Abstract
The radiotracer 3-[18F]fluoro-5-(2-pyridinylethynyl)benzonitrile, or [18F]FPEB, is a promising PET imaging agent for the metabotropic glutamate subtype 5 receptor (mGluR5). In an effort to develop a routine production method of this radiotracer for use in clinical research we adapted its radiosynthesis to an automated chemistry module. In the meanwhile, we also developed a simplified “one-pot” method for the preparation of the nitrobenzonitrile radiolabeling precursor for [18F]FPEB and its reference standard to replace the existing multi-step synthetic approach.
Keywords: [18F]FPEB, mGluR5, Radiotracer synthesis, PET, Suzuki coupling
1. Introduction
The metabotropic glutamate subtype 5 receptor (mGluR5) are believed to be involved in a number of central nervous system (CNS) disorders including autism, Fragile X syndrome, Huntington’s disease, Parkinson’s disease, addiction, anxiety, and depression (Carroll, 2008; Moghaddam, 2004; Spooren et al., 2010; Witkin et al., 2007). 3-[18F]Fluoro-5-(2-pyridinylethynyl)benzonitrile, or [18F]FPEB, was developed for PET imaging of the mGluR5 in vivo. In preclinical studies [18F]FPEB displays several favorable properties for a PET tracer, such as high brain uptake, appropriate tissue kinetics, and high levels of specific binding (Hamill et al., 2005). Recent imaging studies have shown that when translated to humans these characteristics of [18F]FPEB are largely preserved, and that [18F]FPEB displays higher specific binding signals than [11C]ABP688 (Kuwabara et al., 2011; Sullivan et al., 2013; Wong et al., 2013). Hence, it is expected that [18F]FPEB will be an effective PET imaging agent for the investigation of mGluR5 in neuropsychiatric disorders, as well as receptor occupancy studies of emerging therapeutic agents targeting mGluR5.
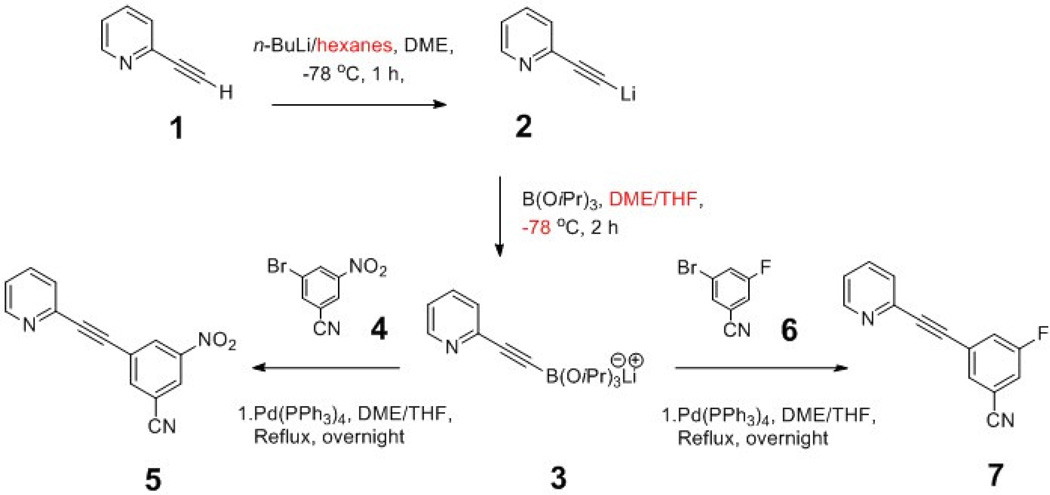
In the initial report by Hamill et al., [18F]FPEB was synthesized from the chlorobenzonitrile precursor and [18F]fluoride with K2CO3/Kryptofix under microwave heating conditions (Hamill et al., 2005). Wong et al. used the same precursor, but with Cs2CO3 instead of K2CO3/Kryptofix as activating agent in the radiosynthesis of [18F]FPEB (Wong et al., 2013), while Wang et al. used the nitrobenzonitrile precursor under conventional heating conditions (Wang et al., 2007). However, these methods for [18F]FPEB radiosynthesis are performed manually. In our effort to validate [18F]FPEB for human use we undertook efforts to adapt the radiosynthesis to an automated chemistry module for reliable production of [18F]FPEB. Synthesis of the chlorobenzonitrile or nitrobenzonitrile precursor was described by Wang et al. and Alagille et al., both of which employed pathways with multiple synthetic steps (Alagille et al., 2011; Wang et al., 2007). Hence, we also sought to develop a simpler and more efficient method for the synthesis of the nitrobenzonitrile precursor and the authentic standard FPEB. Colobert et al. recently reported a methodology of palladium-catalyzed Suzuki-like sp-sp2 coupling of aryl halides with terminal alkynes for the synthesis of 2,3-disubstituted benzo[b]furans, by first deprotonating the alkynes with n-butyl lithium to form an alkynyllithium and then reacting with triisopropylborate to generate a lithiated boronate complex in situ, followed by Pd-catalyzed coupling with arylbromides (Castanet et al., 2000; Colobert et al., 2005). We adopted this methodology for an efficient, one-pot preparation of FPEB and its nitrobenzonitrile radiolabeling precursor from commercially available building blocks. In this paper we report our findings from these experiments.
