Abstract
Background
Increased headache prevalence was recently reported in survivors of childhood ALL. Headache sub types, related morbidity, and effect on quality of life has not been reported thus far
Objective
To study headache prevalence and type, related disability, and quality of life in a cohort of childhood acute lymphoblastic leukemia (ALL) survivors.
Methods
Childhood ALL survivors in at least 1 year of remission and 5 years from diagnosis completed questionnaires and were evaluated by a neurologist. Disability was evaluated with Pediatric Migraine Disability Assessment scale and the Short Form-36 Health Survey assessed quality of life.
Results
Thirty nine of 72 (54%) females and 37 of 90 (41%) males reported headaches. Median time from ALL diagnosis to first headache was 5.2 years and median age at headache onset was 10.1 years in 76 participants with headache. Migraine headaches were diagnosed in 51 (31%) and episodic tension-type headaches in 49 (30%); migraine and tension-type headaches co-existed in 24 (15%) and 18 (11%) participants had chronic daily headaches. Fatigue was associated with migraine headache while hypertension and female gender associated with tension type headache. Headache-related disability was mild in 22 (29%), moderate in 7 (9%), and severe in 5 (7%) survivors, and was absent in the remaining 42 (55%) survivors with headache. Both migraine and tension type headaches associated with reduced mental component scores, while headache related disability associated with a reduced physical component scores.
Conclusions
Headaches are common in ALL survivors but only a minority has significant disability or impairment of quality of life.
Keywords: headaches, morbidity, quality of life, acute lymphoblastic leukemia (ALL), long-term survivor, childhood
Introduction
Acute lymphoblastic leukemia (ALL) is the most common pediatric malignancy and 3000–4000 children are diagnosed with ALL every year.1 Development of more effective therapy, and advances in supportive care have substantially improved the outcomes of children with ALL, with cure rates of >85% even without the use of radiation therapy.2 Nevertheless, all children with ALL are treated with potentially neurotoxic drugs, both intravenous and intrathecal.
As the survival rates for children treated for ALL increase, recognition, prevention and optimal management of late toxicities of therapy becomes even more important. Late effects of therapy for childhood leukemia include both neurotoxicity and psychosocial effects.3 Neurologic complications may include leukemic involvement of the leptomeninges and brain parenchyma, white matter lesions, small-vessel calcifications, cerebrovascular disorders, secondary tumors, and infections.4–6 Late neurologic outcomes among 4,151 adult survivors of childhood ALL were recently reported by the Childhood Cancer Survivor Study (CCSS) and headache was the most common neurologic condition (cumulative incidence of 21% at 20 years).7 Utilization of a self-reported questionnaire and lack of direct physician input prevented CCSS investigators from qualifying different headache syndromes. Additionally, the headache-related disability and association with quality of life could not be explored.
The primary aim of our study was to prospectively evaluate the neurologic symptoms and signs in a large cohort of ALL survivors. Secondary aims included studying risk factors for neurologic symptoms, assessing disability associated with neurologic symptoms, and effect on health related quality of life. In this paper we report results on headache and related morbidity in long-term ALL survivors.
METHODS
Participants
Children treated at St. Jude Children’s Research Hospital (St. Jude) are followed after completion of therapy for at least 10 years and at least until 18 years of age. This prospective cross sectional study was approved by the Institutional Review Board. Study eligibility required cancer to be in remission for a year, treatment on institutional protocol, and at least five years from their original ALL diagnosis. Survivors were recruited during their routine annual follow-up visits. All participants were English speaking and did not have pre-existing neurologic disorder which could have affected study results. Written informed consent was obtained from participants when 18 years of age or older, and from the parents or guardians when younger, with assent from the child participant as appropriate.
