Abstract
Pluripotent stem cells (PSCs) have indisputable cardiomyogenic potential and therefore have been intensively investigated as a potential cardiac regenerative therapy. Current directed differentiation protocols are able to produce high yields of cardiomyocytes from PSCs and studies in small animal models of cardiovascular disease have proven sustained engraftment and functional efficacy. Therefore, the time is ripe for cardiac regenerative therapies using PSC derivatives to be tested in large animal models that more closely resemble the hearts of humans. In this review, we discuss the results of our recent study using human embryonic stem cell derived cardiomyocytes (hESC-CM) in a non-human primate model of ischemic cardiac injury. Large scale remuscularization, electromechanical coupling and short-term arrhythmias demonstrated by our hESC-CM grafts are discussed in the context of other studies using Adult Stem Cells for cardiac regeneration.
Keywords: Heart, Stem Cells, Large animal models, Embryonic Stem Cells, Adult Stem Cells, Cardiac Regeneration
Introduction
Cardiovascular disease is a major cause of morbidity and mortality world-wide (Lozano et al., 2012). Prognosis of heart failure patients admitted to hospital is particularly poor with 5 year mortality approaching 50% (Askoxylakis et al., 2010; Stewart, MacIntyre, Hole, Capewell, & McMurray, 2001). Whilst there has been great progress in recent years in treatment of heart failure and causative cardiac diseases (Yancy et al., 2013) there remains an inexorable decline in cardiac function requiring heart transplantation to avoid death. Unfortunately, demand for donor hearts greatly outstrips supply, such that >99% of potentially eligible patients are not afforded this opportunity. Furthermore, co-morbid non-cardiac disease often precludes recruitment into heart transplantation programs. It is from this unmet need that enormous interest in stem cells for myocardial replacement therapy has rapidly grown. Several cell types including various adult stem cells (ASCs) and pluripotent stem cells (PSCs) have undergone intensive investigation in preclinical models. Many ASCs have also progressed into clinical trials. The first large animal studies using PSCs for cardiac regeneration are now being performed, and PSCs are poised to soon enter the clinical arena for heart repair. This short review will discuss findings of our recent study using human embryonic stem cell (hESC) derived cardiomyocytes (CM) in a non-human primate (NHP) model of ischemic cardiac injury and will discuss these findings in the context of the current wider cardiac regenerative field.
Importance of large animal models
Although large animal models were used commonly in cardiovascular research “back in the day”, in the 1990s there was a marked shift toward mouse models to exploit their genetic malleability. Consequently, much of our more recent knowledge of cardiovascular biology, including initial proofs of concept for novel therapeutics comes from small animals (predominantly mice or rats). However, significant differences in fundamental cardiac characteristics exist between mice, rats and humans (Dixon & Spinale, 2009).
The most obvious differences between these species are the size of their hearts and their heart rates (Table 1) (Gandolfi et al., 2011). Accommodating this range of dynamics are multiple differences at the cellular and molecular level. Differences occur in myocyte size, contractile filament isoforms, ion channels and pumps (Ginis et al., 2004; Haghighi et al., 2003; Rakusan & Nagai, 1994; Stoker, Gerdes, & May, 1982; Wehrens, Kirchhoff, & Doevendans, 2000). Therefore, for research aimed at clinical translation, it is imperative that initial results from rodent studies be confirmed in a large animal model more closely resembling the heart of humans. In studies investigating cell therapy for cardiac regeneration, many different species have been used including pigs (Bolli et al., 2013; Ellison et al., 2011; Hatzistergos et al., 2010; Johnston et al., 2009; Kawamura et al., 2012), dogs (Linke et al., 2005; Perin et al., 2008), sheep (Grieve et al., 2010; Hou et al., 2012; Menard et al., 2005) and monkeys (Bel et al., 2010; Blin et al., 2010). Each of these models has its own advantages and disadvantages which need to be considered, depending on the specific aims of the studies (Gandolfi et al., 2011).
Table 1. Comparison of heart weight, rate and systolic pressure between animals used commonly for models of heart disease.
(Adapted from Gandolfi et al 2011).
| Species | Heart Weight (g) | Heart rate (beat/min) |
Systolic Pressure (mm Hg) |
|---|---|---|---|
| Human | 360–480 | 60–90 | 60–120 |
| Pig | 400–500 | 65–75 | 70–130 |
| Sheep | 240–360 | 70–80 | 80–120 |
| Dog | 160–420 | 60–120 | 120–150 |
| Monkey | 37–52 | 110–140 | 100–130 |
| Rabbit | 9–11 | 120–300 | 70–170 |
| Mouse | 0.14–0.15 | 500–600 | 80–160 |
Large animal models also allow studies using delivery methods that will be used for early stage clinical studies. This is especially important since regulatory bodies will consider the delivery device as part of the treatment to be assessed. Therefore safety and toxicity data using the appropriate delivery method will be required. The most common delivery methods used clinically for cardiac cell therapy are 1) trans-epicardial injection into the myocardium using direct visualization of the heart after thoracotomy (Menasche et al., 2008; Menasche et al., 2003; Mocini et al., 2006), 2) trans-endocardial injection using percutaneous delivery catheters (Heldman et al., 2013; Hendrikx et al., 2006; Perin et al., 2004). These are often used in conjunction with specialized mapping techniques to target areas of the heart with least electromechanical activity 3) intra-coronary infusion usually using an over-the-wire angioplasty balloon (Assmus et al., 2002; Bolli et al., 2013; Janssens et al., 2006; Makkar et al., 2012). (Intravenous delivery has largely been abandoned due low efficiency of homing.) Of these methods the intra-coronary route is the most straightforward and would allow rapid translation by many interventional cardiologists well versed with this technique. The limiting factor for intracoronary delivery is microvascular occlusion, which makes it difficult to restore the billion or so cardiomyocytes lost to serious infarctions. Trans-endocardial injections are more complex, although still reasonably accessible to an experienced interventional cardiologist or electrophysiologist. Trans-epicardial injection is the most invasive delivery approach since it commonly requires thoracotomy, although thoracoscopic injection using a mini-thoracotomy has also been utilized to decrease the invasiveness of the procedure(Ota, Patronik, Schwartzman, Riviere, & Zenati, 2008).
Of these approaches, there is increasing evidence that intramyocardial injection results in greater cell engraftment. This is particularly true for larger cells (MSC, PSC-CM) that are less likely to traverse the vascular barrier at intracoronary injection, compared to, say, bone marrow mononuclear cells.
