Abstract
Background
We recently found marked deficits in sleep spindles, non-rapid eye movement (NREM) sleep oscillations that are generated within the thalamus and then amplified and sustained in the cortex, in patients with schizophrenia compared to both healthy and psychiatric controls. Here, we investigated the thalamic and cortical contributions to these sleep spindle deficits.
Methods
Anatomical Volume of Interest analysis (i.e., thalamic volumes) and electroencephalogram (EEG) source modeling (i.e., spindle-related cortical currents) were performed in patients with schizophrenia and healthy comparison subjects.
Findings
Schizophrenia patients had reduced mediodorsal (MD) thalamic volumes, especially on the left side, compared to healthy controls, whereas whole thalami and lateral geniculate nuclei did not differ between groups. Furthermore, left MD volumes were strongly correlated with the number of scalp-recorded spindles in an anterior frontal region, and cortical currents underlying these anterior frontal spindles were localized in the prefrontal cortex, in Brodmann Area (BA) 10. Finally, prefrontal currents at the peak of spindle activity were significantly reduced in schizophrenia patients and correlated with their performance in an abstraction/working memory task.
Conclusion
Altogether, these findings point to deficits in a specific thalamo-cortical circuitry in schizophrenia, which is associated with some cognitive deficits commonly reported in those patients.
Keywords: schizophrenia, thalamus, prefrontal cortex, MRI, sleep spindles, hd-EEG
1.1 Introduction
Sleep spindles are waxing/waning, fast (11–16 Hz) EEG oscillations which occur during NREM sleep. While the functional role of spindles is still not fully established, they are thought to be implicated in memory consolidation and plasticity(Molle and Born, 2011), and are considered a proxy measure for the individual's learning potential(Fogel and Smith, 2011). This assumption is supported by animal studies, showing that the timing and number of spindle sequences regulate plasticity-related changes in cortical pyramidal neurons(Rosanova and Ulrich, 2005). It is also consistent with findings in healthy humans, which have shown that higher spindle activity is associated with better performance in verbal memory(Schabus et al., 2008), visuo-spatial memory(Clemens et al., 2006), as well as declarative learning tasks(Gais et al., 2002).
In two recent studies we found a marked reduction in several whole night sleep spindle parameters, including duration, amplitude, and number in patients with schizophrenia compared to both healthy and psychiatric controls(Ferrarelli et al., 2007; Ferrarelli et al., 2010). These sleep spindle impairments were established in baseline conditions, given that patients did not perform any cognitive task before sleep, therefore suggesting an intrinsic fundamental deficit of the underlying neuronal circuitry. Deficits in cognitive tasks involving working memory and other learning paradigms have been consistently found in patients with schizophrenia(Kalkstein et al., 2010). Furthermore, accumulating evidence has revealed sleep spindle deficits associated to cognitive performance in schizophrenia patients. Two studies employing a finger-tapping motor sequence task (MST) before and after a night of sleep found that patients with schizophrenia had reduced spindle density(Manoach et al., 2010) and coherence(Manoach et al., 2010; Wamsley et al., 2012) compared to healthy controls, and in one of these studies lower spindle incidence predicted less overnight improvement in the MST(Wamsley et al., 2012). A reduction in spindle density was also established in schizophrenia patients compared to healthy subjects as well as mood disordered patients during a 40 minute nap, and schizophrenia patients showed no after nap improvement in a procedural learning task(Seeck-Hirschner et al., 2010).
Sleep spindles are generated within the thalamus, and are then transferred to the cortex, where these oscillations are amplified and sustained. To further characterize the thalamic and cortical contribution to sleep spindle deficits in schizophrenia, in this study we combined anatomical (i.e., thalamic volumes) and functional (i.e., cortical sources underlying EEG sleep spindles) measures. We also investigated whether some of those biological measures could predict performance in an abstraction/working memory task in schizophrenia patients.
2.1 Methods
2.1.1 Participants
Twenty-one patients with schizophrenia (Mean ± S.D. = 36 ± 10.2; Female = 8) and twenty-one age-matched healthy controls (Mean ± S.D. = 36.2 ± 8.5; Female= 4) participated in this study (Table 1). In these subjects, we performed whole night high-density (hd)-EEG recordings. Furthermore, in all schizophrenics as well as in a subset (N=11) of healthy controls anatomical magnetic resonance imaging (MRI) scans were collected to perform region of interest analyses (ROIs, see below). This subset of healthy controls was also age-matched with the schizophrenia group (Mean ± S.D. was 36.8 ± 8.4 in controls, 36.0 ± 10.2 in patients with schizophrenia). The remaining control subjects had completed their MRI scans prior to an upgrade of the scanner, and had used different acquisition parameters (ie, number and thickness of individual slices) for the anatomical scans, and could not be used in comparisons to the other subjects. Exclusion criteria for enrollment in the study were substance abuse/dependence within the last six months, neurological disorders, and sleep disorders. Among neurological conditions, individuals with a history of recurrent seizures or epilepsy, medical conditions that could increase the chance of having a seizure (e.g. – stroke, aneurysm, brain surgery, brain tumor or other structural lesion), as well as a history of head trauma that caused neurological injury were excluded. Patients with current or past diagnoses of sleep disorders, including sleep apnea and restless leg syndrome, were also excluded. An additional exclusion criterion for healthy subjects was the presence of first-degree relatives with psychiatric diagnoses.
Table 1.
Demographics and clinical ratings of subject groups.
| Healthy Controls (n=21) |
Schizophrenics (n=21) |
|
|---|---|---|
| Clinical Measures | Mean ± S.D. | Mean ± S.D. |
| Age (Mean ± S.D.) | 36.2 ± 8.5 | 36.0 ± 10.2 |
| Body Mass Index (BMI) | 28.1± 5.2 | 29.8 ± 6.9 |
| Antipsychotic dose* (Mean ± S.D.) | 570 ± 430 | |
| Benzodiazepines (Number) | 6 | |
| Antidepressants (Number) | 11 | |
| Mood stabilizers (Number) | 6 | |
| PANSS Positive (Mean ± S.D.) | 21.0± 4.4 | |
| PANSS Negative (Mean ± S.D.) | 20.7± 3.4 | |
| PANSS General (Mean ± S.D.) | 44.5± 7.3 | |
| PANSS Total (Mean ± S.D.) | 86.2± 14.0 | |
PANSS: Positive and Negative Syndrome Scale
Medication doses are expressed as Chlorpromazine equivalent
A psychiatrist (MJP) interviewed all participants and administered the Structured Clinical Interview for the Diagnostic and Statistical Manual of mental disorders (DSMIV) to assess psychiatric diagnoses. Patients with schizophrenia were diagnosed as paranoid (N=11), undifferentiated (N=5), disorganized (N=1), or residual subtype (N=4). All but one were receiving second-generation antipsychotics. Patients with schizophrenia were outpatients with a mean duration of illness of 13 years (SD=7). After a complete description of the study, each participant gave written informed consent. The study was approved by the University of Wisconsin Institutional Review Board.
