Abstract
BACKGROUND
Modulation of the autonomic nervous system has been used to treat refractory ventricular tachycardia (VT). Renal artery denervation (RDN) is under investigation for the treatment of sympathetic-driven cardiovascular diseases.
OBJECTIVE
The purpose of this study was to report the largest case series to date using RDN as adjunctive therapy for refractory VT in patients with underlying cardiomyopathy.
METHODS
Four patients with cardiomyopathy (2 nonischemic, 2 ischemic) with recurrent VT despite maximized antiarrhythmic therapy and prior endocardial (n = 2) or endocardial/epicardial (n = 2) ablation underwent RDN ± repeat VT ablation. RDN was performed spirally along each main renal artery with either a nonirrigated (6 W at 501C for 60 seconds) or an open irrigated ablation catheter (10–12 W for 30–60 seconds). Renal arteriography was performed before and after RDN.
RESULTS
RDN was well tolerated acutely and demonstrated no clinically significant complications during follow-up of 8.8 ± 2.6 months (range 5.0–11.0 months). No hemodynamic deterioration or worsening of renal function was observed. The number of VT episodes was decreased from 11.0 ± 4.2 (5.0–14.0) during the month before ablation to 0.3 ± 0.1 (0.2–0.4) per month after ablation. All VT episodes occurred in the first 4 months after ablation (2.6 ± 1.5 months). The responses to RDN were similar for ischemic and nonischemic patients.
CONCLUSION
This case series provides promising preliminary data on the safety and effectiveness of RDN as an adjunctive therapy in the treatment of patients with cardiomyopathy and VT resistant to standard interventions.
Keywords: Renal denervation, Ventricular tachycardia, Cardiomyopathy, Ventricular tachycardia storm
Introduction
The demonstrated effectiveness of implantable cardioverter-defibrillators (ICDs) for primary and secondary preventions of sudden cardiac death has resulted in an increasing number of patients presenting with recurrent, appropriate ICD shocks for ventricular tachycardia (VT).1-3 Because of the frequently insufficient success of pharmacologic therapy, catheter-based VT ablation is commonly used in these patients but is associated with limited long-term efficacy and significant complications.4 The multicenter ThermoCool VT Ablation Trial reported recurrence of VT in 47% of patients at 6 months and a periprocedural complication rate of 7.3%. Therefore, adjunctive treatment approaches are desirable in this patient population.5
Given the established interaction of ventricular arrhythmias and the autonomic nervous system,6 cardiac sympathetic denervation using left stellate gangliectomy has been tested successfully in patients with long QT syndrome,7 catechola-minergic polymorphic VT,8 and cardiomyopathy and refractory ventricular arrhythmias.9
Renal artery sympathetic denervation (RDN) recently has emerged as a less invasive means for modulating the autonomic nervous system. Endovascular catheter-based ablation of the renal arteries is emerging as a possibly more direct, organ-specific therapeutic strategy. Preclinical swine studies10 and subsequent human studies11,12 have demon-strated catheter-based RDN to be an effective treatment in patients with resistant hypertension, with an excellent safety profile. Consequently, catheter-based RDN is currently being evaluated as a potential adjunctive therapy in a spectrum of sympathetically modulated cardiovascular diseases, including impaired glucose metabolism,13 left ventricular (LV) hypertrophy and diastolic dysfunction,14 congestive heart failure,15 obstructive sleep apnea,16 and atrial fibrillation (AF).17 Importantly, catheter-based RDN recently has been described as a possible treatment strategy in patients with chronic heart failure and recurrent ventricular arrhythmias.18
Here we report the largest case series to date of catheter-based RDN as an adjunctive therapy in patients with refractory VT in the setting of underlying cardiomyopathy.
