Abstract
Calcified coronary arteries are associated with the development of cardiovascular disease and stroke. It is currently unknown whether coronary artery calcium (CAC) is associated with an increased risk of atrial fibrillation (AF). We addressed this question in 6,641 participants (mean age 62 ± 10; 53% women; 62% non-whites) from the Multi-Ethnic Study of Atherosclerosis (MESA) who were free of baseline clinical cardiovascular disease and AF. CAC measurements were assessed by cardiac computed tomography (CT) at study baseline. AF was ascertained by review of hospital discharge records and from Medicare claims data until December 31, 2010. Cox regression was used to compute hazard ratios (HR) and 95% confidence intervals (95%CI) for the association between CAC and AF. During a median follow up of 8.5 years, 308 (4.6%) participants developed AF. In a model adjusted for socio-demographics, cardiovascular risk factors, and potential confounders, higher CAC scores were associated with an increased risk of AF (CAC=0: HR=1.0, referent; CAC=1–100: HR=1.4, 95%CI=1.01, 2.0; CAC=101–300: HR=1.6, 95%CI=1.1, 2.4; CAC>300: 2.1, 95%CI=1.4, 2.9). The addition of CAC to the Framingham Heart Study and the CHARGE AF risk scores yielded an integrated discrimination improvement (IDI) of 0.0033 (95%CI=0.0015, 0.0066) and 0.0028 (95%CI=0.0012, 0.0057) and with relative IDI of 0.10 (95%CI=0.061, 0.15) and 0.077 (95%CI=0.040, 0.11), respectively. In conclusion, CAC is independently associated with an increased risk of AF.
Keywords: coronary calcium, atrial fibrillation, epidemiology
INTRODUCTION
Coronary artery calcium (CAC) measured by cardiac computed tomography (CT) provides an estimate of total coronary plaque burden.1 This technique largely has been studied to identify patients at-risk for obstructive coronary artery disease and has been shown to predict future coronary heart disease events.2–6 The application of CAC to predict conditions that are not limited to the coronary arteries has recently been explored. In a large population-based cohort study, CAC independently predicted stroke events.7 Additionally, highly calcified coronary arteries are associated with larger pulmonary veins and left atria, suggesting an association between CAC and atrial fibrillation (AF).8 However, no studies have examined this potential association. The purpose of this study was to examine the association of CAC with incident AF using data from the Multi-Ethnic Study of Atherosclerosis (MESA).
METHODS
Details of MESA have been reported previously.9 Briefly, between July 2000 and September 2002, a total of 6,814 persons were recruited at 6 field centers (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles, California; New York, New York; and St. Paul, Minnesota). Participants were required to be between 45 and 84 years of age and to have no clinical cardiovascular disease. All participants provided informed consent and the study protocol was approved by the Institutional Review Boards at each participating institution. Our analysis examined the relationship between baseline CAC measurements and incident AF. Participants were excluded if they did not undergo baseline measurement of CAC, a baseline diagnosis of AF was present, baseline characteristics were missing, or follow-up data regarding AF follow-up were missing.
