Abstract
Studies in animals and humans show that blockade of nerve growth factor (NGF) attenuates both malignant and non-malignant skeletal pain. While reduction of pain is important, a largely unanswered question is what other benefits NGF blockade might confer in bone cancer patients. Using a mouse graft model of bone sarcoma, we demonstrate that early treatment with an NGF antibody reduced tumor-induced bone destruction, delayed time to bone fracture, and increased the use of the tumor-bearing limb. Consistent with animal studies in osteoarthritis and head and neck cancer, early blockade of NGF reduced weight loss in mice with bone sarcoma. In terms of the extent and time course of pain relief, NGF blockade also reduced pain 40-70% depending on the metric assessed. Importantly, this analgesic effect was maintained even in animals with late stage disease. Our results suggest that NGF blockade immediately upon detection of tumor metastasis to bone may help preserve the integrity and use, delay the time to tumor-induced bone fracture, and maintain body weight.
Keywords: NGF (nerve growth factor), bone cancer, weight loss, pain, time-to-fracture
Introduction
Cancer in bone tissue is typically the result of tumor metastases from a distant site, and cancers having the highest prevalence of bone metastases (lung, prostate, and breast) are the most frequently diagnosed cancers worldwide (1, 2). In the United States, in 2013, the estimated number of new cases of cancer in the lung (228,190), prostate (238,590) and breast (234,580) (3), combined with the known prevalence of bone metastasis in lung, prostate, and breast cancer (<36%, <90%, 65-75%, respectively)(4), results in the estimate that 460,000 new cases of bone metastases will be diagnosed. Once cancer metastasis to bone (CMB) occurs, it is usually incurable (unless the metastases are limited to very few sites). However, advances in cancer treatments continue to dramatically increase survival rates, thus patients with metastatic disease and suffering with malignant skeletal pain are living years to decades beyond their initial cancer diagnosis. Maintenance of the functional status of patients with CMB requires proactive management as CMB can cause excruciating bone pain, pathologic fractures, spinal cord and nerve compression syndromes, weight loss, derangements of calcium and phosphate homeostasis and decline in the ability to load and use tumor-bearing bones cannot be viewed individually but rather as interactive events ultimately contributing to increased morbidity, mortality, and diminished quality of life (2, 5).
In the past decade, progress has been made in understanding what drives CMB. Bisphosphonates and the RANK ligand inhibitor, Denosumab, have been approved for treatment of both pain and preventing bone fracture (6, 7). A therapy showing promise in blocking pain induced by CMB and non-malignant skeletal pain is inhibition of nerve growth factor (NGF) and its primary receptor, tyrosine kinase receptor type 1 (TrkA) (8-10). In breast, sarcoma, and prostate mouse models of CMB, anti-NGF significantly attenuates bone cancer pain (11-15). Additionally, in humans, anti-NGF attenuates moderate-to-severe skeletal pain due to osteoarthritis or low back pain (16, 17). While animal and human studies suggest that blockade of NGF/TrkA effectively alleviates malignant and non-malignant skeletal pain, most efforts at developing novel therapies to block or sequester NGF or TrkA were not initially developed to treat skeletal pain but rather to block/attenuate tumor growth and metastasis. Extensive in vitro studies show that NGF and/or TrkA drive the growth and metastasis of breast, ovarian, lung, pancreas, and prostate tumor cells (18-20). Moreover, in vivo studies show that anti-NGF inhibits ethylnitrosourea-induced carcinogenesis in mice and rats (21), and either anti-NGF or siRNA against NGF inhibits breast cancer tumor growth and metastasis in a mouse xenograft model (22).
In the present study we directly address these CMB patient issues by using a primarily osteolytic model of bone cancer which drives tumor-induced bone loss, bone fracture, loss of the use of the tumor-bearing limb, and weight loss. We explore whether early administration of anti-NGF can attenuate these pathological features. In addition we have modified and refined our bone disease progression and behavioral endpoints to more closely mirror endpoints used in human clinical studies in patients with CMB.
Materials and Methods
Surgical procedures and drug treatment
Mice
Experiments were conducted with adult C3H/HeJ mice (Jackson Laboratories, Bar Harbor, ME) approximately 4-8 weeks old, weighing 25-30 g at time of tumor cell injection. Mice were housed in accordance with National Institutes of Health guidelines under specific pathogen-free conditions in autoclaved cages maintained at 22°C with a 12-hr alternating light/dark cycle and access to food and water ad libitum. All procedures adhered to the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain (23) and were approved by the Institutional Animal Care and Use Committee at the University of Arizona (Tucson, AZ).Experiments were initiated with 90 mice, out of which a total of 85 mice were included for data analyses:naïve (n=8); sham surgery (n=13); NCTC 2472 (femur-injected) + vehicle (n=36); NCTC 2472 (femur injected) + anti-NGF (n=24); NCTC 2472 + vehicle (muscle-injected) (n=4). To allow for careful attention to both surgical treatments and behavioral pain assessments/analyses, each experiment typically involved 12-16 mice, comprising of different experimental groups.
Cells
NCTC 2472 cells (ATCC® CCL11™, American Type Culture Collection, Manassas, VA), derived from a culture of subcutaneous areolar and adipose connective tissue (taken from a normal 82-day-old male C3H/HeJ mouse), aggressively stimulate osteoclast activity upon intramedullary femoral injection, and produce a bone histopathology resembling that found in human osteolytic bone cancer (24, 25). Authentication/testing carried out by ATCC confirmed the post-freeze viability, growth properties, morphology, absence of mycoplasma, species, sterility and development of sarcomas upon injection into mice. NCTC 2472 cells were stably transfected to express green fluorescent protein (GFP) to enable visualization. In vitro and in vivo tumor cell characteristics of GFP-transfected NCTC 2472 cells (growth rate, bone resorption rate, induction of bone cancer-related pain), were temporally, behaviorally, and physically identical to that of non-transfected NCTC 2472 cells (26). Upon thaw, GFP-transfected NCTC 2472 cells were cultured according to ATCC recommendations, passaged for at least three, but not more than 20 passages (less than three months), and verified mycoplasma-free before injection into mice. Additional information included in Supplemental Material.
Surgery
Injection of NCTC 2472 cells directly into the intramedullary space of the mouse femur was as previously described (13, 27-33). To prevent the patella from becoming displaced post-arthrotomy, muscles were secured back in position using a horizontal mattress suture. In addition, after surgery, animals were individually housed and allowed to recover for one week before being handled for behavioral and radiological assessment. Additional information included in Supplemental Material.
Anti-NGF Treatment
The anti-NGF sequestering antibody (mAb911), kindly provided by Dr. David Shelton (Rinat/Pfizer, San Francisco, CA), blocks the binding of NGF to both TrkA (tyrosine kinase receptor type 1, NTRK1) and p75 (neurotrophin receptor, LNGFR), and inhibits TrkA auto-phosphorylation (34). Anti-NGF has no effect on healthy bone (11-14, 35-37), its plasma half-life is five to six days in the mouse, and it does not appreciably cross the blood-brain barrier (38). In this study, the dose used (10 mg/kg, i.p.) was based on previous studies (11),and it was delivered starting at Day 7 post-cancer cell injection, and every five days thereafter.