Methods and Results
Four patients with cardiomyopathy (2 nonischemic, 2 ischemic) and VT refractory to therapy were recruited from 3 contributing centers. All patients had not responded to antiarrhythmic therapy and had undergone either endocardial catheter ablation (n = 2) or both endocardial/epicardial catheter ablation (n = 2). Given that no U.S. Food and Drug Administration (FDA) approval for RDN was available outside of clinical trials, detailed informed consent was obtained from all patients. In preparation for RDN, extensive discussions were conducted with all patients and/or the patients’ family regarding compassionate off-label use of an FDA-approved product/institutional review board (IRB) consultation/emergency hospital credentialing and/or inclusion of operators with previous experience in RDN. RDN was performed with the patient under general anesthesia, with an end-point of delivery of circumferential lesions from first bifurcation to the os of the renal artery as determined by the operator.
Patient 1
The patient was a 68-year-old obese man with a history of hypertension and AF who presented after an episode of slow VT (left bundle [LB] pattern, left superior [LS] axis with a cycle length [CL] of 495 ms) during anesthesia induction for elective prostate surgery. Transthoracic echocardiography demonstrated mild global hypokinesis with a left ventricular ejection fraction (LVEF) of 45% to 50%. Cardiac catheterization revealed nonobstructive coronary artery disease. Cardiac magnetic resonance imaging (MRI) was significant for an inferobasal septal and focal inferior midseptal scar. Despite maximal medical therapy with amiodarone, lidocaine, procainamide, and esmolol, the patient continued to have frequent recurrences of clinical VT requiring multiple cardioversions for hemodynamic instability.
Two distinct VT morphologies were inducible during a first VT ablation, originating from either the inferior mid-right ventricular (RV) septum (LBLS axis, CL 457 ms, correlating with the clinical VT), or the distal RV apex (LBLS axis, CL 255 ms), correlating with scar seen on MRI. After endocardial ablation targeting best pace-mapping sites, VT was noninducible with up to 3 ventricular extrastimuli.
Two days postablation, 3 previously unobserved VTs occurred spontaneously despite medical therapy with pro-cainamide and amiodarone (LBLS axis, CL 510 ms; LB VT with alternating left superior/inferior axis, CL 460 ms; LB, right superior [RS] axis, CL 290 ms; right bundle [RB] pattern, left inferior [LI] axis, CL 280 ms). Six days after the original procedure, repeat VT ablation only induced 3 previously unobserved VT morphologies (all LBLI axis, CL 330/325/280 ms) originating from midseptal MRI scar. Despite extensive LV and RV endocardial ablation of the septal substrate, VT remained inducible. Given the residual inducibility of VT, bilateral RDN was performed during the same procedure as previously discussed with the patient.
Bilateral renal arteriography was performed through an 8Fr sheath. A 7Fr, 4–mm nonirrigated ablation catheter (Blazer II, Boston Scientific, Natick, MA) was advanced into each renal artery, and delivery of radiofrequency (RF) energy was performed at sequential sites at 6 W and 501C for 60 seconds. No acute or delayed hemodynamic sequelae were seen (pre-RDN blood pressure [BP] 102–106/52–54 mm Hg, heart rate [HR] 60–70 bpm; acute post-RDN BP 103–120/50–56 mm Hg, HR 60–70 bpm; 2–hour post-RDN BP 109– 120/52–59 mm Hg, HR 60–70 bpm, 24–hour post-RDN 110/58 mm Hg, HR 92 bpm). Three days post-RDN, MR angiography of the renal arteries showed no vascular stenosis or dissection. Three days post-RDN, the patient developed slow VT at 110 bpm, which was treated with metoprolol. An ICD was placed for secondary prevention. Sixteen days post-RDN, the patient received antitachycardia pacing (ATP) for VT (CL 285 ms). Additional ATP was programmed, but given the prevalent pacing requirement, he was upgraded to a Bi-V ICD system using the new ICD settings. Medical therapy with beta-blockade and amiodarone was continued. After these cumulative interventions, the patient has received no other device therapies at 10 months post-RDN (Figure 1 and Table 1). Renal function has remained stable over 10–month follow-up (glomerular filtration rate 460, creatinine 0.8–1.0).