2. Materials and Methods
2.1. Reagents and Instrumentation
Reagents and solvents were purchased from commercial suppliers and used without further purification. [18O]H2O was obtained from Huayi Isotopes Co (ON, Canada). 2-Ethynylpyridine were purchased from Sigma-Aldrich (MO, USA), 3-bromo-5-nitrobenzonitrile from Combi-Blocks (CA, USA), Inc., and 3-bromo-5-fluorobenzonitrile from Santa Cruz Biotechnology (TX, USA).
Melting point was measured using an electrothermal MEL-TEMP® melting point apparatus from Barnstead International (IA, USA). 1H and 13C NMR spectra were recorded on either a Bruker Avance or Varian Mercury instrument at the Yale Chemical and Biophysical Instrumentation Center (CBIC). Chemical shifts (δ) are given in ppm, coupling constants (J) in Hz. 1H NMR spectra are relative to tetramethylsilane (δ = 0.00 ppm). 13C NMR spectra are relative to CDCl3 (δ = 77.0 ppm). Mass spectral data were obtained with a 9.4 Tesla Apex-Qe Hybrid Qe-Fourier Transform Ion Cyclotron Resonance (FT-ICR) mass spectrometer from Bruker (Billerica, MA).
A Shimadzu Prominence HPLC system was used for quality control analysis. It consists of a LC-20AT quaternary pump, a DGU-20A degasser, a CBM-20A communications bus module, and a SPD-M20A Photodiode Array (PDA) detector or SPD-20A UV/Vis detector connected in series with a Bioscan Flow-Count gamma-detector.
[18F]Fluoride was produced on a GE PETtrace cyclotron using an 18O(p, n)18F reaction in a GE high yield target assembly. Bombardment at 40–65 µA of beam current for 30–40 min on this target produced 1–3 Ci of radioactivity. Synthesis of [18F]FPEB was carried out with a TRACERLab™ FXF-N module from GE Healthcare (Milwaukee, WI).
2.2.1. One-pot synthesis of 3-nitro-5-[(pyridine-2-yl)ethynyl]benzonitrile (5), precursor for [18F]FPEB
2-Ethynylpyridine 1 (408 mg, 3.96 mmol) was dissolved in anhydrous dimethoxyethane (20 mL) in a 100 mL round-bottomed flask under Argon. The solution was cooled in an acetone-dry ice bath (−78 °C). To this solution was added slowly 2.5 M n-BuLi in hexanes (2.06 mL, 5.15 mmol). After reaction for 1 h at −78 °C, a solution of triisopropylborate (970 mg, 5.15 mmol) in anhydrous THF (2 mL) was added slowly and the reaction mixture stirred for another 2 h at −78 °C. The reaction was then allowed to warm to room temperature, and a solution of Pd(PPh3)4 (140 mg, 0.12 mmol) and 3-bromo-5-nitrobenzonitrile (674 mg, 2.97 mmol) in 1,2-dimethoxyethane (20 mL) was added. The resulting reaction mixture was heated under reflux overnight and then allowed to cool to room temperature. Water (50 mL) was added and the mixture extracted with EtOAc (3 × 30 mL). The combined organic phase was dried with Na2SO4, filtered, and the filtrate evaporated in vacuo. Flash column chromatography on silica gel and elution with 15–30% EtOAc/hexane afforded the product, which was crystallized from MeOH to give compound 5 as a yellowish solid (244 mg, 33%), m.p.: 135 – 138 °C (lit. 133 – 134 °C) (Wang et al., 2007). 1H NMR (500 MHz, CDCl3): δ 8.70 (d, 1H, J = 4.6 Hz), 8.66 (m, 1H), 8.50 (m, 1H), 8.17 (m, 1H), 7.79 (td, 1H, J = 7.7, 1.7 Hz), 7.62 (dt, 1H, J = 7.8, 1.1 Hz), 7.39 (ddd, 1H, J = 7.8, 4.8, 1.1 Hz). 13C NMR (126 MHz, CDCl3): δ 150.50, 148.29, 141.63, 140.11, 136.53, 130.41, 127.71, 126.58, 126.11, 124.13, 115.86, 114.59, 93.34, 83.88. HRMS: m/z [M + H]+ calculated for C14H7N3O2: 250.0611; found: 250.0608.
2.2.2. One-pot synthesis of 3-fluoro-5-[(pyridine-2-yl)ethynyl]benzonitrile (7, FPEB)
Compound 7 was prepared as a yellowish solid in 72% yield (478.2 mg, 2.15 mmol), in a procedure similar to that for compound 5, using 3-bromo-5-fluorobenzonitrile (6) (594.5 mg, 2.97 mmol), instead of 3-bromo-5-nitrobenzonitrile, m.p. 97 – 99 °C (lit. 97 – 98.5 °C) (Wang et al., 2007). 1H NMR (300 MHz, CDCl3): δ 8.66 (d, 1 H, J = 5.0 Hz), 7.74 (br t, 1 H, J = 8.0 Hz), 7.67 (br, 1 H), 7.57-7.51 (m, 2 H), 7.38-7.30 (m, 2 H). 13C NMR (75 MHz, CDCl3): δ 161.88 (d, JC,F = 251.6 Hz), 150.13, 141.92, 136.62, 131.46 (d, JC,F = 3.6 Hz), 127.58, 125.96 (d, JC,F = 10.0 Hz), 123.76, 123.50 (d, JC,F = 22.9 Hz), 119.54 (d, JC,F = 24.7 Hz), 116.76 (d, JC,F = 3.3 Hz), 114.34 (d, JC,F = 10.3 Hz), 91.37, 85.41 (d, JC,F = 3.4 Hz). 19F NMR (282 MHz, CDCl3): δ −108.89 (t, J = 8.3 Hz). HRMS: m/z [M + H]+ calculated for C14H7N2F: 223.0666; found: 223.0663.
