Abstract
Purpose
To compare the efficacy of behavior activation (BA) + low vision rehabilitation (LVR) with supportive therapy (ST) + LVR to prevent depressive disorders in patients with age-related macular degeneration (AMD).
Design
Single-masked, attention-controlled, randomized, clinical trial with outcome assessment at 4 months.
Participants
Patients with AMD and subsyndromal depressive symptoms attending retina practices (n = 188).
Interventions
Before randomization, all subjects had 2 outpatient LVR visits, and were then randomized to in-home BA+LVR or ST+LVR. Behavior activation is a structured behavioral treatment that aims to increase adaptive behaviors and achieve valued goals. Supportive therapy is a nondirective, psychological treatment that provides emotional support and controls for attention.
Main Outcome Measures
The Diagnostic and Statistical Manual IV defined depressive disorder based on the Patient Health Questionnaire-9 (primary outcome), Activities Inventory, National Eye Institute Vision Function Questionnaire–25 plus Supplement (NEI-VFQ), and NEI-VFQ quality of life (secondary outcomes).
Results
At 4 months, 11 BA+LVR subjects (12.6%) and 18 ST+LVR subjects (23.4%) developed a depressive disorder (relative risk [RR], 0.54; 95% CI, 0.27–1.06; P = 0.067). In planned adjusted analyses the RR was 0.51 (95% CI, 0.27–0.98; P = 0.04). A mediational analysis suggested that BA+LVR prevented depression to the extent that it enabled subjects to remain socially engaged. In addition, BA+LVR was associated with greater improvements in functional vision than ST+LVR, although there was no significant between-group difference. There was no significant change or between-group difference in quality of life.
Conclusions
An integrated mental health and low vision intervention halved the incidence of depressive disorders relative to standard outpatient LVR in patients with AMD. As the population ages, the number of persons with AMD and the adverse effects of comorbid depression will increase. Promoting interactions between ophthalmology, optometry, rehabilitation, psychiatry, and behavioral psychology may prevent depression in this population.
Age-related macular degeneration (AMD) is the leading cause of severe vision loss in older adults, with 6.5% having early signs of disease and 0.8% having late disease (i.e., neovascular AMD or geographic atrophy).1 By 2050, 17.8 million persons will have early AMD and 3.8 million will have late AMD.2 This will confront ophthalmologists, health care decision makers, insurers, and family members with the need to care for many visually disabled older persons. Although antiangiogenic treatments have greatly improved the prognosis of neovascular AMD, the majority of treated patients do not regain lost vision.3,4 No medical treatment is available for patients with geographic atrophy. Thus, many patients with AMD experience irreversible vision loss, impaired functional vision, and diminished quality of life.5,6 About 10% to 30% of patients with AMD develop clinically significant depression, which is associated with greater levels of disability, medical costs, and mortality.7–9 Despite the substantial adverse effects of depression, many depressed persons receive no treatment because they perceive depression as a personal failure or an expected part of aging, they are uncertain how to access specialty mental health care, or their physicians lack the expertise or time to effectively diagnose and treat depression.10
To investigate an integrated model of treatment, we conducted the Low VIsion Depression Prevention TriAL (VITAL), a randomized controlled trial that compared the efficacy of behavior activation (BA) + low vision rehabilitation (LVR) with supportive therapy (ST) + LVR to prevent progression to more severe depressive disorders in patients with bilateral AMD and early signs of depression. Before randomization, all subjects had 2 visits with low vision optometrists. Subjects were then randomized to in-home BA or ST. In the former, occupational therapists delivered BA to address depression and functional deficits owing to vision loss. Behavior activation is a structured behavioral treatment that aims to increase adaptive behaviors and achieve valued goals.11 In ST+LVR, masters-level therapists delivered in-home ST, a nondirective, psychological treatment that provides emotional support and controls for attention.12 The primary hypothesis of VITAL was that BA+LVR would be more effective than ST+LVR to prevent depressive disorders and improve functional vision and quality of life.
Methods
Eligibility and Trial Design
Institutional review board/ethics committee approval was obtained to conduct VITAL (clinicaltrials.gov NCT00769015). All subjects provided informed consent; study procedures were compliant with the Health Insurance Portability and Accountability Act and adhered to the tenets of the Declaration of Helsinki. Subjects were recruited from a large private retina practice associated with the Wills Eye Hospital, Philadelphia, Pennsylvania, who met the following inclusion criteria: (1) age >65 years, (2) bilateral AMD (either neovascular disease or geographic atrophy), (3) best-corrected visual acuity <20/70 in the better seeing eye, (4) >5 antiangiogenic injections if the better eye had neovascular disease, or no injections in the previous 3 months, (5) moderate difficulty performing a valued vision-dependent activity, and (6) subthreshold depressive symptoms, defined as a Patient Health Questionnaire-9 score of >5, or depressed mood or anhedonia several days per week.13 The exclusion criteria were (1) ongoing or anticipated antiangiogenic treatment, (2) current Diagnostic and Statistical Manual (DSM) IV-defined depressive disorder,14 (3) uncontrolled glaucoma, diabetic retinopathy, corneal dystrophy, or anticipated cataract surgery, and (4) cognitive impairment on an abbreviated version of the Mini-Mental Status Examination that omits vision-dependent items.15
The study statistician randomized eligible subjects using a random-numbers table, sealed envelopes containing treatment assignments, and a fixed randomization scheme with a 1:1 allocation ratio to the 2 study groups, stratified by severity of vision loss (visual acuity of 20/70–20/100 vs <20/100 in the better eye).