Figure 1.
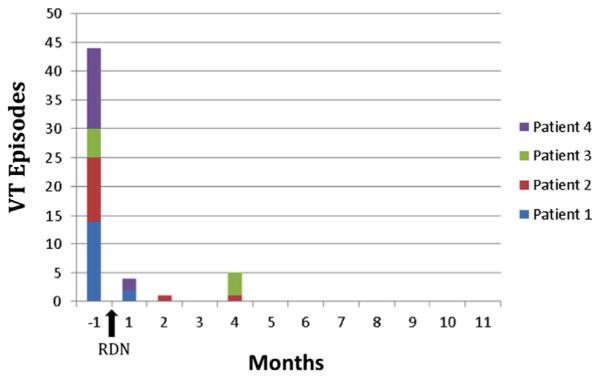
Cumulative ventricular tachycardia (VT) episodes before and after renal denervation (RDN).
Table 1.
Characteristics of patients included in the study
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Age (years) | 68 | 83 | 63 | 60 |
| LEVF (%) | 45–50 | 30–35 | 30 | 15–20 |
| Etiology of Cardiomyopathy |
Nonischemic | Nonischemic | Ischemic | Ischemic |
| Ablation location | Endocardial ×2 | Endocardial ×1 |
Endocardial ×1, Epicardial ×1 |
Endocardial ×2 |
| Timimg of RDN relative to VT ablation |
Simultaneous to ablation no. 2 | 4 weeks | postablation | 1 year postepicardial ablation |
| Simultaneous to ablation no. 2 |
||||
| ICD shocks in the month Pre-RDN |
14 | 11 | 5 | 14 |
| ICD shocks post- RDN |
2 | 2 | 4 | 2 |
| post-RDN Follow-up Months |
10 | 9 | 11 | 14 |
| Pre-Hospitalization anti Arrhythmic Agents |
Metoprolol ER 100 mg P0 daily | Sotalol 80 mg Po bid |
Amiodarone 200 mg Po bid |
Amiodarone 200 mg P0 daily Cervedilol 25 mg Po bid |
| Hospitalization antiarrhythmic agent |
Lidocaine gtt Esmolol gtt Amiodarone gtt (~2 g total) Procainamide gtt Amiodarone 200 mg P0 daily Metoprolol ER 150 mg P0 daily Mexifetine 150 mg P0 tid |
Sotalol 80 mg P0 bid |
Amiodarone 200 mg P0 bid |
Amiodarone gtt (~1 g total) Amiodarone 400 mg P0 daily Metoprolol ER 200 mg P0 daily |
| Posthospitalization antiarrhythmic agents |
Amiodarone 200 mg P0 daily Metoprolol ER 100 mg P0 daily Mexiletine 150 mg P0 tid |
Sotalol 80 mg P0 bid |
Amiodarone 200 mg P0 bid |
Amiodarone 200 mg P0 bid Carvedilol 25 mg P0 bid Metoprolol ER 200 mg P0 daily |
ICD = implantable cardioverter defibrillator; LVEF = left ventricular ejection fraction; RDN = renal artery denervation; VT = ventricular tachycardia.
Patient 2
The patient was an 83-year-old man with a history of paroxysmal AF, nonischemic cardiomyopathy (LVEF 30%–35%), and previous ICD placement. Seven years post-ICD placement, he developed episodes of VT that were treated with ICD shocks and ATP. He was admitted with a slow VT below the rate cutoff of his device (LBRI axis, CL 476 ms). Endocardial mapping revealed a small area of scar at the anterior basal portion of the LV. Pace-mapping localized the VT focus adjacent to the anterior mitral annulus. Ablation rendered the VT noninducible, and sotalol 80 mg twice daily was initiated.