2.1.2 Volume of interest analysis
Anatomical T1-weighted magnetic resonance (MR) images were acquired in a 3 Tesla GE scanner. T1-weighted images used a high resolution three-dimensional gradient echo sequence with 512×512×248 voxels in x-y-z direction, resulting in a resolution of 0.5×0.5×0.8mm. TR was 7.448ms, TE 1.496ms, flip angle 10°. Additionally, we collected T2-weighted images with 39 slices with 256×256 voxels in x-y-z direction, resulting in a resolution of 0.94×0.94mm, slice thickness 3mm. TR was 8000ms, TE 1.68ms, flip angle 90 degrees. All participants included in the study had their MRI performed in the same scanner, using the same acquisition parameters. Scans were collected within a week of the whole night sleep EEG recordings. MR images were bias-corrected with SPM8, and Volumes of Interest analysis (VOIs) were measured manually using the software MRIcroN. We selected whole thalamus (WT), MedioDorsal (MD) nucleus, and Lateral Geniculate Nucleus (LGN) bilaterally. The MD was chosen because it is the most recognizable nucleus of the dorsal thalamus, which is heavily interconnected with thalamic reticular nucleus (TRN), the spindle pacemaker. Anatomical and electrophysiological evidence of this privileged interaction between dorsal thalamus and TRN is presented in two comprehensive review articles(Fuentealba and Steriade, 2005; Pinault, 2004). Moreover, the MD is an anterior nucleus of the thalamus, and in recent work we found that schizophrenia patients showed the strongest reduction in sleep spindles in anterior (frontal-prefrontal), rather than posterior (parieto-occipital) regions(Ferrarelli et al., 2007; Ferrarelli et al., 2010). The LGN was chosen as a control volume because it is a posterior nucleus, and it is not highly inter-connected with the TRN. VOIs were measured three times by two raters (A.B., F.F.). Intra-rater correlations ranged between 0.84 and 0.89, inter-rater correlations ranged from 0.70 to 0.76. The MD thalami were identified as the darker medial structures starting anterior to the nucleus ruber and extending posterior to the medial geniculate. The LGN were identified as a knee-shaped formation localized alongside the middle hippocampus. For all VOIs, the average values obtained across all measurements were utilized for statistical analyses.
2.1.3 Scalp EEG recordings and analysis
Whole-night sleep recordings were performed in schizophrenia patients and healthy controls with 256 electrode high-density (hd)-EEG systems (EGI, Eugene, Oregon). There was no adaptation night specifically for this study. However, all participants in this study had been enrolled in previous sleep recordings and had spend at least a night in the laboratory prior to those sleep hd-EEG recordings. Sleep stages were scored on C3A2 and C4A1 derivations based on the American Academy of Sleep Medicine criteria (AASM) (Silber et al., 2007). The AASM recommends the use of F4, C4 and O2 for staging sleep; however the present study focused on sleep spindle activity, and therefore we utilized the C4 as the main derivation for sleep scoring. Furthermore, we scored the data with 20 second resolution, rather than 30 seconds, to minimize the amount of data rejected throughout the analysis. Artifacts were excluded visually during sleep staging. Additional artifacts were removed automatically, by rejecting epochs that exceeded thresholds based on the mean power for each channel in the 0.75 to 4.5 Hz and 20 to 30 Hz bands. After removing noisy electrodes, which were excluded automatically based on high impedance value (>150 KOhm) as well as by visual inspection, EEG signals were re-referenced to the average of all retained channels, and sleep power spectra as well as spindle detection analysis were performed. These analyses are described in detail elsewhere(Ferrarelli et al., 2007; Ferrarelli et al., 2010). Results from this dataset have been partially presented in a previous publication(Ferrarelli et al., 2010). Here we focused on the correlation between thalamic VOIs and sleep spindles as well as on source modeling scalp EEG spindle activity. All spindle parameters (number, activity) were calculated per minute of NREM sleep.
2.1.4 Source Modeling analysis
EEG data were band-pass filtered in the spindle frequency (11–16 Hz) and Independent Component Analysis (ICA) was used to remove ocular, muscle, and electrocardiographic artifacts. Specifically, ICA components with activity patterns and topographic maps characteristic of artifactual activity were removed. ICA was performed on NREM sleep data, including N2 and N3 epochs, during which sleep spindles occurred in the frontal cluster showing the strongest correlation with MD thalamic volumes in both groups, as shown in Figure 2. Spindles were then detected on EEG scalp data with an in house automatic algorithm. Briefly, for each channel thresholds for detection relative to the mean amplitude of that channel were used, whereas for each spindle the amplitude was the maximum above an upper threshold, while the beginning and end were the points preceding or following this maximum when the amplitude dropped below a lower threshold(Ferrarelli et al., 2010). For source modeling, the length of the analysis window matched the duration of each scalp detected spindle. Sleep spindles were localized individually, and solutions were then averaged across all detections. Those spindles occurred during sleep epochs that were artifact rejected both visually and automatically, and therefore there was no EEG baseline noise. Source localization was performed using a 4-shell head model derived from a template MRI. An MRI-coregistered set of electrodes was used to construct the forward model. The inverse matrix was calculated using the minimum norm least-squares (L2) method, subject to depth weighting, Tikhonov regularization at 10−1, and Low Resolution Electromagnetic Tomography (LORETA) constraint. The value of each source was then standardized (sLORETA). The source space was restricted to 2447 cortical voxels (7 mm3), each assigned to a gyrus based on the Montreal Neurological Institute probabilistic atlas. Source analyses were performed using GeoSource software (EGI, Eugene, Oregon).
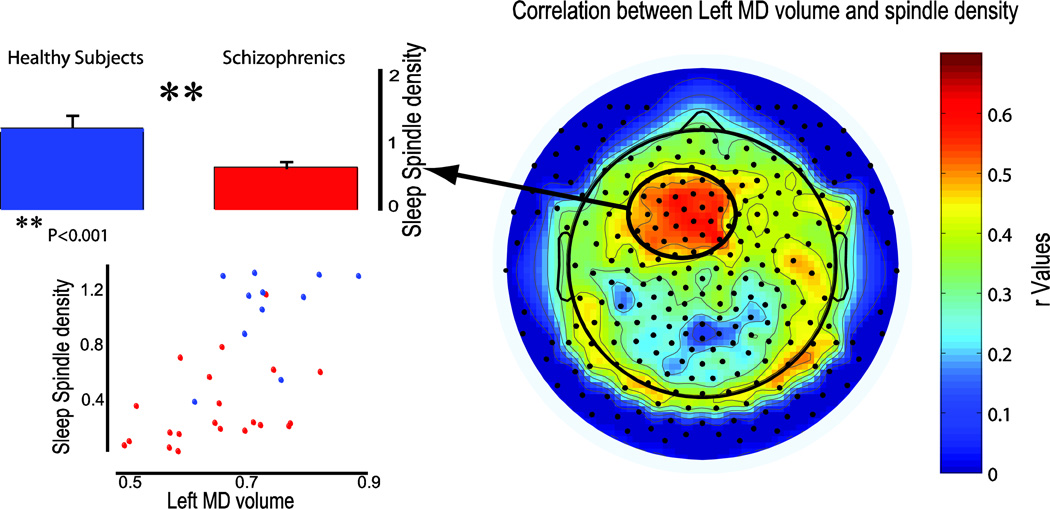
Figure 2. Left MD volume reduction in schizophrenia patients significantly correlated with sleep spindle density in a frontal cluster of electrodes.
