Abstract
Purpose
We sought to examine prognostic and therapeutic implications, including cost-effectiveness, of elective neck dissection in the management of patients with clinically-determined T1N0 oral tongue carcinoma.
Materials and Methods
A retrospective review of patients with cT1N0 oral tongue squamous cell carcinoma who underwent surgical extirpation of primary tumor, with or without elective neck dissection, at UCLA Medical Center from 1990–2009 was performed. Cox proportional hazards regression was used to assess effects of variables on time to first loco-regional recurrence. A healthcare costs analysis of elective neck dissection was performed by querying the SEER-Medicare linked database.
Results
Of the 123 patients identified with cT1N0 squamous cell carcinoma of the oral tongue, 88 underwent elective neck dissection at the time of tumor resection while 35 did not. For all patients, disease-free survival at 3, 5, and 10 years was 93%, 82%, and 79%. Of the 88 patients undergoing elective neck dissection, 20 (23%) demonstrated occult metastatic disease. Male gender, tumor size, perineural invasion, and occult metastatic disease were individually associated with higher rates of loco-regional recurrence. There was no significant difference in loco-regional recurrence between those who underwent elective neck dissection and those who did not (HR=0.76, p=0.52). On cost analysis, neck dissection was not associated with any significant difference in Medicare payments.
Conclusions
The high rate of occult metastasis (23%) following elective neck dissection, which did not confer additional healthcare costs, leads to the recommendation of elective neck dissection in patients with cT1N0 oral tongue squamous cell carcinoma.
1. Introduction
Surgical resection of early primary squamous cell carcinomas (SCC) of the oral tongue has been accepted as the standard of care [1]. However, the question of whether the patient with a clinically negative neck should undergo elective neck dissection versus observation remains unanswered. In particular, T1N0 and T1N1 oral tongue cancers generally portend favorable prognostic outcomes: the five year disease-free outcome of T1N0 and T1N1 oral tongue SCC have been shown to be 76% and 71%, respectively [2]. Recurrence occurs in approximately 23% of T1 oral tongue cancers, and is primarily regional rather than local [3]. Prognosis following recurrence is debated: some report excellent control of nodal recurrence of a T1 primary tumor [4], while others report salvage as the exception rather than the rule [3,5].
The optimal management of a clinically-negative neck in stage I and stage II SCC of the oral tongue has remained controversial over the past three decades. The issue is of more than academic interest since cervical nodal metastasis has been shown to be the most significant prognosticator of survival for patients with SCC of the oral tongue, due to a decrease in survival in patients with cervical metastases as well as poor clinical outcomes with salvage therapy [6,7]. Previous retrospective studies have reported the incidence of neck metastasis and recurrence rates with a wide range of values varying from 6% to 46% [8,9], and 27% to 42% [10,11], respectively.
The decision to treat the neck, therefore, is not made lightly in early-stage SCC of the oral tongue. While primary neck dissection fulfills diagnostic and therapeutic purposes, surgical intervention, which necessarily increases general anesthesia duration, is not without morbidity. It becomes imperative to clarify the role that elective neck dissection may play in patient outcome.
In an attempt to predict the risk of occult cervical metastases further, recent studies have demonstrated the importance of tumor depth, suggesting that the significantly increased risk of occult cervical metastases in tumors with a depth of invasion greater than 4mm should undergo elective neck dissection [12]. However, lack of a consensus toward measurement techniques, study populations, and cut-off values has slowed the adoption of tumor thickness and depth of invasion as a primary decision-making tool for neck dissection [13].
The aim of this study was to evaluate the patterns of recurrence and survival in patients with cT1N0 oral tongue SCC who underwent elective neck dissection with primary surgical resection compared to that of patients who did not undergo initial neck dissection. In order to delineate the clinical course of this disease as stratified by primary management, a retrospective analysis of 123 patients with cT1N0 oral tongue SCC treated at the University of Los Angeles, California (UCLA) Medical Center over an 18-year period was undertaken.
2. Materials and methods
Permission to perform the study was granted by the Institutional Review Board. Patients diagnosed with SCC of the oral tongue during the period of 1992 and 2009 at UCLA Medical Center were considered. Inclusion criteria included patients who presented with clinically-determined T1N0 (cT1N0) disease, as specified by the American Joint Committee on Cancer (AJCC) [14], who underwent primary surgical resection of the tumor with or without neck dissection. Cases of cancer involving the base of tongue or recurrent oral tongue cancer were excluded from the study.