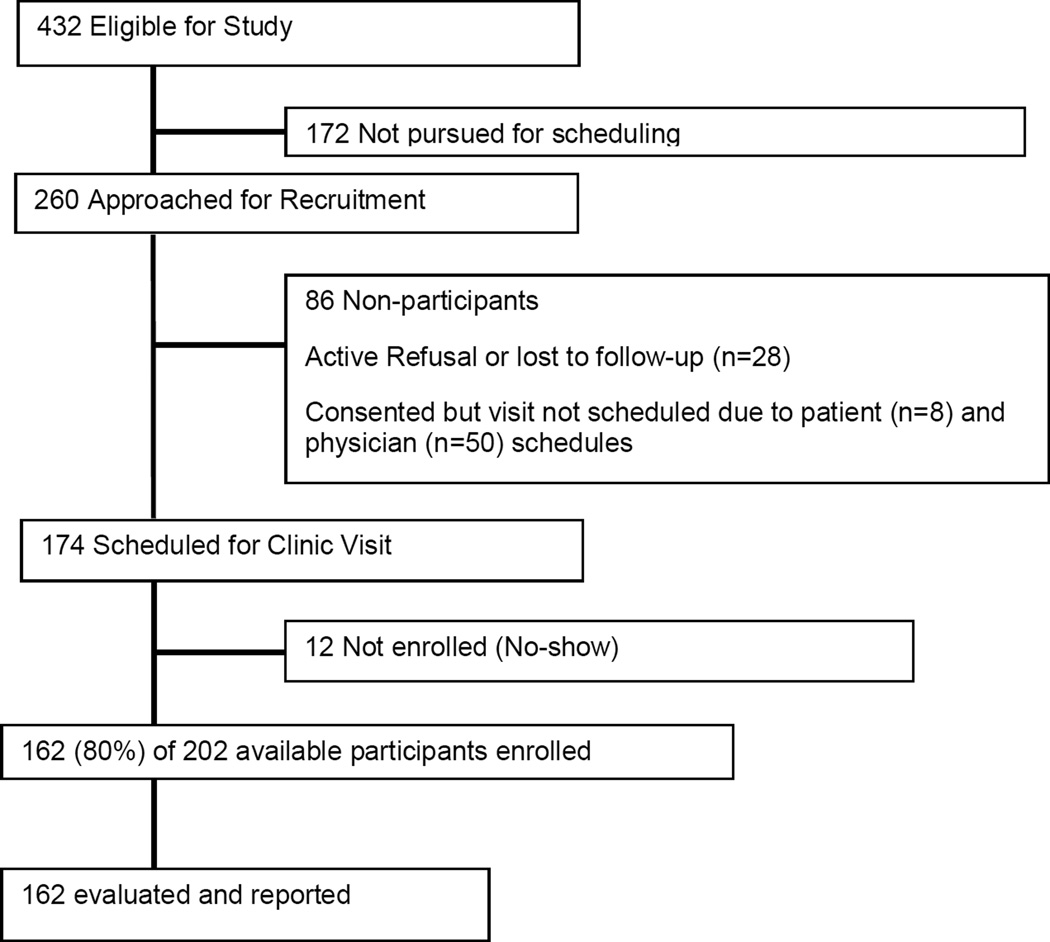
About 432 potential participants visited the institution from December 2005 to October 2008. Of these, 260 could be invited to participate based on the availability of study personnel and coordination of patient schedules and 232 agreed to participate. However, 58 could not be scheduled due to participant’s or physician’s schedule (Figure 1). Thus, 162 (80%) of 202 available survivors could be enrolled over a three year period. As reported previously,8 there were no statistically significant differences in demographic or treatment variables between 162 participants and 270 non-participants.
Figure 1. Recruitment and participation of patients.
Consort diagram of accrual of patients based on eligibility with inclusion criteria for recruitment. The actual evaluable participants are those who agreed to participate, did not have scheduling conflicts, or who did not miss their appointments.
Study Measures
Headache Diagnosis
After enrollment, a trained study personnel administered the questionnaire, with the patient serving as the primary respondent and a parent corroborating information when needed. This was followed by a face to face interview and structured neurologic examination performed by a board-certified neurologist with special expertise in childhood cancer. The questionnaire consisted of items designed to establish the presence of headache, to further characterize the headache, and to determine the degree of impairment resulting from the headache. The International Headache Society criteria were used to classify headache type (Table 1).9 Specific headache syndrome diagnosis was made by study neurologists based upon responses to the questionnaire and face to face assessment. Headaches were divided into three groups: migraine headaches; tension type headaches; and chronic headaches. To be diagnosed as chronic, headache had to be present ≥15 days of each month for the last three consecutive months. Probable migraines (n=12) per criteria were diagnosed as migraine headaches and frequent and infrequent tension type headaches were combined into one group as episodic tension type headaches.
Table 1.
Diagnostic criteria for headache types
| Variable | Migraine headache | Tension-type Headache |
Chronic headache |
|---|---|---|---|
| Number of headaches | >5 | >10 | 15 days/month for 3 months |
| Duration | >1 hour | >30 minutes | >30 minutes |
| LocationA | Unilateral | Bilateral | Any |
| QualityA | Throbbing | Non-throbbing | Any |
| IntensityA | Moderate to severe | Mild to moderate | Any |
| ActivityA | Increases | No affect | Any |
| Nausea / vomitingB | Present | Absent | Any |
| Photo / phonophobiaB | Both present | None or one present | Any |
presence of at least two elements required to diagnose migraine or tension type headaches
One element required to diagnose migraine headaches and both needed to be present for the diagnosis of tension type headache.