This cluster (black-encircled red spot) was significantly reduced in schizophrenics compared to healthy controls (blue and red box plots, p<0.01). On the bottom left, single subject correlation between left MD volume and spindle number for schizophrenia patient (red) and healthy controls (blue).
2.1.5 Cognitive task
A subset of schizophrenia patients (N=12) performed the PENN CNP, a computerized battery that includes several cognitive tests. However, the analysis was focused on the Penn Abstraction, Inhibition and Working Memory (AIM) task. The AIM, like the Wisconsin Card Sorting Test (WCST), is a measure of cognitive executive functions, which has been found to be impaired in schizophrenia patients compared to healthy comparisons(Glahn et al., 2000). More importantly, both anatomical and functional neuro-imaging studies have found that those type of tasks implicate the prefrontal cortex(Berman et al., 1995; Szulc et al., 2012; Yuan and Raz, 2014), which is where we found the peak of spindle activity in both groups. Here we investigated if AIM performance correlated with cortical spindle currents in schizophrenia patients. We also assessed whether this spindle activity could predict the Raven's scores, a non-verbal measure of IQ, in schizophrenia patients.
2.1.6 Statistics
Differences in VOIs and sleep spindles between schizophrenia patients and healthy controls were assessed with unpaired t tests. Because six VOIs (WT, MD, and LGN bilaterally) were measured, threshold for significance was set at p (0.05/6) = 0.0083. Correlation analyses between left MD thalamic volumes and the topography of sleep spindle density was performed. Left MD nuclei were the most markedly and consistently reduced VOIs in schizophrenia patients compared to healthy controls. Furthermore, we chose spindle density, rather than other spindle parameters, since sleep spindles are generated during NREM sleep within the thalamus, and therefore spindle density, which measures the number of spindles occurring per minute of NREM sleep, was the parameter most likely to reflect a thalamic defect in generating sleep spindles.
We also performed unpaired t tests between healthy controls and patients with schizophrenia for the strongest cortical currents. Specifically, the dipoles corresponding to the top 1 % of all cortical currents were organized by Broadmann areas and the most intense currents at the beginning, at the peak, and at the end of spindle activity. Because three measures were computed, threshold for significance was set at p (0.05/3)=0.016. Finally, correlation analyses were performed between medication doses and MD thalamic volumes, EEG frontal spindles, and spindle-related cortical currents.
3.1 Results
3.1.1 Schizophrenics had MD volume reduction compared to healthy controls
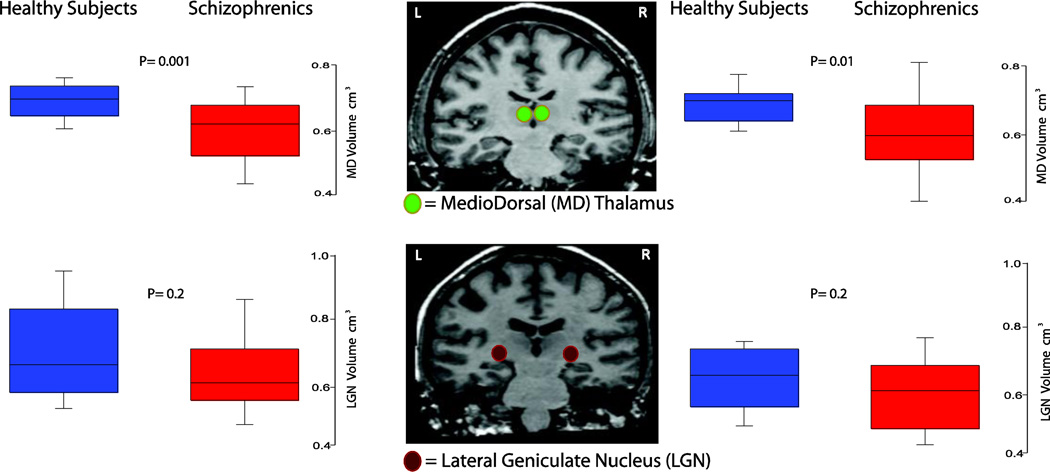
Schizophrenia patients showed a significant reduction in the volume of both the left and right MD nucleus (Figure 1). The left MD volume decrease was significant even after Bonferroni correction for multiple comparisons (Table 2). By contrast, the volume of both left and right LGN was not different between the two groups (Figure 1, Table 2). Furthermore, no difference in either left or right whole thalamus (WT) volumes was found between groups (Table 2, Supp. Figure 1). To assess whether group differences were affected by variability in VOI measurements, we calculated the margin of error (MOE) between raters as the mean difference in VOI measures and found that the largest inter-raters MOE was 0.05 for the left WT and 0.07 for the right WT, whereas group differences for the same VOIs were 0.15 and 0.28 respectively.
Figure 1. ROI's analysis of the MedioDorsal (MD) and Lateral Geniculate Nucleus (LGN) in healthy subjects (blue) and patients with schizophrenia (red).
Schizophrenia patients showed a volume reduction in both left and right MD, but not in the LGN, compared to control subjects. Left MD reduction was significant (p=0.001) after correction for multiple comparison (p threshold =0.0083, or 0.05/6).