A retrospective chart review was performed to determine gender; stage of primary tumor; pathological features, including presence of metastases in neck dissection specimens, if performed; length of follow-up; and status of disease during follow-up.
2.1. Pathological analysis
For initial pathological analysis, all tissues were fixed in buffered formalin and submitted for histology after standard overnight machine processing. After processing, tissues were embedded in paraffin wax. These cassettes were then cut into 5-micron sections, placed on glass slides, and stained with hematoxylin and eosin. Cases were reviewed by a designated Head and Neck pathologist at the David Geffen School of Medicine at UCLA, and the presence of lymph node metastases was reported. Depth of tumor invasion was variably reported in specimens prior to 2000, and therefore, this was not further stratified herein. Variables were independently tested for their relation to recurrence using Cox proportional hazards regression, and Kaplan-Meier curves were generated to assess effects of variables on survival.
2.2. Healthcare costs analysis
To perform a cost analysis, the SEER-Medicare linked database was queried. Records of patients meeting the following inclusion criteria were analyzed in two separate groups: in the first group, patients with T1N0 SCC of the oral tongue who underwent partial glossectomy with neck dissection, and in the second group, patients with T1N0 SCC of the oral tongue who underwent partial glossectomy without neck dissection. Median and mean total Medicare payment amounts were calculated in a manner analogous to previously published work.[15] A literature review yielded additional estimates of healthcare costs associated with radiation therapy.
3. Results
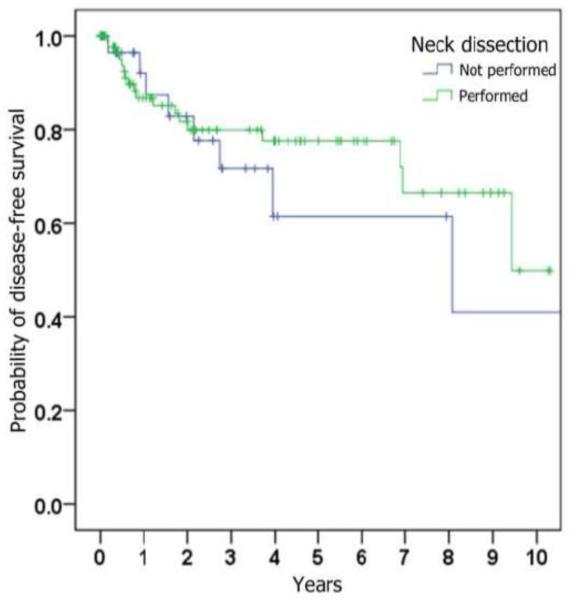
We identified 123 patients with cT1N0 oral tongue SCC who fulfilled all inclusion criteria (Table 1). Sixty-four were male and 59 were female. Age at the time of tumor resection ranged from 27 to 92 years, with a mean of 56 years. Length of follow-up ranged from 1 to 196 months, with a median of 29 months. For the entire study population, disease-free survival at 3, 5, 10 years was 93%, 82%, and 79% respectively (Figure 1).
Table 1.
Patient characteristics.
