Abstract
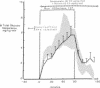
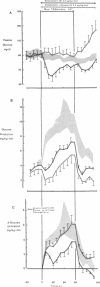
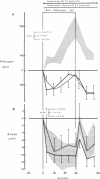
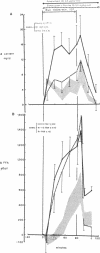
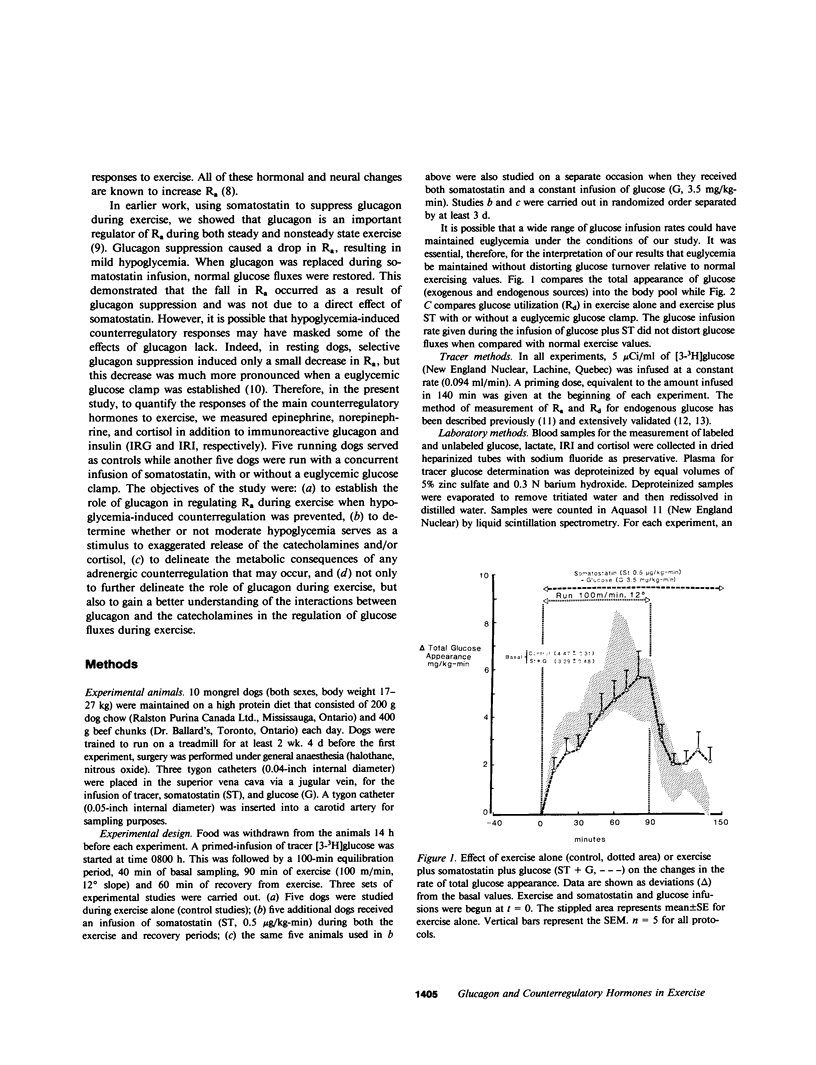
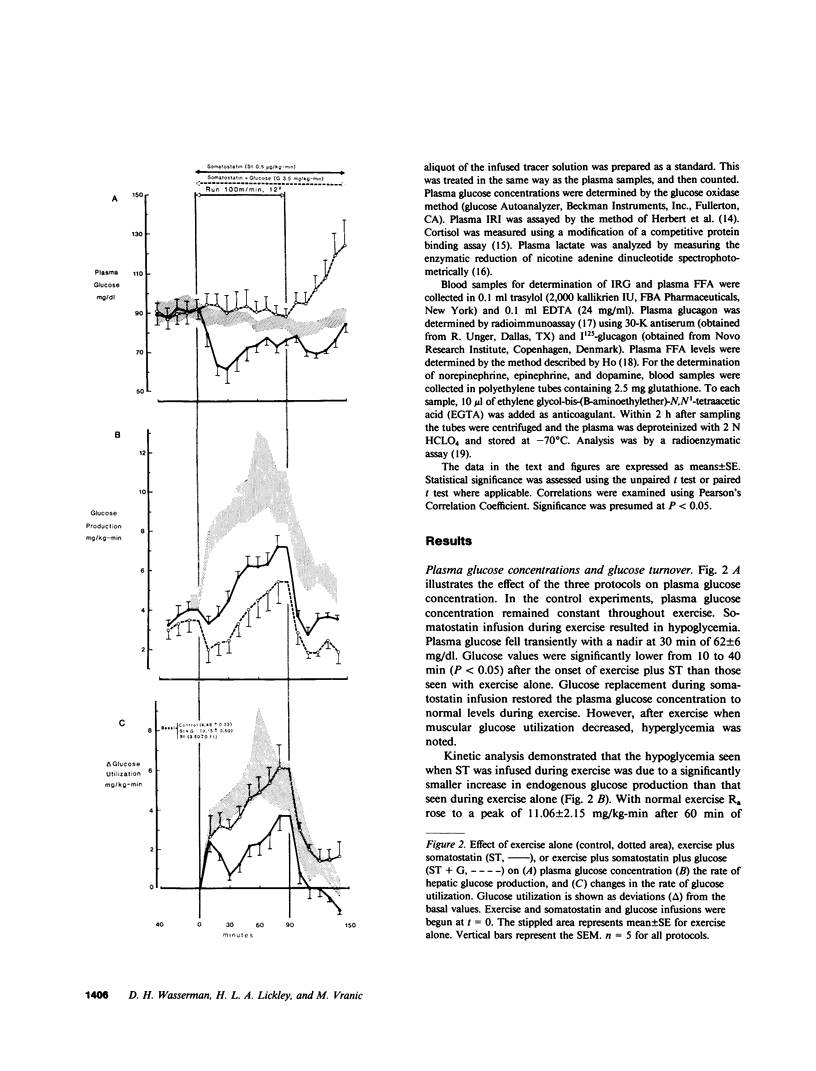
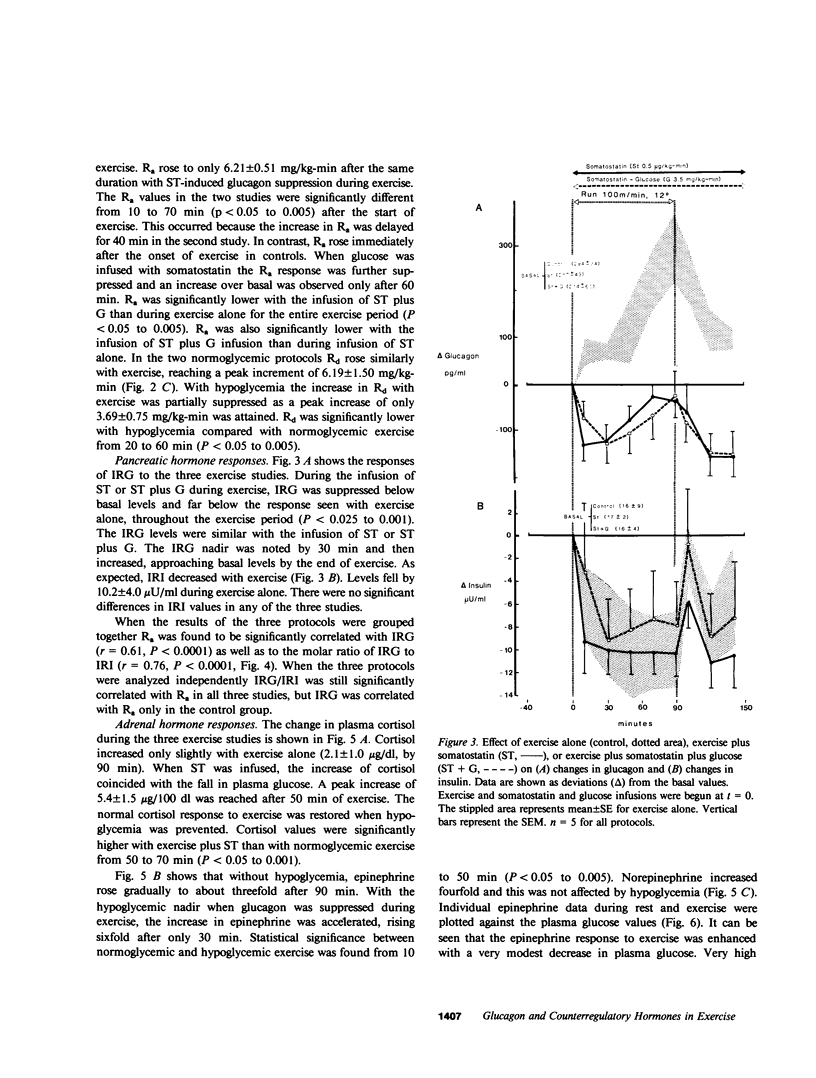
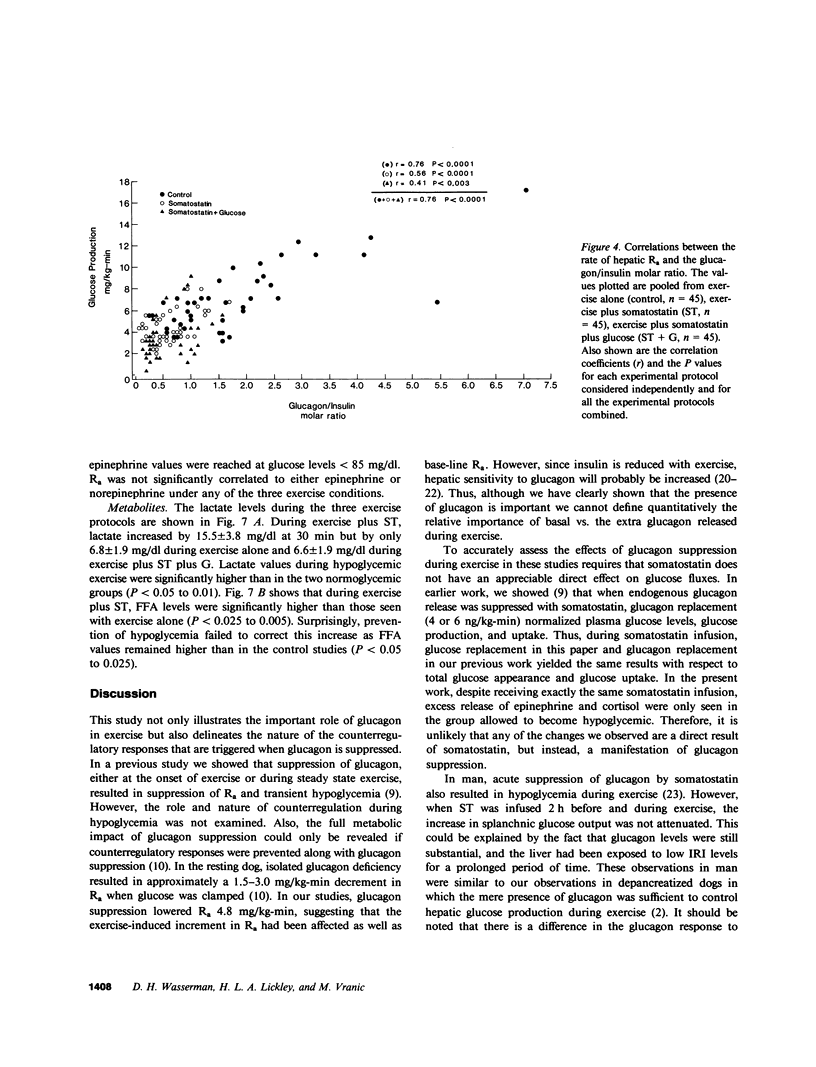
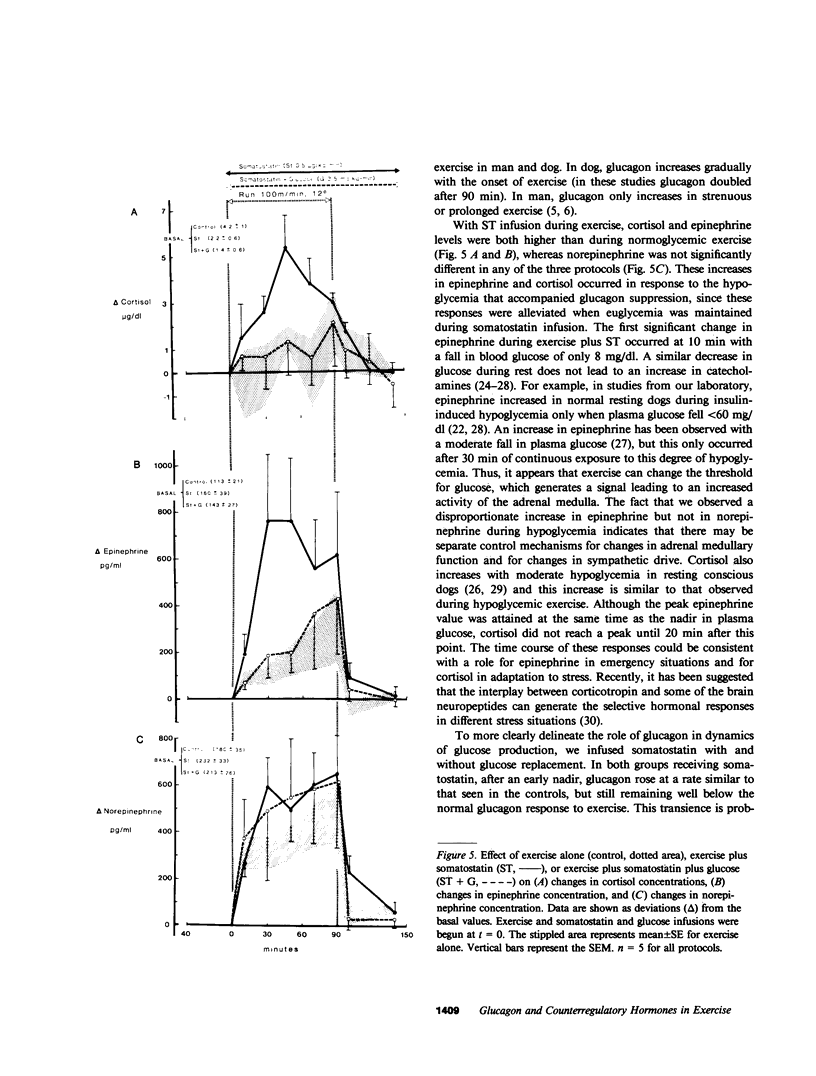
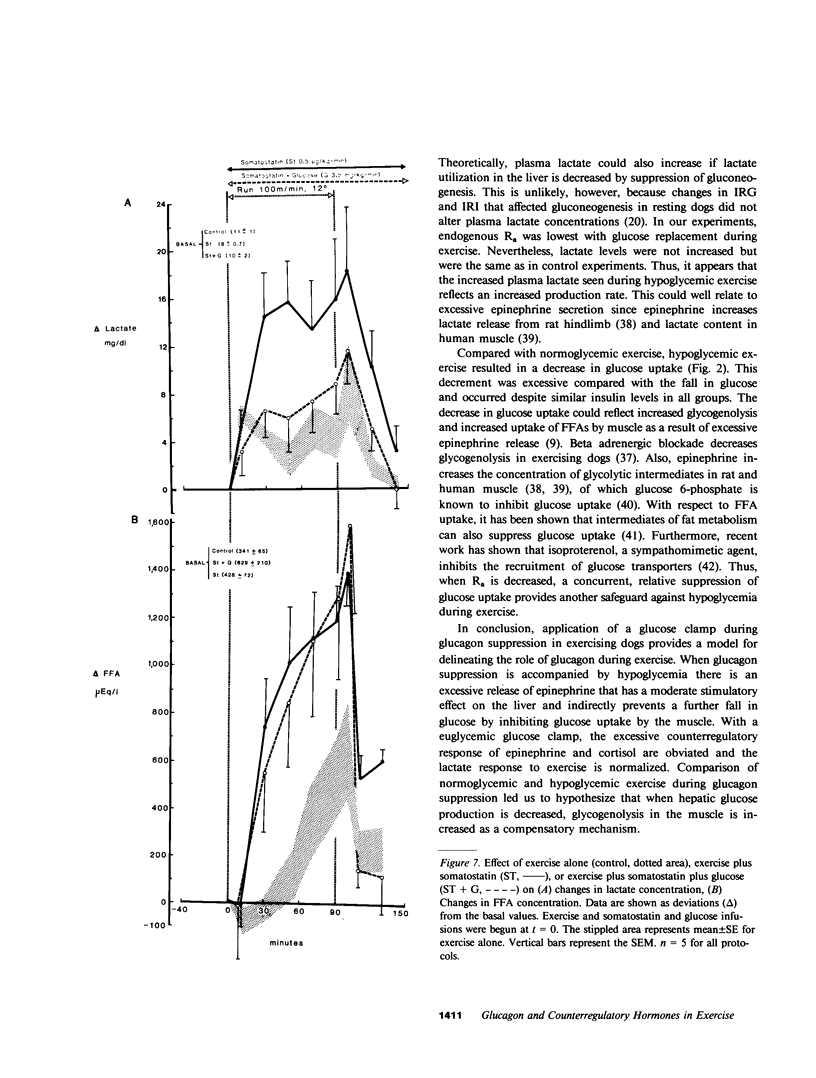
Somatostatin (ST)-induced glucagon suppression results in hypoglycemia during rest and exercise. To further delineate the role of glucagon and interactions between glucagon and the catecholamines during exercise, we compensated for the counterregulatory responses to hypoglycemia with glucose replacement. Five dogs were run (100 m/min, 12 degrees) during exercise alone, exercise plus ST infusion (0.5 micrograms/kg-min), or exercise plus. ST plus glucose replacement (3.5 mg/kg-min) to maintain euglycemia. During exercise alone there was a maximum increase in immunoreactive glucagon (IRG), epinephrine (E), norepinephrine (NE), FFA, and lactate (L) of 306 +/- 147 pg/ml, 360 +/- 80 pg/ml, 443 +/- 140 pg/ml, 541 +/- 173 mu eq/liter, and 6.3 +/- 0.7 mg/dl, respectively. Immunoreactive insulin (IRI) decreased by 10.2 +/- 4 micro/ml and cortisol (C) increased only slightly (2.1 +/- 0.3 micrograms/dl). The rates of glucose production (Ra) and glucose uptake (Rd) rose markedly by 6.6 +/- 2.2 mg/kg-min and 6.2 +/- 1.5 mg/kg-min. In