2.3. Automated radiosynthesis of [18F]FPEB using a GE TRACERLab™ FXF-N module
Radiosynthesis of [18F]FPEB was performed using a computer controlled automated TRACERLab™ FXF-N module. A bolus of [18O]H2O containing [18F]fluoride was loaded onto a Chromafix 30-PS-HCO3 cartridge and eluted into the graphite reaction vessel with a solution of Kryptofix K222 (7 mg) and K2CO3 (0.7 mg) in 1 mL of CH3CN / water (1:0.4, v/v). The solvent was azeotropically evaporated at reduced pressure and under Ar stream. Two more portions of MeCN (1 mL each) were added to the reaction vessel and subsequently evaporated to afford a dry residue. After cooling to 55 °C, a solution of the precursor 3-nitro-5-[(pyridine-2-yl)ethynyl]benzonitrile (5) (1.0 mg) in anhydrous DMSO (1.5 mL) was added. The reaction vessel was sealed, and the reaction mixture was stirred and heated at 150 °C for 15 min. After cooling to 65 °C, the mixture was diluted with H2O (6.5 mL) and loaded onto a Waters C18 Light SepPak cartridge. The reaction vessel was rinsed with a second portion of H2O (6.5 mL) and the solution passed through the same SepPak cartridge. Crude reaction product was eluted off the SepPak with EtOH (1 mL) into a receiving vial preloaded with H2O (2.5 mL). The resulting mixture was then loaded onto a semi-preparative HPLC column (Phenomenex Luna C18(2), 10 µm, 250 × 10 mm) eluting with 38% EtOH/62% H2O at a flow rate of 5 mL/min. The product fraction (eluting at 28–31 min) was collected and diluted with H2O (15 mL) in a round bottom flask. The diluted product solution was then passed through a second C18 Light SepPak cartridge. The SepPak was washed with H2O (15 mL) and dried. The final product was eluted from the second SepPak with EtOH (1 mL), followed by USP saline (3 mL), into a product vial pre-charged with USP saline (7 mL). Finally, the formulated product solution was passed through a membrane filter (Millipore Millex GV, 0.22 µm) into a vented sterile empty vial. A portion of the product solution was then taken for quality control tests.
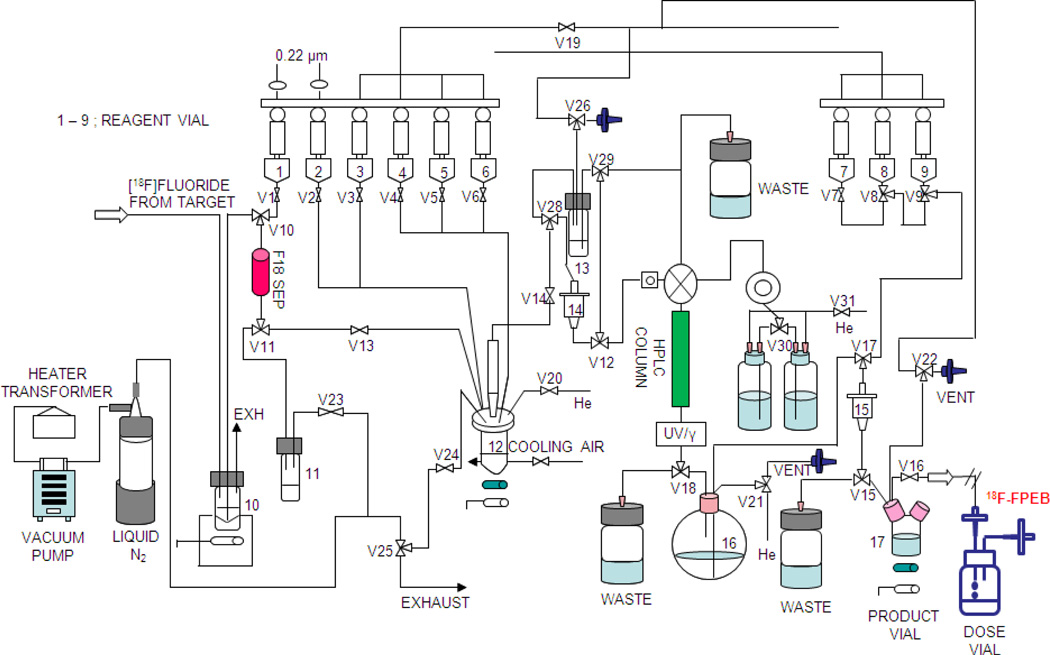
A schematic drawing of the GE Tracerlab FX-FN synthesis module is depicted in Figure 1. Following sections describe the flow of events in the schematic.
Figure 1.