| Age (years) | Mean, 56 |
| Range, 27 to 92 | |
|
| |
| Gender | |
| Male | 64 (52%) |
| Female | 59 (48%) |
|
| |
| Length of follow-up (months) | Mean, 29 |
| Range, 1 to 196 | |
|
| |
| Degree of differentiation of primary tumor | |
| Well differentiated | 23 (19%) |
| Moderately differentiated | 82 (67%) |
| Poorly differentiated | 11 (9%) |
| Not reported | 7 (6%) |
|
| |
| Perineural invasion in primary tumor | |
| Present | 35 (28%) |
| Absent | 37 (30%) |
| Not reported | 51 (41%) |
|
| |
| Ipsilateral neck dissection at time of primary resection | |
| Performed | 88 (72%) |
| Not performed | 35 (28%) |
|
| |
| Occult metastases in neck dissection specimen | |
| Present | 20 (23%) |
| Absent | 68 (77%) |
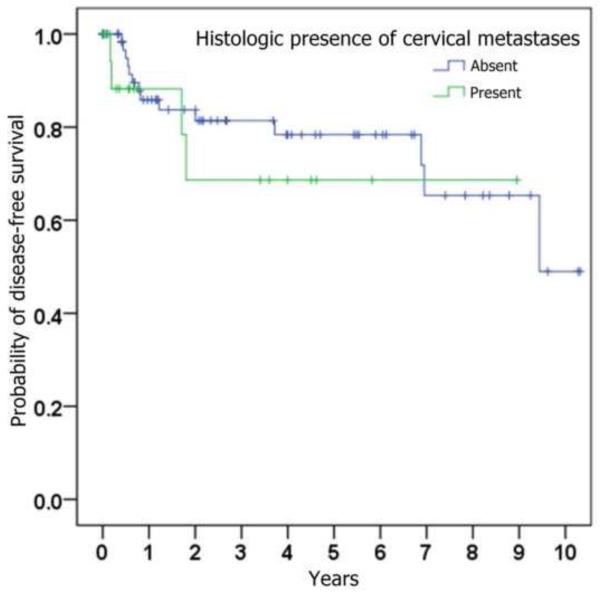
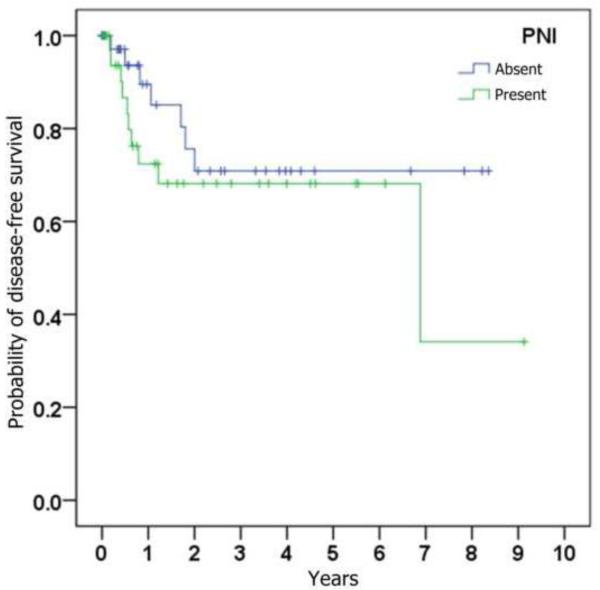
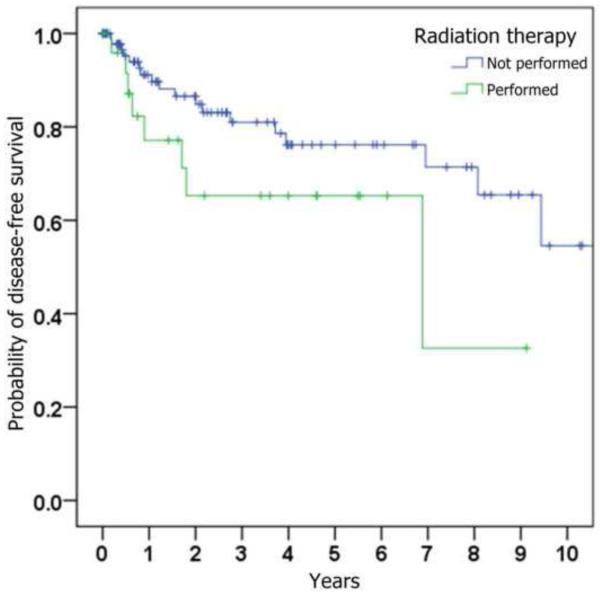
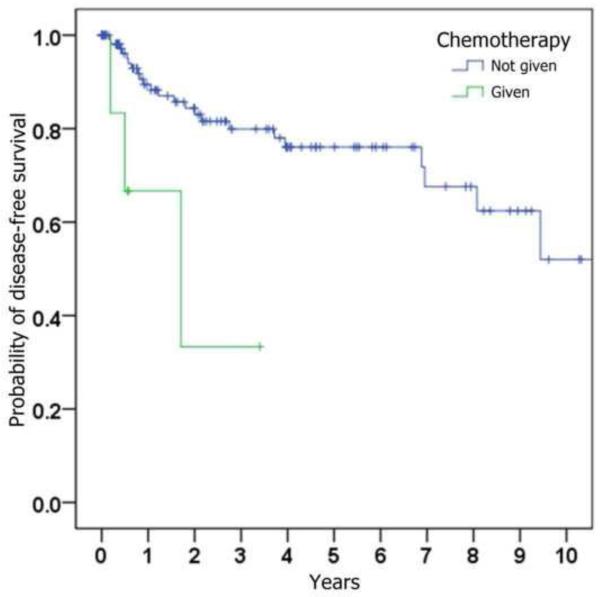
Figure 1.