Participant characteristics were collected during the initial MESA visit. Age, sex, race/ethnicity, income, and education were self-reported. Annual income was categorized as <$20,000, $20,000–$49,999, and ≥ $50,000, and education was categorized as “high school or less,” “some college,” and “college or more.” Smoking was defined as current or ever smoker. Blood samples were obtained after a 12-hour fast and measurements of total cholesterol, high-density lipoprotein (HDL) cholesterol, plasma glucose, and high sensitivity C-reactive protein (hs-CRP) were used. Diabetes was defined as fasting glucose values ≥126 mg/dL or a history of diabetes medication use. Blood pressure was measured for each participant after 5 minutes in the seated position and systolic measurements were recorded 3 separate times and the mean of the last two values was used. Aspirin, statin, antihypertensive, and lipid-lowering medication use were self-reported. Body mass index was computed as the weight in kilograms divided by the square of the height in meters. Left ventricular hypertrophy was defined by the Cornell criteria (R wave amplitude AVL plus S wave amplitude V3 ≥ 2800 mm males and ≥ 2000 mm females) using baseline electrocardiogram data.10 In a subgroup of MESA participants who had cardiac magnetic resonance imaging (MRI) data (N=4,896), left ventricular end-diastolic mass and left ventricular ejection fraction were recorded. Myocardial horizontal and vertical tagging were performed on three left ventricular short-axis slices (base, mid, and apex) by nonselective radiofrequency pulses separated by a spatial modulation of magnetization-encoding gradients. Imaging and analytical methods for this technique have been previously described.11
CAC measurements were assessed by cardiac CT using either cardiac-gated electron-beam CT or multi-detector CT systems depending on the study site.12 The CAC score was computed using the phantom-adjusted Agatston score for 2 consecutive scans for each participant and the mean value was used.13 During the CT examinations, the 2 scans were independently analyzed for CAC by 2 analysts. Interobserver agreement between different CT image analysts who measured CAC on the same cardiac CT image was excellent (κ-statistic, 0.90). Similarly, intraobserver agreement was excellent when the same analyst measured CAC at separate time periods (κ-statistic, 0.93).
Follow-up phone calls to study participants every 9–12 months were used to identify AF events. Medical records, including discharge diagnoses, were obtained for each hospitalization. Additionally, for participants 65 years or older enrolled in fee-for-service Medicare, Medicare claims data were used to identify AF diagnoses in the inpatient setting. Incident AF was defined by International Classification of Disease Ninth Revision codes 427.31 or 427.32.
Categorical variables were reported as frequency and percentage while continuous variables were recorded as mean ± standard deviation. Statistical significance for categorical variables was tested using the chi-square method and the Wilcoxon rank-sum procedure for continuous variables. Kaplan-Meier estimates were used to compute cumulative incidence of AF by CAC score and the differences in incidence estimates were compared using the log-rank procedure.14 Follow-up time was defined as the time between initial visit until the diagnosis of AF or until death, loss to follow-up, or end of follow-up (December 31, 2010). Cox proportional hazards regression was used to compute hazard ratios (HR) and 95% confidence intervals (95%CI) for the association between CAC scores and AF. CAC was examined using predefined categories (0, 1–100, 101–300, and >300).6 Additionally, CAC scores were analyzed as a continuous variable using the base-2 logarithm of the CAC score plus 1 (log2[CAC + 1]) to examine the risk of AF when the CAC score doubles.5 Multivariable models were constructed with incremental adjustments as follows: Model 1 adjusted for age, sex, race/ethnicity, income, and education; Model 2 adjusted for Model 1 covariates plus smoking status, systolic blood pressure, diabetes, BMI, total cholesterol, HDL-cholesterol, aspirin, antihypertensive and lipid-lowering medications, hs-CRP, and left ventricular hypertrophy. The proportional hazards assumption was not violated in our analysis. We tested for interactions between our main effect variable and age, sex, and race/ethnicity. Additionally, we further adjusted for coronary heart disease events as a time-dependent variable to examine whether incident coronary heart disease events mediate the association between CAC and AF. In participants with cardiac MRI data, we further adjusted for left ventricular end-diastolic mass and left ventricular ejection fraction.
We assessed the ability of CAC to predict AF by computing the C-statistic using covariates from the Framingham Heart Study and the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) AF consortium risk models for AF.15,16 The added predictive ability of CAC was compared by the difference in C-statistics of the models before and after CAC inclusion. We also investigated the integrated discrimination improvement (IDI) and relative IDI. The integrated discrimination improvement (IDI) quantifies the increase in the difference between mean predicted risks for participants who do and do not develop AF after adding CAC to the model and this measure also was computed.17,18 Additionally, net reclassification improvement (NRI) which quantifies any desirable change in predicted risk was computed for the following risk categories: <2.5%, 2.5% to 5%, >5%.19 Statistical significance was defined as p < 0.05. SAS Version 9.3 (Cary, NC) was used for all analyses.