Study variables
Demographic and treatment related variables of interest are provided in Table 2. Fatigue was defined as per the criteria suggested by Cella et al for cancer patients.10 This included a screening question if significant fatigue, diminished energy, or increased need to rest that was not related to any recent activity was experienced in at least 15 days of the last month. In case of an affirmative answer, at least 5 of the 10 subsequent questions had to be answered in affirmative for cancer related fatigue diagnosis.
Table 2.
Comparative demographic and clinical features of a cohort of 147 children with and without headache
| Variable | Group | Number | No headache (percentage) |
Migraine (percentage) |
Tension-type (percentage) |
Migraine + Tension-type (percentage) |
|---|---|---|---|---|---|---|
| Gender | Female | 65 | 33(50.8) | 9 (13.8) | 11 (16.9) | 12 (18.5) |
| Male | 82 | 53 (64.6) | 11 (13.4) | 13 (15.9) | 5 (6.1) | |
| Race | White | 131 | 75 (57.3) | 18 (13.7) | 21 (16) | 17 (13) |
| Other races | 16 | 11 (68.8) | 2 (12.5) | 3 (18.8) | 0 (0) | |
| H/O leukemia relapse | No | 140 | 82 (58.6) | 19 (13.6) | 22 (15.7) | 17 (12.1) |
| Yes | 7 | 4 (57.1) | 1 (14.3) | 2 (28.6) | 0 (0) | |
| CNS leukemia | No | 109 | 65 (59.6) | 13 (11.9) | 20 (18.3) | 11 (10.1) |
| Yes | 38 | 21 (55.3) | 7 (18.4) | 4 (10.5) | 6 (15.8) | |
| Radiation | No | 126 | 75 (59.5) | 16 (12.7) | 21 (16.7) | 14 (11.1) |
| Yes | 21 | 11 (52.4) | 4 (19) | 3 (14.3) | 3 (14.3) | |
| BMI | Normal | 69 | 42 (60.9) | 7 (10.1) | 11 (15.9) | 9 (13) |
| Overweight | 36 | 21 (58.3) | 6 (16.7) | 5 (13.9) | 4 (11.1) | |
| Obese | 41 | 22 (53.7) | 7 (17.1) | 8 (19.5) | 4 (9.8) | |
| MTX | <5 gm/m2 | 123 | 74 (60.2) | 17 (13.8) | 17 (13.8) | 15 (12.2) |
| ≥ 5 gm/m2 | 24 | 12 (50) | 3 (12.5) | 7 (29.2) | 2 (8.3) | |
| IT-chemotherapy | <10 | 74 | 44 (59.5) | 11 (14.9) | 8 (10.8) | 11 (14.9) |
| ≥10 | 73 | 42 (57.5) | 9 (12.3) | 16 (21.9) | 6 (8.2) | |
| HTN | No | 126 | 74 (58.7) | 13 (10.3) | 24 (19) | 15 (11.9) |
| Pre or definite | 21 | 12 (57.1) | 7 (33.3) | 0 (0) | 2 (9.5) | |
| Mean age at Dx in yrs (std) | 147 | 5.2 (4.0) | 4.5 (3.1) | 5.3 3(4.0) | ||
| Mean time from Dx in yrs (std) | 147 | 10.8 (4.3) | 10.9 (4.3) | 12 (4.0) | ||
| Mean time from end of Rx in yrs (std) | 147 | 7.9 (4.5) | 8.3 (4.3) | 9.4 (4.1) |
A: tension type headache
CNS:central nervous system
MTX: methotrexate
IT: intrathecal
HTN: hypertension
Dx: ALL diagnosis
std: standard deviation
yrs:years
Rx:cancer treatment.
Overweight is described as a body mass index (BMI) of 25.0 to 29.9 and obese as BMI of 30 or higher. Hypertension is defined as definite when average systolic and diastolic readings are ≥95th percentile for gender, age, and height, and as pre-hypertension when ≥90th percentile but less than the 95th percentile for gender, age, and height.
Participants with headache preceding the diagnosis of ALL (n=15) were not included in this Table.
Pediatric Migraine Disability Assessment Scale (PedMIDAS)
The PedMIDAS tool was used to assess the headache related disability. PedMIDAS is a 6-item questionnaire evaluating the impact of headache on school, home, and social activity.11,12 Headache related disability was calculated as follows: a total score of 0–5 = minimal or infrequent disability; 6–10 = mild disability; 11–20 = moderate disability; and ≥ 21 = severe disability.
The Medical Outcome Survey Short Form-36 (SF-36)
The physical component summary and mental component summary, as well as individual subscales were used to assess headache-related quality of life. The SF-36 is a widely used generic health profile with extensive age and gender specific norms.13 The SF-36 provides subscale scores for 8 domains of quality of life: vitality, social functioning, role emotional (emotional symptoms affecting daily activities and work), and mental health comprise mental component score; while physical functioning, role physical (physical function affecting daily activities and work), bodily pain, and general health comprise physical component score. Raw scores from SF-36 are converted to T-scores with a population mean of 50 and standard deviation of 10. For this study, a T-score ≤40 was considered an indication of an impaired quality of life.