Kaplan-Meier curves depicting probability of disease-free survival (i.e., absence of loco-regional recurrence), stratified by (a) whether ipsilateral neck dissection was performed, (b) histologic presence of metastatic SCC on neck dissection specimen, (c) histologic presence of perineural invasion in the primary tumor, (d) whether radiation was given, and (e) whether chemotherapy was given.
Of the 123 patients, 88 underwent ipsilateral neck dissection at the time of surgical extirpation. The number of lymph nodes found in pathological specimens ranged from 4 to 66 (mean, 25). Cervical lymph nodes were deemed occult metastatic nodes if at least one lymph node in the pathological specimen was found to be positive for squamous cell carcinoma; using this criterion, occult metastases were found in 20 of the 88 patients, conferring a false-negative rate of 23% for the preoperative clinical evaluation of cervical metastases.
Of the patients who underwent neck dissection, adjuvant chemoradiotherapy was performed in 6 (7%) and adjuvant radiotherapy alone was performed in 15 (17%). Of the patients who did not undergo neck dissection, 4 (11%) received adjuvant radiotherapy; none received chemotherapy.
Of the 88 patients who underwent ipsilateral primary neck dissection, 8 patients had subsequent isolated recurrent disease at the primary site, 7 patients had isolated recurrent disease in the neck, and 3 patients presented with recurrence at both the primary and the neck, with a 20% overall loco-regional recurrence rate in this group. Of the 35 patients who did not undergo neck dissection at the time of surgical resection of the primary tumor, 7 patients had isolated recurrent disease at the primary site, 2 patients had isolated recurrent disease in the neck, and 2 patients presented with recurrence at both the primary and the neck, with a 31% overall loco-regional recurrence rate in this group (Table 2).
Table 2.
Incidence of local and regional recurrence as stratified by management of the clinically negative neck.
| Local recurrence only | Regional recurrence only | Both local and regional recurrence | Overall loco-regional recurrence | |
|---|---|---|---|---|
| Ipsilateral primary neck dissection (n = 88) | 8 (9%) | 7 (8%) | 3 (3%) | 18 (20%) |
| No neck dissection (n = 35) | 7 (20%) | 2 (6%) | 2 (6%) | 11 (31%) |
| Total (n = 123) | 15 (12%) | 9 (7%) | 5 (4%) | 29 (24%) |
The correlation of loco-regional recurrence with variables of interest is shown in Table 3. Trends toward a higher risk of loco-regional recurrence were seen with male gender, increasing age, increasing tumor size, perineural invasion, poorer histopathological differentiation, occult cervical metastases, and subsequent treatment with adjuvant radiation and chemotherapy. Statistical significance (p < 0.05) was only reached when comparing chemotherapy status. Although primary neck dissection was found to be associated with a lower risk of loco-regional recurrence (HR = 0.76), this was not statistically significant (p = 0.52).
Table 3.
Hazard ratios of loco-regional recurrence as determined by univariate Cox proportional hazards analysis.
| Variable | HR | 95% CI | p-value |
|---|---|---|---|
|
| |||
| Sex (female vs male) | 1.16 | (0.53,2.53) | 0.70 |
|
| |||
| Age (years) | 1.013 | (0.989,1.037) | 0.29 |
|
| |||
| Ipsilateral primary neck dissection (performed vs not performed) | 0.76 | (0.33,1.75) | 0.52 |
|
| |||
| Radiation (performed vs not performed) | 2.05 | (0.88,4.80) | 0.096 |
|
| |||
| Chemotherapy (given vs not given) | 5.32 | (1.54,18.40) | 0.008* |
|
| |||
| Tumor size (≤ 1 cm versus > 1 cm) | 1.23 | (0.64,2.34) | 0.54 |
|
| |||
| Degree of differentiation | - | - | 0.24 |
| Well differentiated | 0.74 | (0.12,4.62) | |
| Moderately differentiated | 1.98 | (0.58, 6.70) | |
| Poorly differentiated | 2.69 | (0.62, 11.73) | |
|
| |||
| Perineural invasion (present vs absent) | 1.66 | (0.63,4.37) | 0.30 |
|
| |||
| Occult metastases (present vs absent) | 1.34 | (0.98,1.82) | 0.064 |
|
| |||
| Cervical metastases identified in primary neck dissection specimen (present vs absent) | 1.51 | (0.49,4.69) | 0.47 |
3.1. Healthcare costs analysis
To analyze healthcare costs, the December 2012 SEER-Medicare linked database was queried, representing all patients diagnosed with cancer through 2009 with Medicare claims reported through 2010. This yielded 422 patients diagnosed with T1 oral tongue SCC who underwent glossectomy without neck dissection and 305 patients with T1 oral tongue SCC who underwent glossectomy with neck dissection. In the group who did not undergo neck dissection, the mean total Medicare payment was $70070 (median, $34667; σ = $121806), exceeding the mean total payment for analogous patients who underwent neck dissection, $67393 (median, $35328, σ = $86094). A student's t-test revealed that this trend was not statistically significant (t = 0.329, p = 0.742).