Assessment of bone cancer disease progression and pain
Mice were assessed for bone cancer disease progression, functional status, and both spontaneous and movement-evoked pain, to measure endpoints that are clinically relevant to the patient with bone cancer (2, 9). Behavioral testing was performed on the same days as radiological assessment to enable comparison between pain behavior and bone destruction. Each method of behavioral assessment was performed by the same experimenter who was blinded to the drug treatments.
Radiology
High resolution X-ray images of cancer or vehicle-injected femurs were obtained several days before surgery (baseline), and immediately following weekly behavioral assessments, using a Faxitron MX-20 digital cabinet X-ray system (Faxitron/Bioptics, Wheeling, IL). Mice were lightly anesthetized with ketamine/xylazine (0.005 ml/g, 50 mg/10 kg, s.c.) to enable consistent placement of the animal for radiological assessment. Faxitron settings were optimized for radiological assessment of cortical or trabecular bone destruction. Animals were excluded from the study if a patella displacement was identified through radiography (see Supplemental Material, Fig. 1).
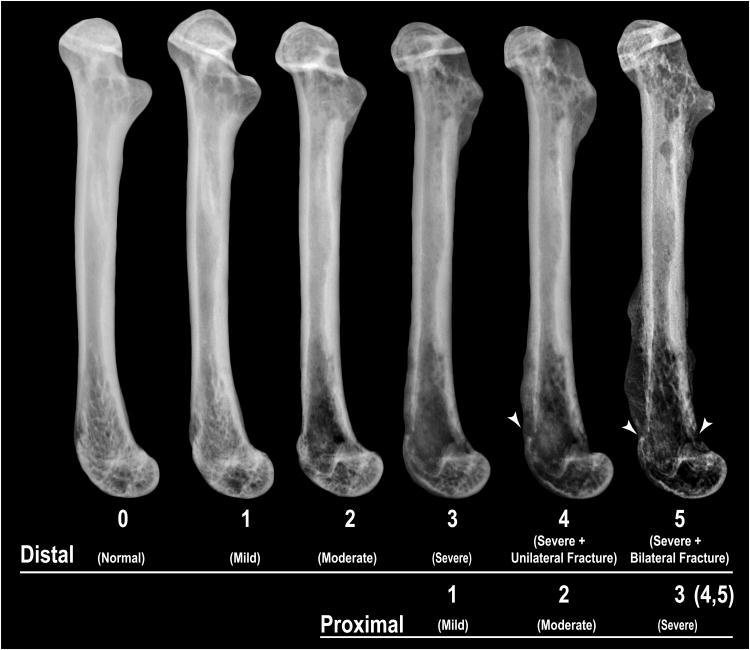
Figure 1. Sarcoma-induced bone destruction.
High-resolution radiographs of the mouse femur illustrate progressive bone destruction following intramedullary injection of sarcoma cells. To measure the extent of bone destruction, each femur was divided into two equal-length aspects (distal and proximal), and bone destruction scores for each aspect were separately determined. For each femoral aspect, radiographs were scored on a 0-5 scale: 0 (normal), no signs of bone destruction; 1 (mild), small pits (1-3 in number) of bone destruction; 2 (moderate), increased pitted appearance (4-6 in number) and loss of medullary bone; 3 (severe), loss of medullary bone and erosion of cortical bone; 4 (severe + unilateral fracture), full thickness unicortical bone loss; 5 (severe + bilateral fracture), full thickness bicortical bone loss.
Bone Scoring
To quantify the extent of bone destruction, and to separately analyze disease progression at the distal and proximal aspects of tumor-bearing femora, a 10-point bone scoring method was used in which the distal and proximal halves of each femur were scored separately on a previously validated scale of 0 to 5 (28, 33), and then the scores for each femur half were summed (maximum possible score of 10). For each femoral aspect, bone scores were defined as: 0, normal bone with no signs of destruction; 1, small pits (1-3 in number) of bone destruction; 2, increased pitted appearance (4-6 in number) and loss of medullary bone; 3, loss of medullary bone and erosion of cortical bone; 4, full thickness unicortical bone loss; 5, full thickness bicortical bone loss and displaced skeletal fracture (Fig. 1).
Time-to-fracture
The radiographic endpoint “time-to-fracture” was defined as the time (day) following surgery when a fracture was identified through radiography.
Spontaneous nocifensive behavior
Mice were placed in small raised Plexiglas chambers (11.5 × 6.8 × 7.5 cm) with a wire grid floor, acclimated for 30 min (until cage exploration and major grooming activities ceased), and then their movements were videotaped from below using Sony Handycam DCR-SR68 cameras. Time spent in “nocifensive” behavior was assessed during a 5-min observation period (between minutes 15-20 of the filmed behavior). Nocifensive behavior was defined as: (a) full guarding (lifting the affected limb and holding it against its body), (b) reduced weight-bearing (affected limb is not completely held up against its body and some weight is borne on it), (c) tending to the affected limb (abnormal grooming behavior directed solely to affected limb), and (d) sporadic hopping (intermittent jumps without utilizing affected limb). See Supplemental Material for additional information, including videos of mice showing different types of spontaneous nocifensive behavior.
Limb Use (movement-evoked pain)
Limb use in an open field was assessed as previously described (28). The mouse was placed in the middle of a large Plexiglas box (40 × 50 × 20 cm) containing a mirror on the end facing the observer (for increased visibility of limb use), and observed while walking/running in a continuous motion over a two-minute period. Movements were categorized as: limping, guarding, flinching, non-use of limb, hunched posture, slow-to-get-up-and-go, walking slow, stiff/uneven gait, tilted stance, splayed limb, dragging of toes, and refusal to move. Movement of the affected limb was rated on the following scale: 0 = normal walking, +1 point assigned for each additional type of abnormal movement or posture observed (up to a total of 12).
Dynamic weight bearing
The percentage of weight borne by each limb of a freely moving animal was measured using a floor-instrumented dynamic weight bearing system (DWB, BioSeb EB Instruments, Pinellas Park, FL). Data is presented as percent weight borne by ipsilateral hind limb of total weight borne by both hind limbs. Additional information included in Supplemental Material.
Exclusion criteria
Animals were observed daily, and criteria for exclusion from the study (and euthanasia) included: rapid weight loss (>20% in one week), patella displacement, lack of intra-femoral cancer cell growth, extra-femoral cancer cell growth, prolonged digestive abnormalities (e.g., diarrhea or vomiting for over three days), severe ulcerative dermatitis or infected tumors, and paralysis.
Statistical analysis
One-way ANOVA was used to compare behavioral and radiological bone scoring results between experimental groups. Significance level was set at P< 0.05.