Statistical Analyses
Means and standard deviations were calculated for age at diagnosis, time from diagnosis, and time from cancer treatment. Contingency tables were developed for demographic, clinical variables and headache issues (any headache, migraine headache, tension type headache, and headache-related disability). Univariable logistic regression analysis was used to assess their associations (Table 3). This study was exploratory in nature and no correction was made for multiple statistical testing. The variables with p-values less than 0.1 were entered in a multivariate logistic regression model and significant variables are presented in Table 4. The 15 survivors who had headache starting before cancer diagnosis were excluded from the above analysis so that cancer related risk factors could be identified. All study participants (n=162) were included in the univariate logistic regression analysis of the association between headache (any headache, migraine headache, tension type headache, and headache-related disability) and quality of life (Table 5). SAS version 9.2 (SAS Institute, Cary, NC) was used for all analyses.
Table 3.
Risk factors for headache and headache-related disability on univariate analyses
| Variable | Any headache Odds Ratio (95% CI) |
Migraine Odds Ratio (95% CI) |
Tension type Odds Ratio (95% CI) |
DisabilityA Odds Ratio (95% CI) |
|---|---|---|---|---|
| Female vs. Male | 1.77 (0.91 – 3.44)* | 1.95 (0.94 – 4.04)* | 1.97 (0.93 – 4.19)* | 1.12 (0.49 – 2.55) |
| Older vs. Younger age at cancer diagnosis | 1.0 (0.92 – 1.09) | 0.93 (0.84 – 1.03) | 1.01 (0.92 – 1.11) | 1.02 (0.92 – 1.13) |
| Leukemia relapse | 1.06 (0.23 – 4.92) | 1.04 (0.19 – 5.56) | 0.48 (0.06 – 4.14) | 1.75 (0.32 – 9.55) |
| CNS leukemia | 1.2 (0.57 – 2.52) | 0.9 (0.39 –2.07) | 1.84 (0.82 – 4.14) | 0.42 (0.13 – 1.29) |
| Radiation treatment | 1.34 (0.53 – 3.38) | 1.04 (0.37 – 2.9) | 1.6 (0.59 – 4.33) | 1.89 (0.66 – 5.42) |
| Methotrexate (≥5 vs. < 5 gm/m2) | 1.51 (0.63 – 3.63) | 1.71 (0.68 – 4.28) | 0.75 (0.26 – 2.17) | 2.0 (0.74 – 5.42) |
| Intrathecal chemotherapy (≥ 10 vs. <10 doses) | 1.08 (0.56 – 2.09) | 1.25 (0.61 – 2.57) | 0.61 (0.29 – 1.3) | 1.45 (0.63 – 3.33) |
| Body mass index (Overweight vs. normal) | 1.11 (0.49 – 2.52) | 0.82 (0.33 – 2.04) | 1.27 (0.51 – 3.19) | 0.63 (0.21 – 1.93) |
| Body mass index (Obese vs. normal) | 1.34 (0.62 – 2.94) | 1.01 (0.43 – 2.37) | 1.22 (0.5 – 2.95) | 1.11 (0.43 – 2.84) |
| HypertensionB (Pre / definite vs. normal) | 1.07 (0.42 – 2.72) | 0.24 (0.05 – 1.06)* | 2.63 (1.0 – 6.86)** | 1.0 (0.31 – 3.24) |
| Seizures | 2.31 (0.78 – 6.87) | 1.33 (0.43 – 4.17) | 1.56 (0.5 – 4.91) | 1.64 (0.48 – 5.58) |
| Fatigue | 2.85 (1.23 – 6.6)** | 2.61 (1.12 – 6.09)** | 1.45 (0.59 – 3.54) | 3.63 (1.46 – 9.01)** |
headache related disability assessed by PedMIDAS
CI: 95% confidence interval
p-value >0.05 and ≤ 0.1
p-value ≤ 0.05
vs:versus
CNS: central nervous system
as diagnosed at the time of enrollment.
Table 4.
Risk factors for headache on multivariate logistic regression analyses
| Variable | Odds ratio (Confidence Interval) |
p-value |
|---|---|---|
| Any headache Fatigue |
2.83 (1.21 – 6.6) |
0.02 |
| Migraine headache Fatigue |
2.54 (1.07 – 6.03) |
0.03 |
| Tension-type headache Female gender Hypertension |
2.56 (1.13 – 5.81) 3.67 (1.3 – 10.35) |
0.02 0.01 |
| Moderate-severe headache disability Fatigue |
3.63 (1.46 – 9.01) |
0.01 |
CNS: central nervous system
Table 5.