4. Discussion
The most common cause of treatment failure following surgical extirpation of early-stage oral tongue cancer is regional recurrence [9,10]. Once regional recurrence occurs, prognosis is significantly worsened. Therefore, it is reasonable to attempt to improve long-term survival of patients with early tongue SCC by optimizing regional control.
In a series of 58 patients surgically treated for early oral tongue SCC, Kaya showed a 0% salvage rate for patients with regional recurrent disease despite aggressive management with surgery, radiotherapy, and chemotherapy [16]. Other series have described a more favorable prognosis, including one that demonstrated that a disease-free interval of greater than one year was a positive predictor for survival [5,17]. The polarity of this published data makes it difficult to draw definitive conclusions, but it is at least agreed that loco-regional recurrence is overall a negative prognostic indicator for eventual survival.
In our series of patients with cT1N0 oral tongue SCC, 88 patients (72%) underwent elective neck dissection, while 35 (28%) did not undergo neck dissection and instead were clinically observed for recurrence. Eighteen of the 88 patients (20%) undergoing elective neck dissection subsequently developed loco-regional recurrence, while 11 of the 35 patients who did not undergo elective neck dissection experienced loco-regional recurrence (31%). This represents a hazard ratio (HR) of 0.76 favoring elective neck dissection, although this finding did not achieve statistical significance.
Similar to our findings, other authors have also reported a high incidence of occult metastases and recurrence in the neck in early-stage oral tongue SCC [18], emphasizing the need for strategic management of the clinically negative neck. Our SEER-Medicare analysis revealed that over a lifetime, patients with T1 SCC of the oral tongue treated with glossectomy and elective neck dissection resulted in lower Medicare payments than analogous patients who did not undergo elective neck dissection when calculated over a lifetime, although this did not reach statistical significance. A plausible explanation is that cervical metastases in patients undergoing glossectomy without neck dissection were later treated with radiation therapy, which has been reported to cost, on average, $18679 per patient in a government payment analysis [19]. Compared to the mean total Medicare payment for patients undergoing glossectomy and neck dissection in our analysis, $70070, this cost is substantial. An elective neck dissection at the time of extirpation of the primary tumor may therefore be an oncologically-sound maneuver that does not increase, and may decrease, healthcare costs.
The current work adds to literature suggesting that small primary tumors in oral tongue SCC carry a moderate to high risk for occult and delayed development of cervical metastatic disease: Teichgraeber found an incidence of 31% occult neck metastasis in T1 oral tongue SCC [20], while Johnson observed a high incidence of occult neck metastasis (36%) in a series of 28 patients with T1 oral tongue SCC and recommended elective neck treatment given the poor outcome after salvage treatment [21]. In this study, we demonstrate a 23% incidence of occult metastasis in T1 oral tongue SCC. This result is significant, given that elective neck treatment is traditionally recommended when the risk of occult cervical metastasis exceeds 20%. Therefore, our data suggest that ipsilateral elective neck treatment is recommended in the management of T1 SCC of the oral tongue.
Tumor size, degree of differentiation, and status of perineural invasion have all been proposed as predictors of regional metastatic disease. In our series, these factors were associated with higher trends in loco-regional recurrence, but failed to demonstrate statistical significance. It is conceivable that this reflects a limitation in sample size, but it also suggests that additional factors may be at play. Other studies have highlighted primary tumor thickness, which was variably reported in our pathological specimens, as well as perineural invasion, as prognostic variables [6,12].