Results
Characterization of bone cancer pain model
Experimental investigation in our optimized model involved first baseline behavioral assessment and X-ray analysis of naïve mice, followed by selection of animals with comparable values and their random assignment to treatment groups (vehicle or therapy). To monitor disease progression, high-resolution radiographs of the mouse femur were taken at various time points post-surgery. Radiological evidence of bone destruction presents as radiolucent areas, typically observed in the distal and proximal aspects of the femur at early time points of disease progression. As time and disease progress, multiple focal radiolucencies are accompanied by loss of medullary bone, erosion of cortical bone, and ultimately, fracture (Fig. 1).
At Day 0 (surgery), arthrotomies were performed, alternately between vehicle- and therapy-designated mice. Post-surgical pain, as exhibited by sham (HBSS-injected) mice, typically peaked at Day 3 following the arthrotomy, and approached baseline values by Day 7 post-surgery (Fig. 2). Spontaneous nocifensive behavior exhibited by NCTC 2472-injected mice was temporally differentiated into “surgical pain” and “bone cancer pain.” Post-surgical pain in NCTC 2472-injected mice was similar to that experienced by sham mice, peaking at Day 3 post-surgery and significantly lessened by Day 5 post-surgery. However, at Day 7 post-surgery, the observed profiles of nocifensive behavior for HBSS- and NCTC 2472-injected mice typically started to diverge, with tumor-bearing mice increasing in nocifensive behavior with time. At Days 10 and 14 post-surgery, nocifensive behavior exhibited by cancer cell-injected mice was approximately three and six times that of Day 7 post-surgery, respectively, and this rapid rise in cancer-induced bone pain extended to Day 18 post-surgery, at which time it was approximately eight-fold higher than that at Day 7 post-surgery. After Day 18 post-surgery, nocifensive behavior in NCTC 2472-injected mice steadily increased, although at a less rapid pace, and was approximately ten times that of Day 7 post-surgery at the end of the study (Day 30). Collectively, these data demonstrated that our bone cancer model enabled the differentiation between surgical and cancer cell-induced bone pain, thereby providing an accurate assessment of cancer cell-induced pain and the efficacy of therapies targeted specifically to attenuate cancer-induced bone pain.
Figure 2. Nocifensive behavior over time in bone cancer model.
Time-course of spontaneous pain-related behaviors following injection of either NCTC 2472 cells in Hanks balanced salt solution (HBSS) (open circles, n=36) or HBSS (sham) (inverted triangles, n=8) into the mouse femur show that spontaneous nocifensive behavior is temporally separated into “surgical (orthopedic) pain” (Day 1-7) and “bone cancer” pain (Day 7-30). Spontaneous nocifensive behavior exhibited by HBSS-injected (sham) mice show that by Day 7 post-surgery, spontaneous pain-related behaviors approach those exhibited by naïve mice (open squares, n=8). By Day 10 post-cancer-cell injection, time spent in nocifensive behavior by sarcoma-injected mice is easily differentiated from that of sham-injected mice. Error bars represent SEM.
To demonstrate the importance of location of cancer cell growth in the development of pain behaviors, we included in our study a group of “muscle-injected” animals wherein NCTC 2472 cells were injected directly into the muscle adjacent to the femur. Despite the development of large tumor volumes, “muscle-injected” animals did not exhibit nocifensive behavior (Fig. 3).
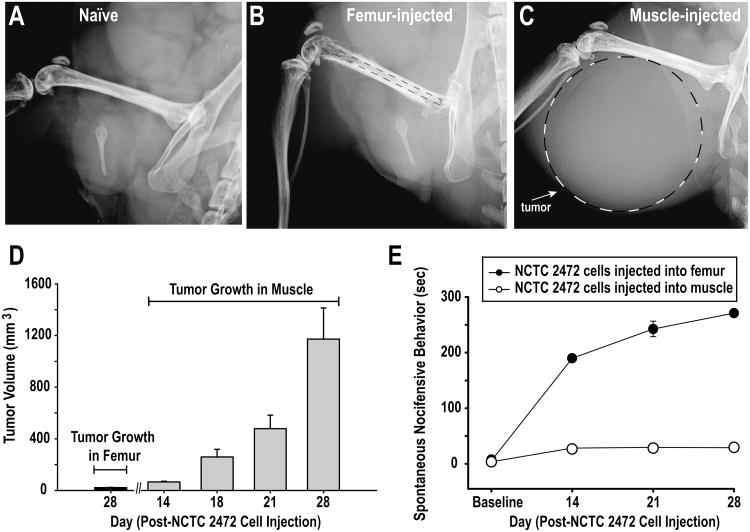
Figure 3. Tumor location (not size) influences cancer pain.
To demonstrate that location of the tumor growth (within the bone or within the muscle adjacent but not involving the bone) is the critical factor in the intensity of bone cancer pain, experiments were performed in which murine NCTC 2472 sarcoma-producing cells were injected either directly into the intramedullary space of the mouse femur, or into the muscle directly adjacent to the femur. Representative high-resolution radiographs of a naïve mouse femur (A) a femur from a bone-injected (B), and a muscle-injected (C) mouse at Day 28 post-cancer cell injection revealed the greater tumor volume present in the muscle-injected mouse than in the femur-injected mouse. (D) Tumor volumes dramatically increased following cancer-cell-injection in the muscle-injected mice over time. (E) Despite harboring greater tumor volumes, the time spent in nocifensive behavior by muscle-injected mice (open circles, n=4) was significantly less than that exhibited by femur-injected mice (closed circles, n=6). Error bars represent SEM.
Anti-NGF therapy attenuates sarcoma-induced bone destruction
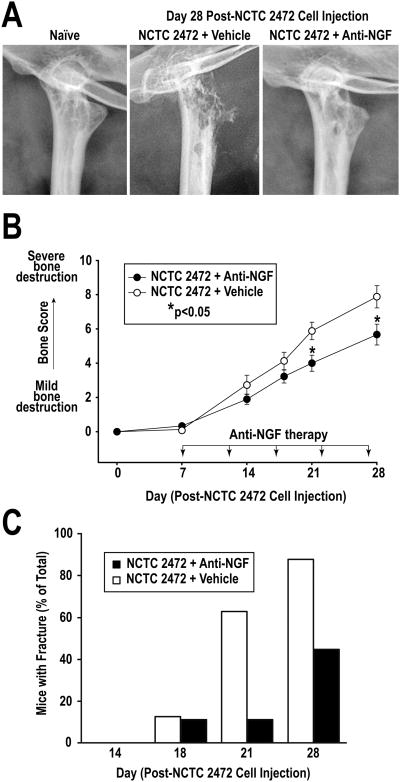
To determine the effect of anti-NGF on NCTC 2472-induced bone destruction, mice were administered injections of either vehicle (phosphate-buffered saline, PBS) or anti-NGF in PBS (10 mg/kg, i.p.). Anti-NGF therapy was initiated at Day 7 post-surgery, and repeated every five days. At Days 7, 14, 21, and 28 post-cancer cell injection, mouse femurs were radiologically assessed for evidence of bone destruction. At Day 7, prior to the administration of the first dose of anti-NGF, minimal bone destruction was observed for both treatment groups. By Day 21 (after three doses of anti-NGF), a significant decrease in bone destruction was achieved with anti-NGF treated animals, and continued through Day 28 post-NCTC 2472 cell injection. Fig. 4A shows representative radiographs of the proximal aspects of mouse femurs of vehicle- and anti-NGF-treated mice at Day 28 post-NCTC 2472 cell injection, and the attenuating effect of anti-NGF on disease progression is striking. Whereas in vehicle-treated mice there was evidence of extreme bone destruction, this was not the case with the anti-NGF-treated mice. Mean bone scores for anti-NGF-treated mice at Days 21 and 28 were 4.0±0.5 and 5.7±0.4, respectively. In contrast, mean bone scores for vehicle-treated mice at Days 21 and 28 were significantly greater, 5.9±0.4 and 7.9±0.4, respectively (Fig 4B). The importance of the attenuating effect of anti-NGF on bone destruction in terms of overall functionality was demonstrated by the fact that at Day 28 post-NCTC 2472 injection, the percent of anti-NGF-treated mice with evidence of fracture was half that of vehicle-treated mice (Fig. 4C).