contrast, when ST was given during exercise, IRG fell transiently by 130 +/- 20 pg/ml, Ra rose by only 3.6 +/- 0.5 mg/kg-min, and plasma glucose decreased by 29 +/- 6 mg/dl. The decrease in IRI was no different than with exercise alone (10.2 +/- 2.0 microU/ml). As plasma glucose fell, C, FFA, and L rose excessively to peaks of 5.4 +/- 1.3 micrograms/dl, 1,166 +/- 182 mu eq/liter and 15.5 +/- 7.0 mg/dl. The peak increment in E (765 +/- 287 pg/ml) coincided with the nadir in plasma glucose and was four times greater than during normoglycemic exercise. Hypoglycemia did not affect the rise in NE. The increase in Rd was attenuated and reached a peak of only 3.7 +/- 0.8 mg/kg-min. During glucose replacement, IRG decreased by 109 +/- 30 pg/ml and the IRI response did not differ from the response to normal exercise. Ra rose minimally by 1.5 +/- 0.3 mg/kg-min. The changes in E, C, Rd, and L were restored to normal, whereas the FFA response remained excessive. In all protocols increments in Ra were directly correlated to the IRG/IRI molar ratio while no correlation could be demonstrated between epinephrine or norepinephrine and Ra. In conclusion, (a) glucagon controlled approximately 70% of the increase of Ra during exercise. This became evident when counterregulatory responses to hypoglycemia (E and C) were obviated by glucose replacement; (b) increments in Ra were strongly correlated to the IRG/IRI molar ratio but not the plasma