5. Conclusion
Recent reports have suggested that nodal recurrence in early-stage oral tongue SCC occurs more commonly than previously thought [18], and furthermore may be recidivistic to salvage neck dissection [4]. In this series, given the relatively high incidence of occult neck metastases (23%), we propose that neck dissection, likely selective, is justified in patients presenting with cT1N0 oral tongue SCC. In the absence of clinical metastasis, a supraomohyoid neck dissection has been shown to be effective and with limited attendant morbidity [22–24]. Further research will focus on exploring the role of tumor thickness, molecular stratification, and additional primary tumor characteristics to predict loco-regional recurrence and survival.
ACKNOWLEDGEMENTS
Funding/support: This work was supported by NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR000124.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no other financial disclosures to report.
Conflicts of interest Disclosures: No conflicts of interest to disclose.
Previous Presentation: Presented in part at the Annual Meeting of the American Academy of Otolaryngology – Head and Neck Surgery, Washington, DC, USA, September 10, 2012. This manuscript has not and will not be published as a result of this meeting presentation.
REFERENCES
- [1].Shah JP, Gil Z. Current concepts in management of oral cancer -- Surgery. Oral oncology. 2009;45(4):394–401. doi: 10.1016/j.oraloncology.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sessions DG, Spector GJ, Lenox J, Haughey B, Chao C, Marks J. Analysis of treatment results for oral tongue cancer. Laryngoscope. 2002 Apr;112(4):616–25. doi: 10.1097/00005537-200204000-00005. [DOI] [PubMed] [Google Scholar]
- [3].Lim YC, Lee JS, Koo BS, Kim SH, Kim YH, Choi EC. Treatment of contralateral N0 neck in early squamous cell carcinoma of the oral tongue: elective neck dissection versus observation. Laryngoscope. 2006 Mar;116(3):461–5. doi: 10.1097/01.mlg.0000195366.91395.9b. [DOI] [PubMed] [Google Scholar]
- [4].Tsang RK, Chung JC, To VS, Chan JY, Ho WK, Wei WI. Efficacy of salvage neck dissection for isolated nodal recurrences in early carcinoma of oral tongue with watchful waiting management of initial N0 neck. Head Neck. 2011 Oct;33(10):1482–5. doi: 10.1002/hed.21643. [DOI] [PubMed] [Google Scholar]
- [5].Wong LY, Wei WI, Lam LK, Yuen AP. Salvage of recurrent head and neck squamous cell carcinoma after primary curative surgery. Head Neck. 2003 Nov;25(11):953–9. doi: 10.1002/hed.10310. [DOI] [PubMed] [Google Scholar]
- [6].Sparano A, Weinstein G, Chalian A, Yodul M, Weber R. Multivariate predictors of occult neck metastasis in early oral tongue cancer. Otolaryngol Head Neck Surg. 2004 Oct;131(4):472–6. doi: 10.1016/j.otohns.2004.04.008. [DOI] [PubMed] [Google Scholar]
- [7].Fakih AR, Rao RS, Patel AR. Prophylactic neck dissection in squamous cell carcinoma of oral tongue: a prospective randomized study. Semin Surg Oncol. 1989;5(5):327–30. doi: 10.1002/ssu.2980050507. [DOI] [PubMed] [Google Scholar]
- [8].Ho CM, Lam KH, Wei WI, Lau SK, Lam LK. Occult lymph node metastasis in small oral tongue cancers. Head Neck. 1992 Sep-Oct;14(5):359–63. doi: 10.1002/hed.2880140504. [DOI] [PubMed] [Google Scholar]
- [9].Yuen AP, Wei WI, Wong YM, Tang KC. Elective neck dissection versus observation in the treatment of early oral tongue carcinoma. Head Neck. 1997 Oct;19(7):583–8. doi: 10.1002/(sici)1097-0347(199710)19:7<583::aid-hed4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- [10].Capote A, Escorial V, Muñoz-Guerra MF, Rodríguez-Campo FJ, Gamallo C, Naval L. Elective neck dissection in early-stage oral squamous cell carcinoma—does it influence recurrence and survival? Head & Neck. 2007;29(1):3–11. doi: 10.1002/hed.20482. [DOI] [PubMed] [Google Scholar]