Headache and headache type as risk factors for quality of life on univariate analysis
| SF 36 scale | Headache type | OR (95% CI) | p-value |
|---|---|---|---|
| Mental Health Component | Any HA | 6.03 (1.24,29.33) | 0.03 |
| Migraine HA | 8.32 (2.05,33.78) | <0.0001 | |
| Tension-type HA | 7.14 (1.77,28.85) | 0.01 | |
| HA disability | 5.31 (1.46,19.22) | 0.01 | |
| Vitality | Any HA | 2.4 (1.07,5.38) | 0.03 |
| Migraine HA | 2.23 (0.97,5.13) | 0.06 | |
| Tension-type HA | 2.52 (1.11,5.71) | 0.03 | |
| HA disability | 2.46 (0.99,6.08) | 0.05 | |
| Social functioning | Any HA | 0.75 (0.31,1.83) | 0.53 |
| Migraine HA | 1.24 (0.48,3.2) | 0.65 | |
| Tension-type HA | 1.23 (0.49,3.08) | 0.66 | |
| HA disability | 1.22 (0.43,3.43) | 0.71 | |
| Role emotional | Any HA | 3.48 (0.87,13.84) | 0.08 |
| Migraine HA | 5.12 (1.39,18.86) | 0.01 | |
| Tension-type HA | 4.43 (1.21,16.25) | 0.02 | |
| HA disability | 2.18 (0.59,8.13) | 0.24 | |
| Physical Component Score | Any HA | 2.17 (0.6,7.87) | 0.24 |
| Migraine HA | 3.38 (0.95,11.97) | 0.06 | |
| Tension-type HA | 1.27 (0.35,4.64) | 0.72 | |
| HA disability | 5.31 (1.46,19.22) | 0.01 | |
| Physical functioning | Any HA | 1.5 (0.43,5.19) | 0.52 |
| Migraine HA | 3.48 (0.99,12.24) | 0.05 | |
| Tension-type HA | 0.44 (0.09,2.16) | 0.31 | |
| HA disability | 2.34 (0.63,8.66) | 0.2 | |
| Role physical | Any HA | 1.9 (0.63,5.73) | 0.25 |
| Migraine HA | 3.34 (1.1,10.11) | 0.03 | |
| Tension-type HA | 2.88 (0.96,8.66) | 0.06 | |
| HA disability | 2.73 (0.87,8.57) | 0.08 | |
| Bodily pain | Any HA | 3.09 (0.91,10.47) | 0.07 |
| Migraine HA | 3.67 (1.17,11.51) | 0.03 | |
| Tension-type HA | 1.64 (0.53,5.1) | 0.4 | |
| HA disability | 6.45 (1.99,20.85) | <0.001 | |
| General health | Any HA | 2.92 (0.85,10.05) | 0.09 |
| Migraine HA | 3.31 (1.03,10.62) | 0.04 | |
| Tension-type HA | 1.96 (0.61,6.25) | 0.26 | |
| HA disability | 3.84 (1.17,12.59) | 0.03 |
Mental health subscale not analyzed because of only 5 participants reporting abnormality.
HA: headache
SF 36:short form 36 quality of life questionnaire
A: ≤40 score of short form 36 measure means impaired quality of life
B: headache related disability as measured by PedMIDAS instrument
OR: odds ratio
CI: 95% confidence interval
RESULTS
Demographics and clinical features
Median age at study enrollment was 15.7 years (range 6.9– 29.0 years) and a median age at ALL diagnosis was 3.9 years (range 0.4–18.6 years). At the time of participation, 29 (17.9%) survivors were at least 21 years of age. Median follow-up since cancer diagnosis was 10.2 years (range 5–22.7 years). Median follow-up since completion of therapy was 7.4 years (range 1.9– 20.3 years). The prevalence rate of headache in 162 participants was 39 of 72 (54%) females and 37 of 90 (41%) males. However, prevalence changed to 32 of 65 (49.2%) females and 29 of 82 (35.4%) males if 15 survivors with headache prior to ALL diagnosis were excluded. The median age at onset of headache was 10.1 years (range 3.9–28 years). Table 2 summarizes the demographic and clinical variables in 147 ALL participating survivors (excluding 15 with headache onset prior to ALL diagnosis). The median time from diagnosis of ALL to the onset of the first headache in the 61 survivors who developed headache after diagnosis of ALL was 5.4 years (range 0.0–15.8 years). Headache onset was more than two years from diagnosis of ALL in 51 (84%) survivors with headache after ALL diagnosis.
