Abstract
Objective
To assess the availability of obstetric institutions, the risk of unplanned delivery outside an institution and maternal morbidity in a national setting in which the number of institutions declined from 95 to 51 during 30 years.
Design
Retrospective population-based, three cohorts and two cross-sectional analyses.
Setting
Census data, Statistics Norway. The Medical Birth Registry of Norway from 1979 to 2009.
Population
Women (15–49 years), 2000 (n = 1 050 269) and 2010 (n = 1 127 665). Women who delivered during the period 1979–2009 (n = 1 807 714).
Methods
Geographic Information Systems software for travel zone calculations. Cross-table and multiple logistic regression analysis of change over time and regional differences. World Health Organization Emergency Obstetric and Newborn Care (EmOC) indicators.
Main outcome measures
Proportion of women living outside the 1-hour travel zone to obstetric institutions. Risk of unplanned delivery outside obstetric institutions. Maternal morbidity.
Results
The proportion of women living outside the 1-hour zone for all obstetric institutions increased from 7.9% to 8.8% from 2000 to 2010 (relative risk, 1.1; 95% confidence interval, 1.11–1.12), and for emergency obstetric care from 11.0% to 12.1% (relative risk, 1.1; 95% confidence interval, 1.09–1.11). The risk of unplanned delivery outside institutions increased from 0.4% in 1979–83 to 0.7% in 2004–09 (adjusted odds ratio, 2.0; 95% confidence interval, 1.9–2.2). Maternal morbidity increased from 1.7% in 2000 to 2.2% in 2009 (adjusted odds ratio, 1.4; 95% confidence interval, 1.2–1.5) and the regional differences increased.
Conclusions
The availability of and access to obstetric institutions was reduced and we did not observe the expected decrease in maternal morbidity following the centralisation.
Keywords: Access, availability, emergency obstetric care indicators, Geographic Information Systems, healthcare quality
Introduction
Caught between high-technology services and the care for normal uncomplicated deliveries, obstetric care has been a core issue in the current health system debate in several high-income countries.1–5 Within other fields in medicine, such as cancer treatment, surgery and intervention cardiology, centralisation to larger units improves patient outcome, although the mechanisms are complex.6–8 In obstetrics, however, delivery in large institutions has been associated with an increased frequency of interventions for low-risk women and the benefit for neonatal outcome in low-risk infants remains a matter of debate.1,9,10 With the exception of access to neonatal intensive care units and neonatal outcome, the availability of and access to obstetric institutions has received little attention in high-income countries.11,12 Treatment of obstetric complications requires skills and medical and technical resources, and thus access to institution-based care.13 The World Health Organization (WHO) has developed tools to monitor emergency obstetric care, including the geographical distribution of institutions, access, utilisation and the type of services provided.14 Registration of severe maternal morbidity adds information about the health service performance in all types of resource settings.15 National policy in Norway has emphasised the need for decentralised care in order to provide safe services of high quality near a woman's home.16 However, the number of obstetric institutions in Norway declined from 95 to 51 between 1979 and 2009.
Knowledge of how centralisation of obstetric services affects availability and access to obstetric institutions is lacking in high-income countries. In particular, the consequences are unclear for maternal outcomes. Our objective was to study the time trends and regional variations in travel distance to institutions, the risk of unplanned delivery outside institutions and maternal morbidity using nationwide population-based registries to design three cohort and two cross-sectional analyses. Our hypothesis was that the centralisation has led to reduced availability of and access to institutions, but a reduced risk of maternal morbidity.
Methods
Core definitions
Basic obstetric care was defined as care for a normal delivery and referral if complications occurred. Emergency obstetric care institutions provided all the nine signal functions outlined in Table1. A travel zone was defined as the geographical area in which all women were estimated to reach the nearest institution within the given time limit. An unplanned delivery outside an institution was defined as delivery at home, during transportation or in a non-obstetric institution (e.g. health centre) for a woman who had planned an institutional delivery. Maternal morbidity from causes related to pregnancy and childbirth was assessed using the following diagnoses or treatment-based categories: maternal intensive care, puerperal sepsis and sepsis during delivery, thromboembolic disease with the exception of peripheral venous thrombophlebitis, eclampsia and haemorrhage >1500 ml or blood transfusion. We defined delivery-related perinatal mortality as intrapartum death or neonatal death within 24 hours at a gestational age of ≥22 weeks or birth weight of ≥500 g.