We recently reported a proof-of-concept study wherein human embryonic stem cell-derived cardiomyocytes (hESC-CMs) were transplanted into the infarcted hearts of non-human primates [ref]. In this study, we elected to use trans-epicardial intramuscular injection of our hESC-CMs after thoracotomy. During pilot experiments we found a significant degree of cell “leakage” from the injection site directly after injection. This was a result of the high pressures within the left ventricular myocardial wall. Therefore, we incorporated a small purse-string suture into our cell delivery technique. The cell injections were made through the ready-placed suture and the purse string tightened around the needle immediately after injection. We demonstrated in pilot studies that this promoted about a 3-fold retention of injectate within the intra-myocardial wall (Chong et al., 2014). We realize that this approach may not ultimately be clinically feasible and further studies utilizing trans-endocardial injection are planned.
Remuscularization of the damaged heart in animal models
Early studies using skeletal myoblasts for cardiac remuscularization have shown that engraftment is possible, however transdifferentiation of myoblasts to cardiomyocytes is not (Murry, Wiseman, Schwartz, & Hauschka, 1996; Reinecke, Poppa, & Murry, 2002). Based on these efforts, investigation of ASCs then PSCs as sources of replacement cardiomyocytes gained momentum. Using small animal models, initial high impact studies suggested that bone marrow (BM) HSCs could transdifferentiate into cardiomyocytes (Orlic et al., 2001). In more rigorous follow up studies, however, genetic lineage tracing showed that bone marrow stem cells differentiated into myeloid cells that did not persist in the infarct; hence marrow-to-myocardium transdifferentiation does not occur (Balsam et al., 2004; Murry et al., 2004). Similarly, although initial reports of BM MSCs being able to generate new myocardium (Shake et al., 2002; Toma, Pittenger, Cahill, Byrne, & Kessler, 2002) subsequent studies have failed to demonstrate significant new donor-derived cadiomyocytes (Dixon et al., 2009). Therefore, it is becoming increasingly accepted that the secretion of various factors and cytokines are responsible for ventricular functional improvements after delivery of these cell types (Mirotsou, Jayawardena, Schmeckpeper, Gnecchi, & Dzau, 2011; Mirotsou et al., 2007; Shintani et al., 2009).
Various endogenous cardiac stem cell (CSC) populations have now been reported to replace damaged myocardium. Early reports suggested engraftment of these cells would lead to robust differentiation and replacement cardiomyocytes (Beltrami et al., 2003; Smith et al., 2007). However, more recent studies show that, like bone marrow mononuclear cells and MSCs, CSCs do not persist long term in the injured heart, and paracrine mechanisms are the predominant mechanism of functional improvement (Li et al., 2012; Malliaras et al., 2012). Indeed, a recent study showed that the leading candidate CSC population, the cell derived from the heart that expresses the c-Kit receptor, does not generate new cardiomyocytes during either normal homeostasis or after infarction [van Berlo, Nature 2014]. This suggests that the c-Kit+ cell is not a stem cell for cardiomyocytes. Large animal studies pivotal in the clinical translational path for CSCs show, at best, low level engraftment of donor cells (Bolli et al., 2013; Johnston et al., 2009). Consequently, the mechanism of functional improvement in clinical trials remains unclear (Bolli et al., 2011; Makkar et al., 2012).
In contrast to ASCs, PSCs possess indisputable potential to form large numbers of spontaneously contracting cardiomyocytes (Burridge, Keller, Gold, & Wu, 2012; Mummery et al., 2012; Murry & Keller, 2008). Furthermore, small animal studies have shown that hESC-CM show long term engraftment in the infarcted heart (Caspi et al., 2007; Fernandes et al., 2010; Laflamme et al., 2005; van Laake et al., 2007) with the cardiomyocyte phenotype being maintained at 3 months after delivery (Fernandes et al., 2010). In addition to hESC-CM, cardiovascular progenitors, also derived from hESC, have shown promising benefits for cardiac regeneration (Bel et al., 2010; Blin et al., 2010; Song et al., 2010).
Despite the robust findings from these small animal studies, large animal experiments using PSC derived CM have been more difficult to accomplish and slower to complete, compared to similar studies using ASC derivatives. The predominant reason for this has been difficulty generating sufficient quantities of CM for such experiments. Whilst efficient methods of undifferentiated hESC propagation and expansion have been available for some time, achieving consistently high purity of cardiomyocytes from resultant differentiation has been more difficult. Early efforts to enhance cardiomyocyte yields used physical purification such as via Percoll-centrifugation (Laflamme et al., 2007). This has proven to be excessively harsh on the treated cells with a drastic reduction in viable cell yields. Since then other strategies to purify CM from differentiation have included live cell sorting for SIPRα+ cells (enriching for CM) (Dubois et al., 2011) and a novel culture based approach exploiting the greater resistance to anaerobic metabolism in CM (Tohyama et al., 2013). In our hands, cell sorting based on SIPRα or selection in a lactate-rich medium has not proven sufficiently scalable for large animal studies, so we have focused on improving our differentiation conditions to the point where we do not need purification. These efforts have proved fruitful. Even without enrichment strategies, current directed differentiation protocols routinely allow the experienced cell biologist to achieve 70–95% CM purity. Furthermore, small molecules modulating the Wnt/β-Catenin pathway show promise in replacing expensive and batch-variable cytokine thereby further improving the reliability and efficient production of hESC-CM (Lian et al., 2012).
Another limitation for any potential cell therapy for cardiac regeneration is low efficiency of donor cell engraftment (Robey, Saiget, Reinecke, & Murry, 2008). The success of engraftment is influenced by the physical retention of cells after delivery, the fraction that dies after being retained, and the proliferation of the surviving fraction. It has long been recognized that there is extensive cell death after transplantation, in part related to ischemia (cell clumps are inherently avascular) and to anoikis (death due to matrix detachment). One strategy developed by our group is to inhibit cell necrosis and apoptosis via multiple pathways by using a “pro-survival cocktail” (Laflamme et al., 2007). Although this cocktail improved survival by several logs, we estimate that ~90% of the retained cells still die post-injection. Hence, there is considerable gain in graft size still possible by addressing the residual mechanisms of cell death.
