Abstract
Background
There are a variety of periprocedural anticoagulation strategies for atrial fibrillation (AF) ablation, including the use of dabigatran. It is unclear which strategy is superior.
Objective
To compare the safety and efficacy of anticoagulation with uninterrupted warfarin, dabigatran, and warfarin with heparin bridging in patients undergoing ablation of AF at four experienced centers.
Methods and Results
In this retrospective analysis, 882 patients (mean age: 61 ± 11 years) underwent ablation of AF using uninterrupted warfarin (n = 276), dabigatran (n = 374), or warfarin with heparin bridging (n = 232) for periprocedural anticoagulation. The rate of total complications was 23/276 (8.3%) in the uninterrupted warfarin group, 30/374 (8.0%) in the dabigatran group, and 29/232 (12.5%) in the bridged group (P = 0.15). Major complications were more frequent in the uninterrupted warfarin group 12/276 (4.3%) compared with 3/374 (0.8%) in dabigatran and 6/232 (2.6%) in the bridged group (P = 0.01). The most common major complication was the need for transfusion or occurrence of major bleeding. Minor complications did not differ among the three groups. On multivariate analysis, female gender (odds ratio [OR] 1.93, confidence interval [CI] 1.16–3.19, P = 0.011), bridging heparin (OR 2.13, CI 1.100–3.941, P = 0.016), use of triple antithrombotic therapy (OR 1.77, CI 1.05–2.98, P = 0.033), and prior myocardial infarction (OR 2.40, CI 1.01–5.67, P = 0.046) independently predicted total complications.
Conclusions
When comparing the use of uninterrupted warfarin, dabigatran, and warfarin with heparin bridging in patients undergoing catheter ablation of AF, dabigatran was not associated with increased risk, major complications were more common in the uninterrupted warfarin group, and after adjustment, warfarin with bridging increased total complications.
Keywords: atrial fibrillation, ablation, pharmacology
Introduction
Atrial fibrillation (AF) is the most common clinical arrhythmia, with an estimated 2.2 million people in the United States and 4.5 million in the European Union afflicted with the disorder.1 Catheter ablation has become an established invasive strategy for drug refractory AF. Approximately 50,000 ablations are performed annually in the United States, and about 60,000 in Europe,2 and favorable outcomes suggest that this approach will remain a popular alternative to chronic drug therapy. There are, however, two feared complications that occur during or shortly after the procedure: thromboembolic events including stroke and major bleeding including cardiac tamponade, with an estimated incidence of 1.33% and 0.94%, respectively.3 The combination of heightened risk of both stroke and hemorrhage is linked to the unique complexity of the AF ablation procedure and to the underlying substrate in patients with AF, and the need to use high-dose periprocedural anticoagulation.
Historically, preprocedure warfarin therapy was discontinued and replaced with bridging low-molecular-weight heparin (LMWH) before and after the ablation, followed by resumption of warfarin at hospital discharge. Although widely adopted throughout the world and endorsed by current formal guidelines, it was recognized that this approach may result in a higher incidence of bleeding complications, especially at the site of vascular access.4,5 All ablation procedures also use intravenous heparin during the procedure. Several studies have suggested that continuation of therapeutic warfarin could reduce thromboembolic complications without increasing the risk of hemorrhagic complications.6–8 The recent 2012 HRS/EHRA/ECAS expert consensus statement notes that uninterrupted warfarin is a potential alternative to bridging with LMWH.5 However, international normalized ratio (INR) levels often fluctuate during warfarin use, and may not be in the optimal therapeutic range in up to >50% of patients.9 Lower or higher INR levels on the day of ablation may increase the risk of complications.
In late 2010, the U.S. Food And Drug Administration approved the first of three novel oral anticoagulants (NOACs) to be used as an alternative to chronic warfarin for stroke prophylaxis in patients with nonvalvular AF. These newer drugs all have favorable pharmacokinetic profiles that lend themselves to use in the periablation period, including rapid onset of therapeutic effect obviating the need for bridging heparin. Most recent studies, with varying sample sizes, control groups, and anticoagulant regimens, have found dabigatran to be equivalent to warfarin.10–14 Only a minority of studies have suggested dabigatran is inferior to warfarin.11
The purpose of this multicenter study was to determine the relative safety and efficacy of anticoagulation with uninterrupted warfarin, dabigatran, and warfarin with bridging LMWH by collecting data from four high-volume and experienced centers in patients undergoing catheter ablation of AF. This study had the following advantages: one of the largest sample sizes in a diverse cohort of patients and centers employing a range of contemporary ablation techniques and anticoagulation regimens.
Methods
Study Protocol
This is a retrospective analysis of consecutive patients undergoing AF ablation at four centers in the United States. All consecutive patients at each site who underwent ablation for any type of AF were included, from a start date of October 2010, the date of dabigatran availability in the United States, until the database was closed in October 2012. Data collection and analysis were approved by each center’s Institutional Review Board.
Patients with all forms of AF, including paroxysmal, persistent, and longstanding persistent, were included. In accordance with current HRS/EHRA/ECAS guidelines,5 all patients had symptomatic AF refractory or intolerant to at least one Class 1 or 3 antiarrhythmic medication, or in some cases, prior to initiation of antiarrhythmic drug therapy.5 Procedures were either primary ablation or redo procedures. The technique of ablation was at the discretion of the investigator and ablation laboratory. The clinical characteristics of patients, the nature of AF, and the technical aspects of the ablation procedure were carefully reviewed from lab and hospital records, and other source documents, and tabulated.