Figure 4. Anti-NGF reduces sarcoma-induced bone destruction.
(A) Representative high-resolution radiographs of the proximal aspect of the femur of a naïve mouse, and of vehicle- and anti-NGF-treated mice at Day 28 post-cancer cell injection. Anti-NGF treatment attenuated sarcoma-induced bone destruction. (B) Anti-NGF significantly reduced the extent of bone destruction in therapy-treated mice (closed circles, n=24) compared to vehicle-treated mice (open circles, n=36) at Days 21 and 28 post-cancer cell injection. Error bars represent SEM; *p<0.05, one-way ANOVA. (C) Anti-NGF therapy resulted in an approximate 50% reduction of the number of mice with fractures at Day 28 post-cancer cell injection. Error bars represent SEM.
Anti-NGF therapy attenuates bone cancer-induced weight loss
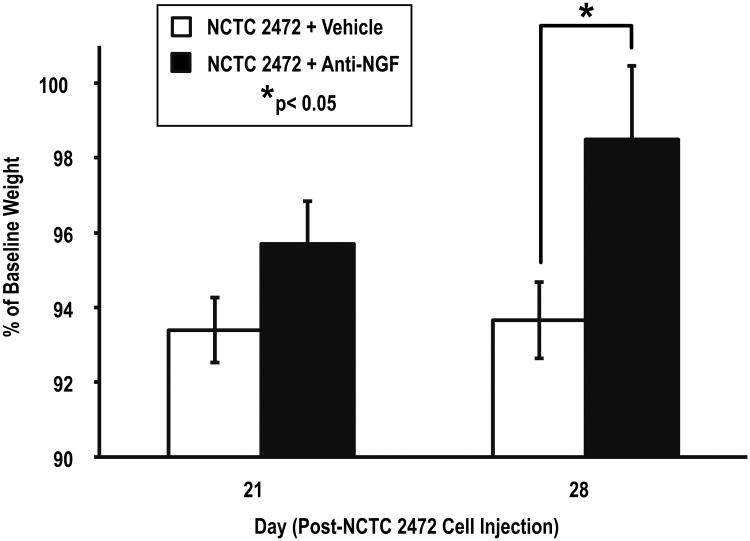
Weight maintenance in cancer patients remains an important clinical challenge. In our pre-clinical model, a loss of 20% of baseline weight was a severe physiological event requiring removal from the study and euthanasia. Our data show that at Day 28 post-cancer cell injection, when there was significant disease progression and bone destruction, mice treated with anti-NGF therapy had only lost ∼2% of their baseline weight, whereas vehicle-treated mice had lost ∼6% of their baseline weight (Fig. 5).
Figure 5. Anti-NGF therapy attenuates tumor-related weight loss.
Mice treated with anti-NGF (closed rectangles, n=24) had significantly less weight loss than vehicle-treated mice (open rectangles, n=36) at Day 28 post-NCTC 2472 cell injection. Error bars represent SEM; *p<0.05, one-way ANOVA.
Anti-NGF attenuates spontaneous and movement-evoked bone cancer pain behaviors
To determine the efficacy of anti-NGF in reducing bone pain in both early and late stage bone cancer disease, spontaneous nocifensive behavior exhibited by vehicle- and anti-NGF-treated mice was analyzed with respect to bone score (Fig. 6A). Mouse movements defined as “nocifensive” were full guarding, reducing weight-bearing and abnormal grooming of the affected limb, and also sporadic hopping using the non-affected limb (see Methods, Supplemental Material videos). Spontaneous nocifensive behavior associated with a bone score of 0 was minimal for both treatment groups (approximately 25-30 sec, 8-10% of the 300-second assessment period), and was reflective of the pain experienced the week following cancer cell injection due to the surgical procedure. This pain was not NCTC 2472-induced bone cancer pain (Fig. 2, 4B).
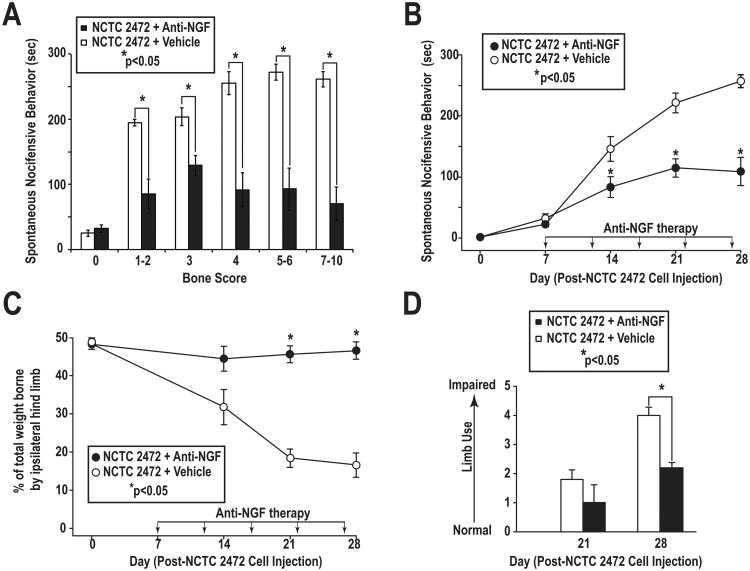
Figure 6. Anti-NGF attenuates spontaneous and movement-evoked bone cancer pain behaviors.
(A) Time spent in spontaneous nocifensive behavior by anti-NGF-treated mice (closed rectangles) with bone scores of 1-2 (minimal bone destruction, early-stage bone cancer disease), 3-4 (moderate bone destruction), and 5-10 (severe to extreme bone destruction with fracture, late-stage bone cancer disease) was significantly reduced as compared to vehicle-treated mice (open rectangles). (B) Anti-NGF-treated mice (closed circles) spent significantly less time in spontaneous nocifensive behavior than vehicle-treated mice (open circles) at Days 14, 21, and 28 post-cancer cell injection. (C) Weight-bearing ability of tumor-bearing hind limbs of anti-NGF-treated mice (closed circles) was preserved throughout the study, whereas the weight-bearing ability of vehicle-treated mice (open circles) was significantly reduced at Days 21 and 28 post-cancer cell injection. (D) Impaired limb use in anti-NGF-treated mice (closed rectangles) was significantly reduced at Day 28 post-cancer cell injection, as compared to vehicle-treated mice (open rectangles). NCTC 2472 + vehicle, n=36; NCTC 2472 + anti-NGF, n=24. Error bars represent SEM; *p<0.05, one-way ANOVA.