catecholamine concentration; (c) the main role of E in hypoglycemia was to limit glucose uptake by the muscle; (d) with glucagon suppression, glucose production was deficient but a further decline of glucose was prevented through the peripheral effects of E, (e) the hypoglycemic stimulus for E secretion was facilitated by exercise; and (f) we hypothesize that an important role of glucagons during exercise could be to spare muscle glycogen by stimulating glucose production by the liver.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergman R. N. Integrated control of hepatic glucose metabolism. Fed Proc. 1977 Feb;36(2):265–270. [PubMed] [Google Scholar]
- Brown M. R., Fisher L. A. Brain peptide regulation of adrenal epinephrine secretion. Am J Physiol. 1984 Jul;247(1 Pt 1):E41–E46. doi: 10.1152/ajpendo.1984.247.1.E41. [DOI] [PubMed] [Google Scholar]
- Böttger I., Schlein E. M., Faloona G. R., Knochel J. P., Unger R. H. The effect of exercise on glucagon secretion. J Clin Endocrinol Metab. 1972 Jul;35(1):117–125. doi: 10.1210/jcem-35-1-117. [DOI] [PubMed] [Google Scholar]
- Chasiotis D., Sahlin K., Hultman E. Regulation of glycogenolysis in human muscle in response to epinephrine infusion. J Appl Physiol Respir Environ Exerc Physiol. 1983 Jan;54(1):45–50. doi: 10.1152/jappl.1983.54.1.45. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Lacy W. W., Chiasson J. L. Effect of glucagon on glucose production during insulin deficiency in the dog. J Clin Invest. 