Schematic diagram of GE FX-FN synthesis module for the radiosynthesis of [18F]FPEB
2.3.1. Preparation of the GE TRACERLab FXFN module for [18F]FPEB synthesis
Before each synthetic run the automated module is thoroughly cleaned. Reagents were then loaded in reagent vials 1–9 (V1–V9): V1: 1 mL of K222-K2CO3 solution, V2: 2 mL of MeCN, V3: 1 mg of precursor in 1.5 mL DMSO, V4: 1 mL of USP absolute EtOH, V5: not used, V6: 13.5 mL of H2O, V7: 3 mL of USP saline, V8: 1 mL of USP absolute EtOH, V9: 15 mL of H2O. The receiving vial 13 and the round bottom flask 16 were pre-filled with 2.5 mL and 15 mL of H2O, respectively. The PRODUCT VIAL was pre-filled with 7 mL of USP saline. An activated Chromafix 30-PS-HCO3 ion exchange cartridge was placed onto the holder between valves v10 and v11. Two activated Waters C18 Light SepPak cartridge (14 and 15) were also put in place. A 10 mL sterile empty vial (DOSE VIAL) attached with a Millipore Millex® GV membrane filter (0.22 µm, 33 mm) and a sterile venting needle was connected to the end of product line from valve v16. A semi-preparative HPLC COLUMN was put in place and equilibrated with the mobile phase for 25 min at a flow rate of 5 mL/min before use.
2.3.2. [18F]Fluoride drying step
When the [18F]fluoride delivery from target was complete, the [18F]fluoride solution in container 10 was drawn through the Chromafix SepPak cartridge (F18SEP) under vacuum. Trapped [18F]fluoride was then eluted with 1 mL of K222-K2CO3 solution from V1 into the reaction vessel 12 and the solvent evaporated under heating (70 °C) and vacuum. MeCN in V2 was added in two portions (1 mL each) to the reaction vessel 12 during the azeotropic evaporation process with the first portion added when the reaction vessel was at 70 °C and the second at 100 °C.
2.3.3. [18F]FPEB labeling step
After completion of the [18F]fluoride drying step, precursor solution in V3 was added to the reaction vessel 12 and the reaction was performed by heating at 150 °C for 15 min. After reaction, the crude reaction mixture was diluted with H2O (6.5 mL) from V6 and the solution passed through the C18 Light SepPak 14. SepPak 14 was washed with the remainder of H2O (~7 mL) from V6. The product captured on SepPak 14 was eluted into the receiving vial 13 with 1 mL of EtOH from V4. During this process, the [18F]FPEB crude product was thoroughly mixed with the pre-filled water (2.5 mL) in receiving vial 13 by argon flow.
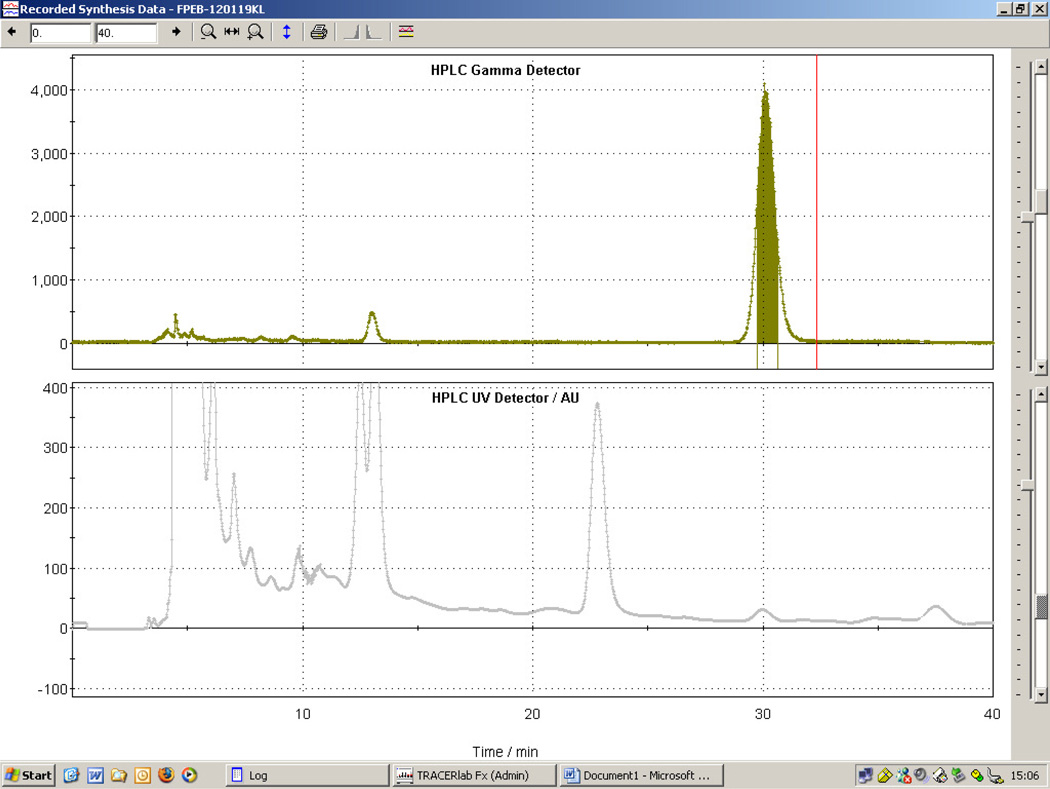
2.3.4. [18F]FPEB semi-preparative HPLC purification step
The crude [18F]FPEB product mixture in receiving vial 13 was loaded into a 5 mL stainless steel loop and injected onto the semi-preparative HPLC COLUMN. The chromatogram was monitored with tandem radioactivity and UV (absorbance at 254 nm) detectors. A typical chromatogram for the semi-preparative HPLC is shown in Figure 2. It shows three main distinctive radioactive peaks, which agree well with the published data by Wang et al. [9]. The [18F]FPEB product fraction eluting between 28–31 min was collected, diluted with H2O (15 mL) in the round bottomed flask 16, and passed through the second C18 Light Sep-Pak 15. The SepPak was rinsed with 15 mL of H2O from vial V9 and dried with helium flow.