Table 1.
World Health Organization (WHO) Emergency Obstetric Care (EmOC) indicators and signal functions
| Indicators (8) |
| Institution availability and geographic distribution. Recommendation: 5 institutions per 500 000 inhabitants including one institution providing comprehensive emergency care |
| Proportion of all births in emergency obstetric care institutions. Recommendation: to be determined locally |
| Met need of emergency obstetric care. The proportion of women with major direct complications who are treated in EmOC facilities. Recommendation: 100% |
| Caesarean section rate as a proportion of all births. Recommendation: 5–15% |
| Direct obstetric case fatality rate. Recommendation: <1% |
| Intrapartum and very early neonatal mortality. Recommendation not given |
| Maternal mortality from indirect causes. Recommendation not given |
| Signal functions (9) |
| Basic emergency obstetric care |
| Perform parenteral administration of antibiotics (1), uterotonic drugs (2) and anticonvulsants (3) |
| Perform manual removal of placenta (4) and removal of retained products (5) |
| Perform assisted vaginal delivery (6) |
| Perform basic neonatal resuscitation (7) |
| Comprehensive emergency obstetric care include the above plus |
| Perform surgery, e.g. hysterectomy and caesarean section (8) |
| Perform blood transfusion (9) |
WHO.14
Availability of institutions
Women of fertile age (15–49 years) who lived more than 1 or 2 hours away from the nearest obstetric institution were counted. Institutions were included if they were registered to provide obstetric care and reported more than 10 deliveries in 2000 or 2009. Cross-sectional assessments were performed for 1 January 2000 (n = 1 050 269 women, 59 institutions) and 1 January 2010 (n = 1 127 665 women, 51 institutions). Four basic obstetric care institutions in the Northern region had fewer than 10 deliveries in 2000 and were therefore excluded.
Since 2000, Statistics Norway has assigned geographical coordinates to individual addresses as part of the census update on 1 January each year. Individual coordinates had been assigned to 98% of the census addresses in 2000 (county range, 95.5–99%), whereas the coverage was 99% in 2010 (county range, 98.2–100%). We registered the institutions with geographical coordinates, and the surrounding travel zones were calculated based on the national road database for the corresponding year. A merged area (polygon) was created for the travel zones, and the number of women registered to live fully within the area was counted. The women were counted in the area of the nearest institution, irrespective of county and health region borders. Estimates were based on registered speed limits and standard duration of ferry/boat journeys, but did not take into account such factors as harbour waiting times, difficult driving conditions or temporary route changes. The estimates thus represented the minimum time for non-emergency transport.
Access to obstetric institutions at the time of delivery, the risk of unplanned delivery outside an institution
We performed a retrospective cohort analysis of unplanned deliveries outside institutions from 1979 to 2009 using data from the Medical Birth Registry of Norway (MBRN). The registry has received mandatory notifications of all births since 1967, both live births and stillbirths from 16 weeks of gestation (12 weeks since 2002). The notification form is standardised and was revised in 1999 to include more information about the mother, the neonate and the birthplace, including planned home deliveries. Notification is given as free text and, after 1999, also as check boxes/predefined variables. Free text is coded at the MBRN using the International Classification of Diseases, 8th Revision for births in 1967–1998 and 10th Revision for births from 1999 onwards. Birth notifications are sent from the institutions to the MBRN at the time of discharge. Inclusion criteria were the known place of birth and either gestational age ≥22 completed weeks or birth weight ≥500 g (n = 1 807 714). Planned home deliveries from 1999 to 2009 were excluded (n = 1267); these constituted 0.2% of the study population during these years. The year of delivery was categorised in 5-year groups; the last group covered 6 years.