Bone scores of 1-2, indicative of mild bone destruction and early stage bone cancer disease, in vehicle-treated mice were associated with a mean spontaneous nocifensive behavior of 195.0±5.3 sec. In contrast, in anti-NGF-treated mice, bone scores of 1-2 were associated with a mean spontaneous nocifensive behavior of 85.0±22.9 sec. Thus, anti-NGF treatment reduced pain in early stage bone cancer by approximately 56%. Bone scores of 3 and 4, indicative of moderate bone destruction and mid-stage bone cancer disease progression, in vehicle-treated mice were associated with mean spontaneous nocifensive behaviors of 203.8±13.5 and 255.3±17.6 sec, respectively. In anti-NGF-treated mice with similar bone scores, were associated with mean spontaneous nocifensive behaviors of 129.2±15.5 and 91.6±26.1 sec, respectively. Taken together, these data demonstrated that anti-NGF treatment reduced pain in mid-stage bone cancer disease by approximately 40-60%.
Bone scores of 5-6 and 7-10, indicative of severe to extreme bone destruction (with fracture), in vehicle-treated mice were associated with mean spontaneous nocifensive behaviors of 272.0±12.0 and 261.3±11.6 sec, respectively. In contrast, in anti-NGF-treated mice, bone scores of 5-6 and 7-10 were associated with mean spontaneous nocifensive behaviors of 93.0±32.4 and 70.4±25.6 sec, respectively. Taken together, these data show that anti-NGF treatment reduced the time spent in spontaneous nocifensive behavior in mice suffering with late stage bone cancer disease by approximately 70%.
The efficacy of anti-NGF in reducing bone pain in both early and late stage bone cancer disease was also revealed by analysis of spontaneous nocifensive behavior exhibited by vehicle- and anti-NGF-treated mice with respect to time (day) following cancer-cell injection (Fig. 6B). At Day 14 post-cancer cell injection, vehicle-treated mice had a mean spontaneous nocifensive behavior of 158.8±20.3 sec. This indicated that approximately 50% of the total 300-second assessment period the mice exhibited pain-related behaviors. In contrast, anti-NGF-treated mice had a mean spontaneous nocifensive behavior of 28.5±6.8 sec, illustrating that cancer-induced bone pain had been reduced six-fold. At Days 21 and 28 post-cancer cell injection, vehicle-treated mice had mean spontaneous nocifensive behaviors of 237.3±14.1 and 244.8±16.5 sec, respectively. However, at these same time points, anti-NGF-treated mice had significantly reduced mean spontaneous nocifensive behaviors of 114.8±14.8 and 102.9±24.3 sec, respectively. Taken together, these data show that at Days 21 and 28, when mice had severe to extreme bone destruction, anti-NGF treatment resulted in mice spending approximately 30-40% of the total 300-second assessment period in spontaneous nocifensive behavior as opposed to approximately 80% as in the case of vehicle-treated mice.
The ability of anti-NGF treatment to reduce pain-related behaviors in both early and late stage bone cancer disease was also revealed by analysis of dynamic weight bearing and limb use in vehicle- and anti-NGF-treated mice with respect to time (day) following cancer-cell injection (Fig. 6C,D). Measurement of dynamic weight bearing in the mouse is equivalent to the human clinical measure of the ability to load the tumor-bearing bone. In vehicle-treated mice, the weight borne for the tumor-bearing limb progressively decreased over the course of the study; at Day 28 post-cancer cell injection the ability of the tumor-bearing hind limb to bear weight had been reduced by over 70% (Fig.6C). In contrast, anti-NGF treatment reduced bone cancer-induced pain enough that the animal was capable of preserving the ability of the tumor-bearing hind limb to bear weight. At Days 21 and 28 post-cancer cell injection, the percent weight borne by tumor-bearing hind limbs of vehicle-treated mice (18.4±2.4, 16.6±3.2, respectively) was significantly less than that of anti-NGF-treated mice (45.7±2.2, 46.6±2.3, respectively). Assessment of mouse limb use in an open field, comparable to assessment of gait, posture and the spontaneous use of the affected limb in humans, demonstrated that anti-NGF treatment prevented limb use impairment. A total of 12 different mouse movements or postures were included as evidence of limb impairment (see Methods). At Day 28 post-cancer cell injection, vehicle-treated mice had significantly more limb impairment, indicated by a higher limb use score (4.0±0.3) than anti-NGF-treated mice (2.2±0.2) (Fig. 6D).
Discussion
In the present study, we used a mouse model of sarcoma-induced bone cancer and showed that early treatment with anti-NGF reduced tumor-induced bone destruction, delayed time-to-fracture, and increased the use of the tumor-bearing limb. Additionally, as suggested in previous studies in animal models of osteoarthritis (39) and head/neck cancer (40), early blockade of NGF also reduced weight loss in animals with bone cancer. In terms of the extent and time course of pain relief, blockade of NGF reduced bone cancer pain by 40-70% depending on the pain measure being assessed, and this analgesic effect was maintained even in animals with late stage disease.
NGF and bone cancer disease progression
In the current study, NGF blockade delayed bone cancer disease progression; measured by time-to-fracture, tumor-induced bone destruction, and tumor-bearing limb use. What the specific mechanisms that maintain the integrity and use of bone remain unclear, but previous data in both older individuals and those with CMB have suggested that exercise and loading of the bone promotes bone health. Previous studies in human clinical trials in patients with moderate-to-severe osteoarthritis pain showed that anti-NGF attenuated this pain by 40-50%. However, within this group a small but significant number of patients receiving the highest dose of anti-NGF and naproxen required earlier-than-expected joint replacement. Whether this earlier-than-expected joint replacement was caused by overuse of the arthritic joints due to the robust pain relief provided by anti-NGF, or was a direct effect of blocking NGF on the maintenance and formation of adult bone remains unknown (10). However, in patients with CMB, fracture of the tumor-bearing bone is a highly undesirable event as active tumor-induced bone destruction makes stabilization and repair of the fractured bone problematic.
NGF and weight loss
A second major finding of the present study is that anti-NGF in this model of sarcoma-induced bone cancer reduced weight loss at late stage time points. This anti-NGF inhibition of weight loss confirms and extends what was previously reported in a rat model of Freund's induced osteoarthritis (39), and a mouse model of head/neck cancer (40), where anti-NGF markedly reduced weight loss. Currently, we do not know whether this inhibition of weight loss in the present study was due to blockade of a circulating anti-cachexia effect or reduction in pain/disease progression which allows the animal to maintain an appetite. Anti-NGF may indirectly affect weight loss by impacting the circulating levels of variety of cytokines (e.g., TNF-alpha, IL-6 and leptin) proposed to be involved in the regulation of body mass and cachexia. Chronic anti-NGF antibody treatment did not promote weight gain in normal rodents (39), thus the relationship between anti-NGF and weight is not straightforward. Given that opiates (commonly used to relieve cancer pain) have not been shown to reduce weight loss in patients (41), pain reduction and weight maintenance in the cancer patient appear to not be directly correlated. However, what is clear is that as CBM progresses, maintenance of body weight and muscle mass has an important impact on the functional status of the patient.