Figure 2.
A typical semi-preparative HPLC chromatogram for [18F]FPEB.
2.3.5. [18F]FPEB formulation step
The product [18F]FPEB was eluted from the Sep-Pak 15 with 1 mL of EtOH from vial V8, followed by 3 mL of USP sterile saline from vial V7 into the PRODUCT VIAL prefilled with 7 mL of USP saline. Finally, the content in the PRODUCT VIAL was passed through a sterile membrane filter (33 mm diameter, 0.22 µm pore size, Millex® GV, Millipore) into a 10 mL sterile DOSE VIAL. The total synthesis time was about 90 min from end of beam (EOB).
2.4. Quality Control for [18F]FPEB
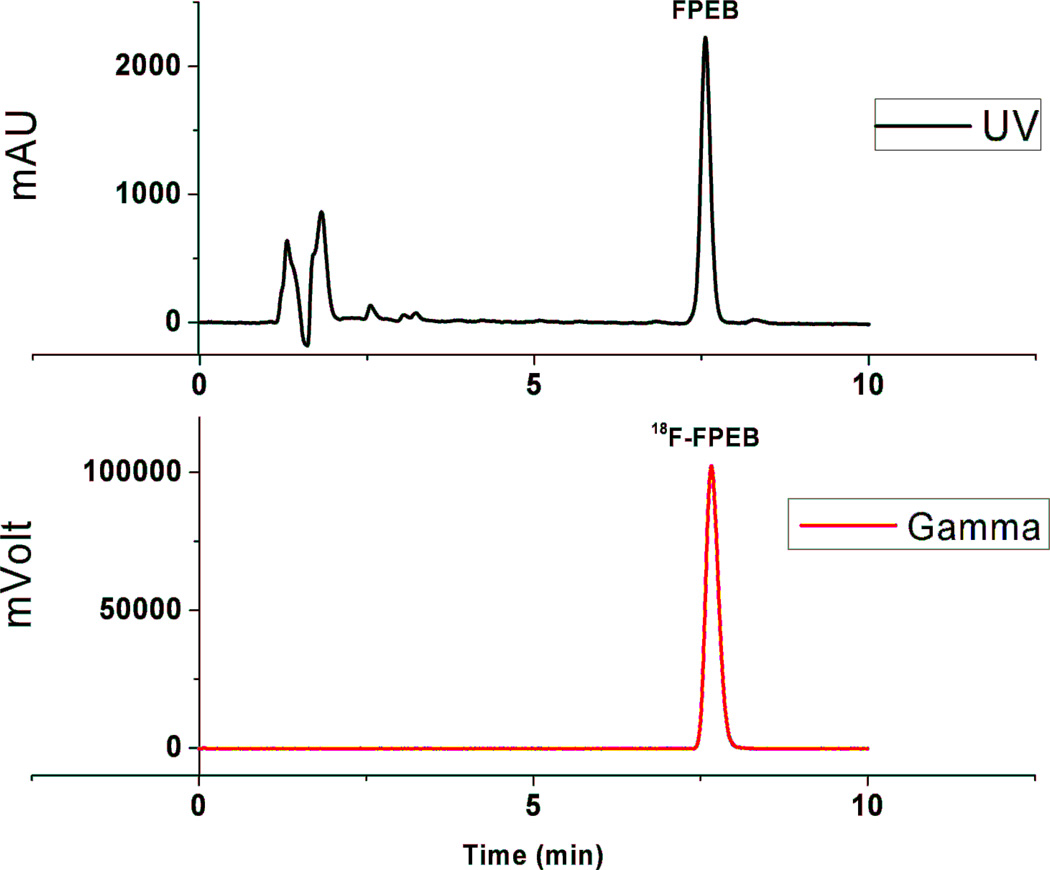
Radiochemical purity, chemical purity and specific activity of [18F]FPEB was determined by analytical HPLC analysis of the final product solution. A Phenomenex Luna C18(2) analytical column (5 µm, 250 × 4.6 mm) was used, and eluted with CH3CN/0.1% trifluoroacetic acid (TFA) (40:60, v/v) at a flow rate of 2 mL/min. The eluent was monitored for radioactivity and UV absorbance at 280 nm (tR = ~7.7 min for [18F]FPEB). A typical chromatogram from the quality control analytical HPLC is shown in Figure 3.
Figure 3.
A typical quality control analytical HPLC chromatogram for [18F]FPEB.
Identity was confirmed by co-injection of the product solution with the reference standard FPEB. The radiochemical purity for [18F]FPEB were greater than 98%. Specific activity was calculated by assaying injected radioactivity and its associated mass determined by the area under UV peak against a standard mass curve.
3. Results and discussion
3.1. One-pot synthesis of the nitrobenzonitrile precursor and reference compound for [18F]FPEB
Both the nitrobenzonitrile precursor and reference compound for [18F]FPEB were successfully synthesized in a one-pot procedure with in-situ generation of an alkynyl borate followed by a Suzuki coupling of the borate with an aryl bromide (Scheme 1). The precursor was prepared in 33% yield and the reference compound in 72% yield. This simple one-pot synthesis uses commercially available starting materials and allows the preparation of both compounds in 2 days.
Scheme 1.