RESULTS
Of the 6,814 participants from the original MESA cohort, 58 participants had a diagnosis of AF before enrollment in MESA. These cases, detected by Centers for Medicare & Medicaid Services linkage, were excluded although they did not have AF in the baseline electrocardiogram. Of those that remained, 6 participants with missing follow-up data and 109 participants missing either baseline characteristics or medication data also were excluded. A total of 6,641 study participants (mean age 62 ± 10; 53% women; 38% whites; 27% blacks; 22% Hispanics; 12% Chinese-Americans) were included in the final analysis.
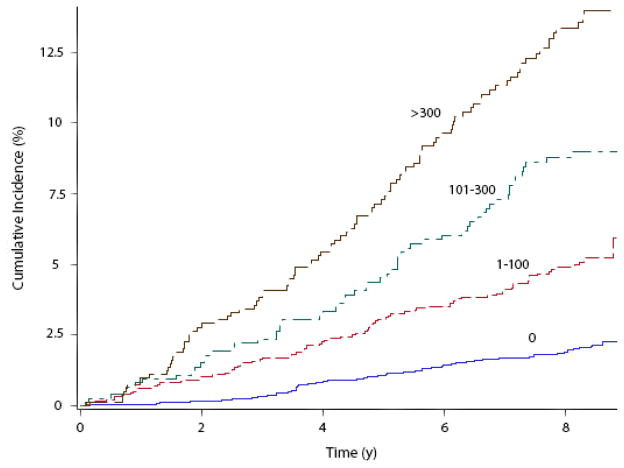
Over a median follow-up of 8.5 years, 308 (4.6%) participants developed AF. Baseline characteristics for study participants stratified by AF are shown in Table 1. The incidence rate of AF increased with increasing CAC scores (Table 2). Unadjusted cumulative incidence curves for AF by CAC scores are shown in Figure 1 (log-rank p<0.0001). In an unadjusted Cox regression model, higher CAC scores were associated with an increased risk of AF (CAC=0: HR=1.0; CAC=1–100: HR=2.5, 95%CI=1.8, 3.4; CAC=101–300: HR=4.3, 95%CI=3.1, 6.1; CAC>300: HR=6.9, 95%CI=5.0, 9.4). Similarly, doubling of the CAC score was associated with a 23% increased risk of AF (p<0.0001). This pattern of increasing risk of AF associated with increasing CAC scores persisted after adjustment for socio-demographics, cardiovascular risk factors, and potential confounders (Table 2). These results were consistent in subgroup analyses by sex and race/ethnicity (Table 3). A significant interaction was observed for age, with the association being slightly stronger for participants less than 62 years (Table 3). The association between CAC and AF remained significant after further adjustment for coronary heart disease events as a time-dependent variable (log2[CAC + 1]; HR=1.1, 95%CI=1.05, 1.12), left ventricular end-diastolic mass (log2[CAC + 1]; HR=1.1, 95%CI=1.05, 1.14), and left ventricular ejection fraction (log2[CAC + 1]; HR=1.1, 95%CI=1.06, 1.15).
Table 1.