Table 2.
| Volumes of Interest (VoIs) |
Mean Volume (±SD) Healthy controls Schizophrencis |
p values (unpaired t test) |
|
|---|---|---|---|
| Left Whole Thalamus (WT) |
6.00 ± 0.91 | 5.85 ± 1.00 | p= n.s. |
| Right Whole Thalamus (WT) |
5.84 ± 1.06 | 5.56 ± 0.77 | p= n.s. |
| Left MedioDorsal (MD) | 0.74 ± 0.05 | 0.64 ± 0.10 | p= 0.001 * |
| Right MedioDorsal (MD) | 0.72 ± 0.05 | 0.62 ± 0.15 | p= 0.01 |
| Left Lateral Geniculate Nucleus (LGN) |
0.72 ± 0.12 | 0.66 ± 0.15 | p= 0.2 |
| Right Lateral Geniculate Nucleus (LGN) |
0.62 ± 0.09 | 0.57 ± 0.11 | p= 0.2 |
Significant after Bonferroni correction for multiple comparisons
3.1.2 MD volume reduction correlated with frontal EEG spindle deficits in schizophrenia
Correlation analysis between left MD volumes and the EEG topography of spindle density in the pooled sample (schizophrenia patients and healthy controls) established that spindles in posterior, parietal-occipital regions showed the lowest correlation values (Figure 2, right). In contrast, frontal-prefrontal areas had higher correlation coefficients, and a left anterior frontal cluster of electrodes (N=23) showed the strongest correlations with the left MD volumes (r≥0.5, p≤0.04). To rule out that the correlation between MD thalamic volume and sleep spindle density was exclusively driven by the group differences on these parameters, we performed a linear regression analysis in which spindle density was the dependent variable whereas MD volume was the independent, predictive variable with group as a categorical independent covariate. We found that MD thalamic volumes could still significantly predict variability in spindle number, even after accounting for the group effect (MD t=2.381, p=0.024). Furthermore, when comparing the incidence of sleep EEG spindles in this left frontal cluster between the two groups it was found that schizophrenics had a marked reduction in spindle density (0.3 ± 0.25) compared to healthy controls (0.9 ± 0.3, p<0.001, Figure 2 left).
3.1.3 Prefrontal cortical currents underlie frontal EEG spindle deficits
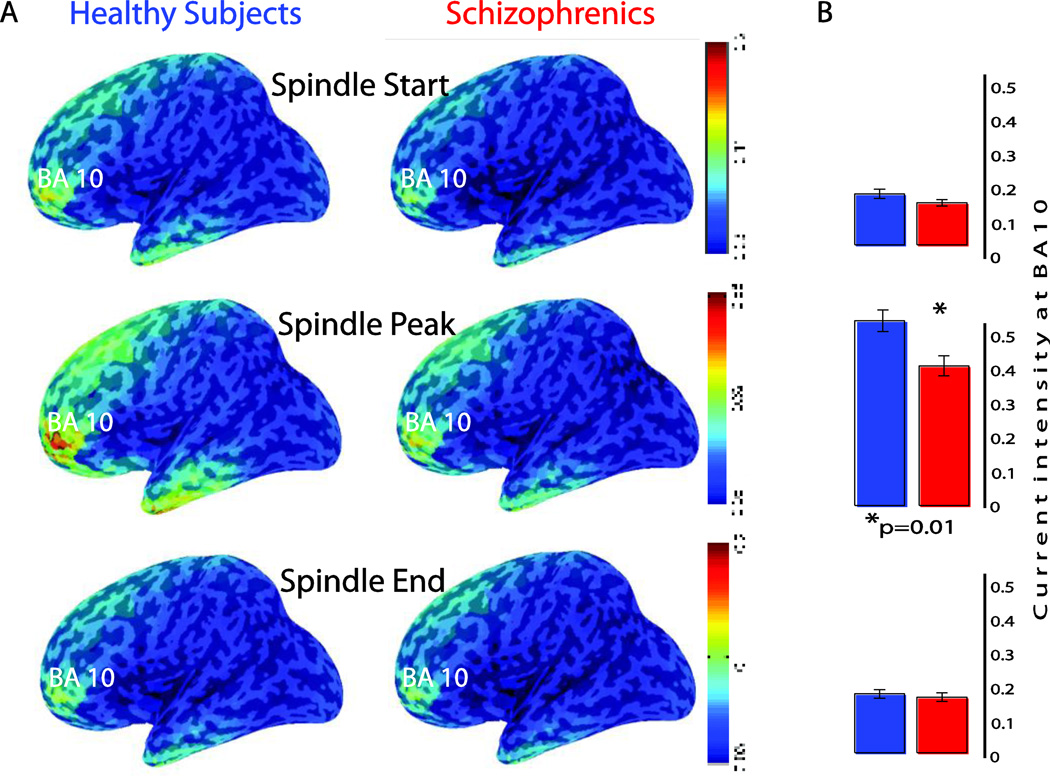
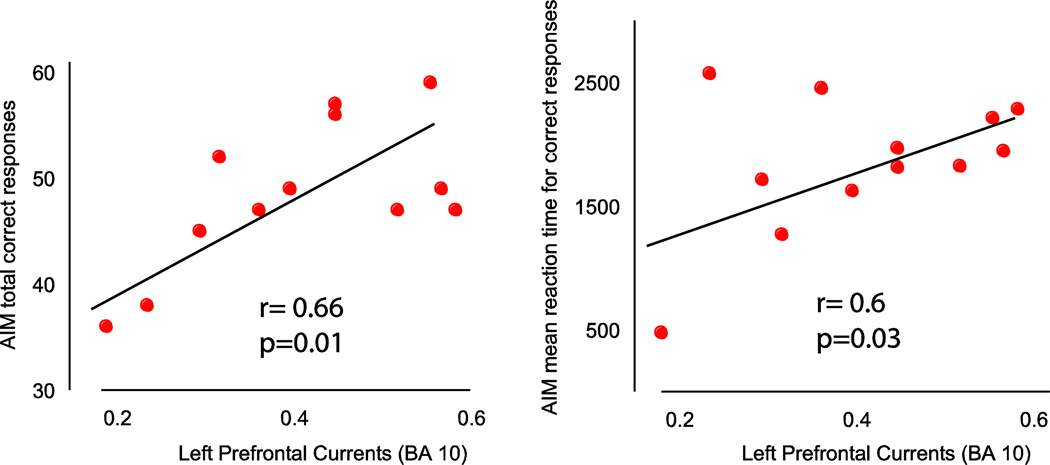
Source modeling of spindle activity occurring in this left frontal cluster established that most of the cortical currents were localized in frontal/prefrontal areas. Moreover, in both patients with schizophrenia and healthy subjects the strongest spindle cortical sources were localized in the prefrontal cortex, in a region overlapping the boundaries between BA 10 and BA 11, with the center of mass in BA 10 (Figure 3 left). BA 10 showed the largest currents at the beginning, at the peak, and at the end of spindle activity in both groups. Furthermore, while both groups had similar current intensities at the beginning and end of sleep spindles, cortical currents at the peak of spindle activity were reduced in schizophrenia patients (0.4 ± 0.02) compared to healthy controls (0.53 ± 0.03, p=0.01, Figure 3 right). Finally, those spindle-related prefrontal cortical currents were significantly correlated with performance in the AIM task (Figure 4); by contrast, spindle prefrontal currents could not predict the Raven's scores in schizophrenia patients (r=0.39; p=0.2). Furthermore, we found no correlation between antipsychotic medication doses, expressed as Chlorpromazine equivalents, and cortical currents, EEG spindle number, as well as MD thalamic volumes (r≥−0.23, p≥0.3).
Figure 3. Source Modeling (sLORETA) of sleep spindles in healthy subjects (blue) and schizophrenia patients (red).