1978 Sep;62(3):664–677. doi: 10.1172/JCI109174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrington A. D., Liljenquist J. E., Shulman G. I., Williams P. E., Lacy W. W. Importance of hypoglycemia-induced glucose production during isolated glucagon deficiency. Am J Physiol. 1979 Mar;236(3):E263–E271. doi: 10.1152/ajpendo.1979.236.3.E263. [DOI] [PubMed] [Google Scholar]
- Christensen N. J., Alberti K. G., Brandsborg O. Plasma catecholamines and blood substrate concentrations: studies in insulin induced hypoglycaemia and after adrenaline infusions. Eur J Clin Invest. 1975 Sep 12;5(5):415–423. doi: 10.1111/j.1365-2362.1975.tb00473.x. [DOI] [PubMed] [Google Scholar]
- Cowan J. S., Hetenyi G., Jr Glucoregulatory responses in normal and diabetic dogs recorded by a new tracer method. Metabolism. 1971 Apr;20(4):360–372. doi: 10.1016/0026-0495(71)90098-9. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Andres R., Bedsoe T. A., Boden G., Faloona G. A., Tobin J. D. A test of the hypothesis that the rate of fall in glucose concentration triggers counterregulatory hormonal responses in man. Diabetes. 1977 May;26(5):445–452. doi: 10.2337/diab.26.5.445. [DOI] [PubMed] [Google Scholar]
- Galbo H., Christensen N. J., Holst J. J. Catecholamines and pancreatic hormones during autonomic blockade in exercising man. Acta Physiol Scand. 1977 Dec;101(4):428–437. doi: 10.1111/j.1748-1716.1977.tb06026.x. [DOI] [PubMed] [Google Scholar]