The choice of anticoagulation regimen was at the discretion of the center/operator. Three broad categories were used:
Uninterrupted warfarin with documented therapeutic INR. Patients were started on warfarin ≥4 weeks prior to the procedure with a therapeutic INR goal of 2.0–3.0. INR levels were measured on the same day of the procedure and were required to be >2.0. If subtherapeutic, the patients were treated with bridging LMWH and included in category #2. Warfarin was administered on the night of the procedure and according to prior regimen thereafter in patients with therapeutic INR.
Pre- and postprocedural dabigatran. Dabigatran was dosed at 150 mg twice daily (bid) for creatinine clearance >30 mL/min and 75 mg bid if creatinine clearance was 15–30 mL/min. Dabigatran was started ≥4 weeks preprocedure with the last dose per institutional protocol, ranging from 12 to 48 hours preprocedure. Dabigatran was resumed in the evening postprocedure and continued during the follow-up period.
Pre- and postprocedure warfarin with periprocedure enoxaparin (LMWH) bridging. Warfarin was started ≥4 weeks preprocedure and held 5 days prior to the procedure. LMWH was dosed at 1 mg/kg 3 days prior to the procedure or when INR was <2.0, and 0.5–0.6 mg/kg postprocedure until a therapeutic INR was documented with warfarin therapy. Warfarin was administered on the night of the procedure according to prior regimen and LMWH was continued until a therapeutic INR was established.
A small number of patients (N = 76) with low CHADS2 score were either on no anticoagulation or on aspirin only preablation and these patients were excluded from the analyses. A small number of patients received rivaroxaban (N = 16) but we anticipated too small a sample size so these patients were a priori excluded as well. In a minority of patients (N = 5) with renal dysfunction, LMWH dose was adjusted for renal function.
Classification and Definition of End Points
Analyses included comparisons between major and minor complications that occurred during and up to 30 days after the catheter ablation procedure. Total complication rate represented the sum of major and minor complications. Complications were based on the 2012 HRS/EHRA/ECAS definitions.5 An event was classified as “major” if a complication resulted in permanent injury or death, required intervention for treatment, or required or prolonged hospitalization for >48 hours. Events in this category included death, stroke, or transient ischemic attack (TIA); cardiac tamponade; bleeding of any kind that necessitated blood transfusion or resulted in a 20% or greater fall in hematocrit; and surgical intervention for any vascular complication. Minor complications included bleeding from any source requiring medical attention but not requiring transfusion or surgery (e.g., groin hematoma not requiring evacuation; pseudoaneurysm not requiring intervention; and pericardial effusion without intervention).
Ablation Procedure
Electrophysiologic study and catheter ablation were performed according to local protocol. Procedures were conducted under moderate or deep sedation using propofol, fentanyl sodium, and midazolam at the direction of an anesthesiologist in two centers and under general anesthesia in two centers. Transesophageal echocardiogram was routinely performed prior to the procedure in three of the four centers, and selectively in one center. Only 18 patients (2%) had the procedure canceled due to presence of left atrial (LA) thrombus. Vascular access was obtained through standard technique and via bilateral femoral veins. Hemodynamic monitoring was performed using either radial or femoral arterial lines.
Intraprocedural unfractionated heparin was administered according to institutional protocol. In three of the four centers, heparin bolus (range 70–100 units/kg) and infusion (100 units/hour) were instituted prior to transseptal puncture, and in one center, heparin bolus (80 units/kg) and infusion (18 units/kg/hour) were initiated immediately following transseptal puncture. Three centers targeted activated clotting time (ACT) 300–350 seconds and one center targeted ACT 300–400 seconds. Protamine was given in all centers after catheters were withdrawn from the left atrium at a dose 0.5–1 mg/100 units of heparin used in the preceding 2 hours.
After transseptal puncture, pulmonary vein isolation (PVI) was performed in all patients with the guidance of a three-dimensional electroanatomical mapping system. All pulmonary veins (PVs) were mapped with a circular mapping catheter. Ablation of complex fractionated atrial electrograms or linear atrial ablation was performed at the discretion of the operator.
All patients were examined and had electrocardiographic monitoring during an overnight hospital stay after the ablation. In patients who had a complication, further diagnostic and therapeutic interventions were performed as clinically appropriate. All patients were seen in an outpatient clinic 4–6 weeks after the procedure or sooner as necessary. Patients self-reported symptoms suggestive of a complication and were seen by a physician to categorize and treat complications as needed.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation and compared by Student’s t-test or Wilcoxon’s rank sum whenever the distribution was not normal. Categorical variables were reported as counts and percentages and compared using χ2 test or Fisher’s exact test as appropriate. A multivariate logistic regression analysis was performed to determine predictors of complications.
All data were analyzed using IBM/SPSS statistical software version 19.0 (IBM Corp., Armonk, NY, USA). A P value of <0.05 indicated statistical significance.