The patient was discharged but was readmitted 8 days postablation with recurrent VT and ICD shocks (LBLI axis, CL 420 ms). During repeat ablation, epicardial mapping demonstrated dense scar below the mitral valve annulus on the anterior wall extending from base to apex. Highly fractionated electrograms (EGMs) were seen at the base opposite of the previous endocardial ablation site. Coronary angiography revealed the ablation catheter to be adjacent to the left anterior descending (LAD)/first diagonal artery branch, and RF ablation was deemed unsafe because of high risk of coronary artery injury.
The patient experienced do further events over the next few weeks, and he was readmitted for RDN. Aortography and selective renal arteriography were performed. The right and left renal arteries each measured approximately 6 mm in diameter. Using an open irrigated ablation catheter (ThermoCool, Biosense Webster, Diamond Bar, CA) up to 12 W for 60 seconds at a flow rate of 17 cc/min, longitudinal and spiral lesions were delivered to the right (n = 4 lesions) and left (n = 5 lesions) renal artery from the bifurcation to the ostium. The final arteriogram revealed minimal irregularities in both renal arteries and a questionable small non–flow-limiting dissection in the right mid-renal artery without need for further intervention. No acute or delayed hemodynamic sequelae were seen (pre-RDN BP 150–170/60–70 mm Hg, HR 60–70 bpm; acute post-RDN BP 150–170/40–70 mm Hg, HR 55 bpm; 24-hour post-RDN 129/64 mm Hg, HR 55 bpm; 9-month post-RDN 118/82 mm Hg, HR 61 bpm). Renal function has remained stable over 6-month follow-up (creatinine 1.2–1.4). He had 2 isolated VT episodes, each treated with ICD therapy at 2 and 4 months post-RDN despite unchanged oral sotalol therapy. He has experienced no other VT episodes at 9 months post-RDN.
Patient 3
The patient was a 63-year-old woman with a history of ischemic cardiomyopathy (LVEF 30%), previous coronary artery bypass grafting surgery, and ICD placement, who eventually underwent destination left ventricular assist device (LVAD) placement. The postoperative course was notable for incessant VT (RBLI axis, CL 490 ms) for which endocardial VT ablation was performed but was unsuccessful. A second ablation attempt was undertaken using open surgical access to evaluate the epicardial surface. The patient’s clinical VT was found to originate on the epicardium and was successfully ablated. After the ablation, the patient was ICD therapy-free for approximately 1 year.
One year later, the patient re-presented with VT storm (RBLI axis, CL 430 ms) and 5 ICD shocks. Comparison of prior electrophysiologic study results with the ECG characteristics of the patient’s clinical VT suggested an epicardial origin. Given her clinical status (i.e., LVAD in place), the previous failure of multiple antiarrhythmic agents, and the likely need for a repeat open surgical procedure to access the epicardial surface, the patient opted for less invasive alternative management.
The patient underwent circumferential bilateral RDN usiing an open irrigated ablation catheter (ThermoCool, Biosense Webster) at up to 10 W for 60 seconds, with a flow rate of 30 cc/min. Electroanatomic maps (NavX, St. Jude Medical, Minnetonka, MN) were created of the renal arteries and associated ablation lesion sets. Evaluation of response to individual ablation lesions with high-frequency pacing could not be performed in this case because the patient had an LVAD that largely limited assessment of hemodynamic response. After the spiral lesion set was delivered to the left renal artery, transient slow flow was seen on repeat angiography. However, this flow quickly improved without sequelae after infusion of nitroglycerin and a IIB/IIIA inhibitor. The patient was monitored post-procedure without significant rise in measures of renal function acutely (glomerular filtration rate 50, creatinine 1.3). Although limited because of the presence of the LVAD, no significant hemodynamic changes could be appreciated. No LVAD setting changes were necessary pre- or post-RDN.
Before the patient was discharged from the hospital, no changes were made to her previous antiarrhythmic regimen of amiodarone 200 mg twice daily.
The patient remained ICD shock-free for 3.5 months of follow-up, at which time she presented with 4 ICD therapies for 2 different VTs (EGM CL 490 ms, CL 350 ms). There have been no further device therapies at 11 months post-RDN.