- Galbo H., Holst J. J., Christensen N. J., Hilsted J. Glucagon and plasma catecholamines during beta-receptor blockade in exercising man. J Appl Physiol. 1976 Jun;40(6):855–863. doi: 10.1152/jappl.1976.40.6.855. [DOI] [PubMed] [Google Scholar]
- Gauthier C., Vranic M., Hetenyi G., Jr Nonhypoglycemic glucoregulation: role of glycerol and glucoregulatory hormones. Am J Physiol. 1983 Apr;244(4):E373–E379. doi: 10.1152/ajpendo.1983.244.4.E373. [DOI] [PubMed] [Google Scholar]
- Gray D. E., Lickley H. L., Vranic M. Physiologic effects of epinephrine on glucose turnover and plasma free fatty acid concentrations mediated independently of glucagon. Diabetes. 1980 Aug;29(8):600–608. doi: 10.2337/diab.29.8.600. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Hetenyi G., Jr, Perez G., Vranic M. Turnover and precursor-product relationships of nonlipid metabolites. Physiol Rev. 1983 Apr;63(2):606–667. doi: 10.1152/physrev.1983.63.2.606. [DOI] [PubMed] [Google Scholar]
- Ho R. J. Radiochemical assay of long-chain fatty acids using 63Ni as tracer. Anal Biochem. 1970 Jul;36(1):105–113. doi: 10.1016/0003-2697(70)90337-4. [DOI] [PubMed] [Google Scholar]
- Häggendal J., Hartley L. H., Saltin B. Arterial noradrenaline concentration during exercise in relation to the relative work levels. Scand J Clin Lab Invest. 1970 Dec;26(4):337–342. doi: 10.3109/00365517009046242. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr, Issekutz A. C., Nash D. Mobilization of energy sources in exercising dogs. J Appl Physiol. 1970 Nov;29(5):691–697. doi: 10.1152/jappl.1970.29.5.691. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr Role of beta-adrenergic receptors in mobilization of energy sources in exercising dogs. J Appl Physiol Respir Environ Exerc Physiol. 1978 Jun;44(6):869–876. doi: 10.1152/jappl.1978.44.6.869. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr, Vranic M. Role of glucagon in regulation of glucose production in exercising dogs. Am J Physiol. 1980 Jan;238(1):E13–E20. doi: 10.1152/ajpendo.1980.238.1.E13. [DOI] [PubMed] [Google Scholar]
- Iversen J. Secretion of glucagon from the isolated, perfused canine pancreas. J Clin Invest. 1971 Oct;50(10):2123–2136. doi: 10.1172/JCI106706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller-Wood M. E., Shinsako J., Keil L. C., Dallman M. F. Insulin-induced hypoglycemia in conscious dogs. I. Dose-related pituitary and adrenal responses. Endocrinology. 1981 Sep;109(3):818–824. doi: 10.1210/endo-109-3-818. [DOI] [PubMed] [Google Scholar]
- Luyckx A. S., Dresse A., Cession-Fossion A., Lefebvre P. J. Catecholamines and exercise-induced glucagon and fatty acid mobilization in the rat. Am J Physiol. 1975 Aug;229(2):376–383. doi: 10.1152/ajplegacy.1975.229.2.376. [DOI] [PubMed] [Google Scholar]