Maternal morbidity and emergency obstetric care indicators
Two national retrospective cohort analyses were performed using all deliveries from 1 January to 31 December 2000 (n = 58 632) and 2009 (n = 61 895). The inclusion criterion was gestational age ≥22 completed weeks or birth weight ≥500 g. Deliveries categorised as unknown birthplace (2000, n = 11; 2009, n = 22) or lacking registered maternal address (2000, n = 103; 2009, n = 33) were excluded from the regional analyses. Population data were obtained from Statistics Norway. We applied the WHO emergency obstetric care signal functions (Table1) to classify institutions, and used the indicators to assess the geographical distribution of institutions, access, use and maternal and neonatal outcomes in 2000 and 2009. The WHO handbook was developed as a tool for low-income countries, but the indicators have also been used to evaluate services in high- and middle-income countries.13 We used the 1-year cohorts rather than the proposed 3 months registration, as some indicators represent rare events. Caesarean section rates were assessed on a national and regional level. Data on maternal deaths were obtained from the Norwegian Cause of Death Registry and from a Norwegian maternal mortality audit study. The Norwegian Air Ambulance records for 2009 documented the number of urgent emergency transports as a result of suspected or diagnosed complications during pregnancy or after delivery. The records included information about indication and whether the transport was from the woman's home (primary) or was a transfer between institutions (secondary).
Direct maternal deaths were rare, and maternal deaths from indirect causes were not registered in Norway. We used maternal morbidity from causes related to pregnancy and childbirth (see Core definitions) as well as the delivery-related perinatal mortality to assess the quality of clinical care according to the WHO guidelines.
Analyses
The cross-sectional travel zone analyses were performed with the Geographic Information Systems (GIS) software Arc Info with Network Analyst (Environmental Systems Research Institute Inc. (Esri), Redlands, CA, USA). The GIS tool integrates hardware, software and data, and is used for the capture, analysis and display of geographically referenced information. Arc Info is the software currently used by Statistics Norway. Travel zones were estimated by combining the institution coordinates with the national road database.17 The number of women living within or outside the zone was counted. The differences in the proportions of women who lived outside the 1-hour and 2-hour travel zones in 2000 and 2010 were calculated by cross tables providing relative risk (RR) with 95% confidence intervals (CIs), using 2000 as the reference year.
Cross tables were used to calculate the risk of unplanned delivery outside an institution in all 5-year groups from 1979–83 to 2004–09, and we evaluated time trends across these groups using logistic regression analyses. Cross tables were also used to calculate odds ratios (ORs) with 95% CIs for maternal morbidity in 2009 relative to 2000. Finally, we analysed regional differences in maternal morbidity and delivery-related perinatal mortality using the region with the lowest risk as reference. Logistic regression analyses were used to adjust for confounding by maternal age (<20, 20–24, 25–29, 30–34, 35 + years), parity (0, 1, 2+), education (<11, 11–14, 14 + years) and partner status (single or married/cohabiting). Maternal morbidity was also adjusted for tobacco use (daily smoking, occasional smoking, or non-smoking). All outcomes were rare and ORs were considered to be close approximations to RRs in these analyses. We used IBM SPSS Statistics version 19 (IBM SPSS Inc., Chicago, IL, USA) for all calculations.
Results
Availability
The proportion of women who lived outside the 1-hour zone of all institutions increased from 7.9% to 8.8% from 2000 to 2010 (RR, 1.11; 95% CI, 1.10–1.12; Table2). The number of counties in which more than 10% of women lived outside the 1-hour zone increased from seven to nine from 2000 to 2010 (Figure1, Appendix S1, see Supporting information). Increases in proportions were observed in counties in which obstetric care institutions closed during this period, whereas decreases related to major infrastructure projects were observed in two counties.
Table 2.