Baseline Characteristics of Study Participants Stratified by Atrial Fibrillation (N=6,641)
| Characteristic | AF (n=308) | No AF (n=6,333) | P-value* |
|---|---|---|---|
| Age, mean ± SD (years) | 70 ± 8.0 | 62 ± 10 | <0.0001 |
| Male | 189 (61%) | 2,941 (46%) | <0.0001 |
| White | 168 (55%) | 2,381 (38%) | |
| Black | 66 (21%) | 1,757 (28%) | |
| Chinese-American | 21 (6.8%) | 773 (12%) | |
| Hispanic | 53 (17%) | 1,422 (22%) | <0.0001 |
| Education | |||
| At least high school | 118 (38%) | 2,299 (36%) | |
| Some college | 78 (25%) | 1,814 (29%) | |
| College or more | 112 (36%) | 2,220 (35%) | 0.45 |
| Annual income | |||
| <$20,000 | 99 (32%) | 1,680 (27%) | |
| $20,000-$49,999 | 106 (34%) | 2,219 (35%) | |
| ≥$50,000 | 103 (33%) | 2,434 (38%) | 0.067 |
| Body mass index, mean ± SD (kg/m2) | 29 ± 5.6 | 28 ± 5.5 | 0.19 |
| Current or former smoker | 180 (58%) | 3,107 (49%) | 0.0013 |
| Diabetes | 51 (17%) | 878 (14%) | 0.24 |
| Systolic blood pressure, mean ± SD (mm Hg) | 135 ± 22 | 126 ± 21 | <0.0001 |
| Total cholesterol, mean ± SD (mg/dL) | 190 ± 36 | 194 ± 36 | 0.011 |
| HDL-cholesterol, mean ± SD (mg/dL) | 50 ± 15 | 51 ± 15 | 0.034 |
| Antihypertensive medications | 177 (57%) | 2,262 (36%) | <0.0001 |
| Statins | 52 (17%) | 924 (15%) | 0.27 |
| Aspirin | 120 (39%) | 1,452 (23%) | <0.0001 |
| Lipid-lowering medications | 56 (18%) | 1,007 (16%) | 0.29 |
| hs-CRP, mean ± SD (mg/L) | 3.9 ± 6.0 | 3.7 ± 5.9 | 0.31 |
| Left ventricular hypertrophy | 15 (4.9%) | 241 (3.8%) | 0.34 |
| Coronary artery calcium score | |||
| 0 | 67 (22%) | 3,283 (52%) | |
| <100 | 84 (27%) | 1,660 (26%) | |
| 100–300 | 60 (19%) | 674 (11%) | |
| >300 | 97 (31%) | 716 (11%) | <0.0001 |
| Mean ± SD | 444 ± 807 | 128 ± 377 | <0.0001 |
Statistical significance for continuous data was tested using Wilcoxon rank-sum procedure and categorical data was tested using the Chi-square test.
AF=atrial fibrillation; HDL=high-density lipoprotein; hs-CRP= high sensitivity C-reactive protein; SD=standard deviation.
Table 2.
Risk of Atrial Fibrillation with Increasing Coronary Artery Calcium Score
| CAC Score | Events/No. at risk | Incidence Rate per 1000 Person-Years (95%CI) | Model 1* HR (95%CI) |
P-value | Model 2† HR (95%CI) |
P-value |
|---|---|---|---|---|---|---|
| 0 | 67/3,350 | 2.6 (2.0, 3.2) | 1.0 | - | 1.0 | - |
| 1–100 | 84/1,744 | 6.4 (5.1, 7.9) | 1.5 (1.04, 2.0) | 0.027 | 1.4 (1.01, 2.0) | 0.046 |
| 101–300 | 60/734 | 11 (8.5, 14) | 1.8 (1.2, 2.6) | 0.0017 | 1.6 (1.1, 2.4) | 0.010 |
| >300 | 97/813 | 17 (14, 21) | 2.4 (1.7, 3.3) | <0.0001 | 2.1 (1.4, 2.9) | <0.0001 |
| Log2(CAC + 1)‡ | 308/6,641 | 6.1 (5.4, 6.8) | 1.1 (1.07, 1.14) | <0.0001 | 1.1 (1.05, 1.13) | <0.0001 |
Adjusted for age, sex, race/ethnicity, income, and education.
Adjusted for Model 1 covariates plus smoking status, systolic blood pressure, diabetes, body mass index, total cholesterol, HDL-cholesterol, aspirin, antihypertensive and lipid-lowering medications, hs-CRP, and left ventricular hypertrophy.
Denotes HR for AF with doubling of CAC score.
AF=atrial fibrillation; CAC=coronary artery calcium; CI=confidence interval; HDL=high-density lipoprotein; hs-CRP= high sensitivity C-reactive protein; HR=hazard ratio.