Whilst the pro-survival cocktail enabled considerable hESC-CM graft size in rodent and guinea pig (Shiba et al., 2012) experiments, the cell numbers required for transplantation into hearts of large animals were calculated to still be at least an order of magnitude greater. The logistics in coordinating the timing of large scale hESC differentiation and complex large animal experiments are extremely difficult and potentially wasteful. Therefore a cryopreservation strategy appeared necessary to uncouple the timing of cell production from cell delivery. Although theoretically possible that cryogenic procedures could prohibit successful hESC-CM engraftment, this has not been the case when directly tested in rodent models (Xu et al., 2011). In our NHP studies we employed and also tested the ability of cryopreserved hESC-CM to engraft in an immune incompetent mouse model (Chong et al., 2014). After demonstrating in mice that engraftment rates of cryopreserved hESC-CM were comparable to those engrafted directly after cell culture, we then employed a strategy of cryopreserving large quantities of hESC-CM after large-scale differentiation efforts. Therefore, the above advances have paved the way for a new wave of large animal experiments investigating the potential of hESC-CM to regenerate the failing/damaged heart.
Remuscularization of the Non-Human Primate Heart
As discussed above, relatively few studies have used PSCs to investigate cardiac regeneration in large animal models. Rhesus monkeys have been used to study the ability of allogeneic NHP ESC derived cardiovascular progenitors transplantation to regenerate infarcted hearts, with (Bel et al., 2010) and without the addition of BM derived MSC (Blin et al., 2010). Recently, human iPSC derived CM have been used in a swine model of MI investigating whether CM cell-sheets induce cardiac regeneration (Kawamura et al., 2012). In these studies however, there were few engrafted cardiomyocytes detected in the hearts of treated animals.
We sought to use hESC-CMs to generate long term grafts of human myocardium in the hearts of non-human primates. Pig-tailed macaques (Macaca nemestrina) were infarcted percutaneously using a balloon catheter, with 90 min of ischemia followed by reperfusion. Nine days later, immunosuppression with cyclosporine A, methylprednisolone and abatacept was commenced and continued until sacrifice. At 2 weeks post-infarction the monkeys were anesthetized and their hearts exposed via left thoracotomy. Under direct visualization we injected 1 billion hESC-CM into the peri-infarct and central ischemic region, using the afore-mentioned purse string technique. Control animals underwent the same myocardial infarction and had “sham” treatment with intra-myocardial injection of vehicle alone (“pro-survival cocktail” and media without cells). Using this model we have shown that clinical scale production and resulting engraftment (Figs 1 and 2) in the NHP infarcted myocardium is possible and reproducible. We enrolled seven macaques into this study and have shown robust graft survival at 3 months (the longest time-point examined). One animal was euthanised prior to cell transplantation due to arterial thrombosis, with the remaining 6 included in data analysis. Myocardial infarcts were consistent (2.5–10.4% of LV), and grafts sizes were large, ranging from 0.7–5.3% of the LV mass (Chong et al., 2014). One monkey had ventricular fibrillation upon myocardial reperfusion and was successfully cardiovereted. The remainder of the animals tolerated the procedure well. To our knowledge, this is by a factor of 10-fold the largest graft of human myocardium that has been achieved, using any animal model and any stem/progenitor cell type. As an indication of progress in this area, figure 3 demonstrates the relative sizes of our hESC-CM grafts in the infarcted rat and macaque hearts.
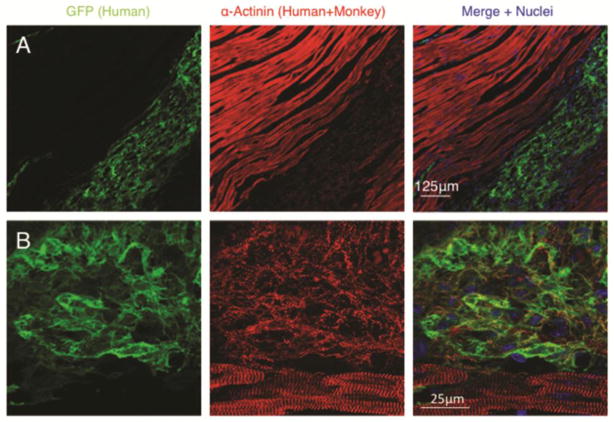
Figure 1. Human cardiomyocyte grafts within injured monkey hearts.
Low power 20× (A) and high power 60× (B) confocal immunofluorescence microscopy of human embryonic stem cell derived cardiomyocytes delivered into the infarcted monkey heart and analysed 14 days after cell delivery. Note the presence of sarcomeric organization and cross-striations present within human cardiomyocytes although much less organized than the host adult monkey cardiomyocytes. GFP= green fluorescent protein.
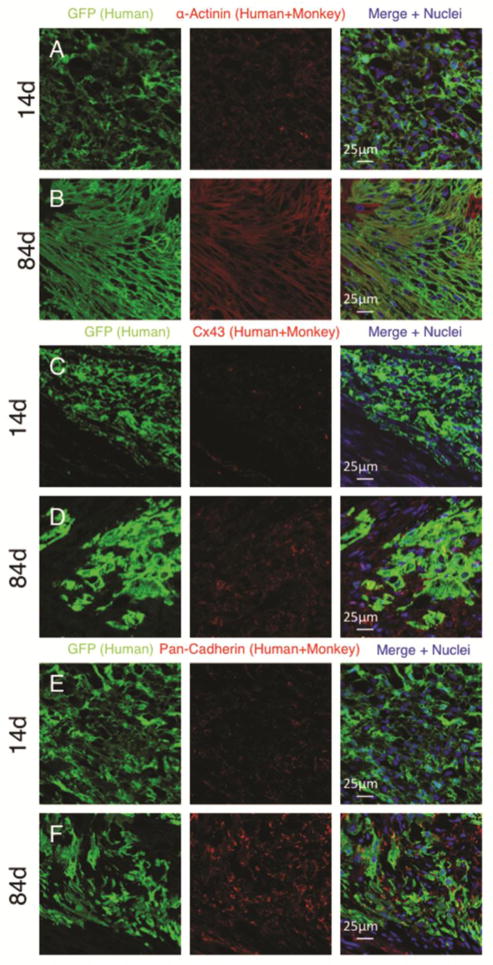
Figure 2. Human cardiomyocyte grafts display maturation with time after delivery into the host heart.
Confocal immunofluorescence of microscopy of human embryonic stem cell derived cardiomyocytes delivered into the infarcted monkey heart. Comparison is made from grafts analysed 14 days (A, C and E) or 84 days (B,D and F) after cell delivery. human cardiomyocytes display increased size and alignment (A–B), increased connexin expression (C–D) and increased cadherin expression (E–F) with time. GFP= green fluorescent protein. Cx43= connexin 43.