Treatment Interventions
Low Vision Optometry
One of 5 community-based low vision optometrists evaluated and treated all subjects before randomization. The 2 clinic visits included assessment of vision function (e.g., visual acuity, refraction), and prescribing devices and providing instruction on their use. The study provided $350 to all subjects to purchase a basic set of optical devices. After these visits, subjects were randomized to BA, which was delivered by 1 of 5 occupational therapists, or ST, which was delivered by 1 of 3 masters-level therapists (e.g., social workers).
BA+LVR
The occupational therapists delivered 6 in-home, 1-hour BA sessions over 8 weeks. Treatment emphasized the link between action, mood, and mastery, and promoted self-efficacy and social connection as ways to improve mood and function and counter self-defeating behaviors (e.g., social withdrawal).11 The occupational therapist suggested environmental modifications to improve function and, with the subject, developed action plans to accomplish valued personal and functional goals. The action plans drew on rehabilitation principles (e.g., breaking down tasks into manageable steps), were integrated into daily routines, and focused on increasing social activities and reducing vision-related task difficulty. The latter was accomplished by increasing magnification, improving lighting, highlighting objects with high-contrast tape, and simplifying routines.
ST+LVR
Supportive therapy therapists delivered 6 in-home, 1-hour sessions over 8 weeks to facilitate discussion of illness, disability, and vision loss. Treatment facilitated personal expression about vision loss and disability and, in this trial, controlled for the nonspecific effects of attention.12
Treatment Fidelity
All sessions were audiotaped and an experienced psychotherapy researcher (M.T.H.) and a certified low vision occupational therapist reviewed one-third of randomly selected tapes. On a scale from 1 to 5, with 5 representing better standing, the global treatment fidelity ratings of the occupational therapists and supportive therapists were above satisfactory (i.e., ≥3) at 3.5 (1.2) and 4.9 (0.80), respectively.
Study Measures
Research assistants evaluated subjects in their homes masked to treatment assignment at baseline and 4 months to assess the following variables.
Depression
The primary outcome was a DSM-IV diagnosis of major or minor depression based on the Patient Health Questionnaire-9 (PHQ-9).13 The PHQ-9 includes the 9 criteria that define DSM-IV diagnoses of depression and is valid in low-vision patients.16 A scoring algorithm determines whether the profile of symptoms meets categorical diagnoses of depression.
Self-reported Functional Vision
This was assessed using the Activities Inventory and the National Eye Institute Vision Function Questionaire-25 (NEI-VFQ) near and distance activities sub-scales.17,18 The Activities Inventory measures the ability to achieve general vision-dependent activity goals, and perform specific vision-dependent cognitive and motor tasks. An overall functional vision variable is estimated by Rasch analysis.19 The NEI-VFQ rates difficulty performing daily activities. Standardized scores range from 0 to 100, with higher scores indicating better function.
Vision-Related Quality of Life
This was a latent variable comprised of the NEI-VFQ social functioning, mental health, role difficulties, and dependency subscales. Standardized scores range from 0 to 100 with higher scores indicating better life quality.
Vision Status
This included standardized measurement of distance and near visual acuity, contrast sensitivity, and the size and location of central scotomas.
Physical Health Status
This was assessed with the Chronic Disease Score and the Medical Outcomes Study-6 (MOS-6). The Chronic Disease Score yields a weighted score based on medication use that reflects severity of medical comorbidity.20 The MOS-6 yields a global index of self-rated physical and mental health.21 Higher scores on both scales reflect worse health status.
Personality
The Revised Neuroticism, Extroversion, Openness Five Factor Inventory was used to assess the personality traits of neuroticism, conscientiousness, and openness to experience.22 Higher scores reflect higher standing on a given trait.
Behavioral Activation for Depression Scale
This scale measures engagement in social and occupational activities.23 Its 4 subscales tap activation, avoidance/rumination, work/school impairment, and social impairment. Scores range from 0 to 42; higher scores reflect worse functioning.
Device Use
Subjects rated their frequency of use of various low vision aids (e.g., task lighting) and devices (e.g., magnifiers) to improve visual ability.
Statistical Methods
A sample of 144 subjects provided 90% power to detect a 50% reduction in depression incidence at 4 months. This calculation assumed equal numbers in the 2 visual acuity strata (with a 60% incidence rate of depression in controls in the worse vision stratum, and 50% in the better vision stratum) using a 2-sided, continuity-corrected Mantel-Haenszel test of the hypothesis that the risk ratio equaled 1. The type I error rate was set at 5%. We planned to recruit an additional 56 subjects to control for possible improvements in visual acuity in subjects who might receive additional antiangiogenic treatments during the study and to account for a 10% attrition rate.