Pain induced by tumor growth in bone vs. muscle
A third aim of the present study was to examine whether tumor growth per se would be correlated with the extent of pain. To explore this possibility, we compared the pain induced by injection of NCTC 2472 cells into the bone versus the pain induced by injection of the same tumor cell line into the overlying muscle. Interestingly, while there was a clear increase in pain when tumor cells were injected, confined and grew within the bone, there was negligible pain following injection of NCTC 2472 cells into the muscle, despite the development of a tumor mass > 50× that in bone. These data extend previous data showing that it is not size, but rather location and specific nerve innervation, that influences tumor-induced pain (8, 9, 11, 42, 43).
Early vs. late administration of anti-NGF following cancer metastasis to bone
Current clinical trials are examining or considering examining the blockade of NGF/TrkA in terms of being an analgesic and not a disease modifying agent. However, the present data suggests that if anti-NGF can reduce tumor-induced bone destruction, time-to-fracture, weight loss, and CMB pain, and also maintain the functional status of patients, then early rather than late administration of anti-NGF following the initial diagnosis of CMB appears warranted. Currently, following diagnosis of CMB, where the initial diagnosis of CMB is frequently mild-to-moderate bone pain, patients usually are put on the WHO “analgesic ladder” for treatment of cancer pain which consists of NSAIDs alone, then NSAIDs + weak opiate, and finally NSAIDs + strong opiate. However, NSAIDs inhibit bone formation, and opiates have numerous side effects including nausea, dizziness, constipation and somnolence (9). In contrast, blockade of NGF or TrkA has remarkable efficacy in attenuating skeletal pain, and a total of 11,000 patients that received anti-NGF for non-metastatic skeletal pain had relatively few side effects (16).
One major concern with administering anti-NGF to humans with late stage disease bone cancer pain is that the patient typically will already be on NSAIDs + strong opiate, making it difficult to discern even an analgesic effect. However, in clinical trials if anti-NGF is given early (immediately upon diagnosis of CMB), and anti-NGF does have the positive effects on maintaining bone health, preventing bone fractures and weight loss, and reducing skeletal pain, early therapy with anti-NGF may significantly help in maintaining the functional status and quality of life of the patient. This would be especially true in a population of breast cancer patients who are young women (20–44 years old), and in the early or midstage of their careers and/or raising a family. As survival times of these young women with metastatic breast cancer have significantly increased (44), an important and unmet objective is to maintain their functional status and quality of life without the unwanted side effects of currently available therapies.
Pre-clinical vs. clinical measures of bone cancer disease progression and pain
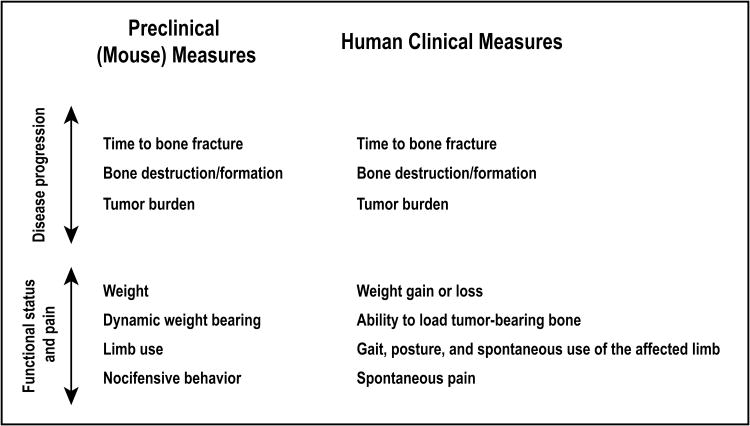
Essential to the translational success of an emerging therapy is its validation in a pre-clinical animal model that incorporates endpoints directly translatable to those that can be used in human clinical trials. In the clinic, commonly-measured endpoints for bone cancer disease progression and functional status include radiological assessment of bone destruction and time-to-fracture (5), weight loss (45), evaluation of gait and weight-bearing (46, 47), and pain (48-50). As illustrated in Fig. 7, preclinical (mouse) measures incorporated in the present study to monitor disease progression, functional status, and pain closely mirror the human clinical measures used.
Figure 7. Preclinical and clinical measures of bone cancer disease progression, functional status, and cancer-induced bone pain.
To facilitate the bench-to-beside translation of preclinical data examining the efficacy of a therapy targeting human bone cancer, it is critical that the pre-clinical animal model incorporate endpoints that are directly translatable to endpoints that can be used in human clinical trials. To that end, we have included in our bone cancer model a variety of preclinical measures of disease progression, functional status, and pain that might be used in human clinical trials involving cancer patients.
Limitations and conclusions of the study
To provide a clinically-relevant animal model of bone cancer pain, we originally developed and have recently optimized a mouse model in which mouse NCTC 2472 osteolytic sarcoma-producing cells are injected directly into the intramedullary space of the femur, following which the injection site is sealed with bone cement (13, 27-33). Surgical implantation of NCTC 2472 cells directly into the femoral intramedullary space enables a pre-clinical animal model that mirrors the development of bone cancer pain and bone remodeling in humans. The critical advantage of this method of bone cancer induction is that tumor cells are confined within the marrow space of the femur and prevented from invading surrounding soft tissue. However, this surgical procedure itself causes nocifensive behavior that is observed in the first week following surgery, and consequently, an important consideration in optimizing our bone cancer model was to ensure that an appropriate number of NCTC 2472 cells were injected such that assessment of sarcoma-induced bone cancer pain was not confounded by the presence of post-surgical (orthopedic) pain. Injection of too high a concentration of NCTC 2472 cells would induce significant tumor burden and bone destruction within a few days post-surgery and preclude differentiation between the pain caused by the arthrotomy from that of cancer-induced changes in bone remodeling.
Additional limitations of the current study are that the in vivo analysis was only performed using one tumor cell line, and that a single species and gender was used in the present study. However, previous work from our laboratory has investigated the effect of anti-NGF on bone cancer pain using implantation of breast cancer and prostate cancer cells (11, 12, 14, 15). These previous studies and the current study show anti-NGF effectively attenuates bone pain in all three mouse models of bone cancer pain.
Concerning the potential for observer bias in the analysis of pain behavior, we believe that due to the preventive measures taken in this work we have precluded, as much as humanly possible, the occurrence of subjective and biased pain measurements. Personnel measuring pain behaviors and analyzing x-rays were scrupulously blinded to the therapy treatments. Parameters for spontaneous nocifensive pain and limb use were specifically delineated prior to the start of this work, and videotaping of mice enabled careful (and repeat) analysis of limb movements. Use of a commercial, floor-instrumented dynamic weight bearing system enables objective measurement of weight borne by both ipsilateral and contralateral hind limbs.