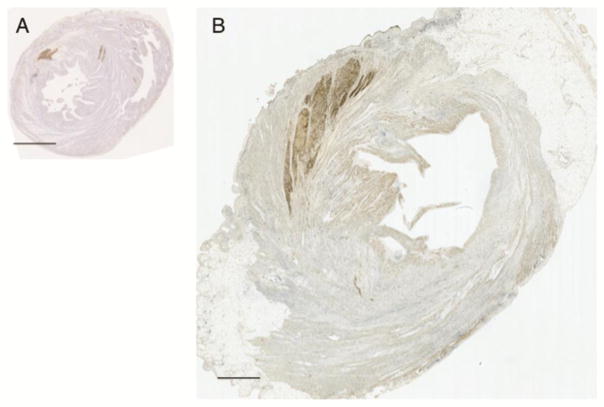
Figure 3. Relative size of human cardiomyocyte grafts in the infarcted rat and monkey hearts.
Human embryonic stem cell derived cardiomyocyte (hESC-CM) grafts in the infarcted rat (A) and monkey (B) hearts. hESC-CM graft is detected by anti-green fluorescent protein primary antibody with 3,3’-Diaminobenzidine detection of secondary antibody (brown). Scale bar = 2.5mm.
One potential limitation of using PSCs to produce CMs for heart failure treatment is that the resulting CMs possess an immature phenotype (Kehat et al., 2001). Whilst this may actually prove beneficial for graft survival, since fetal cells are relatively insensitive to hypoxia, the immature phenotype may not impart the necessary force generation for functional cardiac improvement. Furthermore, the immature calcium handling (Dolnikov et al., 2006; Liu, Fu, Siu, & Li, 2007), slower conduction velocity and residual pacemaking currents may result in arrhythmogenesis after engraftment. Interestingly, in our NHP experiments, we observed structural maturation in the transplanted CMs. From 2 weeks to three months, human cardiomyocyte diameter increased from 5.92 ± 1.1 to 10.9 ± 1.8 μm, equivalent to the size of adult monkey cardiomyocytes and only slightly smaller than adult human cardiomyocytes (Hoshino, Fujiwara, Kawai, & Hamashima, 1983). The graft cardiomyocytes showed increased alignment of their contractile cytoskeletons, elongated to typical rod shape, and showed polarized expression of the gap junction protein, connexin43, and the adherens junction protein, N-cadherin, at intercalated disks by 3 months (Fig. 2). Thus, hESC-derived cardiomyocytes appear capable of developing to an adult phenotype in the proper environment. We speculate that this maturation will increase functional improvements.
Electromechanical integration of stem cell derived cardiomyocyte grafts
Studies from multiple groups have demonstrated effective engraftment of hESC-CM and positive functional effects on the infarcted heart. However, until recently the mechanism underpinning the functional improvements was unknown. Although it appears intuitive that functional hPSC-CMs should electromechanically couple to the host heart after transplantation, this had not been conclusively demonstrated. Early studies indirectly supported the coupling of ESC-CM to the host heart (Gepstein et al., 2010; Kehat et al., 2004; Xue et al., 2005). Recently, together with the laboratory of Michael Laflamme, we used a guinea-pig model of MI to show that hESC-CM can electromechanically couple to the injured host heart (Shiba et al., 2012). In these experiments a line of hESC-CM expressing a calcium indicator protein, GCAMP3, was created using zinc-finger nuclease technology. These CMs flash green fluorescence with each calcium transient, allowing the identification of every contraction of human CM graft within the guinea pig heart. By correlating the fluorescence transients with the host electrocardiogram (measuring depolarization over the whole heart) we showed that most hESC-CM grafts were electromechanically coupled to the host. Although this does not exclude cardiac functional improvement due to other paracrine effects (by which various ASCs exert their functional benefits), the proof that hESC-CM act functionally and synchronously with hearts in which they were transplanted was a major step forward for the field.
In our recent NHP study, we again used the same GCAMP3 expressing hESC-CM cell line and adapted the Langendorff ex vivo perfusion model for the large animal heart. Whilst some hESC-CM grafts in the guinea pig model did not demonstrate electromechanical coupling, in the macaque, all identified hESC-CM grafts were perfectly coupled to the host myocardium. Reasons for the improved electromechanical integration may relate to the difference in heart rates of these species or the patchier infarcts caused by the ischemia-reperfusion model in monkeys, which may give more opportunities to couple than the confluent cryoinjuries used in guinea pigs.
Arrhythmogenesis
Early clinical studies using skeletal myoblasts for cardiac regeneration/repair were complicated by ventricular arrhythmias in cell-treated subjects (Menasche et al., 2003; Siminiak et al., 2004; Smits et al., 2003). These findings, together with the failure of myoblasts to improve systolic function, led to a rapid decline in interest for these cells. Although arrhythmogenesis was a highly feared possible complication of the later trials using BM derived mononuclear cells or MSCs (Assmus et al., 2002; Hare et al., 2009; Heldman et al., 2013; Hendrikx et al., 2006; Perin et al., 2003), increased frequency of ventricular arrhythmias in treated subjects has not occurred.
The explanation for the contrasting experiences in these clinical trials can be explained by key differences among the ASC derivatives engrafted within recipients’ hearts. Firstly, differentiated skeletal muscle cells lack gap junctions and therefore cannot electrically couple to the host heart into which they are delivered (Gepstein et al., 2010; Reinecke, MacDonald, Hauschka, & Murry, 2000; Roell et al., 2007). Studies using programmed electrical stimulation have demonstrated that this large graft of uncouple cells creates a conduction block and serves as a substrate for ventricular arrhythmias (Roell et al., 2007). Furthermore, over-expression of connexin43, the major gap junction protein facilitating electrical conduction between cardiomyocytes, ameliorated this increased arrhythmogenesis. In contrast, BM derived mononuclear cells and MSCs do not show sustained engraftment in the host heart, so of course they cannot leave an electrically uncoupled substrate as an arrhythmogenic source.
PSC on the other hand, display sustained engraftment within infarcted hearts and are capable of spontaneous electrical activity as well as responding to endogenous pacing. Therefore, ventricular arrhythmias after delivery into an infarcted ventricle have been an important and valid concern. Using programmed electrical stimulation, we showed that hESC-CM decrease the propensity for ventricular arrhythmias in the guinea pig heart (Shiba et al., 2012). Since hESC-CM can electromechanically couple to the host heart, it seems likely that hESC-CMs create a new conduction bridge across an otherwise electrically silent scar. This would decrease the arrhythmogenic substrate and therefore decrease arrhythmogenicity. Although very promising, as discussed above, large animal models are required to confirm such findings in structurally larger hearts that possess lower spontaneous heart rates. This is particularly important with respect to ventricular arrhythmias since the faster heart rate of smaller animals may be able to “pace terminate” re-entrant circuits or overdrive suppress ectopic pacemaking. Furthermore, larger grafts required in larger hearts may have increased arrhythmogenicity due to larger areas of slowed wave front propagation.