Continuous baseline demographic and clinical characteristics were summarized using mean values and standard deviations, and categorical variables using counts and percentages. For the primary efficacy analysis, we calculated stratum-specific relative risks (RR) and 95% CIs for the incident depressive disorder at 4 months using Mantel-Haenszel methods. Poisson regression with robust standard errors was used to compute estimates of the intervention's effect on depression incidence adjusted for important baseline variables.24 The stratification variable (visual acuity) and baseline depression score (PHQ-9) were included as adjustment covariates in all models. Other baseline covariates considered were related to the outcome at the bivariate level with P < 0.10. Linear mixed effects models were used to analyze all available Activities Inventory, NEI-VFQ, and Behavioral Activation for Depression Scale data at baseline and 4 months. We extended the mixed effects model to jointly analyze the 4 NEI-VFQ quality-of-life subscales at baseline and 4 months to account for correlation among the 4 subscales and allow for a multivariate test of group differences in change over time.25,26 Mediation analysis was performed using structural equation models.27
Results
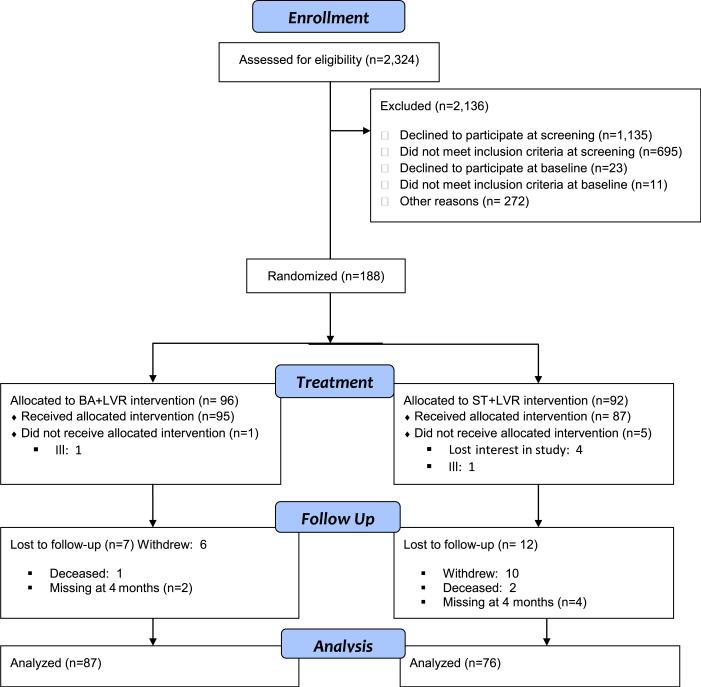
Figure 1 depicts the study flow chart. From July 2009 to February 2013, we reviewed the records of 2324 potentially eligible patients. Of them, 1158 (49.8%) declined participation, 706 (30.4%) were ineligible, and 272 (11.8%) could not be reached. There were no differences between enrolled subjects and eligible patients who declined participation with regard to age, sex, or visual acuity (data not shown). Baseline assessments were conducted on 222 subjects. Of them, 23 subjects declined further participation and 11 were ineligible. Thus, 188 subjects were randomized to the 2 study interventions. Their average age was 84.0 years (standard deviation 6.94); 70.2% were women and 50.0% lived alone. As shown in Table 1, the demographic and clinical characteristics of subjects in the 2 treatment groups were similar, except that BA+LVR subjects were somewhat older and more often married.
Figure 1.
Low VIsion Depression Prevention TriAL (VITAL) cohort participation. BA = behavior activation; LVR = low vision rehabilitation visits; ST = supportive therapy.
Table 1.
Baseline Demographic and Clinical Characteristics of Subjects by Treatment Group
| Demographic Characteristics | BAD LVR (n = 96), Mean (SD) or N (%) | ST D LVR (n = 92), Mean (SD) or N (%) |
|---|---|---|
| Age (y) | 85.2 (6.6) | 82.7 (6.9) |
| Sex (female) | 70 (72.9%) | 62 (67.4%) |
| Education (y) | 13.4 (2.9) | 13.1 (3.0) |
| Marital status (married) | 36 (37.5%) | 27 (29.3%) |
| Living situation (lives alone) | 50 (52.1%) | 43 (46.7) |
| Medical characteristics | ||
| Chronic disease score* | 5.5 (3.0) | 5.8 (2.8) |
| Medical Outcomes Study-6† | 13.0 (4.3) | 12.9 (4.0) |
| Ophthalmologic characteristics | ||
| Best eye distance acuity (logMAR) | 0.68 (0.40) | 0.65 (0.34) |
| Worse eye distance acuity (logMAR) | 1.36 (0.66) | 1.39 (0.65) |
| Best eye contrast sensitivity (log CS) | 1.47 (0.57) | 1.62 (0.48) |
| Worse eye contrast sensitivity (log CS) | 1.00 (0.47) | 0.94 (0.46) |
| Best eye scotoma size (no. of squares) | 85.7 (125.8) | 109.8 (148.6) |
| Worse eye scotoma size (no. of squares) | 246.6 (160.7) | 249.3 (157.4) |
| Vision severity strata | ||
| Best eye acuity, 20/70–20/100 | 48 (50.0%) | 47 (51.1%) |
| Best eye acuity, <20/100 | 58 (50.0%) | 45 (48.9%) |
| Previous anti-VEGF treatment | 49 (51.0%) | 42 (45.7%) |
| Activities Inventory‡ | 0.50 (0.70) | 0.49 (0.73) |
| Vision-specific quality of life§ | ||
| NEI-VFQ: Mental Health | 50.3 (27.1) | 43.0 (27.8) |
| NEI-VFQ: Role Functioning | 44.3 (19.0) | 45.3 (19.2) |
| NEI-VFQ: Dependency | 52.9 (31.2) | 53.7 (33.5) |
| NEI-VFQ: Social Functioning | 64.3 (26.9) | 66.6 (29.4) |
| Behavioral Activation for Depression Scale∥ | ||
| Work/school impairment | 15.7 (3.7) | 16.3 (4.3) |
| Activation | 28.2 (8.1) | 29.5 (9.2) |
| Avoidance/rumination | 36.5 (6.4) | 35.1 (5.5) |
| Social impairment | 28.4 (3.6) | 28.0 (3.6) |
| Personality traits# | ||
| Neuroticism | 20.5 (5.8) | 20.2 (7.1) |
| Conscientiousness | 32.0 (5.0) | 32.4 (4.1) |
| Openness | 26.0 (5.5) | 25.3 (4.8) |
| Depressive symptoms (PHQ-9)** | 5.5 (2.5) | 5.6 (2.2) |
BA = Behavior Activation; log CS = log contrast sensitivity; logMAR = logarithm of the minimum angle of resolution; LVR = low vision rehabilitation visits; NEI-VFQ = National Eye Institute Visual Functioning Questionnaire; PHQ-9 = Patient Health Questionnaire-9; SD = standard deviation; ST = supportive therapy; VEGF = vascular endothelial growth factor.