The major conclusions of the present study are that early, sustained sequestration of NGF reduces tumor-induced bone destruction, delays time-to-fracture, increases use of the tumor-bearing limb, and reduces weight loss in animals with bone cancer. Blockade of NGF reduced bone cancer pain by 40-70% depending on the pain measure being assessed, and this analgesic effect was maintained even in animals with late stage disease. Given the potential effects of sequestration of NGF on disease progression, weight loss and pain, and the side effect profile observed with the 11,000 individuals who have received anti-NGF therapy to date (16), early rather than late blockade of NGF may improve the functional status and quality of life of patients with CMB.
Supplementary Material
Acknowledgments
Financial support: This work was supported by the National Institutes of Health (grants CA154550, NS023970).
Footnotes
Disclosure of Potential Conflicts of Interest: The authors disclose no potential conflicts of interest.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim T, Farolfi A, Mercatali L, Ricci M, Amadori D. Metastatic bone disease in the era of bone-targeted therapy: clinical impact. Tumori. 2013;99:1–9. doi: 10.1177/030089161309900101. [DOI] [PubMed] [Google Scholar]
- 3.Cancer.org. American Cancer Society, Inc.; 2014. [updated 2013 Dec 31; cited 2013 Apr 5]. Internet. Available from: http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2013/index. [Google Scholar]
- 4.Farrell C. Bone metastases: assessment, management and treatment options. Br J Nurs. 2013;22:S4, S6, S8–11. doi: 10.12968/bjon.2013.22.Sup7.S4. [DOI] [PubMed] [Google Scholar]
- 5.von Moos R, Sternberg C, Body JJ, Bokemeyer C. Reducing the burden of bone metastases: current concepts and treatment options. Support Care Cancer. 2013;21:1773–83. doi: 10.1007/s00520-013-1755-1. [DOI] [PubMed] [Google Scholar]
- 6.Tolia M, Zygogianni A, Kouvaris JR, Meristoudis C, Margari N, Karakitsos P, et al. The key role of bisphosphonates in the supportive care of cancer patients. Anticancer Res. 2014;34:23–37. [PubMed] [Google Scholar]
- 7.Rolfo C, Raez LE, Russo A, Reguart N, Campelo RG, Bronte G, et al. Molecular target therapy for bone metastasis: starting a new era with denosumab, a RANKL inhibitor. Expert Opin Biol Ther. 2014;14:15–26. doi: 10.1517/14712598.2013.843667. [DOI] [PubMed] [Google Scholar]
- 8.Mantyh PW. The neurobiology of skeletal pain. Eur J Neurosci. 2014;39:508–19. doi: 10.1111/ejn.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantyh P. Bone cancer pain: Causes, consequences, and therapeutic opportunities. Pain. 2013;154:S54–62. doi: 10.1016/j.pain.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 10.Seidel MF, Wise BL, Lane NE. Nerve growth factor: an update on the science and therapy. Osteoarthritis Cartilage. 2013;21:1223–8. doi: 10.1016/j.joca.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jimenez-Andrade JM, Ghilardi JR, Castaneda-Corral G, Kuskowski MA, Mantyh PW. Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain. Pain. 2011;152:2564–74. doi: 10.1016/j.pain.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mantyh WG, Jimenez-Andrade JM, Stake JI, Bloom AP, Kaczmarska MJ, Taylor RN, et al. Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience. 2010;171:588–98. doi: 10.1016/j.neuroscience.2010.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sevcik MA, Ghilardi JR, Peters CM, Lindsay TH, Halvorson KG, Jonas BM, et al. Anti-NGF therapy profoundly reduces bone cancer pain and the accompanying increase in markers of peripheral and central sensitization. Pain. 2005;115:128–41. doi: 10.1016/j.pain.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Halvorson KG, Kubota K, Sevcik MA, Lindsay TH, Sotillo JE, Ghilardi JR, et al. A blocking antibody to nerve growth factor attenuates skeletal pain induced by prostate tumor cells growing in bone. Cancer Res. 2005;65:9426–35. doi: 10.1158/0008-5472.CAN-05-0826. [DOI] [PubMed] [Google Scholar]
- 15.Bloom AP, Jimenez-Andrade JM, Taylor RN, Castaneda-Corral G, Kaczmarska MJ, Freeman KT, et al. Breast cancer-induced bone remodeling, skeletal pain, and sprouting of sensory nerve fibers. J Pain. 2011;12:698–711. doi: 10.1016/j.jpain.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leite VF, Buehler AM, El Abd O, Benyamin RM, Pimentel DC, Chen J, et al. Anti-nerve growth factor in the treatment of low back pain and radiculopathy: a systematic review and a meta-analysis. Pain Physician. 2014;17:E45–60. [PubMed] [Google Scholar]
- 17.Tiseo PJ, Kivitz AJ, Ervin JE, Ren H, Mellis SJ. Fasinumab (REGN475), an antibody against nerve growth factor for the treatment of pain: results from a double-blind, placebo-controlled exploratory study in osteoarthritis of the knee. Pain. 2014;155:1245–52. doi: 10.1016/j.pain.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Molloy NH, Read DE, Gorman AM. Nerve growth factor in cancer cell death and survival. Cancers (Basel) 2011;3:510–30. doi: 10.3390/cancers3010510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hondermarck H. Neurotrophins and their receptors in breast cancer. Cytokine Growth Factor Rev. 2012;23:357–65. doi: 10.1016/j.cytogfr.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Kruttgen A, Schneider I, Weis J. The dark side of the NGF family: neurotrophins in neoplasias. Brain Pathol. 2006;16:304–10. doi: 10.1111/j.1750-3639.2006.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vinores SA, Perez-Polo JR. Nerve growth factor and neural oncology. J Neurosci Res. 1983;9:81–100. doi: 10.1002/jnr.490090110. [DOI] [PubMed] [Google Scholar]
- 22.Adriaenssens E, Vanhecke E, Saule P, Mougel A, Page A, Romon R, et al. Nerve growth factor is a potential therapeutic target in breast cancer. Cancer Res. 2008;68:346–51. doi: 10.1158/0008-5472.CAN-07-1183. [DOI] [PubMed] [Google Scholar]
- 23.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 24.Clohisy DR, Ogilvie CM, Ramnaraine ML. Tumor osteolysis in osteopetrotic mice. J Orthop Res. 1995;13:892–7. doi: 10.1002/jor.1100130613. [DOI] [PubMed] [Google Scholar]
- 25.Clohisy DR, Ogilvie CM, Carpenter RJ, Ramnaraine ML. Localized, tumor-associated osteolysis involves the recruitment and activation of osteoclasts. J Orthop Res. 1996;14:2–6. doi: 10.1002/jor.1100140103. [DOI] [PubMed] [Google Scholar]