- Murphy B. E. Some studies of the protein-binding of steroids and their application to the routine micro and ultramicro measurement of various steroids in body fluids by competitive protein-binding radioassay. J Clin Endocrinol Metab. 1967 Jul;27(7):973–990. doi: 10.1210/jcem-27-7-973. [DOI] [PubMed] [Google Scholar]
- Ohneda A., Aguilar-Parada E., Eisentraut A. M., Unger R. H. Control of pancreatic glucagon secretion by glucose. Diabetes. 1969 Jan;18(1):1–10. doi: 10.2337/diab.18.1.1. [DOI] [PubMed] [Google Scholar]
- Perez G., Kemmer F. W., Lickley H. L., Vranic M. Importance of glucagon in mediating epinephrine-induced hyperglycemia in alloxan-diabetic dogs. Am J Physiol. 1981 Oct;241(4):E328–E335. doi: 10.1152/ajpendo.1981.241.4.E328. [DOI] [PubMed] [Google Scholar]
- RANDLE P. J., SMITH G. H. Regulation of glucose uptake by muscle. 2. The effects of insulin, anaerobiosis and cell poisons on the penetration of isolated rat diaphragm by sugars. Biochem J. 1958 Nov;70(3):501–508. doi: 10.1042/bj0700501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziuk J., Norwich K. H., Vranic M. Experimental validation of measurements of glucose turnover in nonsteady state. Am J Physiol. 1978 Jan;234(1):E84–E93. doi: 10.1152/ajpendo.1978.234.1.E84. [DOI] [PubMed] [Google Scholar]
- Richter E. A., Ruderman N. B., Gavras H., Belur E. R., Galbo H. Muscle glycogenolysis during exercise: dual control by epinephrine and contractions. Am J Physiol. 