In both groups the strongest cortical currents were localized in the prefrontal cortex (BA 10), and these prefrontal currents were significantly weaker in schizophrenia compared to healthy controls at the spindle activity peak.
Figure 4. Cortical Prefrontal currents (BA 10) correlated with performance (left) as well as reaction time (right) in an executive function task (AIM from PENN CNP).
4.1 Discussion
Variations in thalamic gray matter volumes have been previously investigated in schizophrenia. Some studies found WT reductions in schizophrenia patients(Ettinger et al., 2007; van Haren et al., 2007), whereas other studies reported no WT differences between schizophrenics and healthy subjects(Yoshihara et al., 2008). In another recent work LGN volumes of schizophrenics, health subjects and depressed patients were comparable in size(Dorph-Petersen et al., 2009). We found no difference in WT as well as LGN volumes between schizophrenics and control subjects. By contrast, schizophrenia patients showed a volume reduction in MD nuclei, more prominently on the left side. A stronger left-sided reduction in MD volumes in schizophrenia has been reported in previous structural MRI (Byne et al., 2001) as well as neuropathological, post-mortem(Danos et al., 2003) studies. Furthermore, in the few studies measuring both WT and MD volumes it was found that WT was reduced only in one study and on one side(Shimizu et al., 2008), whereas schizophrenics had consistently smaller left MD nuclei volumes compared to healthy controls(Kemether et al., 2003; Shimizu et al., 2008). The MD nucleus is a major higher order (HO) thalamic relay that receives driver inputs from cortical layer V neurons, whereas first order (FO) thalamic nuclei, such as the lateral geniculate nuclei, receive most of their driver inputs from subcortical sources(Sherman and Guillery, 2002). HO thalamic nuclei are thought to be implicated in transferring information from one cortical area to another, and also receive a copy of the motor message that is sent from the cortex to lower, mostly motor centers in the central nervous system (Guillery and Sherman, 2002). Thus, it has been proposed that HO thalamic nuclei may play a role in internal motor monitoring mechanisms, also known as efference copy or corollary discharge mechanisms, and a recent study found that such mechanisms are impaired in schizophrenia(Spering et al., 2013). The reduction in volume of the MD nuclei reported here may be implicated in the efference copy failure found in schizophrenia patients.
Here left MD volumes were correlated with left frontal EEG spindles in both healthy and schizophrenic subjects. Our previous findings of spindle deficits in schizophrenia (Ferrarelli et al., 2007; Ferrarelli et al., 2010) point to a defect in the thalamus, and particularly in the TRN, the spindle pacemaker. Because measuring the volume of the TRN with current anatomical neuro-imaging techniques is a practical impossibility, we exploited the location of the TRN, which covers most of the dorsal thalamus, as well as its connectivity with dorsal thalamic nuclei, of which the MD is the most recognizable structure. Smaller MD volumes reflect reduced neurons and connections between those neurons and the TRN, which are likely to affect the incidence of sleep spindles. Consistent with this prediction, we found a strong correlation between MD volumes and spindle density in scalp-recorded spindles, in a cortical region known to be anatomically connected to the MD nucleus.
This finding suggests that the MD nucleus contributes to the incidence of frontal spindles, and is consistent with electrophysiological studies in animals demonstrating that spindles are initiated within the thalamus by the interplay of the TRN with (mostly) dorsal thalamic nuclei(Fuentealba and Steriade, 2005), as well as with electro-corticographic recordings in humans showing an implication of MD and TRN in spindle generation(Nakamura et al., 2003). Furthermore, electrophysiological recordings in awake and attentive primates have demonstrated that MD nucleus and the pulvinar, both HO thalamic nuclei, have much greater rebound burst firing activity compared to FO thalamic nuclei, including the LGN(Ramcharan et al., 2005), and this higher burst propensity could be related to greater expression of voltage-dependent transient (T-type) calcium channels(Wei et al., 2011). Intriguingly, a gene encoding a T-type calcium channel (CACNA1I, which encodes CaV3,3), has been recently implicated in schizophrenia by two large genetic studies(2012; 2014), whereas another study has shown that CaV3.3 calcium channel, which is highly expressed in the TRN, is the major sleep spindle pacemaker in the thalamus(Astori et al., 2011).
We also established that EEG frontal spindles showing a significant correlation with MD volumes originated from the anterior prefrontal cortex (BA 10). Anatomical and immune-histological studies in primates have demonstrated that MD thalamus receives topographically organized fibers from the TRN, the spindle pacemaker, and MD thalamo-cortical neurons are heavily interconnected with prefrontal cortical neurons(Barbas et al., 2011). Moreover, recent f-MRI experiments during sleep spindles found a strong blood-oxygen level dependent (BOLD) activation in the MD thalamus, which correlated with the activity in prefrontal cortex(Schabus et al., 2007).
In this study we found that spindle-associated cortical prefrontal currents were decreased in schizophrenic compared to healthy individuals, and this reduction predicted impaired performance in an abstraction/working memory task in schizophrenia patients. It has been shown that higher spindle activity predict better performances in verbal memory, visuo-spatial memory, as well as declarative memory tasks(Fogel and Smith, 2011). Furthermore, recent studies have reported that schizophrenia patients had reduced sleep spindle density compared to healthy controls, and this reduction predicted worse postsleep performance in procedural learning tasks(Seeck-Hirschner et al., 2010; Wamsley et al., 2012). Our findings not only confirm previous data indicating executive function deficits in those patients(Glahn et al., 2000), but also points to defects in a TRN-MD nucleus-prefrontal cortex circuitry in schizophrenia. Intriguingly, we established that prefrontal spindle currents correlated with performance in the AIM task, but not with the Raven's scores. While this finding does not rule out that other tests may be significantly associated to spindle deficits in schizophrenia (i.e., verbal IQ tasks), it suggests that the reduction in prefrontal spindle activity established here is specifically related to the AIM performance.
We found no associations between cognitive performance and MD thalamic volumes. However, MD volumes predicted a reduced spindle incidence in anterior frontal regions. We think that this is due to the fact that a defect within the thalamus, which includes the MD and the TRN, will result in a reduced incidence of sleep spindles in anterior frontal regions. At the same time, when spindles do occur, the overall level of activation of the prefrontal cortex, which is critical in performing the AIM task correctly, is reduced in patients with schizophrenia compared to healthy controls, and this reduction is associated with an impaired performance in this cognitive task.