Figure 1. Unadjusted Cumulative Incidence of Atrial Fibrillation by Coronary Artery Calcium Score (N=6,641)*.
*Cumulative incidence curves are different between all groups (log-rank p <0.0001).
y=years.
Table 3.
Risk of Atrial Fibrillation with Increasing Coronary Artery Calcium Score Stratified by Age, Sex, and Race/Ethnicity*
| Subgroup | Events/No. at risk | Unadjusted HR (95%CI) |
P-value | Model 1† HR (95%CI) |
P-value | Model 2‡ HR (95%CI) |
P-value | Interaction P-value§ |
|---|---|---|---|---|---|---|---|---|
| Age, years || | ||||||||
| <62 | 43/3,235 | 1.3 (1.2, 1.4) | <0.0001 | 1.3 (1.1, 1.4) | <0.0001 | 1.2 (1.1, 1.3) | 0.0004 | 0.048 |
| ≥62 | 265/3,406 | 1.15 (1.1, 1.2) | <0.0001 | 1.1 (1.07, 1.15) | <0.0001 | 1.1 (1.06, 1.14) | <0.0001 | |
| Sex | ||||||||
| Female | 119/3,511 | 1.2 (1.19, 1.3) | <0.0001 | 1.1 (1.06, 1.2) | <0.0001 | 1.1 (1.05, 1.2) | 0.0005 | 0.28 |
| Male | 189/3,130 | 1.2 (1.16, 1.3) | <0.0001 | 1.1 (1.05, 1.14) | 0.0001 | 1.1 (1.03, 1.13) | 0.0015 | |
| Race/Ethnicity | ||||||||
| White | 168/2,549 | 1.2 (1.15, 1.25) | <0.0001 | 1.1 (1.03, 1.13) | 0.0026 | 1.1 (1.02, 1.12) | 0.010 | 0.11 |
| Non-White | 140/4,092 | 1.3 (1.2, 1.31) | <0.0001 | 1.1 (1.08, 1.19) | <0.0001 | 1.1 (1.05, 1.17) | <0.0001 | |
HRs presented are for the continuous variable, log2(CAC + 1).
Adjusted for age, sex, race/ethnicity, income, and education.
Adjusted for Model 1 covariates plus smoking status, systolic blood pressure, diabetes, body mass index, total cholesterol, HDL-cholesterol, aspirin, antihypertensive and lipid-lowering medications, hs-CRP, and left ventricular hypertrophy.
Interactions tested using Model 2.
Dichotomized at the median age for study participants.
CAC=coronary artery calcium; CI=confidence interval; HDL=high-density lipoprotein; HR=hazard ratio; hs-CRP= high sensitivity C-reactive protein; y=years.
The addition of CAC to the Framingham Heart Study and CHARGE AF risk scores improved the C-statistic from 0.771 to 0.784 (p=0.0048) and 0.789 to 0.798 (p=0.0025), respectively. Also, the addition of CAC to the Framingham Heart Study and the CHARGE AF risk scores yielded an IDI of 0.0033 (95%CI=0.0015, 0.0066) and 0.0028 (95%CI=0.0012, 0.0057) and with relative IDI of 0.10 (95%CI=0.061, 0.15) and 0.077 (95%CI=0.040, 0.11), and a categorical NRI of 0.051 (95%CI=0.015, 0.089) and 0.039 (95%CI=0.0068, 0.075), respectively.
DISCUSSION
In this analysis from MESA, increasing CAC scores were independently associated with the development of AF. This association was consistent across subgroups stratified by sex and race/ethnicity. To our knowledge, this is the first study to show an association between CAC scores and incident AF.
The multi-ethnic population of MESA allowed us to examine if racial differences exist between the association of AF and CAC scores. The prevalence and severity of CAC has been shown to vary among the ethnic groups of MESA.20 However, our results suggest that racial differences do not exist between CAC and AF.