Results
Study Subjects
A total of 882 patients were analyzed. Table I summarizes the baseline demographics and clinical characteristics of these patients. Patients were 61 ± 11 years of age and predominantly male. The groups were generally well balanced; however, the dabigatran group had slightly lower frequency of hypertension, diabetes, and congestive heart failure, and slightly lower body mass index. The majority of patients had paroxysmal AF (58%) or persistent AF (39%); only a few had longstanding persistent AF. Left ventricular (LV) function was normal and moderate LA enlargement was present but a bit lower in the bridged group. Among all procedures, 40.1% were redo ablations. The mean CHADS2 score was 1.2 ± 1.2, and the dabigatran group had the lowest overall CHADS2 score. The mean HASBLED score was 2.3 ± 1.3, and the uninterrupted warfarin group had the lowest overall HASBLED score.
Table I.
Patient Characteristics
| Uninterrupted Warfarin (n = 276) | Dabigatran (n = 374) | Bridged (n = 232) | P Value | |
|---|---|---|---|---|
| Age (years) | 62 ± 11 | 61 ± 12 | 61 ± 11 | 0.14 |
| Male | 178 (64.5%) | 266 (71.1%) | 160 (69.0%) | 0.19 |
| BMI | 31 ± 6 | 29 ± 6 | 31 ± 6 | 0.024 |
| Diabetes | 56 (20.3%) | 45 (12.0%) | 39 (16.8%) | 0.016 |
| Hypertension | 183 (66.3%) | 214 (57.2%) | 158 (68.1%) | 0.010 |
| CHF | 54 (19.6%) | 38 (10.2%) | 27 (11.6%) | 0.002 |
| History of stroke/TIA | 22 (7.9%) | 39 (10.4%) | 17 (7.3%) | 0.49 |
| CAD | 51 (18.5%) | 58 (15.5%) | 48 (20.7%) | 0.25 |
| Prior MI | 16 (5.8%) | 16 (4.3%) | 8 (3.4%) | 0.43 |
| LVEF (%) | 55 ± 11 | 56 ± 10 | 55 ± 9 | 0.21 |
| LA dimension (mm) | 46 ± 10 | 45 ± 9 | 42 ± 6 | <0.001 |
| Type of AF | 0.19 | |||
| Paroxysmal | 174 (63.0%) | 208 (55.6%) | 130 (56.0%) | 0.190 |
| Persistent | 98 (35.5%) | 156 (41.7%) | 93 (40.1%) | 0.190 |
| Longstanding persistent | 4 (1.5%) | 10 (2.7%) | 9 (3.9%) | 0.190 |
| CHADS2 score | 1.4 ± 1.4 | 1.1 ± 1.1 | 1.2 ± 1.1 | <0.001 |
| HASBLED Score | 2.1 ± 1.5 | 2.2 ± 1.3 | 2.8 ± 1.0 | <0.0001 |
Boldface indicates statistically significance. AF = atrial fibrillation; BMI = body mass index; CAD = coronary artery disease; CHF = congestive heart failure; LA = left atrial; LVEF = left ventricular ejection fraction; MI = myocardial infarction; TIA = transient ischemic attack.
Selected medication usage is summarized in Table II. Triple therapy (aspirin, thienopyridine, and an anticoagulant) was used in 21.4% and concomitant aspirin alone in 22.0%.
Table II.
Medical Therapy
| Uninterrupted Warfarin (N = 276) | Dabigatran (N = 374) | Bridged (N = 232) | P Value | |
|---|---|---|---|---|
| Thienopyridine | 18 (6.5%) | 25 (6.7%) | 9 (3.9%) | 0.31 |
| Aspirin | 39 (14.1%) | 89 (23.8%) | 66 (28.4%) | 0.0001 |
| Triple therapy | 54 (19.6%) | 107 (28.6%) | 28 (12.1%) | 0.005 |
| Statin | 137 (49.6%) | 206 (55.1%) | 127 (54.7%) | 0.34 |
| Fish oil | 54 (19.6%) | 122 (32.6%) | 74 (31.9%) | <0.0001 |
| Proton pump inhibitors | 104 (37.7%) | 152 (40.6%) | 64 (27.6%) | 0.004 |
Boldface indicates statistically significance.
Anticoagulation Treatment
The distribution of the three predefined (see above) anticoagulation regimens were: 276 (31.2%) on uninterrupted warfarin, 374 (42.5%) on dabigatran, and 232 (26.3%) patients with a bridged regimen. The INR in the three groups was 2.4 ± 0.4 in the uninterrupted warfarin group, 1.3 ± 0.3 in the dabigatran group, and 1.2 ± 0.2 in the bridged group (P<0.0001). Dabigatran was stopped 12–48 hours preablation; the mean time of dabigatran discontinuation was 20 ± 8 hours, and 299/374 (80%) had dabigatran stopped for <24 hours preablation and 75/374 (20%) had dabigatran stopped for >24 hours preablation. Dabigatran was resumed 3–20 hours postablation, mean time 11 ± 8 hours, and in 340/374 (90%) dabigatran was resumed <12 hours postablation. The intraprocedural anticoagulation was unfractionated heparin in all patients. The mean intraprocedural ACT was similar across groups. Complete PVI was achieved or re-established in 860 (97.5%) patients after a procedure duration of 224 ± 63 minutes. Table III summarizes the procedural details.
Table III.