At this stage, we can only speculate about the molecular mechanisms underlying this defective thalamo-cortical circuitry. One mechanism may involve reduced binding or expression of thalamo-cortical N-methyl-D-aspartate (NMDA) glutamate receptors. Postmortem studies found reduced NMDA glutamate receptors in both MD thalamus and prefrontal cortex in schizophrenia patients(Pakkenberg et al., 2009). Pharmacological manipulations with NMDA antagonists, including ketamine and phencyclidine (PCP), produce schizophrenia-like psychosis in humans, and animal studies have shown that asenapine and clozapine, two second-generation antipsychotics, could revert a PCP-induced hypoactivity of NMDA receptors in both MD thalamus(Santana et al., 2011) and prefrontal cortex(Jardemark et al., 2010). Injections of NMDA antagonists in the TRN rat brain trigger delta-range rhythmic bursting, thus suggesting that NMDA hypofunction underlie TRN-generated delta band EEG oscillations, a waking thalamo-cortical dysrhythmia established in schizophrenia(Zhang et al., 2009). Moreover, 2-deoxyglucose imaging data in mice characterizing the acute effects of ketamine on brain functional connectivity found ketamine-induced impairments in a circuitry involving TRN, MD thalamus, and prefrontal cortex(Dawson et al., 2011).
Another molecular mechanism implicate deficits in the gamma-aminobutyric acid (GABA) thalamo-cortical system. The TRN consists of GABA-ergic neurons, and electrophysiological experiments have shown that TRN GABA release plays a critical role in regulating sensory gating(Krause et al., 2003), which has been found to be defective in schizophrenia(Light and Braff, 1999). The presence of GABA impairments in schizophrenia is also suggested by data from postmortem studies, which found a reduction in glutamate decarboxylase 67, an enzyme involved in GABA synthesis, and in GABA membrane transporter density in cortical interneurons in schizophrenia patients(Lewis et al., 2005). Additionally, treatment studies have shown that Clozapine, one of the most effective antipsychotics, is associated with enhanced thalamo-cortical GABA activity in schizophrenia patients, and the beneficial effects of Electroconvulsive therapy (ECT) and Transcranial Magnetic Stimulation (TMS) are related to increased GABA-mediated inhibitory neurotransmission on excitatory cortical neurons(Daskalakis et al., 2008).
Schizophrenia patients were medicated when data were collected. They were all taking second generation antipsychotics, with the exception of one patient who was on Fluphenazine. Ten patients were on Clozapine, five on Aripripazole, five on Risperidone, three on Quetiapine, one on Olanzapine and one on Ziprasidone. Six patients were on two antipsychotic medications. However, medications are unlikely to account for these findings. Neuro-imaging anatomical studies on the effects of atypical antipsychotics on the thalamus have reported a bilateral increase in thalamic volumes in both first episode psychosis(Dazzan et al., 2005) and chronic patients with schizophrenia, whereas other studies found a slight increase in the right WT and no changes in the left WT(Deng et al., 2009; Tomelleri et al., 2009). Here we reported no difference in bilateral WT, whereas we found a marked decrease in the left MD volumes of schizophrenia patients compared to healthy controls. Moreover, in a recent study we found that non-schizophrenia patients taking antipsychotic medications had no spindle deficits compared to healthy controls(Ferrarelli et al., 2010) and in this study we found that medication doses did not correlate with any of the measures defective in schizophrenia patients, including left MD volumes, EEG frontal spindles, and spindle-related prefrontal cortical currents.
By collecting both anatomical and functional measures in the same group of patients, we identified deficits in a specific thalamo-cortical circuitry underlying sleep spindle activity in schizophrenia. Future studies are needed to fully characterize the involvement of this circuitry in the neurobiology of this disorder. Replicating these findings in larger groups of patients will help to clarify the contribution of different clusters of symptoms to these deficits. Here we found that patients with paranoid schizophrenia had smaller MD thalamic volumes compared to the other schizophrenia patients, but this reduction was not significant. Furthermore, simultaneous fMRI/hd-EEG recordings will allow assessing more directly the reduction in activation of the thalamus, and especially TRN/MD thalamic nuclei, and prefrontal cortex during sleep spindles in schizophrenia. As a first step in this direction, in a recent fMRI/TMS study schizophrenics showed reduced thalamic and anterior frontal activation following direct perturbation of the frontal cortex, and connectivity analyses revealed weaker thalamus-superior frontal cortex in schizophrenia patients compared to healthy controls(Guller et al., 2012). Future studies could also contribute to assess the implication of this thalamo-cortical circuit in the symptoms of schizophrenia, as recently suggested(Lisman, 2011). Finally, improvements in some measures of this thalamo-cortical circuitry may be utilized to establish the effectiveness of both pharmacological and non-pharmacological interventions in schizophrenia. For example, it was recently found that Eszopiclone significantly increased sleep spindles in schizophrenia patients, and this increase correlated with overnight motor sequence task improvement(Wamsley et al., 2013), whereas a recent fMRI study showing a significant improvement in reality monitoring in schizophrenics after 80 hours of computerized training, which correlated with increased activity in the prefrontal cortex(Subramaniam et al., 2012).
5.1 Conclusion
In this study we collected both structural (i.e., MRI-based VOI analysis of thalamic volumes) and functional (i.e., sleep spindle-related cortical currents) measures in patients with schizophrenia and healthy comparison subjects. We found that schizophrenia patients had reduced mediodorsal (MD) thalamic volumes, that MD volumes were strongly correlated with the number of scalp-recorded anterior frontal spindles, and that cortical currents underlying those frontal spindles were localized in the prefrontal cortex (BA 10). Finally, we established that prefrontal currents at BA 10 were significantly reduced in schizophrenia patients and predicted heir performance in an abstraction/working memory task. Altogether, these findings point to deficits in a specific thalamo-cortical circuitry in schizophrenia, which may underlie some cognitive impairments commonly reported in those patients.
Supplementary Material
Highlights.