1982 Jan;242(1):E25–E32. doi: 10.1152/ajpendo.1982.242.1.E25. [DOI] [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Sacca L., Hendler R., Sherwin R. S. Hyperglycemia inhibits glucose production in man independent of changes in glucoregulatory hormones. J Clin Endocrinol Metab. 1978 Nov;47(5):1160–1163. doi: 10.1210/jcem-47-5-1160. [DOI] [PubMed] [Google Scholar]
- Santiago J. V., Clarke W. L., Shah S. D., Cryer P. E. Epinephrine, norepinephrine, glucagon, and growth hormone release in association with physiological decrements in the plasma glucose concentration in normal and diabetic man. J Clin Endocrinol Metab. 1980 Oct;51(4):877–883. doi: 10.1210/jcem-51-4-877. [DOI] [PubMed] [Google Scholar]
- Sole M. J., Hussain M. N. A simple specific radioenzymatic assay for the simultaneous measurement of picogram quantities of norepinephrine, epinephrine, and dopamine and in plasma and tissues. Biochem Med. 1977 Dec;18(3):301–307. doi: 10.1016/0006-2944(77)90064-3. [DOI] [PubMed] [Google Scholar]
- Vranic M., Kawamori R., Pek S., Kovacevic N., Wrenshall G. A. The essentiality of insulin and the role of glucagon in regulating glucose utilization and production during strenuous exercise in dogs. J Clin Invest. 1976 Feb;57(2):245–255. doi: 10.1172/JCI108275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J., Felig P., Ahlborg G., Jorfeldt L. Glucose metabolism during leg exercise in man. J Clin Invest. 1971 Dec;50(12):2715–2725. doi: 10.1172/JCI106772. [DOI] [PMC free article] [PubMed] [Google Scholar]