Headache characteristics
Migraine headaches were diagnosed in 51 (31%) of the 162 participants, of whom 24 (15%) experienced an aura as well. Auras were visual in 18, vertigo in 11, and sensorimotor in 4; more than one type of aura was experienced by 13 participants. Autonomic symptoms such as redness or droopiness of eye, lacrimation and rhinorrhea were reported by 3 survivors while 20 experienced frequent nausea during a headache. No survivor reported syncope with their headaches. Episodic tension-type headaches were diagnosed in 49 (30%) survivors. In 76 survivors with headache, both migraine headaches and episodic tension-type headaches co-existed in 24 (15%) survivors and 18 (11%) survivors had chronic headaches; 10 had tension-type and 8 migraine type chronic headache.
Headache lasted less than 4 hours when not using abortive treatment in 55 survivors (72% of headache patients). Non-steroidal anti-inflammatory drugs for abortive treatment were used by 49 participants: acetaminophen by 46, triptans by 3, and other over-the-counter medications by 6; bed rest was additionally employed by 65. With abortive treatment, 59 (78% of headache survivors) reported resolution within 2 hours of headache onset.
Of the 76 survivors who experienced headaches, 51 (67%) had never consulted a physician for their headaches and 6 (8%) had visited hospital emergency room for severe headache over the last 12 months. On a pain scale of 1–10, 36 (47% of headache patients) reported an intensity of 7–8 for their worst headaches and 31 (41% of headache patients) an intensity of 9–10. Finally, a maternal family history of headache was reported by 34 (45% of headache patients), paternal family history in 9 (12% of headache patients), and both in 6 (8% of headache patients).
Risk factors for headache
Cancer related risk factors for headache and related disability were studied in survivors developing headache after ALL diagnosis (n=61) and are provided in Tables 3 and 4. In univariate analyses, fatigue was associated with presence of any headache, migraine headaches, and increased headache related disability. Presence of hypertension was associated with tension type headaches. Female sex trended to be associated with any headache, migraine, and tension type headaches. In multivariate analyses, fatigue was significantly associated with any headache, migraine headaches, and increased headache-related disability (Table 4). Female gender and hypertension increased the risk of tension type headaches. Multivariate logistic regression analyses showed no significant association between presence of headache and its related disability with age at cancer diagnosis, history of leukemia relapse, use of high dose methotrexate (>5 gm/m2), number of intrathecal chemotherapy infusions, and body mass index.
Headache-related disability
Disability was studied in all 76 survivors with headaches: 13 (17%) reported a frequent need to interrupt activities during a headache, 36 patients (47%) occasionally interrupted activities during a headache, and 27 (36%) did not need to restrict physical activity during a headache. The PedMIDAS score of the 76 survivors with headaches revealed no headache-related disability in 42 (55%), mild disability in 22 (29%), moderate disability in 7 (9%), and severe disability in 5 (7%) survivors. Presence of fatigue was associated with moderate to severe headache-related disability in both univariate and multivariate analysis.
Headache-related quality of life
Data on quality of life was collected on 141 of 162 survivors (Table 5). Overall, the mean mental component summary T-score was 53.8 (range 21.4 to 67.7) and 11 survivors had a score ≤40 indicating impaired quality of life. Mean physical component summary T-score was 52.4 (range 27.3 to 62) with 11 participants scoring ≤40.
Presence of any headache, migraine, tension-type headache and increased headache disability associated with a decrease in the mental component score and mental subscales of vitality, role emotional, and mental health; social functioning subscale was not affected (Table 5). Only increased headache related disability associated with a reduced physical component score. Migraine headaches did not associate with physical component score but did associate with impaired subscales of physical functioning, role physical, bodily pain, and general health. General health subscale was also affected by increased headache-related disability.
DISCUSSION
This is the first study of headache in cancer survivors where an investigator administered questionnaire was followed by a face to face evaluation by a neurologist. This makes the results of this study much more robust compared to studies that rely only on self-reported questionnaires. Under or over reporting of a symptom is a recognized weakness of self-reported questionnaire methodology.14 Administration of the questionnaire by an investigator and evaluation by a neurologist also helped define headache syndromes. Results of this study are also unique as they not only define headache syndromes in ALL survivors, but also their impact on participant’s day to day activities and quality of life.