Procedural Data
| Uninterrupted Warfarin (N = 276) | Dabigatran (N = 374) | Bridged (N = 232) | P Value | |
|---|---|---|---|---|
| INR | 2.4 ± 0.4 | 1.3 ± 0.3 | 1.2 ± 0.2 | <0.0001 |
| Intraprocedural ACT (seconds) | 336 ± 24 | 358 ± 42 | 345 ± 35 | 0.26 |
| ≥300 seconds ACT target achieved | 262 (95.0%) | 366 (97.9%) | 231 (99.6%) | 0.24 |
| Redo procedure | 86 (31.2%) | 191 (51.1%) | 88 (37.9%) | <0.0001 |
| PVI achieved | 270 (97.8%) | 363 (97.1%) | 227 (97.8%) | 0.77 |
| Non-PVI ablation | 75 (27.2%) | 112 (29.9%) | 54 (23.3%) | 0.02 |
| Procedure time (minutes) | 218 ± 61 | 225 ± 66 | 226 ± 60 | 0.33 |
Boldface indicates statistically significant. ACT = activated clotting time; PVI = pulmonary vein isolation.
Total Complications
The prevalence of total complications was similar among the three groups: 23/276 (8.3%) for uninterrupted warfarin, 30/374 (8.0%) for dabigatran, and 29/232 (12.5%) for the bridged group (P = 0.15; Table IV). Some patients had more than one complication within the category of major or minor complications; therefore, the sum of complications is greater than the total number of patients within each category.
Table IV.
Total Complications
| Uninterrupted Warfarin (n = 276) | Dabigatran (n = 374) | Bridged (n = 232) | P Value | |
|---|---|---|---|---|
| Total complications | 23 (8.3%) | 30 (8.0%) | 29 (12.5%) | 0.15 |
| Major complications | 12 (4.3%) | 3 (0.8%) | 6 (2.6%) | 0.01 |
| Stroke or TIA | 4 (1.4%) | 1 (0.3%) | 2 (0.9%) | 0.48 |
| Tamponade | 4 (1.4%) | 2 (0.5%) | 2 (0.9%) | 0.48 |
| Transfusion or major bleeding | 6 (2.1%) | 0 (0.0%) | 3 (1.2%) | 0.04 |
| Surgical intervention | 3 (1.1%) | 0 (0.0%) | 1 (0.4%) | 0.13 |
| Minor complications | 19 (6.9%) | 28 (7.5%) | 25 (10.8%) | 0.23 |
| Hematoma | 14 (5.1%) | 23 (6.1%) | 20 (8.6%) | 0.26 |
| Pseudoaneurysm | 4 (1.4%) | 0 (0.0%) | 1 (0.4%) | 0.04 |
| Pericardial effusion | 4 (1.4%) | 5 (1.3%) | 8 (3.4%) | 0.15 |
Boldface indicates statistically significant. Some patients had more than one complication within the category of major or minor complications, therefore the sum of complications is greater than the total number of patients within each category. TIA = transient ischemic attack.
Major Complications
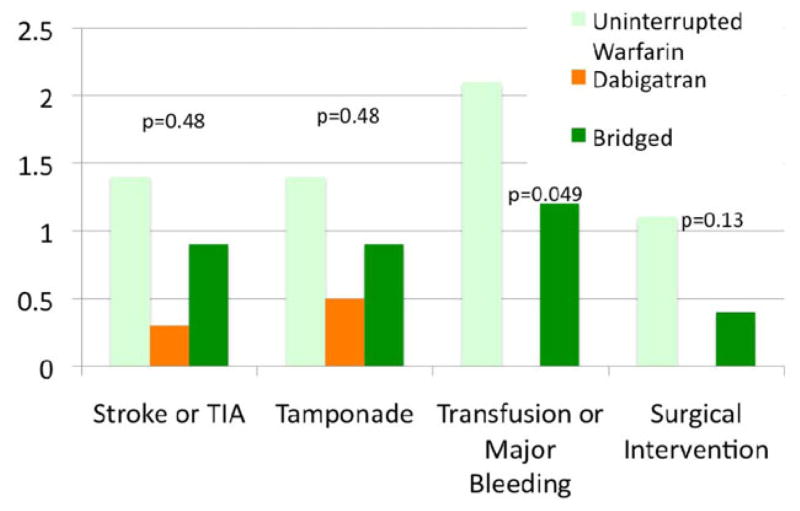
Major complications occurred more frequently in the uninterrupted warfarin group (12/276, 4.3%) versus the dabigatran (3/374, 0.8%) and the bridged groups (6/232, 2.6%; P = 0.01). Major complications were more frequent in the uninterrupted warfarin group for every type of major complication, but due to small numbers, only reached statistical significance for major bleeding requiring transfusion (Fig. 1). The uninterrupted warfarin group had a higher incidence of major complications in each category, including that of stroke and TIA (4/276, 1.4%), tamponade (4/276, 1.4%), and need for surgical intervention (3/276, 1.1%) compared to the other two anticoagulation strategies.
Figure 1.
Incidence of major complications comparing anticoagulation strategies.