National and regional numbers and proportions of women living outside the 1-hour zone of all institutions and emergency obstetric care institutions in 2000 and 2010. Based on institution data from the Medical Birth Registry of Norway, population data from Statistics Norway and the Norwegian road database
| Total population of women 15–49 years* | All institutions** | Emergency obstetric care institutions*** | ||||||
|---|---|---|---|---|---|---|---|---|
| 2000 | 2010 | 2000 | 2010 | Relative risk (95% CI) | 2000 | 2010 | Relative risk (95% CI) | |
| Outside 1 hour (%) | Outside 1 hour (%) | Outside 1 hour (%) | Outside 1 hour (%) | |||||
| Norway | 1 050 269 | 1 127 665 | 82 671 (7.9) | 98 720 (8.8) | 1.1 (1.10–1.12) | 115 701 (11.0) | 136 208 (12.1) | 1.1 (1.09–1.11) |
| Eastern region | 386 227 | 426 030 | 7682 (2.0) | 11 001 (2.6) | 1.3 (1.26–1.34) | 11 341 (2.9) | 18 419 (4.3) | 1.5 (1.44–1.51) |
| Southern region | 200 868 | 211 541 | 5029 (2.5) | 11 985 (5.7) | 2.3 (2.19–2.34) | 11 438 (5.7) | 14 849 (7.0) | 1.2 (1.20–1.26) |
| Western region | 214 827 | 236 258 | 21 640 (10.1) | 25 374 (10.7) | 1.07 (1.05–1.09) | 21 640 (10.1) | 30 749 (13.0) | 1.3 (1.27–1.31) |
| Central region | 142 830 | 150 868 | 23 161 (16.2) | 24 983 (16.6) | 1.02 (1.01–1.04) | 29 208 (20.5) | 26 035 (17.3) | 0.8 (0.83–0.86) |
| Northern region | 105 517 | 102 968 | 25 159 (23.8) | 25 377 (24.7) | 1.03 (1.02–1.05) | 42 074 (39.9) | 46 156 (44.8) | 1.1 (1.11–1.14) |
Women 15–49 years with registered address on 1 January 2000 and 1 January 2010.
Institutions provided only basic obstetric care or all nine emergency obstetric care signal functions; 59 institutions in 2000 and 51 institutions in 2009.
Institutions provided all the nine signal functions; intravenous administration of drugs, removal of placenta/retained products, assisted vaginal delivery, basic neonatal resuscitation, surgery and blood transfusion; 47 institutions in 2000 and 41 institutions in 2009.
Figure 1.
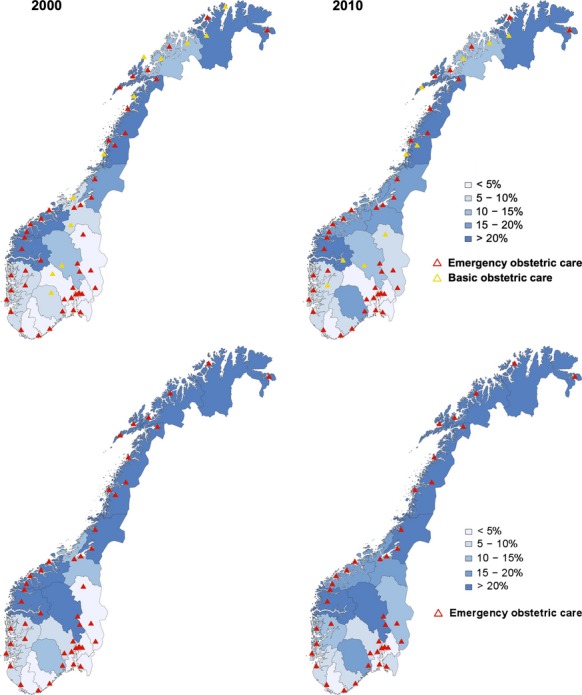
Travel time to all institutions and emergency obstetric care institutions. The proportion of women living outside the 1-hour zone in the 19 counties on 1 January 2000 and 2010 (%) is shown in the background colour scale for all institutions (top) and emergency obstetric care institutions (bottom). The institutions are marked according to the level of care. Based on census data from Statistics Norway and the Norwegian road database.
The availability of emergency obstetric care institutions was also reduced. The proportion of women living outside the 1-hour zone for emergency obstetric care institutions increased from 11.0% to 12.1% from 2000 to 2010 (RR, 1.1; 95% CI, 1.09–1.11; Table2). The number of counties in which more than 10% of women lived outside the 1-hour zone increased from nine to 11 (Figure1, Appendix S2, see Supporting information). Although the absolute numbers were low, the proportion of women living outside the 2-hour zone increased from 3.4% to 4.8% nationally (RR, 1.4; 95% CI, 1.39–1.43), from 0.29% to 1.6% in the Eastern region (RR, 5.6; 95% CI, 5.2–5.9), from 0.81% to 2.9% in the Southern region (RR, 3.6; 95% CI, 3.4–3.8) and from 21% to 28% in the Northern region (RR, 1.3; 95% CI, 1.28–1.32).