Patient 4
The patient was a 60-year-old man with a history of ischemic cardiomyopathy (LVEF 15%–20%), previous coronary artery bypass grafting, and ICD placement, who presented with recurrent VT/ICD shocks (EGM CL 370 ms) despite medical therapy with beta-blockade and amiodarone. Cardiac positron emission tomographic scan demonstrated scar in the LAD vascular territory (mid-to-distal anterior, apical, and distal septum). During the first VT ablation, 6 different VT morphologies were induced (4 LB morphology, two RB morphology, CL 355–646 ms including presumed clinical VT: LBLS, CL 371 ms). LV endocardial ablation was performed at the best pace-mapping sites (LV mid-distal inferoseptum) along the scar border zone. Reinducibility was not assessed because of hemodynamic instability.
Two days postablation, the patient developed recurrence of the clinical VT, which required 3 ICD shocks despite additional intravenous amiodarone and metoprolol. During the second VT ablation, 10 previously unobserved VTs were induced (5 with LB morphology, 5 with RB morphology, CL 262–431 ms). The clinical VT remained inducible with origination from the distal infero–midmyocardial septum, and extensive ablation of the approximated RV and LV endocardial breakout sites was performed. Because of the inability to achieve noninducibility of VT, RDN was performed at the end of the ablation procedure.
Bilateral renal arteriography was performed through an 8Fr sheath. An open irrigated ablation catheter (Thermocool, Biosense Webster) was advanced into each renal artery, and delivery of RF energy was performed spirally at up to 10 W for 30 seconds at a flow rate of 15 cc/min. No acute or delayed hemodynamic sequelae were seen (pre-RDN BP 91/49 mm Hg, HR 90 bpm; acute post-RDN BP 95/44 mm Hg, HR 69 bpm; 2-hour post-RDN BP 92/44 mm Hg, HR 69 bpm; 5-day post-RDN BP 87/56 mm Hg, HR 67 bpm). Post-RDN arteriograms showed “dimpling” of the renal arteries, thought to represent appropriate response to thermal injury (Figure 2) bilaterally without stenosis or dissection. Renal function remained stable throughout hospitalization. Three days post-RDN, the patient received ICD therapy for VT despite continued medical therapy with beta-blockade and amiodarone (EGM CL 324 ms). Three weeks post-RDN, he received unsuccessful ATP therapy for VT (EGM CL 441 ms) that spontaneously converted into normal sinus rhythm. He has experienced no further episodes at 5 months post-RDN.
Figure 2.
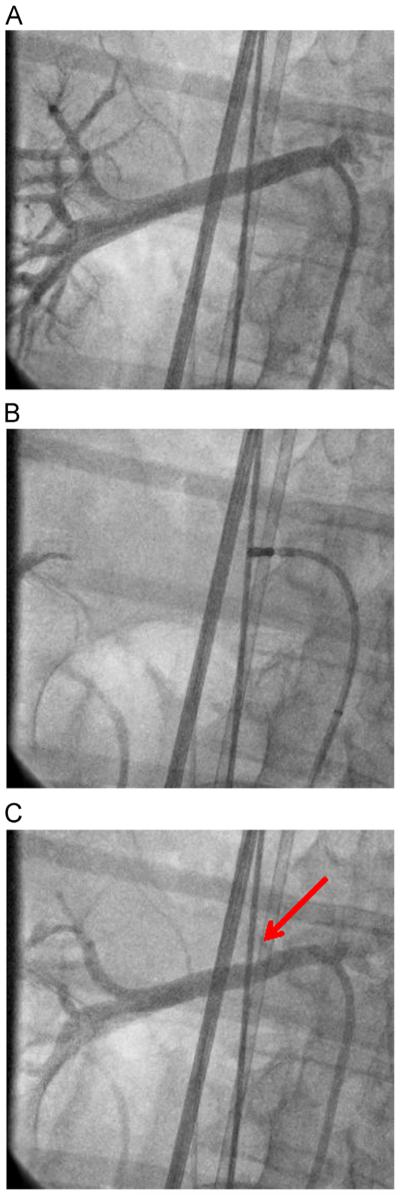
Selective right renal artery angiograms. A: Before radio-frequency ablation. B: During radiofrequency ablation. C: After radio-frequency ablation, with “dimpling” at ablation site (red arrow).