In this light, it may not be surprising that our macaque studies demonstrated transient, non-fatal ventricular arrhythmias in hESC-CM treated animals (Fig. 4). In fact all animals that received hESC-CM had sustained ventricular arrhythmias recorded on telemetric electrocardiography. No ventricular arrhythmias were observed in any of the “sham” treated animals. These were most commonly a wide complex rhythm with heart rate similar to the animal’s resting heart rate. Although atrial depolarization with slowed AV nodal or His-Purkinje conduction can cause a wide QRS complex rhythm on electrocardiographs, the presence of occasional capture beats, fusion beats and sudden change of cardiac axis all suggest that the observed rhythms were ventricular in origin. Fortunately, the arrhythmias peaked and declined over a 2–3 week period, and the monkey studied out to 3 months had no arrhythmias for the last 2 months. This suggests that the grafts have a period of “growing pains”, after which they electrically adapt to the host heart.
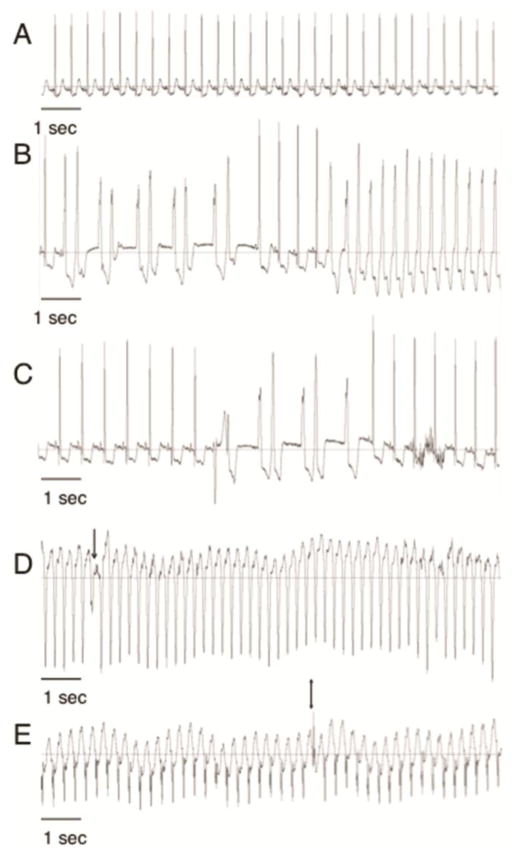
Figure 4. Arrhythmias occur early after engraftment of human cardiomyocytes in the infarcted monkey heart.
Monkeys treated with human embryonic stem cell derived cardiomyocytes (hESC-CM) had telemetric electrocardiographic monitoring devices implanted before myocardial infarction. Electrocardiogram traces are shown from the same animal after engraftment of hESC-CM. Sinus rhythm (A) was seen during continuous telemetric recordings before and after myocardial infarction. Early after hESC-CM engraftment non-sustained (duration greater than 3 beats but less than 30 seconds, B–C) and sustained ( duration greater than 30 seconds, D–E) ventricular arrhythmias were observed. Note the different rates. Ventricular tachycardia was defined as a ventricular rhythm with rate of more than 180 beats/min. Slower ventricular rhythms were defined as Accelerated Idioventricular Rhythms. Arrow in (D) shows fusion beat and Double arrow in (E) shows capture beat both of which demonstrate atrioventricular dissociation proving the ventricular origin of the wide-QRS complex rhythm.
In general terms, arrhythmias can result from enhanced automaticity or from reentrant mechanisms. Enhanced automaticity, in turn, can result from ectopic pacemaking or from triggered activity, e.g. due to early or delayed after-depolarizations. At present we do not know which of these mechanisms underlies arrhythmias in our monkeys, or whether different mechanisms may underlie the ventricular tachycardia vs. the idioventricular rhythm. The cardiomyocytes transplanted have automaticity in culture due to pacemaking currents (Laflamme et al., 2005; Zhu, Santana, & Laflamme, 2009) and others have reported after-depolarizations in these cells due to low expression of the iK1 channel (Lieu et al., 2013). Our initial experiments in optical mapping suggest that the human cardiac grafts have slower conduction than the surrounding host myocardium (Shiba et al., 2012), and this could predispose to unidirectional block and re-entry. Studies are underway to sort these possibilities out. All of these pro-arrhythmic properties are related to the immature state of differentiation that these cardiomyocytes show (Yang, Pabon, & Murry, 2014). If their maturation could be advanced prior to transplantation, it is possible that the arrhythmias would be reduced or disappear.
Why is it that arrhythmias were seen in the monkey heart, when an anti-arrhythmic effect was seen in the guinea pig? The same hESC-CM cell lines and differentiation protocols were used for both, and comparable CM purities were achieved. Therefore, the difference is unlikely to be due to the cells delivered. A more likely explanation is that differences in the host hearts of the respective species were the cause. In this regard, there are three likely possibilities 1) method of myocardial infarct creation, 2) resting heart rate 3) size of the host heart.
With respect to all of the three points, there are important implications for future treatment of human patients. Firstly, myocardial injury caused by cryo-injury (guinea pig model) may affect electrophysiological properties of the infarcted heart in a different manner to ischemia-reperfusion (monkey model). Myocardial infarcts suffered by human patients are mechanistically different from both of these models. Myocardial infarction resulting from ruptured atherosclerotic plaques will have systemic and microvascular effects not present in these animal models and the effects on their may be subsequent influences on any resulting arrhythmias. Secondly, the resting heart rate of humans is approximately 70 bpm, almost half of the pig tailed macaque heart rate. Finally, whilst the macaque hearts were much larger than guinea-pig hearts of the prior study, they are 10 times smaller than the average adult human heart. For all of these reasons ventricular arrhythmias may be increased if the same approach used in the experimental animals is used in clinical practice. Therefore, further assessment of the resulting arrhythmias is required.
Next steps for cardiac repair with pluripotent stem cell derived cardiomyocytes
The study of hESC-CM remuscularization of the NHP heart discussed above has advanced the translational progress of hESC-CM in several ways. Firstly, these experiments have shown that clinical scale production of hESC-CM is possible and viable. Whilst many groups have demonstrated efficient differentiation of relatively pure CMs from hESC and advanced differentiation methods with use of small molecules alone (without need of human cytokines), our NHP experiments are the first to produce, cryopreserve and deliver 1 billion hESC-CM to the infarcted heart of any large animal. Future improvements will be required in terms of further increasing the scale of hESC-CM production. Increased use of bioreactors and automated technologies will be a useful tool in developing reproducible clinical grade hESC-CM batches. Together these advances will ultimately lead to a decrease in cost that will enable a viable model of clinical scale hESC-CM production for future human trials.