One-pot synthesis scheme for [18F]FPEB precursor and FPEB
3.2. Automated synthesis of 3-[18F]fluoro-5-[(pyridine-2-yl)ethynyl]benzonitrile (8, [18F]FPEB)
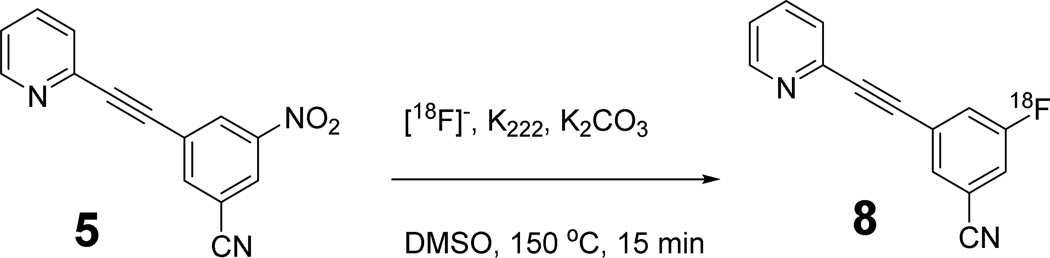
Synthesis of [18F]FPEB is depicted in Scheme 2. [18F]FPEB is produced by nucleophilic displacement of the nitro group in the nitrobenzonitrile precursor with [18F]fluoride under conventional heating conditions. Initial synthesis of [18F]FPEB used the chlorobenzonitrile precursor and [18F]fluoride with kryptofix-222 under microwave heating conditions. Radiochemical yield of ~4% was achieved. Wong et al. used the same precursor with cesium [18F]fluoride under the microwave heating condition (50 W, 4 min), and obtained an average of 45.6 mCi of [18F]FPEB at the end of synthesis. No radiochemical yield was reported. Using the nitrobenzonitrile precursor, Wang et al. reported the preparation of [18F]FPEB in ~5% radiochemical yield under conventional heating condition (110 °C, 10 min). However, heating at 110 °C gave little product when the synthesis was carried out in the automated module. When the temperature was raised to 150 °C and reaction carried out for 15 min, we were able to obtain [18F]FPEB in sufficiently high yield for routine use in clinical research. By using this automated synthesis procedure we have produced over 100 batches of [18F]FPEB with an average radiochemical yield of 2.6% and a highest yield of 9.2%. Radiosynthesis carried out with the GE automated chemistry module FX-FN produced [18F]FPEB in >98% radiochemical purity. The amount of final [18F]FPEB product was 46.1 ± 29.8 mCi (n = 114), enough for use in PET imaging experiments in one or two human subjects. Total synthesis time was ~90 min and specific activity was 5.32 ± 2.47 mCi/nmol at the end of synthesis (n = 114).
Scheme 2.
Radiosynthesis of [18F]FPEB
The final [18F]FPEB solution produced from the automated synthesis met all quality specifications for clinical doses. Table 1 summarizes the quality specifications for [18F]FPEB, test methods and quality control analysis results from three consecutive production batches. For each batch of [18F]FPEB product solution, radiochemical purity, chemical purity, and specific activity were determined by HPLC analysis. Solution pH was measured by spotting on a strip of narrow range pH paper. Limit test for residual organic solvents was conducted with GC analysis on a Restek Rtx-200 capillary column (60 m × 0.53 mm ID). Endotoxin level was assayed using LAL test catridge on the Endosafe-PTS device (Charles River Laboratories, Charleston, SC). Post-release sterility tests were carried out by incubation of the product solution with culture media for 14 days. During the validation stage of the production process the stability of [18F]FPEB was measured in three consecutive batches. The final formulated [18F]FPEB product solution was found to be stable for at least 8 h without any changes in radiochemical purity, chemical purity or solution pH.
Table 1.
Summary of quality specifications and test methods for [18F]FPEB, and quality control analysis results from three consecutive production batches
| Test # |
Test parameters |
Specifications | Test Methods |
Test Results* | ||
|---|---|---|---|---|---|---|
| Batch #1 | Batch #2 | Batch #3 | ||||
| 1 | Dose Vial Integrity |
Vial & septum intact |
Visual inspection |
Intact | Intact | Intact |
| 2 | Product Appearance |
Clear, no presence of particulate matters |
Visual inspection |
Clear, no presence of particulate matters |
Clear, no presence of particulate matters |
Clear, no presence of particulate matters |
| 3 | Product pH | 4.5 – 8.5 | pH paper | 6.0 | 6.0 | 6.5 |
| 4 | Radiochemical Purity |
≥ 90% | HPLC | 99.3% | 99.5% | 98.7% |
| 5 | Chemical Purity |
≥ 90% | HPLC | 98.7% | 97.9% | 100% |
| 6 | Radiochemical Identity |
Radioactive peak within ±10% of UV peak retention time |
HPLC | 2.2 % difference |
1.5 % difference |
1.4 % difference |
| 7 | Specific Activity |
- | Calculation against a standard mass curve |
5.10 mCi/nmol |
3.73 mCi/nmol |
14.6 mCi/nmol |
| 8 | Radionuclide Identity |
t½ = 98.8 – 120.8 min |
Counting in a dose calibrator |
t½ = 109.9 min |
t½ = 111.7 min |
t½ = 117.5 min |
| 9 | Membrane Integrity |
Bubble point ≥ 50 psi for Millipore GV filter |
Bubble point test |
≥ 50 psi | ≥ 50 psi | ≥ 50 psi |
| 10 | Endotoxin Level |
≤ 17.5 EU/mL in a 10 mL dose volume |
Charles River Endo- safe PTS |
≤ 5 EU/mL | ≤ 5 EU/mL | ≤ 5 EU/mL |
| 11 | Volatile Organic Impurities |
MeCN < 0.41 mg/mL DMSO < 5.0 mg/mL |
GC | MeCN < 0.41 mg/mL DMSO < 5.0 mg/mL |
MeCN < 0.41 mg/mL DMSO < 5.0 mg/mL |
MeCN < 0.41 mg/mL DMSO < 5.0 mg/mL |
| 12 | Residual Kryptofix K222 |
< 0.05 mg/mL | TLC | < 0.05 mg/mL |
< 0.05 mg/mL |
< 0.05 mg/mL |
| 13 | Sterility Test | Sterile, no growth at 14 days of incubation in media |
Incubation with test media for 14 days |
No evidence of growth at 14-day incubation |
No evidence of growth at 14-day incubation |
No evidence of growth at 14-day incubation |
| Yield (mCi) at EOS (Decay-uncorrected) - |
70.2 mCi | 42.9 mCi | 108.1 mCi | |||
| Yield (%) at EOS (Decay-uncorrected) - |
2.8 % | 1.8 % | 3.8 % | |||
Quality control tests were conducted with an aliquot of [18F]FPEB product solution taken from the dose vial at the end of synthesis, and according to specifications and test methods defined in the approved Drug Master File (DMF) of [18F]FPEB for Injection at Yale University School of Medicine.