A higher score is worse physical health.
Scores range from 6 to 31, with lower scores indicating better functioning.
Scores range from 0 to 100, with higher scores indicating better functional vision.
Scores range from 0 to 100, with higher scores indicating better quality of life.
Scores range from 0 to 100, with higher scores indicating better coping skills and/or less impairment.
Scores range from 0 to 48, with higher scores indicating higher standing on the trait.
Scores range from 0 to 27, with higher scores indicating worse depression.
From baseline to 4 months, 19 subjects (10.1%) dropped from the trial (7 BA, 12 ST). These subjects had higher baseline Chronic Disease Scores (i.e., worse medical status) and worse visual acuity than retained subjects but did not differ in PHQ-9 or MOS-6 scores (data not shown). After 4 months, there were no within- or between-group changes in visual acuity, contrast sensitivity, scotoma size, Chronic Disease Score, or Behavioral Activation for Depression Scale scores (data not shown). The mean number of treatment sessions that BA+LVR and ST+LVR subjects received were 5.7 (1.1) and 5.0 (1.9), respectively.
Table 2 shows that 11 BA+LVR subjects (12.6%) and 18 ST+LVR subjects (23.4%) developed a depressive disorder by 4 months (RR, 0.54; 95% CI, 0.27–1.06; P = 0.067). The treatment effect was more evident in subjects in the worse vision stratum (RR, 0.37; 95% CI, 0.14–0.96) than in subjects in the better vision stratum (RR, 0.80; 95% CI, 0.29–2.18). Overall, the absolute risk reduction was 11% and the number needed to treat (NNT), or number of patients who need to be treated to prevent 1 additional case of depression, was 9. For subjects with worse vision, the risk reduction was 20% and the NNT was 5. For subjects with better vision, the risk reduction was 3.4% and the NNT was 29. Baseline covariates that were associated with incident depression were higher MOS-6 score (i.e., worse self-rated health) and NEO-PPI neuroticism score (i.e., the trait tendency to experience negative affects).
Table 2.
Rates of Incident Depression at 4 Months by Treatment Group and Vision Stratum
| BAD LVR (N = 87) | ST D LVR (N = 76) | Stratum-Specific Adjusted RR (CI), P | Overall Stratum-Adjusted RR (CI), P | Adjusted RR (CI), P* | |
|---|---|---|---|---|---|
| Incident depressive disorder, n (%) | 11 (12.6) | 18 (23.7) | — | 0.53 (0.27–1.04), 0.063 | 0.51 (0.27–0.97), 0.037* |
| Better vision stratum | 6 (13.6) | 7 (17) | 0.80 (0.29–2.18) | ||
| Worse vision stratum | 5 (11.6) | 11 (31.4) | 0.37 (0.14–0.96) |
BA = behavior activation; CI = confidence interval; LVR = low vision rehabilitation visits; RR = relative risk; ST = supportive therapy.
Adjusted for vision severity stratum, and baseline neuroticism, Patient Health Questionnaire-9, and Medical Outcomes Study-6 scores.
Table 3 shows the results of an adjusted regression analysis that included treatment group, vision stratum, and baseline better eye scotoma size and PHQ-9, MOS-6, and neuroticism scores. The regression revealed that BA+LVR subjects were significantly less likely to develop a depressive disorder than ST+LVR subjects after adjustment for the covariates (RR, 0.51; 95%+CI, 0.27–0.98; P = 0.037). Higher MOS-6 score remained an independent predictor of incident depression (RR, 1.13; 95% CI, 1.04–1.21, for each 1-point increase; P = 0.014).
Table 3.
Adjusted Estimates of the Relative Risk of Incident Depression
| Variable | Comparison | Relative Risk (95% CI) | P Value |
|---|---|---|---|
| Randomization assignment | BA+LVR vs ST+LVR | 0.51 (0.27–0.97) | 0.037 |
| Stratum | 20/70–20/100 vs <20/100 | 0.76 (0.39–1.48) | 0.42 |
| Patient Health Questionnaire-9 | 1-point increase | 1.05 (0.90–1.23) | 0.53 |
| NEO-FFI neuroticism score | 1-point increase | 1.04 (1.00–1.08) | 0.071 |
| Medical Outcomes Study-6 | 1-point increase | 1.13 (1.04–1.21) | 0.014 |
BA = behavior activation; CI = confidence interval; LVR = low vision rehabilitation; NEO-FFI = Neuroticism, Extroversion, Openness Five Factor Inventory; ST = supportive therapy.
To examine the potential impact of attrition, we conducted 3 separate sensitivity analyses. In the first analysis, all subjects with missing data who were alive at 4 months were considered as depressed. The stratum-adjusted RR was 0.56 (95% CI, 0.34–0.92; P = 0.018). In the second analysis, all were considered not depressed. The stratum-adjusted RR was 0.58 (95% CI, 0.29–1.16; P = 0.12). In the third analysis, we used multiple imputation to create 100 data sets with imputed depression status for patients alive but without follow-up data. The imputation model included vision stratum, PHQ-9 score, and other baseline covariates that were significantly related to depression incidence. The RR of incident depression was 0.56 (95% CI, 0.29–1.10; P = 0.083). These analyses suggest that attrition did not impact the observed treatment effect to a substantial degree.
