appear to be common findings following intravitreal ocriplasmin injection and potentially interrelated. Retinal alterations appear to nearly normalize by 3 months, but the long-term implications of these findings need further prospective research.
Supplementary Material
Acknowledgments
NIH/NEI K23-EY022947-01A1 (JPE); Ohio Department of Development TECH-13-059 (JPE, SKS); Research to Prevent Blindness (PKK)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
YI: None; PKK: Thrombogenics (C), Alcon (C), Novartis (C), Allegro (C); RPS: Thrombogenics (C), Alcon (C); SKS: Bausch and Lomb (C), Allergan (R), Bioptigen (P), Leica (C), Synergetics (P); JPE: Bioptigen (P), Synergetics (P), Leica (C), Zeiss (C), Thrombogenics (C, S); Regeneron (S)
References
- 1.Stalmans P, Benz MS, Gandorfer A, et al. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N Engl J Med. 2012 Aug 16;367(7):606–615. doi: 10.1056/NEJMoa1110823. [DOI] [PubMed] [Google Scholar]
- 2.Singh RP, Li A, Bedi R, Srivastava S, et al. Anatomical and visual outcomes following ocriplasmin treatment for symptomatic vitreomacular traction syndrome. Br J Ophthalmol. 2014 Mar;98(3):356–360. doi: 10.1136/bjophthalmol-2013-304219. [DOI] [PubMed] [Google Scholar]
- 3.Freund KB, Shah SA, Shah VP. Correlation of transient vision loss with outer retinal disruption following intravitreal ocriplasmin. Eye (Lond) 2013 Jun;27(6):773–774. doi: 10.1038/eye.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fahim AT, Khan NW, Johnson MW. Acute Panretinal Structural and Functional Abnormalities After Intravitreous Ocriplasmin Injection. JAMA Ophthalmol. 2014 Feb;132(4):484–486. doi: 10.1001/jamaophthalmol.2013.8142. [DOI] [PubMed] [Google Scholar]
- 5.Hibi N, Ueno S, Ito Y, et al. Relationship between retinal layer thickness and focal macular electroretinogram components after epiretinal membrane surgery. Invest Ophthalmol Vis Sci. 2013 Nov 1;54(12):7207–7214. doi: 10.1167/iovs.13-12884. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.