Risk of unplanned delivery outside an institution
During 1979–2009, the number of institutions declined from 95 to 51, and 11 537 deliveries outside an institution were registered among the included deliveries (n = 1 807 714). On a national level, the risk of unplanned delivery outside an institution was doubled in 2004–09 relative to 1979–83 (Table3). The risk increased successively from 0.4% in 1979–83 to 0.8% in 1994–98 and to 0.7% in 1999–2003 and 2004–09 (test for trend [Wald]: P < 0.001). During 1979 to 1998, we were unable to exclude planned home deliveries from these figures (approximately 0.2% per 5-year period after 1999). The geographical variation increased, and the risk in different counties ranged from 0.1% to 0.7% in the first period and from 0.3% to 1.8% in the last period. Two counties experienced a fivefold increase in risk. We observed that the risk of unplanned delivery outside an institution was higher in counties with a decentralised population pattern (Figure2). However, even in urban counties, where <1% of women lived outside the 1-hour zone, the risk more than doubled (counties 2, 3 and 7; Table3, Appendix S1).
Table 3.
Risk of unplanned delivery outside an institution in 2004–09 versus 1979–83. Data from the Medical Birth Registry of Norway on deliveries at gestational age ≥22 weeks or birth weight ≥500 g
| Region and country number | 1979–83 | 2004–09 | Odds ratio, crude | 95% CI | Odds ratio, adjusted*** | 95% CI | |||
|---|---|---|---|---|---|---|---|---|---|
| Total deliveries* | Outside institution** (%) | Total deliveries* | Outside institution** (%) | ||||||
| Norway | 252 621 | 984 (0.39) | 409 432 | 2832 (0.69) | 1.8 | 1.6–1.9 | 2.0 | 1.9–2.2 | |
| Eastern region | 1 | 12 768 | 18 (0.14) | 20 447 | 131 (0.64) | 4.5 | 2.8–7.4 | 5.7 | 3.1–10 |
| 2 | 21 629 | 51 (0.24) | 42 682 | 232 (0.54) | 2.3 | 1.6–3.1 | 2.3 | 1.7–3.2 | |
| 3 | 25 910 | 35 (0.13) | 66 818 | 229 (0.34) | 2.5 | 1.7–3.7 | 2.6 | 1.7–3.8 | |
| 4 | 9246 | 28 (0.30) | 12 604 | 80 (0.63) | 2.1 | 1.4–3.2 | 2.3 | 1.4–3.7 | |
| 5 | 9329 | 56 (0.60) | 12 641 | 101 (0.79) | 1.3 | 0.96–1.8 | 1.7 | 1.2–2.5 | |
| Southern region | 6 | 11 901 | 40 (0.33) | 19 709 | 107 (0.54) | 1.6 | 1.1–2.3 | 1.9 | 1.3–2.9 |
| 7 | 10 343 | 17 (0.16) | 16 739 | 73 (0.43) | 2.7 | 1.6–4.4 | 2.8 | 1.6–4.9 | |
| 8 | 9087 | 42 (0.46) | 11 694 | 110 (0.93) | 2.0 | 1.4–2.9 | 2.3 | 1.6–3.4 | |
| 9 | 5856 | 12 (0.20) | 8462 | 45 (0.53) | 2.6 | 1.4–4.9 | 2.8 | 1.4–5.4 | |
| 10 | 9685 | 39 (0.40) | 14 812 | 99 (0.66) | 1.7 | 1.1–2.4 | 2.0 | 1.3–3.0 | |
| Western region | 11 | 23 663 | 101 (0.43) | 40 629 | 235 (0.58) | 1.4 | 1.1–1.7 | 1.6 | 1.2–2.0 |
| 12 | 26 680 | 103 (0.38) | 42 132 | 340 (0.80) | 2.1 | 1.7–2.7 | 2.1 | 1.7–2.7 | |
| 14 | 6945 | 45 (0.64) | 8476 | 113 (1.32) | 2.1 | 1.5–2.9 | 2.1 | 1.4–3.1 | |
| Central region | 15 | 15 622 | 99 (0.63) | 19 425 | 190 (0.97) | 1.5 | 1.2–1.9 | 2.0 | 1.5–2.6 |
| 16 | 15 484 | 56 (0.36) | 25 176 | 199 (0.78) | 2.2 | 1.6–2.9 | 2.6 | 1.9–3.5 | |
| 17 | 7771 | 53 (0.68) | 10 073 | 141 (1.38) | 2.1 | 1.5–2.8 | 2.5 | 1.8–3.5 | |