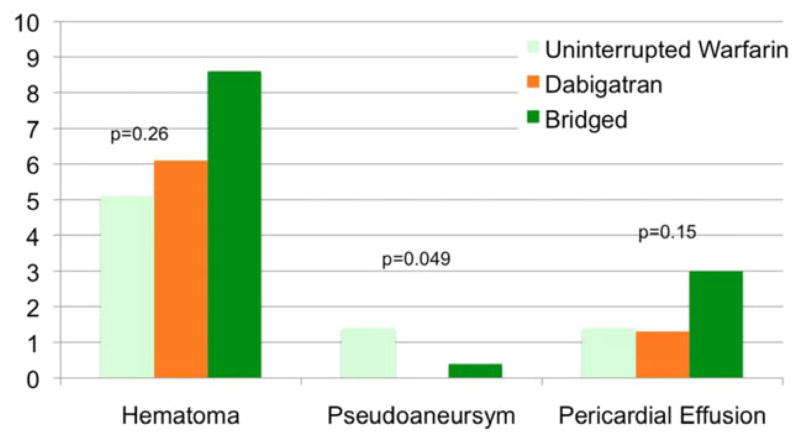
Minor bleeding complications were similar among the three groups: 19/276 (6.9%) for uninterrupted warfarin, 28/374 (7.5%) for dabigatran, and 25/232 (10.8%) for the bridged group (P = 0.23). Hematoma was the most common minor complication in all three groups. Pseudoaneursym was more likely for uninterrupted warfarin (4/276, 1.4%) versus dabigatran (0/374, 0.0%) and bridged groups (1/232, 0.4%; P = 0.049; Fig. 2).
Figure 2.
Incidence of minor complications comparing anticoagulation strategies.
Predictors of Complications
Factors related to total complications (major and minor) on multivariate analysis were female gender (odds ratio [OR] 1.93, confidence interval [CI] 1.16–3.19, P = 0.011), bridging strategy (OR 2.13, CI 1.10–3.94, P = 0.016), use of triple therapy (OR 1.77, CI 1.05–2.98, P = 0.033), and prior myocardial infarction (OR 2.40, CI 1.01–5.67, P = 0.046) after controlling for age, hypertension, diabetes, congestive heart failure, renal disease, prior stroke, type of AF, CHADS2 and HASBLED scores, redo procedure, non-PV ablation, and LV ejection fraction (Table V). Because there were so few bleeding events, a separate stable logistic regression analysis could not be performed. The timing of the last dose of dabigatran preprocedure did not predict complications.
Table V.
Multivariate Predictors of Total Complications
| Adjusted OR | 95% CI | P Value | |
|---|---|---|---|
| Female gender | 1.93 | 1.16–3.19 | 0.011 |
| Bridged versus dabigatran | 2.13 | 1.10–3.94 | 0.016 |
| Triple therapy | 1.77 | 1.05–2.98 | 0.033 |
| Prior MI | 2.40 | 1.01–5.67 | 0.046 |
MI = myocardial infarction.
Discussion
We report a multicenter experience with 882 patients who were treated at four high-volume, geographically diverse centers in the United States. The sampling from several centers was designed to provide a mix of anticoagulation protocols and to represent the range of commonly used procedure practices that can be quite diverse in the current ablation environment. In the absence of randomized clinical trial data, observational series represent a valuable tool to provide contemporaneous data regarding the association of complications with anticoagulation regimens that are employed to reduce thromboembolic events during and after complex ablation procedures. Our study found no statistically significant difference for overall complications after catheter ablation between uninterrupted warfarin, dabigatran, and a bridging strategy. However, a bridging strategy was found to be predictive of total complications after accounting for baseline differences between groups. Major complications occurred more frequently in the uninterrupted warfarin group but were not predictive in the multivariable model for total complications. Minor complications were similar among the three groups. No deaths occurred in any patient group. The increase in major complications associated with uninterrupted warfarin would seem to be a clinically more relevant issue than the increase in the less serious total complications associated with the bridged warfarin group given current trends in periablation anticoagulation.
Historically catheter ablation for AF has been associated with an important serious complication rate, even as experience has increased and techniques have evolved. In the first worldwide survey15 report, overall incidence of major complications was 524/8,745 (6%). Out of the four reported deaths (0.05%), two were caused by massive cerebral thromboembolism. A rate of tamponade of 1.2% was reported, and was the most common major complication.15 When the worldwide survey was updated in 2010,3 the overall incidence of major complications was 741/16,309 (4.5%), slightly lower but still noteworthy. Tamponade remained the most frequent major complication, occurring in 1.3%, and death occurred in 25 patients (0.15%).3 The overall complication rate was similar in the two surveys.
Until recently, warfarin was the mainstay of anticoagulation and two strategies were employed. Historically, warfarin was discontinued before the procedure, and patients were “bridged” with heparin before and after the ablation procedure. Although widely adopted, it was recognized that this approach resulted in a high incidence of bleeding complications, especially at the site of vascular access.16–18 More recently, many AF ablation procedures have been performed under continuous therapeutic anticoagulation with uninterrupted warfarin.6,8,19–23 In a recent large systematic review including nine studies and a total of 27,402 patients, there was a dramatic reduction of periprocedural thromboembolism when catheter ablation was performed with uninterrupted warfarin (n = 6,400) as compared with warfarin discontinuation and heparin bridging (n = 21,002; OR = 0.10; 95% CI, 0.05–0.23; P < 0.001). This analysis failed to disclose any increase in the risk of major bleeding in the therapeutic warfarin group (OR = 0.67; 95% CI, 0.31–1.43; P = 0.30). Minor bleeding complications were also significantly reduced in patients undergoing ablation under therapeutic warfarin (OR = 0.38; 95% CI, 0.21–0.71; P = 0.002).20 In contrast to most previously published studies, we noted an increase in major complications without a reduction in stroke rate associated with the use of uninterrupted warfarin therapy. The reason for these differences in our study is unclear. One possibility is the fact that in all of our patients warfarin therapy was conducted concurrently. It is likely that in many of the previously published studies, the patients may have been on bridged warfarin several years before the patients on uninterrupted warfarin and that the two groups were sequential rather than concurrent. In this case, overall improvement in procedural techniques may have accounted for the apparent benefit of uninterrupted warfarin.