Table 4 shows change in Activities Inventory, NEI-VFQ functional vision and quality of life, and Behavioral Activation for Depression Scale subscale scores at 4 months by treatment group. Activities Inventory scores improved in both treatment groups. Although the effect was larger in BA+LVR (effect size = 0.72) than ST+LVR (effect size = 0.56), there was no statistically significant difference between groups. On the NEI-VFQ, BA+LVR subjects had a statistically significant improvement in near activities (P = 0.007), whereas ST+LVR subjects did not (P = 0.20). Despite this within-group difference, there was no statistically significant between-group difference (P = 0.34). There were no significant within-group changes or between-group differences in distant activities or quality of life. The BA+LVR subjects used a greater number of low vision devices+ than ST+LVR subjects (3.7 ± 1.5 vs 2.9 ± 1.6; P = 0.003).
Table 4.
Changes in Functional Vision, Quality of Life, and Behavior Activation over 4 Months by Treatment Group
| BA+LVR, Within-Group Comparison, Least-squares Means (CI); P | ST+LVR Within-Group Comparison, Least-squares Means (CI); P | BA+LVR vs ST+LVR, Between-Group Comparison, Least-squares Means (CI); P | |
|---|---|---|---|
| Change in activities inventory* | 0.51 (0.36, 0.65); <0.0001 | 0.43 (0.27, 0.58); <0.0001 | 0.08 (–0.13, 0.29); 0.47 |
| Change in NEI-VFQ near activities† | 4.78 (1.34, 8.21); 0.007 | 2.36 (–1.27, 5.99); 0.20 | 2.42 (–2.58, 7.41); 0.34 |
| Change in NEI-VFQ distance activities† | 0.31 (–3.25, 3.87); 0.86 | 1.12 (–2.54, 4.78); 0.55 | –0.81 (–5.92, 4.30); 0.75 |
| Change in NEI-VFQ quality-of-life variables‡ | |||
| Dependency | 2.07 (–4.03, 8.18); 0.50 | 5.54 (–0.73, 11.81); 0.08 | 3.47 (–12.22, 5.29) P = 0.68 |
| Mental health | 2.23 (–3.2, 7.66); 0.42 | 4.25 (–1.32, 9.81); 0.13 | –2.02 (–9.80, 5.76) |
| Role functioning | 2.33 (–1.46, 6.12); 0.23 | 0.93 (–2.96, 4.82); 0.64 | 1.39 (–4.04, 6.82) |
| Social functioning | –0.38 (–5.59, 3.84); 0.89 | –2.00 (–7.46, 3.47); 0.72 | 1.62 (–5.94, 9.17) |
| Change in BADS Subscales§ | |||
| Activation | –1.88 (–3.80, 0.03); 0.05 | –0.41 (–2.37, 1.56); 0.68 | –1.48 (–4.22, 1.27); 0.29 |
| Work/school | 0.18 (–0.71, 1.06); 0.70 | –0.77 (–1.68, 0.13); 0.09 | 0.95 (–0.32, 2.22); 0.14 |
| Avoidance/rumination | –0.11 (–1.41, 1.19); 0.87 | –0.62 (–1.95, 0.71); 0.36 | 0.51 (–1.35, 2.37); 0.59 |
| Social | 0.10 (–0.81, 1.02); 0.82 | –1.14 (–2.08, 0.21); 0.02 | 1.25 (–0.07, 2.56); 0.06 |
BA = behavior activation; BADS = Behavioral Activation for Depression Scale; CI = confidence interval; LVR = low vision rehabilitation visits; NEI-VFQ = National Eye Institute Visual Function Questionnaire; ST = supportive therapy.
Results from mixed effects model using all available month 0 and month 4 data adjusted for vision severity stratum.
Results from mixed effects model using all available month 0 and month 4 data adjusted for vision severity stratum.
Results from mixed effects model simultaneously modeling 4 Quality of Life subscales using all available month 0 and month 4 data. Group test p-value is for the multivariate comparison.
Results from analysis of covariance of change (month 4 – month 0) adjusted for baseline BADS score and vision severity stratum.
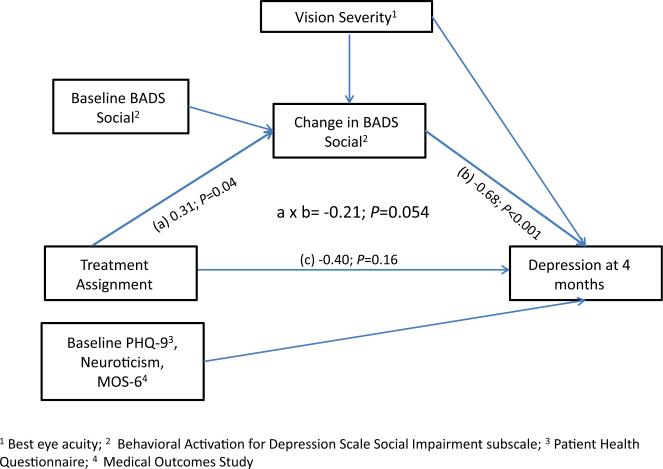
The ST+LVR patients had significant declines in the Behavioral Activation for Depression Scale Social Impairment (mean change, –1.14 [95% CI, –2.08, 0.21]; P = 0.02), whereas BA+LVR subjects had no decline. Figure 2 shows a significant effect of treatment on change in the social impairment (a), a significant association between change in social impairment and incidence of depression (b), and a nearly significant indirect effect of treatment on depression (a × b). These data suggest that change in social impairment at least partially mediated the relationship between treatment group and incident depression, such that BA+LVR prevented depression to the extent that it enabled treated subjects to remain socially engaged.