- [11].Cunningham MJ, Johnson JT, Myers EN, Schramm VL, Jr., Thearle PB. Cervical lymph node metastasis after local excision of early squamous cell carcinoma of the oral cavity. Am J Surg. 1986 Oct;152(4):361–6. doi: 10.1016/0002-9610(86)90305-3. [DOI] [PubMed] [Google Scholar]
- [12].O'Brien CJ, Lauer CS, Fredricks S, Clifford AR, McNeil EB, Bagia JS, et al. Tumor thickness influences prognosis of T1 and T2 oral cavity cancer--but what thickness? Head Neck. 2003 Nov;25(11):937–45. doi: 10.1002/hed.10324. [DOI] [PubMed] [Google Scholar]
- [13].Pentenero M, Gandolfo S, Carrozzo M. Importance of tumor thickness and depth of invasion in nodal involvement and prognosis of oral squamous cell carcinoma: a review of the literature. Head Neck. 2005 Dec;27(12):1080–91. doi: 10.1002/hed.20275. [DOI] [PubMed] [Google Scholar]
- [14].Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. Springer; New York: 2010. [Google Scholar]
- [15].Lang K, Menzin J, Earle CC, Jacobson J, Hsu MA. The economic cost of squamous cell cancer of the head and neck: findings from linked SEER-Medicare data. Arch Otolaryngol Head Neck Surg. 2004 Nov;130(11):1269–75. doi: 10.1001/archotol.130.11.1269. [DOI] [PubMed] [Google Scholar]
- [16].Kaya S, Yilmaz T, Gursel B, Sarac S, Sennaroglu L. The value of elective neck dissection in treatment of cancer of the tongue. Am J Otolaryngol. 2001 Jan-Feb;22(1):59–64. doi: 10.1053/ajot.2001.20681. [DOI] [PubMed] [Google Scholar]
- [17].Agra IM, Carvalho AL, Pinto CA, Martins EP, Filho JG, Soares FA, et al. Biological markers and prognosis in recurrent oral cancer after salvage surgery. Arch Otolaryngol Head Neck Surg. 2008 Jul;134(7):743–9. doi: 10.1001/archotol.134.7.743. [DOI] [PubMed] [Google Scholar]
- [18].Ganly I, Goldstein D, Carlson DL, Patel SG, O'Sullivan B, Lee N, et al. Long-term regional control and survival in patients with “low-risk,” early stage oral tongue cancer managed by partial glossectomy and neck dissection without postoperative radiation: the importance of tumor thickness. Cancer. 2013 Mar 15;119(6):1168–76. doi: 10.1002/cncr.27872. [DOI] [PubMed] [Google Scholar]
- [19].Moore EJ, Hinni ML, Olsen KD, Price DL, Laborde RR, Inman JC. Cost considerations in the treatment of oropharyngeal squamous cell carcinoma. Otolaryngol Head Neck Surg. 2012 Jun;146(6):946–51. doi: 10.1177/0194599812437534. [DOI] [PubMed] [Google Scholar]
- [20].Teichgraeber JF, Clairmont AA. The incidence of occult metastases for cancer of the oral tongue and floor of the mouth: treatment rationale. Head Neck Surg. 1984 Oct;7(1):15–21. doi: 10.1002/hed.2890070105. [DOI] [PubMed] [Google Scholar]
- [21].Johnson JT, Leipzig B, Cummings CW. Management of T1 carcinoma of the anterior aspect of the tongue. Arch Otolaryngol. 1980 May;106(5):249–51. doi: 10.1001/archotol.1980.00790290001001. [DOI] [PubMed] [Google Scholar]
- [22].Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972 Jun;29(6):1446–9. doi: 10.1002/1097-0142(197206)29:6<1446::aid-cncr2820290604>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- [23].Medina JE, Byers RM. Supraomohyoid neck dissection: rationale, indications, and surgical technique. Head Neck. 1989 Mar-Apr;11(2):111–22. doi: 10.1002/hed.2880110203. [DOI] [PubMed] [Google Scholar]
- [24].Yu S, Li J, Li Z, Zhang W, Zhao J. Efficacy of supraomohyoid neck dissection in patients with oral squamous cell carcinoma and negative neck. Am J Surg. 2006 Jan;191(1):94–9. doi: 10.1016/j.amjsurg.2005.10.008. [DOI] [PubMed] [Google Scholar]