Secondly, these studies have shown that hESC-CM can engraft and remuscularize large areas of infarcted myocardium. This had not been demonstrated previously (as discussed above). It is true that intense immune suppression was required in this xenogenic model to achieve the high degree of engraftment. Next steps will require finding a lower threshold of immune suppression that will still support significant engraftment. Clinical hematology and solid-organ transplantation programs have added to our immunological understanding of graft-host interactions. Having an allogeneic model for cell therapy, as opposed to a xenogeneic model, would allow us to study the immune response more meaningfully. We are optimistic that low dose immune suppression or potentially graft tolerance induction will enable hESC-CM replacement therapy without the side effects of long term immune suppression used for current cadaveric heart transplants.
Finally, the most important next steps required will be to thoroughly investigate the mechanism of the arrhythmias observed in our NHP studies. Whilst the results appear at first glance to indicate a formidable barrier impeding clinical translation, we do not feel that this barrier is insurmountable. Firstly, we have shown that the ventricular arrhythmias appear to be transient, with a reduction in frequency after two weeks from engraftment. Preliminary analysis also suggests a cellular adaptation occurring during this two week period with an up regulation of crucial intercellular proteins including cadherins and gap junction proteins. Furthermore, clinical cardiology has already given us a number of tools with which to deal with ventricular arrhythmias. These methods may differ depending on the underlying mechanisms but could include altering the host arrhythmogenic substrate by radiofrequency ablation, placement of implantable cardioverter defibrillators or altering the hESC-CM to be delivered (e.g. in vitro maturation).
It is an exciting period in the history of cardiac regenerative cell therapies with a new chapter heralding the eminent utilization of PSCs as cardiomyocyte replacement therapy.
Highlights.
Large animals cardiac disease models are crucial to confirm results of small animal studies
Pluripotent Stem Cells (PSCs) have indisputable cardiomyogenic properties
PSC-cardiomyocytes (CM) can remuscularize infarcted non-human primate hearts
Large PSC-CM grafts are electromechanically coupled to the host heart
Acknowledgments
This work was supported by NIH grants P01HL094374, R01HL084642, U01HL100405, and P01GM08619 and an Institute of Translational Health Sciences/Primate Center Ignition Award. J.C. was supported by National Health and Medical Research Council of Australia Overseas Training and Australian-American Fulbright Commission Fellowships.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Askoxylakis V, Thieke C, Pleger ST, Most P, Tanner J, Lindel K, Bischof M. Long-term survival of cancer patients compared to heart failure and stroke: a systematic review. BMC Cancer. 2010;10:105. doi: 10.1186/1471-2407-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, Zeiher AM. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106(24):3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428(6983):668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- Bel A, Planat-Bernard V, Saito A, Bonnevie L, Bellamy V, Sabbah L, Menasche P. Composite cell sheets: a further step toward safe and effective myocardial regeneration by cardiac progenitors derived from embryonic stem cells. Circulation. 2010;122(11 Suppl):S118–123. doi: 10.1161/CIRCULATIONAHA.109.927293. [DOI] [PubMed] [Google Scholar]
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114(6):763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- Blin G, Nury D, Stefanovic S, Neri T, Guillevic O, Brinon B, Puceat M. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J Clin Invest. 2010;120(4):1125–1139. doi: 10.1172/JCI40120. 40120 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378(9806):1847–1857. doi: 10.1016/S0140-6736(11)61590-0. S0140-6736(11)61590-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bolli R, Tang XL, Sanganalmath SK, Rimoldi O, Mosna F, Abdel-Latif A, Kajstura J. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation. 2013;128(2):122–131. doi: 10.1161/CIRCULATIONAHA.112.001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge PW, Keller G, Gold JD, Wu JC. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 2012;10(1):16–28. doi: 10.1016/j.stem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, Gepstein L. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50(19):1884–1893. doi: 10.1016/j.jacc.2007.07.054. S0735-1097(07)02635-6 [pii] [DOI] [PubMed] [Google Scholar]
- Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Murry CE. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014 doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JA, Gorman RC, Stroud RE, Bouges S, Hirotsugu H, Gorman JH, 3rd, Spinale FG. Mesenchymal cell transplantation and myocardial remodeling after myocardial infarction. Circulation. 2009;120(11 Suppl):S220–229. doi: 10.1161/CIRCULATIONAHA.108.842302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JA, Spinale FG. Large animal models of heart failure: a critical link in the translation of basic science to clinical practice. Circ Heart Fail. 2009;2(3):262–271. doi: 10.1161/CIRCHEARTFAILURE.108.814459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolnikov K, Shilkrut M, Zeevi-Levin N, Gerecht-Nir S, Amit M, Danon A, Binah O. Functional properties of human embryonic stem cell-derived cardiomyocytes: intracellular Ca2+ handling and the role of sarcoplasmic reticulum in the contraction. Stem Cells. 2006;24(2):236–245. doi: 10.1634/stemcells.2005-0036. [DOI] [PubMed] [Google Scholar]
- Dubois NC, Craft AM, Sharma P, Elliott DA, Stanley EG, Elefanty AG, Keller G. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat Biotechnol. 2011;29(11):1011–1018. doi: 10.1038/nbt.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison GM, Torella D, Dellegrottaglie S, Perez-Martinez C, Perez de Prado A, Vicinanza C, Nadal-Ginard B. Endogenous cardiac stem cell activation by insulin-like growth factor-1/hepatocyte growth factor intracoronary injection fosters survival and regeneration of the infarcted pig heart. J Am Coll Cardiol. 2011;58(9):977–986. doi: 10.1016/j.jacc.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Fernandes S, Naumova AV, Zhu WZ, Laflamme MA, Gold J, Murry CE. Human embryonic stem cell-derived cardiomyocytes engraft but do not alter cardiac remodeling after chronic infarction in rats. J Mol Cell Cardiol. 2010;49(6):941–949. doi: 10.1016/j.yjmcc.2010.09.008. S0022-2828(10)00339-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandolfi F, Vanelli A, Pennarossa G, Rahaman M, Acocella F, Brevini TA. Large animal models for cardiac stem cell therapies. Theriogenology. 2011;75(8):1416–1425. doi: 10.1016/j.theriogenology.2011.01.026. S0093-691X(11)00063-X [pii] [DOI] [PubMed] [Google Scholar]