An interaction by age was observed with a significantly stronger association in participants less than 62 years of age. The level of CAC varies with age and older persons have been shown to have increased CAC scores compared with younger persons.21 However, young to early middle-aged persons with increased risk for coronary heart disease events also have increased levels of CAC.22 Potentially, the stronger association of CAC with AF among younger study participants reflects a population subgroup with an increased predisposition for cardiovascular disease, including arrhythmias such as AF.
There are several explanations for the association between CAC and AF. Potentially, the association between CAC and AF is mediated by coronary heart disease events.2–6,23,24 However, the association between CAC and AF remained significant after adjusting these events. Persons with increased levels of CAC have enlarged left atria and pulmonary veins and both structures are associated with AF.8,25 Although unable to directly adjust for these structures, our results remained significant after adjusting for surrogate markers (electrocardiogram left ventricular hypertrophy and MRI left ventricular mass and function). Additionally, higher levels of inflammation are associated with the development of CAC and AF.26,27 It is plausible that dysfunctional regulation of this biological process associated with CAC increases one’s risk for AF. However, our results remained significant after adjustment for markers of inflammation (i.e. hs-CRP), suggesting that the relationship between CAC and AF unlikely is to be fully explained by inflammation. Nonetheless, the findings of this study suggest an association between CAC and AF and further studies are needed to determine the underlying pathophysiologic link between CAC and AF.
Our results show that CAC improves discrimination and reclassification of AF beyond variables included in the Framingham Heart Study and CHARGE AF risk scores for AF.15,16 Cardiac CT is a relatively non-invasive technique that can easily detect the presence and severity of CAC.1 Potentially, this imaging modality is able to improve the prediction of AF risk and identify persons who are high-risk for the development of AF. However, further research is needed to determine which populations will benefit from such screening as this was beyond the scope of the current study.
Our results should be read in the context of certain limitations. Paroxysmal cases of AF possibly were missed due to the time-dependent nature of this condition. Incident AF cases were ascertained from hospitalization discharge records and inpatient Medicare claims using International Classification of Disease codes which possibly resulted in misclassification. However, these codes have adequate positive predictive value for the identification of AF events.28 The ability of CAC to predict AF may vary by anatomic location and this was not examined. Additionally, cardiac CT is unable to distinguish medial and intimal calcification and results may vary by the location of calcium within the coronary artery.29 We included several covariates in our multivariate models that likely are to influence the development of AF. However, we acknowledge that residual confounding remains a possibility.
Acknowledgments
This research was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-RR-024156 and UL1-RR-025005 from NCRR. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. WTO had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Disclosure: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ghadri JR, Pazhenkottil AP, Nkoulou RN, Goetti R, Buechel RR, Husmann L, Herzog BA, Wolfrum M, Wyss CA, Templin C, Kaufmann PA. Very high coronary calcium score unmasks obstructive coronary artery disease in patients with normal SPECT MPI. Heart. 2011;97:998–1003. doi: 10.1136/hrt.2010.217281. [DOI] [PubMed] [Google Scholar]
- 2.Folsom AR, Kronmal RA, Detrano RC, O’Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, Tracy RP, Watson KE, Burke GL. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA) Arch Intern Med. 2008;168:1333–1339. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain A, McClelland RL, Polak JF, Shea S, Burke GL, Bild DE, Watson KE, Budoff MJ, Liu K, Post WS, Folsom AR, Lima JA, Bluemke DA. Cardiovascular imaging for assessing cardiovascular risk in asymptomatic men versus women: the multi-ethnic study of atherosclerosis (MESA) Circ Cardiovasc Imaging. 2011;4:8–15. doi: 10.1161/CIRCIMAGING.110.959403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman AB, Naydeck BL, Ives DG, Boudreau RM, Sutton-Tyrrell K, O’Leary DH, Kuller LH. Coronary artery calcium, carotid artery wall thickness, and cardiovascular disease outcomes in adults 70 to 99 years old. Am J Cardiol. 2008;101:186–192. doi: 10.1016/j.amjcard.2007.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 6.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 7.Hermann DM, Gronewold J, Lehmann N, Moebus S, Jockel KH, Bauer M, Erbel R Heinz Nixdorf Recall Study Investigative G. Coronary artery calcification is an independent stroke predictor in the general population. Stroke. 2013;44:1008–1013. doi: 10.1161/STROKEAHA.111.678078. [DOI] [PubMed] [Google Scholar]