- 26.Sabino MA, Ghilardi JR, Jongen JL, Keyser CP, Luger NM, Mach DB, et al. Simultaneous reduction in cancer pain, bone destruction, and tumor growth by selective inhibition of cyclooxygenase-2. Cancer Res. 2002;62:7343–9. [PubMed] [Google Scholar]
- 27.Schwei MJ, Honore P, Rogers SD, Salak-Johnson JL, Finke MP, Ramnaraine ML, et al. Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J Neurosci. 1999;19:10886–97. doi: 10.1523/JNEUROSCI.19-24-10886.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luger NM, Honore P, Sabino MA, Schwei MJ, Rogers SD, Mach DB, et al. Osteoprotegerin diminishes advanced bone cancer pain. Cancer Res. 2001;61:4038–47. [PubMed] [Google Scholar]
- 29.Lozano-Ondoua AN, Wright C, Vardanyan A, King T, Largent-Milnes TM, Nelson M, et al. A cannabinoid 2 receptor agonist attenuates bone cancer-induced pain and bone loss. Life Sci. 2010;86:646–53. doi: 10.1016/j.lfs.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghilardi JR, Freeman KT, Jimenez-Andrade JM, Mantyh WG, Bloom AP, Kuskowski MA, et al. Administration of a tropomyosin receptor kinase inhibitor attenuates sarcoma-induced nerve sprouting, neuroma formation and bone cancer pain. Mol Pain. 2010;6:87. doi: 10.1186/1744-8069-6-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sevcik MA, Ghilardi JR, Halvorson KG, Lindsay TH, Kubota K, Mantyh PW. Analgesic efficacy of bradykinin B1 antagonists in a murine bone cancer pain model. J Pain. 2005;6:771–5. doi: 10.1016/j.jpain.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Honore P, Rogers SD, Schwei MJ, Salak-Johnson JL, Luger NM, Sabino MC, et al. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98:585–98. doi: 10.1016/s0306-4522(00)00110-x. [DOI] [PubMed] [Google Scholar]
- 33.Honore P, Luger NM, Sabino MA, Schwei MJ, Rogers SD, Mach DB, et al. Osteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain and pain-related neurochemical reorganization of the spinal cord. Nat Med. 2000;6:521–8. doi: 10.1038/74999. [DOI] [PubMed] [Google Scholar]
- 34.Hongo JS, Laramee GR, Urfer R, Shelton DL, Restivo T, Sadick M, et al. Antibody binding regions on human nerve growth factor identified by homolog- and alanine-scanning mutagenesis. Hybridoma. 2000;19:215–27. doi: 10.1089/02724570050109611. [DOI] [PubMed] [Google Scholar]
- 35.Ghilardi JR, Freeman KT, Jimenez-Andrade JM, Coughlin KA, Kaczmarska MJ, Castaneda-Corral G, et al. Neuroplasticity of sensory and sympathetic nerve fibers in a mouse model of a painful arthritic joint. Arthritis Rheum. 2012;64:2223–32. doi: 10.1002/art.34385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koewler NJ, Freeman KT, Buus RJ, Herrera MB, Jimenez-Andrade JM, Ghilardi JR, et al. Effects of a monoclonal antibody raised against nerve growth factor on skeletal pain and bone healing after fracture of the C57BL/6J mouse femur. J Bone Miner Res. 2007;22:1732–42. doi: 10.1359/jbmr.070711. [DOI] [PubMed] [Google Scholar]
- 37.Jimenez-Andrade JM, Martin CD, Koewler NJ, Freeman KT, Sullivan LJ, Halvorson KG, et al. Nerve growth factor sequestering therapy attenuates non-malignant skeletal pain following fracture. Pain. 2007;133:183–96. doi: 10.1016/j.pain.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 38.Shelton DL, Zeller J, Ho WH, Pons J, Rosenthal A. Nerve growth factor mediates hyperalgesia and cachexia in auto-immune arthritis. Pain. 2005;116:8–16. doi: 10.1016/j.pain.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 39.Iwakura N, Ohtori S, Orita S, Yamashita M, Takahashi K, Kuniyoshi K. Role of low-affinity nerve growth factor receptor inhibitory antibody in reducing pain behavior and calcitonin gene-related Peptide expression in a rat model of wrist joint inflammatory pain. J Hand Surg Am. 2010;35:267–73. doi: 10.1016/j.jhsa.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 40.Ye Y, Dang D, Zhang J, Viet CT, Lam DK, Dolan JC, et al. Nerve growth factor links oral cancer progression, pain, and cachexia. Mol Cancer Ther. 2011;10:1667–76. doi: 10.1158/1535-7163.MCT-11-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reddy A, Yennurajalingam S, de la Cruz M, Palla SL, Wang X, Kwon JH, et al. Factors associated with survival after opioid rotation in cancer patients presenting to an outpatient supportive care center. J Pain Symptom Manage. 2014;48:92–8. doi: 10.1016/j.jpainsymman.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 42.Sabino MA, Luger NM, Mach DB, Rogers SD, Schwei MJ, Mantyh PW. Different tumors in bone each give rise to a distinct pattern of skeletal destruction, bone cancer-related pain behaviors and neurochemical changes in the central nervous system. Int J Cancer. 2003;104:550–8. doi: 10.1002/ijc.10999. [DOI] [PubMed] [Google Scholar]
- 43.Lozano-Ondoua AN, Hanlon KE, Symons-Liguori AM, Largent-Milnes TM, Havelin JJ, Ferland HL, 3rd, et al. Disease modification of breast cancer-induced bone remodeling by cannabinoid 2 receptor agonists. J Bone Miner Res. 2013;28:92–107. doi: 10.1002/jbmr.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howlader N, Noone A, Krapcho M, Garshell J, Neyman N, Altekruse S, et al., editors. SEER Cancer Statistics Review, 1975-2010. National Cancer Institute; 2013. [updated Apr 2013; cited 2013 May 1]. Internet. nAvailable from: http://seer.cancer.gov/csr/1975_2010/ [Google Scholar]
- 45.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–95. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 46.Hustedt JW, Blizzard DJ, Baumgaertner MR, Leslie MP, Grauer JN. Current advances in training orthopaedic patients to comply with partial weight-bearing instructions. Yale J Biol Med. 2012;85:119–25. [PMC free article] [PubMed] [Google Scholar]
- 47.Coutts F. Gait analysis in the therapeutic environment. Man Ther. 1999;4:2–10. doi: 10.1016/s1356-689x(99)80003-4. [DOI] [PubMed] [Google Scholar]
- 48.Baldwin K, Yannascoli SM, Namdari S, Spiegel DA, Keenan MA. What's new in orthopaedic rehabilitation. J Bone Joint Surg Am. 2013;95:2071–7. doi: 10.2106/JBJS.M.01037. [DOI] [PubMed] [Google Scholar]
- 49.Mercadante S. Managing breakthrough pain. Curr Pain Headache Rep. 2011;15:244–9. doi: 10.1007/s11916-011-0191-5. [DOI] [PubMed] [Google Scholar]
- 50.Brown DC, Boston R, Coyne JC, Farrar JT. A novel approach to the use of animals in studies of pain: validation of the canine brief pain inventory in canine bone cancer. Pain Med. 2009;10:133–42. doi: 10.1111/j.1526-4637.2008.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.