Figure 2.
Mediational analysis delineating mechanism by which Behavior Activation (BA) + low vision rehabilitation visits (LVR) prevent depression. BADS = Behavioral Activation for Depression Scale Social Impairment subscale; MOS = Medical Outcomes Study-6; PHQ-9 = Patient Health Ques tionnaire-9.
Discussion
We found that an integrated mental health and low vision intervention halved the incidence of depressive disorders (i.e., 12.6% vs 23.4%) relative to standard outpatient LVR in a high-risk population of patients with AMD. Previous studies indicate that the incidence of depression in the absence of any rehabilitative treatment in patients with AMD ranges from 20% to 28%.28,29 The preventive efficacy of BA+LVR was strong, with an NNT of 9 to prevent 1 case of depression. For subjects with worse vision, the NNT was 5, a remarkably good result. By comparison, the NNT is 38 for intensive glycemic control over 4 years to prevent 1 case of clinically important diabetic retinopathy.30 A mediation model suggested that social activation accounted for BA+LVR's therapeutic effect.
In addition, BA+LVR was associated with improved near functional vision. Although ST+LVR was associated with some but lesser improvement, the observed differences were not significant. The low vision optometry treatment that all subjects received likely accounts for improvements in both groups. We found no changes in distance functional vision because BA+LVR focused on near activities. The quality-of-life measures failed to show a statistically or clinically significant change, likely reflecting the insensitivity of the measures to change.31,32 We also found that worse self-rated health was associated with incident depression independent of treatment. This finding indicates that patients with worse health perceptions require more intensive interventions.33
VITAL is the first clinical trial to test a collaborative mental health care model that was integrated into an ophthalmologic setting. The strengths of this trial include systematic recruitment, successful randomization, low attrition, high subject adherence to protocol-driven treatments, maintenance of treatment fidelity, and control for attention. Generalizability and durability of treatment effects are uncertain, however, given the unique characteristics of the sample, the high refusal rate, and the relatively short follow-up period. A second limitation is reliance on the PHQ-9 for depression diagnosis rather than on a clinical interview. Despite these limitations, VITAL contributes to the growing literature on the benefits of LVR. The Low Vision Intervention Trial demonstrated the efficacy of outpatient LVR to improve reading, mobility, information processing, and visual motor skills.34 Horowitz et al35 found that optical device use reduced functional disability and depressive symptoms in low vision patients. Brody et al36 found that a psychological self-management intervention improved well-being in patients with AMD, and we previously demonstrated the benefits of problem solving therapy in patients with AMD.28,36,37 These studies indicate that LVR programs, especially those that emphasize social engagement, benefit patients with chronic vision loss.
Although depression is an understandable reaction to AMD, its high prevalence, persistence, associated disability, costs, and suicide risk make it a formidable problem.38–40 The 24% incidence rate of depression that we observed in controls substantiates this. Unfortunately, there are no established mechanisms to treat depression in ophthalmo-logic settings. If depression were recognized, referral to primary care physicians alone would not meet patients’ vision rehabilitative needs. We developed a treatment alternative based on evidenced-based practice that screened for depression, increased linkages with LVR, and trained occupational therapists to deliver BA. We standardized the intervention to facilitate its dissemination and drew on current Medicare reimbursement policies to support it, although Medicare does not reimburse for vision assistive equipment.41 In its current form, BA+LVR can serve as an initial treatment model to prevent depression in vision-impaired populations. Few occupational therapists, however, receive formal training in psychotherapies like BA to counter depression, and many ophthalmologists fail to refer patients to LVR. Thus, treatments like BA+LVR are not currently available. To become part of routine ophthalmologic care would require a commitment to comprehensive interdisciplinary care and financial investment to support standardized depression screening, psychiatric consultation, care coordination, and clinical and administrative staff training.
The cost savings of preventing depression are substantial because patients with depression have significantly greater total health care costs than nondepressed patients ($20 046 vs $11 956).42 In this context, BA LVR aligns with the intent of the Affordable Care Act,+capitation-based contracts, and pay-for-performance reimbursement strategies that support cost-lowering and quality-improving interprofessional interventions. As the population ages and the number of persons with AMD increases, the personal losses, disability, and costs of AMD will rise. This clinical trial suggests that increasing interactions between ophthalmology, optometry, rehabilitation, psychiatry, and behavioral psychology can improve how we deliver care and achieve better outcomes for patients with AMD.
Supplementary Material
Acknowledgments
A Data and Safety Monitoring Committee ensured subject safety and supervised recruitment, data collection and analyses, and preparation of this manuscript. The committee members were Sheryl F. Kelsey, PhD, Chair (University of Pittsburgh, Pittsburgh, PA); Dianne M. Bartels, RN, MA, PhD (University of Minnesota, Minneapolis, MN); Donald C. Fletcher, MD (California Pacific Medical Center/Smith-Kettlewell Eye Research Institute, San Francisco, CA); Michele Melia, ScM (Jaeb Center for Health Research, Tampa, FL); David C. Steffens, MD, MHS (University of Connecticut Health Center, Farmington, CT), and Eleanor B. Schron, PhD, and Natalie Kurinji, PhD (National Eye Institute, Rockville, MD). The Wills Eye AMD Study Group provided assistance with recruitment of the sample and data collection.