Discussion
This case series demonstrates the feasibility of catheter-based RDN as a rescue procedure for patients with systolic heart failure and recurrent VT despite previous antiarrhythmic drug and ablation treatment. In this small series of unstable patients, RDN was safe and potentially provided antiarrhythmic modulation of the VT pattern.
The first studies to use RDN in humans were the SYMPLICITY HTN-1 (n = 45) and SYMPLICITY HTN-2 (n = 52) trials, which evaluated catheter-based RDN in patients with resistant hypertension.11,12 Both studies reported a significant decrease in systolic/diastolic BPs after 6 months (mean –22/–11 mm Hg and –32/–12 mm Hg, respectively). In addition, the patients in SYMPLICITY HTN-1 had a sustained significant BP reduction of 33/19 mm Hg at 3 years post-RDN.
Further studies (partially overlapping subjects enrolled for SYMPLICITY HTN-1 and HTN-2) demonstrated 3-month post-RDN improvements in glucose metabolism13 as well as reduced LV mass and improved diastolic function at 6 months.14 In patients with chronic systolic heart failure (mean LVEF 43%), RDN improved symptoms and exercise capacity (+27.1 m in 6-minute walk test at 6 months).15
In published studies, RDN appears to be relatively safe and well tolerated. Of the 97 patients in SYMPLICITY HTN 1-and HTN-2, acute complications consisted of 1 renal artery dissection, 4 femoral artery pseudoaneurysms at the access site, and 7 patients with intraprocedural bradycardia requiring atropine. The most common long-term outcome in patients with resistant hypertension who underwent RDN was postprocedural BP reduction requiring desired medication changes. Interestingly, post-RDN hypotension requiring medication reduction was not observed in any of the trials involving heart failure patients, who had lower baseline BP. This is consistent with the hemodynamic effect observed in our patient series. A possible explanation is differential effect of RDN in patients with sympathetically driven hypertension.18
It is important to note that there has not been a single uniform ablation approach. The Medtronic Symplicity RDN system has been used in many studies,11-16,18 but RDN has also been safely performed using an off-the-shelf open irrigated RF ablation catheter17,19 or nonirrigated RF ablation catheter, as demonstrated in our case series. With regard to ablation settings, nonirrigated catheter setups (Symplicity and off-the-shelf) used no more than 8 W of power for r2 minutes. For irrigated catheters, up to 20 W of power for <90 seconds has been reported,20 but these power settings are higher than most use. Low power settings likely are critical to avoid long-term adverse effects.21
Use of RDN in arrhythmia management is of great interest. Recently, a prospective randomized study assessed the impact of RDN in patients with drug-resistant hyper-tension and refractory AF. Patients treated with pulmonary vein isolation (PVI) and RDN had a significant reduction in systolic and diastolic BPs (systolic BP from 181 ± 7 mm Hg to 156 ± 5 mm Hg; diastolic BP from 97 ± 6 mm Hg to 87 ± 4 mm Hg) compared to no reduction in the PVI group. Importantly, there was a significant reduction in AF recurrence with the addition of RDN to PVI at 12 months (29% vs 69%).17 A multicenter, randomized trial is currently in enrollment to further assess the role of RDN at the time of PVI in the treatment of recurrent AF.19
Ukena et al18 reported the first successful use of catheter-based RDN in the treatment of 2 patients with nonischemic cardiomyopathies and therapy-resistant electrical storm. Both patients had a significant reduction in subsequent ventricular arrhythmias. A patient with hypertrophic obstructive cardiomyopathy experienced 594 ATP-treated VT episodes pre-RDN, 57 episodes at 1 week post-RDN, and 1 episode at 4 weeks post-RDN. A second patient with dilated cardiomyopathy experienced 28 episodes of polymorphic VT/ventricular fibrillation pre-RDN, 12 episodes at 1 day post-RDN, and 0 episodes out to 24 weeks post-RDN. Importantly, neither patient had any complications from RDN.