We measured thalamic VOIs and cortical sources of sleep spindles in schizophrenia
MD thalamus VOIs were reduced in schizophrenia patients compared to healthy controls
MD volumes correlated with EEG frontal spindles, which originated in cortical BA 10
BA 10 prefrontal spindle currents predicted performance in an abstraction/WM task
We found thalamo-cortical deficits related to cognitive dysfunction in schizophrenia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Genome-wide association study implicates HLA-C*01:02 as a risk factor at the major histocompatibility complex locus in schizophrenia. Biol Psychiatry. 2012;72:620–628. doi: 10.1016/j.biopsych.2012.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astori S, Wimmer RD, Prosser HM, Corti C, Corsi M, Liaudet N, Volterra A, Franken P, Adelman JP, Luthi A. The Ca(V)3.3 calcium channel is the major sleep spindle pacemaker in thalamus. Proc Natl Acad Sci U S A. 2011;108:13823–13828. doi: 10.1073/pnas.1105115108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Zikopoulos B, Timbie C. Sensory pathways and emotional context for action in primate prefrontal cortex. Biol Psychiatry. 2011;69:1133–1139. doi: 10.1016/j.biopsych.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Berman KF, Ostrem JL, Randolph C, Gold J, Goldberg TE, Coppola R, Carson RE, Herscovitch P, Weinberger DR. Physiological activation of a cortical network during performance of the Wisconsin Card Sorting Test: a positron emission tomography study. Neuropsychologia. 1995;33:1027–1046. doi: 10.1016/0028-3932(95)00035-2. [DOI] [PubMed] [Google Scholar]
- Byne W, Buchsbaum MS, Kemether E, Hazlett EA, Shinwari A, Mitropoulou V, Siever LJ. Magnetic resonance imaging of the thalamic mediodorsal nucleus and pulvinar in schizophrenia and schizotypal personality disorder. Arch Gen Psychiatry. 2001;58:133–140. doi: 10.1001/archpsyc.58.2.133. [DOI] [PubMed] [Google Scholar]
- Clemens Z, Fabo D, Halasz P. Twenty-four hours retention of visuospatial memory correlates with the number of parietal sleep spindles. Neurosci Lett. 2006;403:52–56. doi: 10.1016/j.neulet.2006.04.035. [DOI] [PubMed] [Google Scholar]
- Danos P, Baumann B, Kramer A, Bernstein HG, Stauch R, Krell D, Falkai P, Bogerts B. Volumes of association thalamic nuclei in schizophrenia: a postmortem study. Schizophr Res. 2003;60:141–155. doi: 10.1016/s0920-9964(02)00307-9. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Moller B, Fountain SI, Chen R. Increased cortical inhibition in persons with schizophrenia treated with clozapine. J Psychopharmacol. 2008;22:203–209. doi: 10.1177/0269881107084002. [DOI] [PubMed] [Google Scholar]
- Dawson N, Morris BJ, Pratt JA. Subanaesthetic Ketamine Treatment Alters Prefrontal Cortex Connectivity With Thalamus and Ascending Subcortical Systems. Schizophr Bull. 2011 doi: 10.1093/schbul/sbr144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzan P, Morgan KD, Orr K, Hutchinson G, Chitnis X, Suckling J, Fearon P, McGuire PK, Mallett RM, Jones PB, Leff J, Murray RM. Different effects of typical and atypical antipsychotics on grey matter in first episode psychosis: the AESOP study. Neuropsychopharmacology. 2005;30:765–774. doi: 10.1038/sj.npp.1300603. [DOI] [PubMed] [Google Scholar]
- Deng MY, McAlonan GM, Cheung C, Chiu CP, Law CW, Cheung V, Sham PC, Chen EY, Chua SE. A naturalistic study of grey matter volume increase after early treatment in anti-psychotic naive, newly diagnosed schizophrenia. Psychopharmacology (Berl) 2009;206:437–446. doi: 10.1007/s00213-009-1619-z. [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Caric D, Saghafi R, Zhang W, Sampson AR, Lewis DA. Volume and neuron number of the lateral geniculate nucleus in schizophrenia and mood disorders. Acta Neuropathol. 2009;117:369–384. doi: 10.1007/s00401-008-0410-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger U, Picchioni M, Landau S, Matsumoto K, van Haren NE, Marshall N, Hall MH, Schulze K, Toulopoulou T, Davies N, Ribchester T, McGuire PK, Murray RM. Magnetic resonance imaging of the thalamus and adhesio interthalamica in twins with schizophrenia. Arch Gen Psychiatry. 2007;64:401–409. doi: 10.1001/archpsyc.64.4.401. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, Riedner BA, Watson A, Bria P, Tononi G. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164:483–492. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Peterson MJ, Sarasso S, Riedner BA, Murphy MJ, Benca RM, Bria P, Kalin NH, Tononi G. Thalamic dysfunction in schizophrenia suggested by whole-night deficits in slow and fast spindles. Am J Psychiatry. 2010;167:1339–1348. doi: 10.1176/appi.ajp.2010.09121731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel SM, Smith CT. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev. 2011;35:1154–1165. doi: 10.1016/j.neubiorev.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Fuentealba P, Steriade M. The reticular nucleus revisited: intrinsic and network properties of a thalamic pacemaker. Prog Neurobiol. 2005;75:125–141. doi: 10.1016/j.pneurobio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Gais S, Molle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. J Neurosci. 2002;22:6830–6834. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Cannon TD, Gur RE, Ragland JD, Gur RC. Working memory constrains abstraction in schizophrenia. Biol Psychiatry. 2000;47:34–42. doi: 10.1016/s0006-3223(99)00187-0. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- Guller Y, Ferrarelli F, Shackman AJ, Sarasso S, Peterson MJ, Langheim FJ, Meyerand ME, Tononi G, Postle BR. Probing thalamic integrity in schizophrenia using concurrent transcranial magnetic stimulation and functional magnetic resonance imaging. Arch Gen Psychiatry. 2012;69:662–671. doi: 10.1001/archgenpsychiatry.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardemark K, Marcus MM, Shahid M, Svensson TH. Effects of asenapine on prefrontal N-methyl-D-aspartate receptor-mediated transmission: involvement of dopamine D1 receptors. Synapse. 2010;64:870–874. doi: 10.1002/syn.20803. [DOI] [PubMed] [Google Scholar]
- Kalkstein S, Hurford I, Gur RC. Neurocognition in schizophrenia. Curr Top Behav Neurosci. 2010;4:373–390. doi: 10.1007/7854_2010_42. [DOI] [PubMed] [Google Scholar]
- Kemether EM, Buchsbaum MS, Byne W, Hazlett EA, Haznedar M, Brickman AM, Platholi J, Bloom R. Magnetic resonance imaging of mediodorsal, pulvinar, and centromedian nuclei of the thalamus in patients with schizophrenia. Arch Gen Psychiatry. 2003;60:983–991. doi: 10.1001/archpsyc.60.9.983. [DOI] [PubMed] [Google Scholar]
- Krause M, Hoffmann WE, Hajos M. Auditory sensory gating in hippocampus and reticular thalamic neurons in anesthetized rats. Biol Psychiatry. 2003;53:244–253. doi: 10.1016/s0006-3223(02)01463-4. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Light GA, Braff DL. Human and animal studies of schizophrenia-related gating deficits. Curr Psychiatry Rep. 1999;1:31–40. doi: 10.1007/s11920-999-0008-y. [DOI] [PubMed] [Google Scholar]