Financial Disclosure(s):
This work was supported by NEI grant U01 EY018819.
Abbreviations and Acronyms
- AMD
age-related macular degeneration
- BA
behavior activation
- DSM
Diagnostic and Statistical Manual
- LVR
low vision rehabilitation
- MOS-6
Medical Outcomes Study-6
- NEI-VFQ
National Eye Institute Vision Function Questionnaire
- NNT
number needed to treat
- PHQ-9
Patient Health Questionnaire-9
- RR
relative risk
- ST
supportive therapy
- VITAL
Low VIsion Depression Prevention TriAL
Footnotes
The authors have no proprietary or commercial interest in any materials discussed in this article.
Members of the Data and Safety Monitoring Committee and the Wills Eye Study Group are: The Wills Eye AMD Study Group: William E. Benson, MD, Gary C. Brown, MD, Jay L. Federman, MD, Mitchell S. Fineman, MD, David H. Fischer, MD, Sunir J. Garg, MD, Allen C. Ho, MD, Jason Hsu, MD, Richard S. Kaiser, MD, Alfred C. Lucier, MD, Joseph I. Maguire, MD, J. Arch McNamara, MD,† Carl H. Park, MD, Carl D. Regillo, MD, Lov K. Sarin, MD, Arunan Sivalingam, MD, Marc J. Spirn, MD, and James F. Vander, MD.
Deceased.
References
- 1.Klein R, Chou CF, Klein BE, et al. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011;129:75–80. doi: 10.1001/archophthalmol.2010.318. [DOI] [PubMed] [Google Scholar]
- 2.Rein DR, Wittenborn JS, Zhang X, et al. Vision Health Cost-Effectiveness Study Group. Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Arch Ophthalmol. 2009;127:533–40. doi: 10.1001/archophthalmol.2009.58. [DOI] [PubMed] [Google Scholar]
- 3.Rosenfeld P, Brown D, Heier J, et al. MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 4.Sloan FA, Hanrahan BW. The effects of technological advances on outcomes for elderly persons with exudative age-related macular degeneration. JAMA Ophthalmol. 2014;132:456–63. doi: 10.1001/jamaophthalmol.2013.7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soubrane S, Cruess A, Lotery A, et al. Burden and health care resource utilization in neovascular age-related macular degeneration. Arch Ophthalmol. 2007;125:1249–54. doi: 10.1001/archopht.125.9.1249. [DOI] [PubMed] [Google Scholar]
- 6.Wysong A, Lee PP, Sloan FA. Longitudinal incidence of adverse outcomes of age-related macular degeneration. Arch Ophthalmol. 2009;127:320–7. doi: 10.1001/archophthalmol.2008.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casten RC, Rovner BW. Update on depression and age-related macular degeneration. Curr Opin Ophthalmol. 2013;24:239–43. doi: 10.1097/ICU.0b013e32835f8e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horowitz A, Reinhardt JP, Kennedy GJ. Major and sub-threshold depression among older adults seeking vision rehabilitation services. Am J Geriatr Psychiatry. 2005;13:180–7. doi: 10.1176/appi.ajgp.13.3.180. [DOI] [PubMed] [Google Scholar]
- 9.Hamer M, Bates CJ, Mishra GD. Depression, physical function, and risk of mortality: National Diet and Nutrition Survey in adults older than 65 years. Am J Geriatr Psychiatry. 2011;19:72–8. doi: 10.1097/JGP.0b013e3181df465e. [DOI] [PubMed] [Google Scholar]
- 10.Lebowitz B, Pearson J, Schneider L, et al. Diagnosis and treatment of depression in late life: consensus statement and update. JAMA. 1997;278:1186–90. [PubMed] [Google Scholar]
- 11.Kanter JW, Manos RC, Bowe WM, et al. What is behavioral activation? A review of the empirical literature. Clin Psychol Rev. 2010;30:608–20. doi: 10.1016/j.cpr.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Novalis PN, Rojcewicz SJ, Peele R. Clinical Manual of Supportive Psychotherapy. American Psychiatric Press; Washington, DC: 1993. pp. 3–7. [Google Scholar]
- 13.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diagnostic and Statistical Manuel of Mental Disorders: DSM IV. 4th ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 15.Reischies FM, Geiselmann B. Age-related cognitive and vision impairment affecting the detection of dementia syndrome in old age. Br J Psychiatry. 1997;171:449–51. doi: 10.1192/bjp.171.5.449. [DOI] [PubMed] [Google Scholar]
- 16.Lamoureux EL, Tee HW, Pesudovs K, et al. Can clinicians use the PHQ-9 to assess depression in people with vision loss? Optom Vis Sci. 2009;86:139–45. doi: 10.1097/OPX.0b013e318194eb47. [DOI] [PubMed] [Google Scholar]
- 17.Massof RW, Ahmadian L, Grover LL, et al. The Activity Inventory: an adaptive visual function questionnaire. Optom Vis Sci. 2007;84:763–74. doi: 10.1097/OPX.0b013e3181339efd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mangione CM, Lee PP, Gutierrez PR, et al. National Eye Institute Visual Function Questionnaire Field Test Investigators. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050–8. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 19.Massof RW, Stelmack JA. Interpretation of low-vision rehabilitation outcome measures. Optom Vis Sci. 2013;90:788–98. doi: 10.1097/OPX.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Von Korff M, Wagner EH, Saunders K. A chronic disease score from automated pharmacy data. J Clin Epidemiol. 1992;45:197–203. doi: 10.1016/0895-4356(92)90016-g. [DOI] [PubMed] [Google Scholar]