CCSS reported an increased headache prevalence relative to control group in long-term survivors of childhood ALL.7 This report confirms CCSS report of high headache prevalence in ALL survivors and also adds to the CCSS study by differentiating headache syndromes, by defining headache-related disability and its impact on quality of life. The difference in prevalence between the two studies may be explained by several methodological differences. The CCSS questionnaire began with the screening question “Have you ever been told by a doctor or other health care professional that you have or have had a headache?” A majority (67%) of survivors with headache in our study never sought physician assistance for headache diagnosis or management, which suggests that lack of physician contact may have resulted in under diagnosis of headaches in CCSS participants. CCSS also reported only severe headache and we also included mild and moderate headaches as well. Additionally, differences in cohort size, age of participants (15.7 versus 20.2 years) and the length of follow-up (10.2 versus 14.1 years) may account for some of the disparity in headache prevalence. Similar to the CCSS, the majority of our participants also developed late-onset headaches. Lack of physician contact in most epidemiological studies of headache in general population also makes comparison difficult.
Prevalence of any headache in the current study is not much different to that reported in the general population without cancer.15 However, migraine headaches are much more prevalence in our cohort is much high compared to general population for (31% versus 7–9%),15,16 tension-type headaches (30% versus 10–25%),17 and chronic headaches (11% versus 3.5%).18 White and gray matter changes are well recognized in cancer survivors treated with chemotherapy with or without radiation treatment.19,20 Higher prevalence of headache in ALL survivors suggests that there may be unrecognized cancer and its treatment-related factors that influence the development of headaches, possibly by altering headache triggering structures in the central nervous system.
Mental and emotional quality of life was affected by migraine and tension-type headaches, as well as severe headache related disability, while only severe headache related disability associated with impairment of physical quality of life. However, migraine headaches did associate with impairment of subscales of physical component score of SF36. Our study also suggests that majority of childhood ALL survivors have generally low headache related disability and fairly preserved quality of life, and they mostly do not discuss their headaches with their physicians.
Although CCSS found an increased risk of headache with prior radiation therapy in ALL survivors and younger age (less than 10 years) at diagnosis of ALL,7,21 we did not find headaches associating with any specific treatment modality. Our finding of possible association of female gender with headaches is consistent with their results. We did find a strong association between presence of fatigue and all modalities of headache and related disability, we are not certain whether there is a cause and effect relationship, or it merely represents a co-existence of two common neurologic symptoms in ALL survivors. Hypertension correlated with tension type headaches and relationship between headaches and hypertension is also recognized in non-cancer population.22 Underlying mechanism is unclear and may be related to analgesia overuse.23 Unfortunately, data regarding frequency of analgesia use was not collected in this study.
Results of our study should be interpreted with caution because of relatively small cohort when compared to epidemiological studies of headache in general population. Lack of racial diversity, absence of control group, and being a single institution study limits its generalizability as well. P-values >0.01 should also be read with caution as we did not correct for multiple statistical testing. However, the high prevalence of headache that we observed is consistent with the results of CCSS and this suggests that our results may be generalizable to other ALL survivors. Our study also makes an important contribution to the literature by qualifying headache types and their impact on survivor’s quality of life.
CONCLUSION
As seen from this study, migraine and tension-type headaches are common in ALL survivors, many of whom experience both. Headache related disability and impairment of quality of life is absent or mild in a majority of childhood ALL survivors. Caregivers should actively seek headache symptoms in childhood ALL survivors, ascertain its severity, and offer appropriate therapy once a specific headache syndrome is recognized. This study confirms earlier finding of higher prevalaence of headache in childhood ALL survivors. Further research is needed to understand cancer and its treatment related factors that contribute to pathophysiology of headaches in ALL survivors.
Acknowledgements
Study Funding: Supported by Cancer Center Support (CORE) Grant CA21765 from the National Cancer Institute, and by the American Lebanese Syrian Associated Charities (ALSAC).
Abbreviations
- ALL
acute lymphoblastic leukemia
- CCSS
childhood cancer survivor study
- PedMIDAS
pediatric migraine disability assessment scale
- SF-36
the medical outcome survey short form-36
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors disclosure, conflict of interest: The authors have no disclosures to report. There are no commercial, financial, or other associations that could pose a conflict of interest in connection with this submitted article.
Authorship Contribution: Dr. Khan conceived and wrote the protocol. Drs. Hudson, Hinds, Crom, Howard and Pui helped in developing the protocol. Miss Ledet and Miss Browne administered the questionnaire, Dr. Khan and Dr. Morris were the study neurologists, Dr. Ness managed and cleaned the data, Dr. Sadighi helped interpreting data and writing the manuscript. Drs. Zhu and Srivastava are the statisticians and all authors helped in reviewing data and editing of the manuscript.