- Gepstein L, Ding C, Rahmutula D, Wilson EE, Yankelson L, Caspi O, Olgin JE. In vivo assessment of the electrophysiological integration and arrhythmogenic risk of myocardial cell transplantation strategies. Stem Cells. 2010;28(12):2151–2161. doi: 10.1002/stem.545. [DOI] [PubMed] [Google Scholar]
- Ginis I, Luo Y, Miura T, Thies S, Brandenberger R, Gerecht-Nir S, Rao MS. Differences between human and mouse embryonic stem cells. Dev Biol. 2004;269(2):360–380. doi: 10.1016/j.ydbio.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Bhindi R, Seow J, Doyle A, Turner AJ, Tomka J, Figtree GA. Microvascular obstruction by intracoronary delivery of mesenchymal stem cells and quantification of resulting myocardial infarction by cardiac magnetic resonance. Circ Heart Fail. 2010;3(3):e5–6. doi: 10.1161/CIRCHEARTFAILURE.109.931360. [DOI] [PubMed] [Google Scholar]
- Haghighi K, Kolokathis F, Pater L, Lynch RA, Asahi M, Gramolini AO, Kranias EG. Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J Clin Invest. 2003;111(6):869–876. doi: 10.1172/JCI17892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Sherman W. A Randomized, Double-Blind, Placebo-Controlled, Dose-Escalation Study of Intravenous Adult Human Mesenchymal Stem Cells (Prochymal) After Acute Myocardial Infarction. Journal of the American College of Cardiology. 2009;54(24):2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107(7):913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldman AW, Difede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Hare JM. Transendocardial Mesenchymal Stem Cells and Mononuclear Bone Marrow Cells for Ischemic Cardiomyopathy: The TAC-HFT Randomized Trial. JAMA. 2013 doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrikx M, Hensen K, Clijsters C, Jongen H, Koninckx R, Bijnens E, Rummens JL. Recovery of regional but not global contractile function by the direct intramyocardial autologous bone marrow transplantation: results from a randomized controlled clinical trial. Circulation. 2006;114(1 Suppl):I101–107. doi: 10.1161/CIRCULATIONAHA.105.000505. [DOI] [PubMed] [Google Scholar]
- Hoshino T, Fujiwara H, Kawai C, Hamashima Y. Myocardial fiber diameter and regional distribution in the ventricular wall of normal adult hearts, hypertensive hearts and hearts with hypertrophic cardiomyopathy. Circulation. 1983;67(5):1109–1116. doi: 10.1161/01.cir.67.5.1109. [DOI] [PubMed] [Google Scholar]
- Hou X, Appleby N, Fuentes T, Longo LD, Bailey LL, Hasaniya N, Kearns-Jonker M. Isolation, Characterization, and Spatial Distribution of Cardiac Progenitor Cells in the Sheep Heart. J Clin Exp Cardiolog. 2012:S6. [PMC free article] [PubMed] [Google Scholar]
- Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Van de Werf F. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367(9505):113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- Johnston PV, Sasano T, Mills K, Evers R, Lee ST, Smith RR, Marban E. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120(12):1075–1083. 1077. doi: 10.1161/CIRCULATIONAHA.108.816058. following 1083. CIRCULATIONAHA.108.816058 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T, Sawa Y. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation. 2012;126(11 Suppl 1):S29–37. doi: 10.1161/CIRCULATIONAHA.111.084343. [DOI] [PubMed] [Google Scholar]
- Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108(3):407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, Gepstein L. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22(10):1282–1289. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25(9):1015–1024. doi: 10.1038/nbt1327. nbt1327 [pii] [DOI] [PubMed] [Google Scholar]
- Laflamme MA, Gold J, Xu C, Hassanipour M, Rosler E, Police S, Murry CE. Formation of human myocardium in the rat heart from human embryonic stem cells. Am J Pathol. 2005;167(3):663–671. doi: 10.1016/S0002-9440(10)62041-X. S0002-9440(10)62041-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TS, Cheng K, Malliaras K, Smith RR, Zhang Y, Sun B, Marban E. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol. 2012;59(10):942–953. doi: 10.1016/j.jacc.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Palecek SP. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A. 2012;109(27):E1848–1857. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu DK, Fu JD, Chiamvimonvat N, Tung KC, McNerney GP, Huser T, Li RA. Mechanism-based facilitated maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Arrhythm Electrophysiol. 2013;6(1):191–201. doi: 10.1161/CIRCEP.111.973420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Anversa P. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci U S A. 2005;102(25):8966–8971. doi: 10.1073/pnas.0502678102. 0502678102 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Fu JD, Siu CW, Li RA. Functional sarcoplasmic reticulum for calcium handling of human embryonic stem cell-derived cardiomyocytes: insights for driven maturation. Stem Cells. 2007;25(12):3038–3044. doi: 10.1634/stemcells.2007-0549. [DOI] [PubMed] [Google Scholar]
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Murray CJ. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. S0140-6736(12)61728-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379(9819):895–904. doi: 10.1016/S0140-6736(12)60195-0. S0140-6736(12)60195-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malliaras K, Li TS, Luthringer D, Terrovitis J, Cheng K, Chakravarty T, Marban E. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation. 2012;125(1):100–112. doi: 10.1161/CIRCULATIONAHA.111.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard C, Hagege AA, Agbulut O, Barro M, Morichetti MC, Brasselet C, Menasche P. Transplantation of cardiac-committed mouse embryonic stem cells to infarcted sheep myocardium: a preclinical study. Lancet. 2005;366(9490):1005–1012. doi: 10.1016/S0140-6736(05)67380-1. [DOI] [PubMed] [Google Scholar]
- Menasche P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, Hagege AA. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117(9):1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. CIRCULATIONAHA.107.734103 [pii] [DOI] [PubMed] [Google Scholar]
- Menasche P, Hagege AA, Vilquin JT, Desnos M, Abergel E, Pouzet B, Duboc D. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003;41(7):1078–1083. doi: 10.1016/s0735-1097(03)00092-5. S0735109703000925 [pii] [DOI] [PubMed] [Google Scholar]