In our study, we found a significant reduction in VT burden from 11.0 ± 4.2 (5.0–14.0) during the month before ablation to 0.3 ± 0.1 (0.2–0.4) per month after RDN in 2 patients with nonischemic cardiomyopathy and 2 patients with ischemic cardiomyopathy (Figure 1 and Table 1). Although the possible mechanisms remain unclear, RDN-mediated reduction of renal norephinephrine spillover by 47%11 and muscle sympathetic nerve activity by 37%22 has previously been implicated. Interestingly, the pattern of arrhythmia suppression post-RDN observed in our series appears to be similar to the report by Ukena et al18 in which possible delayed arrhythmia suppression is seen after weeks to months. All VT episodes in our series were observed in the first 4 months after ablation (2.6 ± 1.5 months). Although these findings are encouraging, assumptions of therapeutic efficacy should be approached with caution, because all of the patients also underwent other periprocedural interventions (e.g., medication changes, catheter ablation, biventricular pacing). Still, in most clinical scenarios, a multimodality approach is necessary to achieve adequate arrhythmia suppression in this critically ill patient population.
Importantly, all of our patients tolerated RDN both acutely and at follow-up. No significant changes in clinical status or renal function were seen . One patient with previously elevated BP experienced normalization postprocedure while taking stable medications. These findings are consistent with the safety profile reported for other studies.
The series suggests that RDN can be performed in this high-risk patient population with relative safety using off-the-shelf irrigated or nonirrigated ablation catheters. Significant reductions of ventricular arrhythmia burden were seen in our patients using renal denervation as adjunctive treatment. Larger observational and randomized studies are required to further assess the therapeutic effect and safety profile in this patient population.
Conclusion
This case series provides promising data on the safety and effectiveness of RDN as an adjunctive therapy in the treatment of patients with systolic cardiomyopathy and treatment-resistant VT. These results should be considered hypothesis-generating, and further trials are needed to assess the effectiveness of RDN in this high-risk population.
Acknowledgments
Dr. Mittal is supported by a research grant from Biosense Webster. Dr. Shivkumar is supported by Grant R01HL084261 from the National Heart, Lung, and Blood Institute of the National Institutes of Health. Dr. Steinberg is supported by research grants from Biosense Webster and Medtronic and is a clinical consultant to Biosense Webster, Philips, Boston Scientific, and Medtronic. Dr. Dickfeld is supported by a research grants from Biosense Webster and GE Healthcare and is a clinical consultant to Biosense Webster.