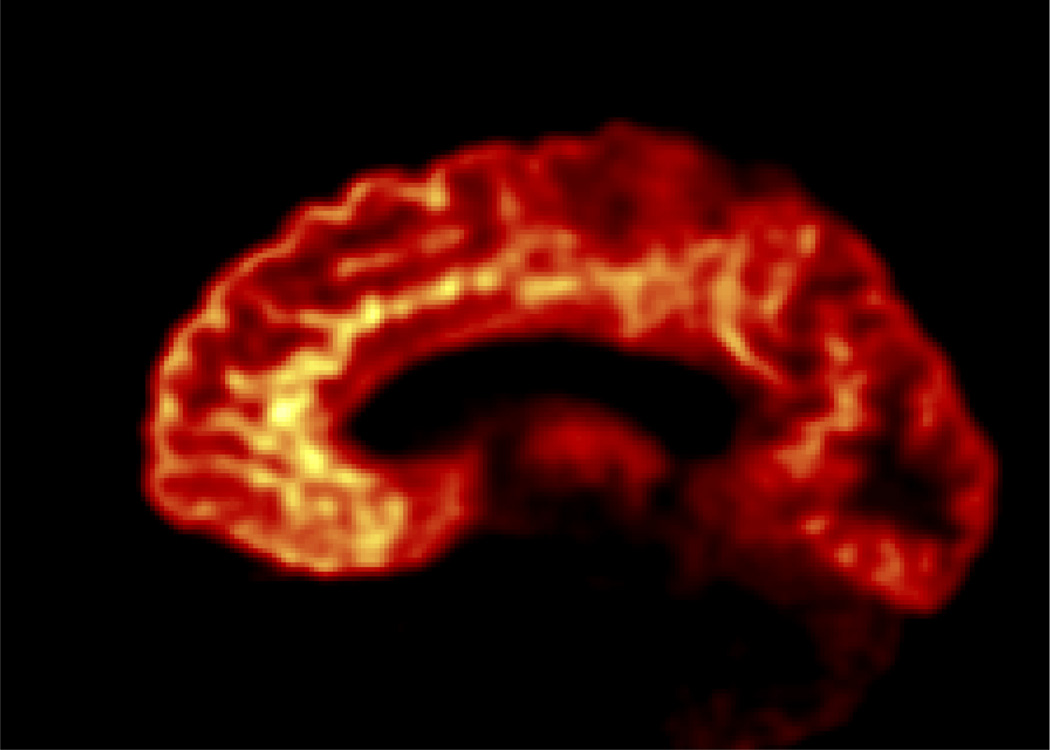
[18F]FPEB produced by the automated synthetic method is being used at our Center in multiple clinical research projects. Presented in Figure 4 is a PET image obtained in a human subject with [18F]FPEB on the High Resolution Research Tomograph (HRRT). Results from our first imaging study with [18F]FPEB in healthy human subjects have been published (Sullivan et al., 2013). Compared with the other mGluR5 radiotracer [11C]ABP688, the F-18 radionuclide in [18F]FPEB provides distinct advantages such as better counting statistics due to its longer half-life and enhanced resolution and image quality due to its shorter positron range, which allows the exquisite visualization and delineation of brain structures as demonstrated in Figure 4. In addition, [18F]FPEB gives higher specific binding signals in vivo (Kuwabara et al. 2011). Taken together, [18F]FPEB appears to be a superior radiotracer for the imaging and quantification of mGluR5 in humans.
Figure 4.
PET image from a healthy human subject obtained with [18F]FPEB on the High Resolution Research Tomograph (HRRT) showing the high resolution and exquisite delineation of brain structure.