Another emerging anticoagulation strategy involves the use of the NOACs, including the direct thrombin inhibitor dabigatran and the Factor Xa inhibitors rivaroxaban and apixaban, all of which are approved for systemic anticoagulation of patients with nonvalvular AF. The predictable anticoagulant effect of these new agents obviates the need for routine coagulation monitoring. However, the optimal timing for holding and resumption of dabigatran and the other NOACs in the ablation setting is unknown.
Clinical experience with these new anticoagulation agents in association with an AF ablation procedure at the present time is limited.10–15,21 In a case-control comparison, Lakkireddy et al. described a several-fold higher rate of major bleeding complications for dabigatran versus warfarin, (6% vs 1%, P = 0.01).11 Specifically, all major bleeding in the dabigatran group was pericardial tamponade requiring pericardiocentesis. The composite of bleeding and embolic complications was also more frequently observed in the dabigatran group than in the uninterrupted warfarin group (16% vs 6%, P = 0.009). On the basis of these observations, Lakkireddy et al. concluded that in patients undergoing AF ablation, continuation of dabigatran during the periprocedural period is associated with an increased risk. In this study, dabigatran was discontinued on the morning of the procedure and resumed within 3 hours of hemostasis, possibly contributing to the higher than expected bleeding rate. The unusually high tamponade rate was notable as well and is unexplained but contributed to the imbalance in complications.
In contrast to the study of Lakkireddy, most other published studies have shown that dabigatran is equivalent to warfarin as a periprocedural anticoagulant for AF ablation. Kim et al. studied 763 consecutive patients who underwent ablation of AF, including 572 patients on uninterrupted warfarin and 191 on dabigatran.12 Dabigatran was held after the morning dose on the day before the procedure and resumed 4 hours after vascular hemostasis was achieved. The prevalence of major (2.1%) and minor (2.1%) bleeding complications in the dabigatran group was similar to the uninterrupted warfarin group. Kaseno et al. studied 211 patients and found no difference in death and symptomatic thromboembolism between the two regimens and a total bleeding rate of 4.5% on dabigatran and 12.9% (P < 0.05) on uninterrupted warfarin.13 Snipelisky et al. studied 156 patients and found no major complications periablation but a 19.3% minor complication rate on dabigatran-treated patients versus 16.8% on uninterrupted warfarin (P = 0.73).14 Bassiouny et al. examined 999 patients, 276 on dabigatran and 623 on uninterrupted warfarin.23 In this study, dabigatran was held one to two doses prior to PVI and restarted at the conclusion of the procedure or as soon as patients were transferred to the nursing floor. Propensity score matching was applied to generate a cohort of 344 patients in each group with balanced baseline data. Total hemorrhagic and thromboembolic complications were similar in both groups before (3.2% vs 3.9%; P = 0.59) and after (3.2% vs 4.1%; P = 0.53) matching. Major hemorrhage occurred in 1.1% versus 1.6% (P = 0.48) before, and 1.2% versus 1.5% (P = 0.74) after matching in the dabigatran versus warfarin group, respectively. A single thromboembolic event occurred in each of the dabigatran and warfarin groups. Haines et al. studied a total of 202 patients who received dabigatran as part of their periprocedural anticoagulation regimen at the time of initial or redo catheter ablation. A comparison group of 202 patients treated with warfarin was randomly selected from patients undergoing AF ablation during the same time period. Time to first dose of dabigatran postprocedure was 12.2 ± 10.3 hour. Two dabigatran and no warfarin-treated patients had systemic thromboembolism (P = NS); five dabigatran and three warfarin-treated patients had bleeding complications (P = NS, combined end point P = 0.116). One dabigatran patient had severe pericardial bleeding.24
There have been two recent meta-analyses examining periablation anticoagulation strategies and outcomes. Musat et al. evaluated 694 patients on dabigatran and 1,181 patients on uninterrupted warfarin and found no difference in bleeding (OR = 0.75, 95% CI 0.49–1.13) and embolic complications (OR = 2.39, 95% CI 0.84–6.80) between the two regimens.25 Steinberg et al. evaluated a total of 1,501 patients receiving dabigatran and 2,356 receiving warfarin. The dabigatran group demonstrated a numerical excess of neurological events 10/1,501 (0.7%) versus 4/2,356 (0.2%), but equivalent major bleeding outcomes 24/1,501 (1.6%) versus 40/2,356 (1.7%).26
The timing of dabigatran discontinuation preprocedure and resumption postprocedure was variable among (and often within) the published studies as well as ours, and may account for some of the different observations. In our study, dabigatran was stopped preprocedure 12–48 hours preablation based on the individual center’s protocol and resumed after vascular hemostasis was achieved 3–20 hours postprocedure. This variability highlights that there is still lack of uniformity in how dabigatran is used, but based on the findings described across all studies, holding dabigatran 12–24 hours preablation with resumption at least 3 or more hours after hemostasis is achieved may be the most balanced and prudent approach.