ABBREVIATIONS
- AF
atrial fibrillation
- ATP
antitachycardia pacing
- BP
blood pressure
- CL
cycle length
- EGM
electrogram
- FDA
Food and Drug Administration
- HR
heart rate
- ICD
implantable cardioverter-defibrillator
- LAD
left anterior descending
- LB
left bundle
- LI
left inferior
- LS
left superior
- LV
left ventricle
- LVAD
left ventricular assist device
- LVEF
left ventricular ejection fraction
- MRI
magnetic resonance imaging
- PVI
pulmonary vein isolation
- RB
right bundle
- RDN
renal artery denervation
- RF
radiofrequency
- RS
right superior
- RV
right ventricle
- VT
ventricular tachycardia
References
- 1.Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted Defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 2.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a Defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 3.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-Defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 4.Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) J Am Coll Cardiol. 2006;48:e247–e346. doi: 10.1016/j.jacc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Stevenson WG, Wilber DJ, Natale A, et al. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: the Multicenter ThermoCool Ventricular Tachycardia Ablation Trial. Circulation. 2008;118:2773–2782. doi: 10.1161/CIRCULATIONAHA.108.788604. [DOI] [PubMed] [Google Scholar]
- 6.Vaseghi M, Shivkumar K. The role of the autonomic nervous system in sudden cardiac death. Prog Cardiovasc Dis. 2008;50:404–419. doi: 10.1016/j.pcad.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz PJ, Priori SG, Cerrone M, et al. Left cardiac sympathetic denervation in the management of high-risk patients affected by the long-QT syndrome. Circulation. 2004;109:1826–1833. doi: 10.1161/01.CIR.0000125523.14403.1E. [DOI] [PubMed] [Google Scholar]
- 8.Wilde AA, Bhuiyan ZA, Crotti L, et al. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N Engl J Med. 2008;358:2024–2029. doi: 10.1056/NEJMoa0708006. [DOI] [PubMed] [Google Scholar]
- 9.Bourke T, Vaseghi M, Michowitz Y, et al. Neuraxial modulation for refractory ventricular arrhythmias: value of thoracic epidural anesthesia and surgical left cardiac sympathetic denervation. Circulation. 2010;121:2255–2262. doi: 10.1161/CIRCULATIONAHA.109.929703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rippy MK, Zarins D, Barman NC, et al. Catheter-based renal sympathetic denervation: chronic preclinical evidence for renal artery safety. Clin Res Cardiol. 2011;100:1095–1101. doi: 10.1007/s00392-011-0346-8. [DOI] [PubMed] [Google Scholar]
- 11.Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 12.Esler MD, Krum H, Sobotka PA, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 13.Mahfoud F, Schlaich M, Kindermann I, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation. 2011;123:1940–1946. doi: 10.1161/CIRCULATIONAHA.110.991869. [DOI] [PubMed] [Google Scholar]
- 14.Brandt MC, Mahfoud F, Reda S, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol. 2012;59:901–909. doi: 10.1016/j.jacc.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 15.Davies JE, Manisty CH, Petraco R, et al. First-in-man safety evaluation of renal denervation for chronic systolic heart failure: primary outcome from REACH-Pilot study. Int J Cardiol. 2013;162:189–192. doi: 10.1016/j.ijcard.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Witkowski A, Prejbisz A, Florczak E, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension. 2011;58:559–565. doi: 10.1161/HYPERTENSIONAHA.111.173799. [DOI] [PubMed] [Google Scholar]
- 17.Pokushalov E, Romanov A, Corbucci G, et al. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol. 2012;60:1163–1170. doi: 10.1016/j.jacc.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 18.Ukena C, Bauer A, Mahfoud F, et al. Renal sympathetic denervation for treatment of electrical storm: first-in-man experience. Clin Res Cardiol. 2012;101:63–67. doi: 10.1007/s00392-011-0365-5. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed H, Miller MA, Dukkipati SR, et al. Adjunctive renal sympathetic denervation to modify hypertension as upstream therapy in the treatment of atrial fibrillation (H-FIB) study: clinical background and study design. J Cardiovasc Electrophysiol. 2013;24:503–509. doi: 10.1111/jce.12095. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed H, Neuzil P, Skoda J, et al. Renal sympathetic denervation using an irrigated radiofrequency ablation catheter for the management of drug-resistant hypertension. JACC Cardiovasc Interv. 2012;5:758–765. doi: 10.1016/j.jcin.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 21.Kaltenbach B, Id D, Franke JC, et al. Renal artery stenosis after renal sympathetic denervation. J Am Coll Cardiol. 2012;60:2694–2695. doi: 10.1016/j.jacc.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 22.Hering D, Lambert EA, Marusic P, et al. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension. 2013;61:457–464. doi: 10.1161/HYPERTENSIONAHA.111.00194. [DOI] [PubMed] [Google Scholar]