- 21.Ware JE, Nelson EC, Sherbourne CD, Stewart AL. Preliminary tests of a 6-item general health survey: A patient application. In: Stewart AL, Ware JE Jr, editors. Measuring Functioning and Well-Being: The Medical Outcomes Study Approach. Duke University Press; Durham, NC: 1992. pp. 291–308. [Google Scholar]
- 22.McCrae RR, Costa PT. Personality in Adulthood. New York: Guilford Press. 1990:41–51. [Google Scholar]
- 23.Kanter JW, Mulick PS, Busch AM, et al. The Behavioral Activation for Depression Scale (BADS): psychometric properties and factor structure. J Psychopathol Behav Assess. 2007;29:191–202. [Google Scholar]
- 24.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 25.Galecki AT. General class of covariance structures for two or more repeated factors in longitudinal data analysis. Commun Stat Theory Methods. 1994;23:3105–19. [Google Scholar]
- 26.Thiébaut R, Jacqmin-Gadda H, Chêne G, et al. Bivariate linear mixed models using SAS Proc MIXED. Comput Methods Programs Biomed. 2002;69:249–56. doi: 10.1016/s0169-2607(02)00017-2. [DOI] [PubMed] [Google Scholar]
- 27.Iacobucci D, Saldanha N, Deng X. A meditation on mediation: evidence that structural equations models perform better than regressions. J Consum Psychol. 2007;17:139–53. [Google Scholar]
- 28.Rovner BW, Casten RJ, Hegel MT, et al. Preventing depression in age-related macular degeneration. Arch Gen Psychiatry. 2007;64:886–92. doi: 10.1001/archpsyc.64.8.886. [DOI] [PubMed] [Google Scholar]
- 29.Casten R, Rovner BW, Lieby BE, Tasman W. Depression despite antievascular endothelial growth factor treatment of age-related macular degeneration [letter]. Arch Ophthalmol. 2010;128:506–7. doi: 10.1001/archophthalmol.2010.24. [DOI] [PubMed] [Google Scholar]
- 30.San Laureano JA, Briganti EM, Colville DJ. Number needed to treat: a useful new method of assessing the magnitude of treatment effect and its application to the management of diabetic retinopathy. Aust N Z J Ophthalmol. 1999;27:137–42. doi: 10.1046/j.1440-1606.1999.00177.x. [DOI] [PubMed] [Google Scholar]
- 31.Stelmack JA, Stelmack TR, Massof RW. Measuring low-vision rehabilitation outcomes with the NEI VFQ-25. Invest Ophthalmol Vis Sci. 2002;43:2859–68. [PubMed] [Google Scholar]
- 32.Marella M, Pesudovs K, Keeffe JE, et al. The psychometric validity of the NEI VFQ-25 for use in a low-vision population. Invest Ophthalmol Vis Sci. 2010;51:2878–84. doi: 10.1167/iovs.09-4494. [DOI] [PubMed] [Google Scholar]
- 33.Montlahuc C, Soumaré A, Dufouil A, et al. Self-rated health and risk of incident dementia: a community-based elderly cohort, the 3C Study. Neurology. 2011;77:1457–64. doi: 10.1212/WNL.0b013e31823303e1. [DOI] [PubMed] [Google Scholar]
- 34.Stelmack JA, Tang XC, Reda DJ, et al. LOVIT Study Group. Outcomes of the Veterans Affairs Low Vision Intervention Trial (LOVIT). Arch Ophthalmol. 2008;126:608–17. doi: 10.1001/archopht.126.5.608. [DOI] [PubMed] [Google Scholar]
- 35.Horowitz A, Brennan M, Reinhardt JP, MacMillan T. The impact of assistive device use on disability and depression among older adults with age-related vision impairments. J Gerontol B Psychol Sci Soc Sci. 2006;61:S274–80. doi: 10.1093/geronb/61.5.s274. [DOI] [PubMed] [Google Scholar]
- 36.Brody BL, Roch-Levecq AC, Gamst AC, et al. Self-management of age-related macular degeneration and quality of life. Arch Ophthalmol. 2002;120:1477–83. doi: 10.1001/archopht.120.11.1477. [DOI] [PubMed] [Google Scholar]
- 37.Rovner BW, Casten RJ, Hegel MT, et al. Improving function in age-related macular degeneration: a randomized clinical trial. Ophthalmology. 2013;120:1649–55. doi: 10.1016/j.ophtha.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cuijpers P, Beekman AT, Reynolds CF., III Preventing depression: a global priority. JAMA. 2012;307:1033–4. doi: 10.1001/jama.2012.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lam BL, Christ SL, Lee DJ, et al. Reported visual impairment and risk of suicide: the 1986-1996 National Health Interview surveys. Arch Ophthalmol. 2008;126:975–80. doi: 10.1001/archopht.126.7.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waern M, Rubenowitz E, Runeson B, et al. Burden of illness and suicide in elderly people: case-control study. BMJ. 2002;324:1355. doi: 10.1136/bmj.324.7350.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Centers for Medicare and Medicaid Services CMS Manual System. Pub 100-02 Medicare Benefit Policy: transmittal 5, Change request 2859 & 2779. [May 8, 2014];220–Coverage of outpatient physical therapy, occupational therapy, and speech-language pathology services under medical insurance. 2009 Jan 4; Available at: https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/2004-Transmittals-Items/CMS044028.html.
- 42.Unützer J, Schoenbaum M, Katon WJ, et al. Healthcare costs associated with depression in medically ill fee-for-service Medicare participants. J Am Geriatr Soc. 2009;57:506–10. doi: 10.1111/j.1532-5415.2008.02134.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.