- 8.Pan NH, Tsao HM, Chang NC, Lee CM, Chen YJ, Chen SA. Dilated left atrium and pulmonary veins in patients with calcified coronary artery: a potential contributor to the genesis of atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20:153–158. doi: 10.1111/j.1540-8167.2008.01290.x. [DOI] [PubMed] [Google Scholar]
- 9.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 10.Devereux RB, Casale PN, Eisenberg RR, Miller DH, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy using echocardiographic determination of left ventricular mass as the reference standard. Comparison of standard criteria, computer diagnosis and physician interpretation. J Am Coll Cardiol. 1984;3:82–87. doi: 10.1016/s0735-1097(84)80433-7. [DOI] [PubMed] [Google Scholar]
- 11.Fernandes VR, Cheng S, Cheng YJ, Rosen B, Agarwal S, McClelland RL, Bluemke DA, Lima JA. Racial and ethnic differences in subclinical myocardial function: the Multi-Ethnic Study of Atherosclerosis. Heart. 2011;97:405–410. doi: 10.1136/hrt.2010.209452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 13.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 14.Gray RJ, Tsiatis AA. A linear rank test for use when the main interest is in differences in cure rates. Biometrics. 1989;45:899–904. [PubMed] [Google Scholar]
- 15.Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D’Agostino RB, Sr, Newton-Cheh C, Yamamoto JF, Magnani JW, Tadros TM, Kannel WB, Wang TJ, Ellinor PT, Wolf PA, Vasan RS, Benjamin EJ. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens AC, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Agarwal SK, McManus DD, Ellinor PT, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kaab S, Couper D, Harris TB, Soliman EZ, Stricker BH, Gudnason V, Heckbert SR, Benjamin EJ. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2:e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 18.Kerr KF, McClelland RL, Brown ER, Lumley T. Evaluating the incremental value of new biomarkers with integrated discrimination improvement. Am J Epidemiol. 2011;174:364–374. doi: 10.1093/aje/kwr086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, Ouyang P, Jackson S, Saad MF. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 21.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2006;113:30–37. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 22.Okwuosa TM, Greenland P, Ning H, Liu K, Lloyd-Jones DM. Yield of screening for coronary artery calcium in early middle-age adults based on the 10-year Framingham Risk Score: the CARDIA study. JACC Cardiovasc Imaging. 2012;5:923–930. doi: 10.1016/j.jcmg.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Malley PG, Taylor AJ, Jackson JL, Doherty TM, Detrano RC. Prognostic value of coronary electron-beam computed tomography for coronary heart disease events in asymptomatic populations. Am J Cardiol. 2000;85:945–948. doi: 10.1016/s0002-9149(99)00906-6. [DOI] [PubMed] [Google Scholar]
- 24.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 25.Schwartzman D, Lacomis J, Wigginton WG. Characterization of left atrium and distal pulmonary vein morphology using multidimensional computed tomography. J Am Coll Cardiol. 2003;41:1349–1357. doi: 10.1016/s0735-1097(03)00124-4. [DOI] [PubMed] [Google Scholar]
- 26.Jenny NS, Brown ER, Detrano R, Folsom AR, Saad MF, Shea S, Szklo M, Herrington DM, Jacobs DR., Jr Associations of inflammatory markers with coronary artery calcification: results from the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2010;209:226–229. doi: 10.1016/j.atherosclerosis.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 28.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rist C, Nikolaou K, Flohr T, Wintersperger BJ, Reiser MF, Becker CR. High-resolution ex vivo imaging of coronary artery stents using 64-slice computed tomography--initial experience. Eur Radiol. 2006;16:1564–1569. doi: 10.1007/s00330-006-0186-5. [DOI] [PubMed] [Google Scholar]