- Lisman J. Excitation, inhibition, local oscillations, or large-scale loops: what causes the symptoms of schizophrenia? Curr Opin Neurobiol. 2011 doi: 10.1016/j.conb.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Thakkar KN, Stroynowski E, Ely A, McKinley SK, Wamsley E, Djonlagic I, Vangel MG, Goff DC, Stickgold R. Reduced overnight consolidation of procedural learning in chronic medicated schizophrenia is related to specific sleep stages. J Psychiatr Res. 2010;44:112–120. doi: 10.1016/j.jpsychires.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molle M, Born J. Slow oscillations orchestrating fast oscillations and memory consolidation. Prog Brain Res. 2011;193:93–110. doi: 10.1016/B978-0-444-53839-0.00007-7. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Uchida S, Maehara T, Kawai K, Hirai N, Nakabayashi T, Arakaki H, Okubo Y, Nishikawa T, Shimizu H. Sleep spindles in human prefrontal cortex: an electrocorticographic study. Neurosci Res. 2003;45:419–427. doi: 10.1016/s0168-0102(03)00007-5. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Scheel-Kruger J, Kristiansen LV. Schizophrenia; from structure to function with special focus on the mediodorsal thalamic prefrontal loop. Acta Psychiatr Scand. 2009;120:345–354. doi: 10.1111/j.1600-0447.2009.01447.x. [DOI] [PubMed] [Google Scholar]
- Pinault D. The thalamic reticular nucleus: structure, function and concept. Brain Res Brain Res Rev. 2004;46:1–31. doi: 10.1016/j.brainresrev.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Ramcharan EJ, Gnadt JW, Sherman SM. Higher-order thalamic relays burst more than first-order relays. Proc Natl Acad Sci U S A. 2005;102:12236–12241. doi: 10.1073/pnas.0502843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanova M, Ulrich D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J Neurosci. 2005;25:9398–9405. doi: 10.1523/JNEUROSCI.2149-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana N, Troyano-Rodriguez E, Mengod G, Celada P, Artigas F. Activation of thalamocortical networks by the N-methyl-D-aspartate receptor antagonist phencyclidine: reversal by clozapine. Biol Psychiatry. 2011;69:918–927. doi: 10.1016/j.biopsych.2010.10.030. [DOI] [PubMed] [Google Scholar]
- Schabus M, Dang-Vu TT, Albouy G, Balteau E, Boly M, Carrier J, Darsaud A, Degueldre C, Desseilles M, Gais S, Phillips C, Rauchs G, Schnakers C, Sterpenich V, Vandewalle G, Luxen A, Maquet P. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proc Natl Acad Sci U S A. 2007;104:13164–13169. doi: 10.1073/pnas.0703084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabus M, Hoedlmoser K, Pecherstorfer T, Anderer P, Gruber G, Parapatics S, Sauter C, Kloesch G, Klimesch W, Saletu B, Zeitlhofer J. Interindividual sleep spindle differences and their relation to learning-related enhancements. Brain Res. 2008;1191:127–135. doi: 10.1016/j.brainres.2007.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeck-Hirschner M, Baier PC, Sever S, Buschbacher A, Aldenhoff JB, Goder R. Effects of daytime naps on procedural and declarative memory in patients with schizophrenia. J Psychiatr Res. 2010;44:42–47. doi: 10.1016/j.jpsychires.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc Lond B Biol Sci. 2002;357:1695–1708. doi: 10.1098/rstb.2002.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M, Fujiwara H, Hirao K, Namiki C, Fukuyama H, Hayashi T, Murai T. Structural abnormalities of the adhesio interthalamica and mediodorsal nuclei of the thalamus in schizophrenia. Schizophr Res. 2008;101:331–338. doi: 10.1016/j.schres.2007.12.486. [DOI] [PubMed] [Google Scholar]
- Silber MH, Ancoli-Israel S, Bonnet MH, Chokroverty S, Grigg-Damberger MM, Hirshkowitz M, Kapen S, Keenan SA, Kryger MH, Penzel T, Pressman MR, Iber C. The visual scoring of sleep in adults. J Clin Sleep Med. 2007;3:121–131. [PubMed] [Google Scholar]
- Spering M, Dias EC, Sanchez JL, Schutz AC, Javitt DC. Efference copy failure during smooth pursuit eye movements in schizophrenia. J Neurosci. 2013;33:11779–11787. doi: 10.1523/JNEUROSCI.0578-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam K, Luks TL, Fisher M, Simpson GV, Nagarajan S, Vinogradov S. Computerized Cognitive Training Restores Neural Activity within the Reality Monitoring Network in Schizophrenia. Neuron. 2012;73:842–853. doi: 10.1016/j.neuron.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulc A, Galinska-Skok B, Tarasow E, Konarzewska B, Waszkiewicz N, Hykiel R, Walecki J. Clinical and cognitive correlates of the proton magnetic resonance spectroscopy measures in chronic schizophrenia. Med Sci Monit. 2012;18:CR390–CR398. doi: 10.12659/MSM.882909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomelleri L, Jogia J, Perlini C, Bellani M, Ferro A, Rambaldelli G, Tansella M, Frangou S, Brambilla P. Brain structural changes associated with chronicity and antipsychotic treatment in schizophrenia. Eur Neuropsychopharmacol. 2009;19:835–840. doi: 10.1016/j.euroneuro.2009.07.007. [DOI] [PubMed] [Google Scholar]
- van Haren NE, Hulshoff Pol HE, Schnack HG, Cahn W, Mandl RC, Collins DL, Evans AC, Kahn RS. Focal gray matter changes in schizophrenia across the course of the illness: a 5-year follow-up study. Neuropsychopharmacology. 2007;32:2057–2066. doi: 10.1038/sj.npp.1301347. [DOI] [PubMed] [Google Scholar]
- Wamsley EJ, Shinn AK, Tucker MA, Ono KE, McKinley SK, Ely AV, Goff DC, Stickgold R, Manoach DS. The effects of eszopiclone on sleep spindles and memory consolidation in schizophrenia: a randomized placebo-controlled trial. Sleep. 2013;36:1369–1376. doi: 10.5665/sleep.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley EJ, Tucker MA, Shinn AK, Ono KE, McKinley SK, Ely AV, Goff DC, Stickgold R, Manoach DS. Reduced sleep spindles and spindle coherence in schizophrenia: mechanisms of impaired memory consolidation? Biol Psychiatry. 2012;71:154–161. doi: 10.1016/j.biopsych.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Bonjean M, Petry HM, Sejnowski TJ, Bickford ME. Thalamic burst firing propensity: a comparison of the dorsal lateral geniculate and pulvinar nuclei in the tree shrew. J Neurosci. 2011;31:17287–17299. doi: 10.1523/JNEUROSCI.6431-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara Y, Sugihara G, Matsumoto H, Suckling J, Nishimura K, Toyoda T, Isoda H, Tsuchiya KJ, Takebayashi K, Suzuki K, Sakahara H, Nakamura K, Mori N, Takei N. Voxel-based structural magnetic resonance imaging (MRI) study of patients with early onset schizophrenia. Ann Gen Psychiatry. 2008;7:25. doi: 10.1186/1744-859X-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Raz N. Prefrontal cortex and executive functions in healthy adults: A meta-analysis of structural neuroimaging studies. Neurosci Biobehav Rev. 2014;42C:180–192. doi: 10.1016/j.neubiorev.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Llinas RR, Lisman JE. Inhibition of NMDARs in the Nucleus Reticularis of the Thalamus Produces Delta Frequency Bursting. Front Neural Circuits. 2009;3:20. doi: 10.3389/neuro.04.020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.