- Mirotsou M, Jayawardena TM, Schmeckpeper J, Gnecchi M, Dzau VJ. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol. 2011;50(2):280–289. doi: 10.1016/j.yjmcc.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104(5):1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocini D, Staibano M, Mele L, Giannantoni P, Menichella G, Colivicchi F, Santini M. Autologous bone marrow mononuclear cell transplantation in patients undergoing coronary artery bypass grafting. Am Heart J. 2006;151(1):192–197. doi: 10.1016/j.ahj.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res. 2012;111(3):344–358. doi: 10.1161/CIRCRESAHA.110.227512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132(4):661–680. doi: 10.1016/j.cell.2008.02.008. S0092-8674(08)00216-X [pii] [DOI] [PubMed] [Google Scholar]
- Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428(6983):664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- Murry CE, Wiseman RW, Schwartz SM, Hauschka SD. Skeletal myoblast transplantation for repair of myocardial necrosis. J Clin Invest. 1996;98(11):2512–2523. doi: 10.1172/JCI119070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- Ota T, Patronik NA, Schwartzman D, Riviere CN, Zenati MA. Minimally invasive epicardial injections using a novel semiautonomous robotic device. Circulation. 2008;118(14 Suppl):S115–120. doi: 10.1161/CIRCULATIONAHA.107.756049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Mesquita CT, Willerson JT. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107(18):2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Silva GV, Willerson JT. Improved exercise capacity and ischemia 6 and 12 months after transendocardial injection of autologous bone marrow mononuclear cells for ischemic cardiomyopathy. Circulation. 2004;110(11 Suppl 1):II213–218. doi: 10.1161/01.CIR.0000138398.77550.62. [DOI] [PubMed] [Google Scholar]
- Perin EC, Silva GV, Assad JA, Vela D, Buja LM, Sousa AL, Willerson JT. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. J Mol Cell Cardiol. 2008;44(3):486–495. doi: 10.1016/j.yjmcc.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Rakusan K, Nagai J. Morphometry of arterioles and capillaries in hearts of senescent mice. Cardiovasc Res. 1994;28(7):969–972. doi: 10.1093/cvr/28.7.969. [DOI] [PubMed] [Google Scholar]
- Reinecke H, MacDonald GH, Hauschka SD, Murry CE. Electromechanical coupling between skeletal and cardiac muscle. Implications for infarct repair. J Cell Biol. 2000;149(3):731–740. doi: 10.1083/jcb.149.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecke H, Poppa V, Murry CE. Skeletal muscle stem cells do not transdifferentiate into cardiomyocytes after cardiac grafting. J Mol Cell Cardiol. 2002;34(2):241–249. doi: 10.1006/jmcc.2001.1507. [DOI] [PubMed] [Google Scholar]
- Robey TE, Saiget MK, Reinecke H, Murry CE. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45(4):567–581. doi: 10.1016/j.yjmcc.2008.03.009. S0022-2828(08)00353-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roell W, Lewalter T, Sasse P, Tallini YN, Choi BR, Breitbach M, Fleischmann BK. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature. 2007;450(7171):819–824. doi: 10.1038/nature06321. [DOI] [PubMed] [Google Scholar]
- Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, Martin BJ. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg. 2002;73(6):1919–1925. doi: 10.1016/s0003-4975(02)03517-8. discussion 1926. [DOI] [PubMed] [Google Scholar]
- Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Laflamme MA. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489(7415):322–325. doi: 10.1038/nature11317. nature11317 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani Y, Fukushima S, Varela-Carver A, Lee J, Coppen SR, Takahashi K, Suzuki K. Donor cell-type specific paracrine effects of cell transplantation for post-infarction heart failure. J Mol Cell Cardiol. 2009;47(2):288–295. doi: 10.1016/j.yjmcc.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Siminiak T, Kalawski R, Fiszer D, Jerzykowska O, Rzezniczak J, Rozwadowska N, Kurpisz M. Autologous skeletal myoblast transplantation for the treatment of postinfarction myocardial injury: phase I clinical study with 12 months of follow-up. Am Heart J. 2004;148(3):531–537. doi: 10.1016/j.ahj.2004.03.043. [DOI] [PubMed] [Google Scholar]
- Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115(7):896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- Smits PC, van Geuns RJ, Poldermans D, Bountioukos M, Onderwater EE, Lee CH, Serruys PW. Catheter-based intramyocardial injection of autologous skeletal myoblasts as a primary treatment of ischemic heart failure: clinical experience with six-month follow-up. J Am Coll Cardiol. 2003;42(12):2063–2069. doi: 10.1016/j.jacc.2003.06.017. [DOI] [PubMed] [Google Scholar]
- Song H, Yoon C, Kattman SJ, Dengler J, Masse S, Thavaratnam T, Zandstra PW. Interrogating functional integration between injected pluripotent stem cell-derived cells and surrogate cardiac tissue. Proc Natl Acad Sci U S A. 2010;107(8):3329–3334. doi: 10.1073/pnas.0905729106. 0905729106 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJ. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail. 2001;3(3):315–322. doi: 10.1016/s1388-9842(00)00141-0. [DOI] [PubMed] [Google Scholar]
- Stoker ME, Gerdes AM, May JF. Regional differences in capillary density and myocyte size in the normal human heart. Anat Rec. 1982;202(2):187–191. doi: 10.1002/ar.1092020203. [DOI] [PubMed] [Google Scholar]
- Tohyama S, Hattori F, Sano M, Hishiki T, Nagahata Y, Matsuura T, Fukuda K. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell. 2013;12(1):127–137. doi: 10.1016/j.stem.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105(1):93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- van Laake LW, Passier R, Monshouwer-Kloots J, Verkleij AJ, Lips DJ, Freund C, Mummery CL. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res. 2007;1(1):9–24. doi: 10.1016/j.scr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Kirchhoff S, Doevendans PA. Mouse electrocardiography: an interval of thirty years. Cardiovasc Res. 2000;45(1):231–237. doi: 10.1016/s0008-6363(99)00335-1. [DOI] [PubMed] [Google Scholar]
- Xu C, Police S, Hassanipour M, Li Y, Chen Y, Priest C, Gold JD. Efficient generation and cryopreservation of cardiomyocytes derived from human embryonic stem cells. Regen Med. 2011;6(1):53–66. doi: 10.2217/rme.10.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue T, Cho HC, Akar FG, Tsang SY, Jones SP, Marban E, Li RA. Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: insights into the development of cell-based pacemakers. Circulation. 2005;111(1):11–20. doi: 10.1161/01.CIR.0000151313.18547.A2. [DOI] [PubMed] [Google Scholar]
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH American Heart Association Task Force on Practice G. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Yang X, Pabon L, Murry CE. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Res. 2014;114(3):511–523. doi: 10.1161/CIRCRESAHA.114.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WZ, Santana LF, Laflamme MA. Local control of excitation-contraction coupling in human embryonic stem cell-derived cardiomyocytes. PLoS One. 2009;4(4):e5407. doi: 10.1371/journal.pone.0005407. [DOI] [PMC free article] [PubMed] [Google Scholar]