In our study, the complication rates we observed, across all groups and in the three anticoagulant arms, were in the range of other contemporary studies and surveys.10–15,22–24 Hematoma was the most frequent observation. For example, the total complication rate in the entire cohort was 9.3%, within the range of 4.7–14% previously published. Similarly, the major complication rate was 2.4%, also within the range of 2.1–8.1% previously described, as was the most serious observations of tamponade and stroke/TIA.
We found dabigatran to be safe and comparable to uninterrupted warfarin or bridging in a diverse cohort of patients undergoing ablation for AF, despite a higher overall use of thienopyridines, aspirin, and triple therapy. In an effort to account for differences in clinical characteristics among groups, we performed a multivariable analysis to arrive at our conclusions. Major complications were more frequent in the uninterrupted warfarin group, principally due to a higher occurrence of major bleeding or need for transfusion. Minor complications were comparable among the three groups.
Factors related to total complications on a multivariate model were female gender, bridging, use of triple therapy, and prior myocardial infarction, after controlling for differences in baseline variables. This is clinically important since bridging predicts risk of total complications and minor complications and is a more expensive and cumbersome strategy compared to the other periablation strategies. Compared to the other multivariate predictors of risk of complications, bridging strategy is easiest to change to an alternate anticoagulation regimen to decrease periablation risk. Avoiding triple antiplatelet/anticoagulant therapy would also be judicious if feasible.
Study Limitations
This was an observational nonrandomized case series, and the groups were not balanced in all clinical characteristics. There may be unmeasured differences that influenced outcomes. Although ablation protocols may have differed slightly among centers, the global ablation strategies were similar. The optimal timing for holding and resumption of dabigatran in the ablation setting is unknown, and thus varied among centers. Although timing of dabigatran did not predict complications, the sample size may have precluded a comparative analysis. We did not have sufficient sample size to test the other NOACs besides dabigatran.
Conclusions
When comparing the use of uninterrupted warfarin, dabigatran, and warfarin with heparin bridging in patients undergoing catheter ablation of AF, dabigatran was not associated with increased risk, major complications were more common in the uninterrupted warfarin group, and after adjustment, warfarin with bridging increased total complications.
References
- 1.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation: An update of the 2010 ESC guidelines for the management of atrial fibrillation–developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14:1385–1413. doi: 10.1093/europace/eus305. [DOI] [PubMed] [Google Scholar]
- 2.Gerstenfeld EP, Callans D, Dixit S, Lin D, Cooper J, Russo AM, Verdino R, et al. Characteristics of patients undergoing atrial fibrillation ablation: Trends over a seven-year period 1999–2005. J Cardiovasc Electrophysiol. 2007;18:23–28. doi: 10.1111/j.1540-8167.2006.00662.x. [DOI] [PubMed] [Google Scholar]
- 3.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32–38. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 4.Lip GY, Proclemer A, Dagres N, Bongiorni MG, Lewalter T, Blomstrom-Lundqvist C. Periprocedural anticoagulation therapy for devices and atrial fibrillation ablation. Europace. 2012;14:741–744. doi: 10.1093/europace/eus105. [DOI] [PubMed] [Google Scholar]
- 5.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9:632–696. e21. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Di Biase L, Burkhardt JD, Mohanty P, Sanchez J, Horton R, Gallinghouse GJ, Lakkireddy D, et al. Periprocedural stroke and management of major bleeding complications in patients undergoing catheter ablation of atrial fibrillation: The impact of periprocedural therapeutic international normalized ratio. Circulation. 2010;121:2550–2556. doi: 10.1161/CIRCULATIONAHA.109.921320. [DOI] [PubMed] [Google Scholar]
- 7.Hussein AA, Martin DO, Saliba W, Patel D, Karim S, Batal O, Banna M, et al. Radiofrequency ablation of atrial fibrillation under therapeutic international normalized ratio: A safe and efficacious periprocedural anticoagulation strategy. Heart Rhythm. 2009;6:1425–1429. doi: 10.1016/j.hrthm.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Wazni OM, Beheiry S, Fahmy T, Barrett C, Hao S, Patel D, Di Biase L, et al. Atrial fibrillation ablation in patients with therapeutic international normalized ratio: Comparison of strategies of anticoagulation management in the periprocedural period. Circulation. 2007;116:2531–2534. doi: 10.1161/CIRCULATIONAHA.107.727784. [DOI] [PubMed] [Google Scholar]
- 9.Connolly SJ, Pogue J, Eikelboom J, Flaker G, Commerford P, Franzosi MG, Healey JS, et al. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008;118:2029–2037. doi: 10.1161/CIRCULATIONAHA.107.750000. [DOI] [PubMed] [Google Scholar]