References
- 1.Ries LAG, Smith MA, Gurney J, et al. Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995. National Cancer Institute (NCI); 1999. [Google Scholar]
- 2.Richards S, Pui CH, Gayon P Childhood Acute Lymphoblastic Leukemia Collaborative Group (CALLCG) Systematic review and meta-analysis of randomized trials of central nervous system directed therapy for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60:185–195. doi: 10.1002/pbc.24228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fulbright JM, Raman S, McClellan WS, August KJ. Late effects of childhood leukemia therapy. Curr Hematol Malig Rep. 2011;6:195–205. doi: 10.1007/s11899-011-0094-x. [DOI] [PubMed] [Google Scholar]
- 4.Inaba H, Khan RB, Laningham FH, et al. Clinical and radiological characteristics of methotrexate-induced acute encephalopathy in pediatric patients with cancer. Ann Oncol. 2008;19:178–184. doi: 10.1093/annonc/mdm466. [DOI] [PubMed] [Google Scholar]
- 5.Laningham FH, Kun LE, Reddick WE, et al. Childhood central nervous system leukemia: historical perspectives, current therapy, and acute neurological sequelae. Neuroradiology. 2007;49:873–888. doi: 10.1007/s00234-007-0300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuskonmaz B, Unal S, Gumruk F, et al. The neurologic complications in pediatric acute lymphoblastic leukemia patients excluding leukemic infiltration. Leuk Res. 2006;30:537–541. doi: 10.1016/j.leukres.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Goldsby RE, Liu Q, Nathan PC, et al. Late-occurring neurologic sequelae in adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Cli Oncol. 2010;28:324–331. doi: 10.1200/JCO.2009.22.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krull KR, Khan RB, Ness KK, et al. Symptoms of attention-deficit/hyperactivity disorder in long-term survivors of childhood leukemia. Pediatr Blood Cancer. 2011;57:1191–1196. doi: 10.1002/pbc.22994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Headache Classification Subcommittee of the International Headache Society. The international classification of headache disorders. Cephalagia. 2004;24(suppl 1):1–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 10.Cella D, Davis K, Breitbart W, Curt G Fatigue Coalition. Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol. 2001;19:3385–3391. doi: 10.1200/JCO.2001.19.14.3385. [DOI] [PubMed] [Google Scholar]
- 11.Hershey AD, Powers SW, Vockell AL, et al. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57:2034–2039. doi: 10.1212/wnl.57.11.2034. [DOI] [PubMed] [Google Scholar]
- 12.Hershey AD, Powers SW, Vockell AL, et al. Development of a patient-based grading scale for PedMIDAS. Cephalalgia. 2004;24:844–849. doi: 10.1111/j.1468-2982.2004.00757.x. [DOI] [PubMed] [Google Scholar]
- 13.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Morales NA, Romano MA, Michael Cummings K, et al. Accuracy of self-reported tobacco use in newly diagnosed cancer patients. Cancer Causes Control. 2013;24:1223–1230. doi: 10.1007/s10552-013-0202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wöber-Bingöl C. Epidemiology of migraine and headache in children and adolescents. Curr Pain Headache Rep. 2013;17:341. doi: 10.1007/s11916-013-0341-z. [DOI] [PubMed] [Google Scholar]
- 16.Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646–657. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- 17.Anttila P. Tension-type headache in childhood and adolescence. Lancet Neurol. 2006;5:268–274. doi: 10.1016/S1474-4422(06)70376-3. [DOI] [PubMed] [Google Scholar]
- 18.Lipton RB, Manack A, Ricci JA, et al. Prevalence and burden of chronic migraine in adolescents: results of the chronic daily headache in adolescents study (C-dAS) Headache. 2011;51:693–706. doi: 10.1111/j.1526-4610.2011.01885.x. [DOI] [PubMed] [Google Scholar]
- 19.Reddick WE, Shan ZY, Glass JO, et al. Smaller white-matter volumes are associated with larger deficits in attention and learning among long-term survivors of acute lymphoblastic leukemia. Cancer. 2006;106:941–949. doi: 10.1002/cncr.21679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeller B, Tamnes CK, Kanellopoulos A, et al. Reduced neuroanatomic volumes in long-term survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2013;31:2078–2085. doi: 10.1200/JCO.2012.47.4031. [DOI] [PubMed] [Google Scholar]
- 21.Lu Q, Krull KR, Leisenring W, et al. Pain in long-term adult survivors of childhood cancers and their siblings: a report from the Childhood Cancer Survivor Study. Pain. 2011;152:2616–2624. doi: 10.1016/j.pain.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buse DC, Manack A, Serrano D, Turkel C, Lipton RB. Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J Neurol Neurosurg Psychiatry. 2010;81:428–432. doi: 10.1136/jnnp.2009.192492. [DOI] [PubMed] [Google Scholar]
- 23.Sudano I, Flammer AJ, Roas S, Enseleit F, Noll G, Ruschitzka F. Nonsteroidal antiinflammatory drugs, acetaminophen, and hypertension. Curr Hypertens Rep. 2012;14:304–309. doi: 10.1007/s11906-012-0274-7. [DOI] [PubMed] [Google Scholar]