In summary, we have developed and validated an automated radiosynthetic method for the routine production of the mGluR5 tracer [18F]FPEB for clinical imaging applications. Ongoing clinical imaging projects at our Center include the use of [18F]FPEB to investigate the role of mGluR5 in a number of neurological and psychiatric disorders such as epilepsy, depression, and addictions. Human imaging studies using this novel radiotracer promise to add to our understanding of mGluR5 in the pathophysiology of neuropsychiatric diseases and to assist in the development of novel therapeutic approaches to these disorders.
4. Conclusion
We have developed a novel “one-pot” methodology for the synthesis of precursor and reference compound for the mGluR5 PET tracer [18F]FPEB. We have also adopted the synthesis of [18F]FPEB to an automated module and successfully carried out routine production of [18F]FPEB for use in clinical research using the nitrobenzonitrile precursor.
Highlights.
Radiosynthesis of [18F]FPEB was performed in a Tracerlab FX-FN automated module
The radiolabeling precursor was prepared from a “one-pot” Suzuki coupling method
Total synthesis time from EOB to a final injectable dose was about 90 min.
The procedure was applied in the routine preparation of [18F]FPEB for human use.
Acknowledgement
The authors thank the staff at the Yale University PET Center for their technical expertise and assistance. The authors also thank Dr. Gilles Tamagnan for providing compounds for our initial radiosynthetic tests of [18F]FPEB and helpful discussions. This publication was also made possible by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Science (NCATS), components of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alagille D, DaCosta H, Chen Y, Hemstapat K, Rodriguez A, Baldwin RM, Conn PJ, Tamagnan GD. Potent mGluR5 antagonists: pyridyl and thiazolyl-ethynyl-3,5-disubstituted-phenyl series. Bioorg Med Chem Lett. 2011;21:3243–3247. doi: 10.1016/j.bmcl.2011.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll FI. Antagonists at metabotropic glutamate receptor subtype 5: structure activity relationships and therapeutic potential for addiction. Ann N Y Acad Sci. 2008;1141:221–232. doi: 10.1196/annals.1441.015. [DOI] [PubMed] [Google Scholar]
- Castanet AS, Colobert F, Schlama T. Suzuki-Miyaura coupling of alkynylboronic esters generated in situ from acetylenic derivatives. Organic letters. 2000;2:3559–3561. doi: 10.1021/ol006439d. [DOI] [PubMed] [Google Scholar]
- Colobert F, Castanet AS, Abillard O. Palladium-catalyzed Suzuki coupling with terminal alkynes - Application to the synthesis of 2,3-disubstituted benzo[B]furans. Eur J Org Chem. 2005:3334–3341. [Google Scholar]
- Hamill TG, Krause S, Ryan C, Bonnefous C, Govek S, Seiders TJ, Cosford NDP, Roppe J, Kamenecka T, Patel S, Gibson RE, Sanabria S, Riffel K, Eng W, King C, Yang X, Green MD, O'Malley SS, Hargreaves R, Burns HD. Synthesis, characterization, and first successful monkey imaging studies of metabotropic glutamate receptor subtype 5 (mGluR5) PET radiotracers. Synapse. 2005;56:205–216. doi: 10.1002/syn.20147. [DOI] [PubMed] [Google Scholar]
- Kuwabara H, Chamroonrat W, Mathews W, Waterhouse R, Brasic JR, Guevara MR, Kumar A, Hamill T, Mozley PD, Wong DF. Evaluation of 11C-ABP688 and 18FFPEB for imaging mGluR5 receptors in the human brain. J Nucl Med. 2011;52:390. [Google Scholar]
- Moghaddam B. Targeting metabotropic glutamate receptors for treatment of the cognitive symptoms of schizophrenia. Psychopharmacology (Berl) 2004;174:39–44. doi: 10.1007/s00213-004-1792-z. [DOI] [PubMed] [Google Scholar]
- Spooren W, Lesage A, Lavreysen H, Gasparini F, Steckler T. Metabotropic glutamate receptors: their therapeutic potential in anxiety. Current topics in behavioral neurosciences. 2010;2:391–413. doi: 10.1007/7854_2010_36. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Lim K, Labaree D, Lin SF, McCarthy TJ, Seibyl JP, Tamagnan G, Huang Y, Carson RE, Ding YS, Morris ED. Kinetic analysis of the metabotropic glutamate subtype 5 tracer [(18)F]FPEB in bolus and bolus-plus-constant-infusion studies in humans. J Cereb Blood Flow Metab. 2013;33:532–541. doi: 10.1038/jcbfm.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JQ, Tueckmantel W, Zhu A, Pellegrino D, Brownell AL. Synthesis and preliminary biological evaluation of 3-[(18)F]fluoro-5-(2-pyridinylethynyl)benzonitrile as a PET radiotracer for imaging metabotropic glutamate receptor subtype 5. Synapse. 2007;61:951–961. doi: 10.1002/syn.20445. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Marek GJ, Johnson BG, Schoepp DD. Metabotropic glutamate receptors in the control of mood disorders. CNS Neurol Disord Drug Targets. 2007;6:87–100. doi: 10.2174/187152707780363302. [DOI] [PubMed] [Google Scholar]
- Wong DF, Waterhouse R, Kuwabara H, Kim J, Brasic JR, Chamroonrat W, Stabins M, Holt DP, Dannals RF, Hamill TG, Mozley PD. 18F-FPEB, a PET radiopharmaceutical for quantifying metabotropic glutamate 5 receptors: a first-in-human study of radiochemical safety, biokinetics, and radiation dosimetry. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2013;54:388–396. doi: 10.2967/jnumed.112.107995. [DOI] [PMC free article] [PubMed] [Google Scholar]