- 10.Winkle RA, Mead RH, Engel G, Kong MH, Patrawala RA. The use of dabigatran immediately after atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2012;23:264–268. doi: 10.1111/j.1540-8167.2011.02175.x. [DOI] [PubMed] [Google Scholar]
- 11.Lakkireddy D, Reddy YM, Di Biase L, Vanga SR, Santangeli P, Swarup V, Pimentel R, et al. Feasibility and safety of dabigatran versus warfarin for periprocedural anticoagulation in patients undergoing radiofrequency ablation for atrial fibrillation: Results from a multicenter prospective registry. J Am Coll Cardiol. 2012;59:1168–1174. doi: 10.1016/j.jacc.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Kim JS, She F, Jongnarangsin K, Chugh A, Latchamsetty R, Ghanbari H, Crawford T, et al. Dabigatran vs warfarin for radiofrequency catheter ablation of atrial fibrillation. Heart Rhythm. 2012;10:483–489. doi: 10.1016/j.hrthm.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Kaseno K, Naito S, Nakamura K, Sakamoto T, Sasaki T, Tsukada N, Hayano M, et al. Efficacy and safety of periprocedural dabigatran in patients undergoing catheter ablation of atrial fibrillation. Circ J. 2012;76:2337–2342. doi: 10.1253/circj.cj-12-0498. [DOI] [PubMed] [Google Scholar]
- 14.Snipelisky D, Kauffman C, Prussak K, Johns G, Venkatachalam K, Kusumoto F. A comparison of bleeding complications post-ablation between warfarin and dabigatran. J Interv Card Electrophysiol. 2012;35:29–33. doi: 10.1007/s10840-012-9708-z. [DOI] [PubMed] [Google Scholar]
- 15.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100–1105. doi: 10.1161/01.CIR.0000157153.30978.67. [DOI] [PubMed] [Google Scholar]
- 16.Haines DE, Stewart MT, Dahlberg S, Barka ND, Condie C, Fiedler GR, Kirchhof NA, et al. Microembolism and catheter ablation I: A comparison of irrigated radiofrequency and multielectrode-phased radiofrequency catheter ablation of pulmonary vein ostia. Circ Arrhythm Electrophysiol. 2013;6:16–22. doi: 10.1161/CIRCEP.111.973453. [DOI] [PubMed] [Google Scholar]
- 17.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation): Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–e354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 18.Abhishek F, Heist EK, Barrett C, Danik S, Blendea D, Correnti C, Khan Z, et al. Effectiveness of a strategy to reduce major vascular complications from catheter ablation of atrial fibrillation. J Interv Card Electrophysiol. 2011;30:211–215. doi: 10.1007/s10840-010-9539-8. [DOI] [PubMed] [Google Scholar]
- 19.Gopinath D, Lewis WR, Di Biase L, Natale A. Pulmonary vein antrum isolation for atrial fibrillation on therapeutic coumadin: Special considerations. J Cardiovasc Electrophysiol. 2011;22:236–239. doi: 10.1111/j.1540-8167.2010.01940.x. [DOI] [PubMed] [Google Scholar]
- 20.Santangeli P, Di Biase L, Horton R, Burkhardt JD, Sanchez J, Al-Ahmad A, Hongo R, Beheiry S, et al. Ablation of atrial fibrillation under therapeutic warfarin reduces periprocedural complications: Evidence from a meta-analysis. Circ Arrhythm Electrophysiol. 2012;5:302–311. doi: 10.1161/CIRCEP.111.964916. [DOI] [PubMed] [Google Scholar]
- 21.Santangeli P, Di Biase L, Burkhardt JD, Natale A. Catheter ablation of atrial fibrillation under therapeutic warfarin should be adopted worldwide: Let’s stop waiting for Gadot. J Cardiovasc Electrophysiol. 2013;24:516–518. doi: 10.1111/jce.12092. [DOI] [PubMed] [Google Scholar]
- 22.Kuwahara T, Takahashi A, Yoshihide T, Kobori A, Miyazaki S, Takei A, Fujino T, et al. Prevention of periprocedural ischemic stroke and management of hemorrhagic complications in atrial fibrillation ablation under continuous warfarin administration. J Cardiovasc Electrophysiol. 2013;24:510–515. doi: 10.1111/jce.12069. [DOI] [PubMed] [Google Scholar]
- 23.Bassiouny M, Saliba W, Rickard J, Shao M, Sey A, Diab M, Martin DO, et al. Use of dabigatran for peri-procedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol. 2013;6:460–466. doi: 10.1161/CIRCEP.113.000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haines DE, Mead-Salley M, Salazar M, Marchlinski FE, Zado E, Calkins H, Yarmohmohmaddi H, et al. Dabigatran versus warfarin anticoagulation before and after catheter ablation for the treatment of atrial fibrillation. J Interv Card Electrophysiol. 2013;37:233–239. doi: 10.1007/s10840-013-9800-z. [DOI] [PubMed] [Google Scholar]
- 25.Musat DL, Mittal S, Cox S, Ferrara M, Arshad A, Preminger M, Sichrovsky T, et al. Frequency, characteristics, and timing of CIED remote monitoring detected urgent and emergent event notifications. Proceedings of the Heart Rhythm 2012 33rd Annual Scientific Sessions; 2012. [Google Scholar]
- 26.Steinberg BA, Hasselblad V, Atwater BD, Bahnson TD, Washam JB, Alexander JH, Daubert JP, et al. Dabigatran for periprocedural anticoagulation following radiofrequency ablation for atrial fibrillation: A meta-analysis of observational studies. J Interv Card Electrophysiol. 2013;37:213–221. doi: 10.1007/s10840-013-9813-7. [DOI] [PMC free article] [PubMed] [Google Scholar]