| Northern region | 18 | 15 472 | 99 (0.64) | 17 498 | 180 (1.02) | 1.6 | 1.3–2.1 | 2.0 | 1.5–2.6 |
| 19 | 9873 | 68 (0.68) | 13 125 | 118 (0.89) | 1.3 | 0.97–1.8 | 1.4 | 1.0–1.9 | |
| 20 | 5303 | 21 (0.39) | 5897 | 105 (1.75) | 4.5 | 2.8–7.2 | 5.4 | 3.2–8.8 | |
Deliveries with known place of birth; planned home deliveries were excluded in 2004–09.
Delivery at home, during transportation or in a non-obstetric institution.
Adjusted for maternal age, parity, education level and partner status.
Figure 2.
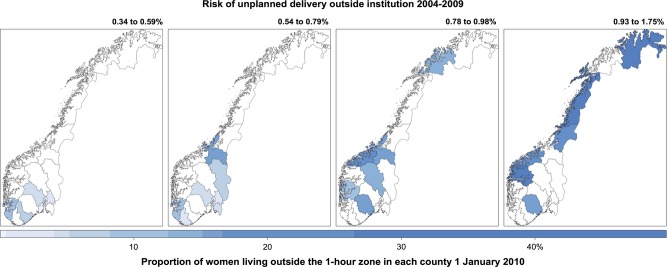
Risk of unplanned delivery outside institutions and travel time to institutions. The counties were sorted into four levels of risk based on the period 2004–09. The colour scale shows the proportion of women living outside the 1-hour zone in each county (%) on 1 January 2010. Based on data from the Medical Birth Registry of Norway, and on census data from Statistics Norway combined with the Norwegian road database.
Emergency obstetric care indicators and maternal morbidity
From 2000 to 2009, the total population increased from 4 478 497 to 4 858 159, whereas the number of emergency obstetric care institutions decreased from 47 to 41 (Table4). Thus, the national number of institutions was lower than the estimated need in 2009. At the regional level, the number of emergency obstetric care institutions was lower than the estimated need in the Southern and Eastern regions in 2000. The coverage in these regions declined further during the decade. The Western region also had fewer institutions than the estimated need in 2009. From 2000 to 2009, the proportion of deliveries in institutions with more than 3000 births per year increased from 34% in four institutions to 46% in five institutions. A total of 31 institutions with fewer than 500 births per year provided care for 10% of all deliveries in 2000, whereas the corresponding numbers were 21 institutions and 9.0% of all deliveries in 2009. The national average caesarean section rate was 14% in 2000 and 17% in 2009, with a regional range of 11–15% in the first period and 13–19% in the latter (Table4). There were 12 institutions that provided only basic obstetric care in 2000 and 10 in 2009. The majority of basic obstetric care institutions were rural and had a helicopter response time exceeding 20 minutes and a road ambulance transfer time of 1–3 hours to the nearest emergency institution. The Norwegian Air Ambulance recorded 444 transports related to pregnancy and childbirth in 2009: 257 primary transports from home to institution and 187 secondary transports between institutions (P. Madsen, the Norwegian Air Ambulance, personal communication, 2011). The maternal death audit identified five direct maternal deaths in 2009, and the direct maternal mortality rate was 8.1 per 100 000 live births (5/61 674). Transport delay was not a major factor in any of these deaths (S. Vangen, University of Oslo, personal communication, 2012). As shown in Table4, the delivery-related perinatal death rate declined from 2.1 per 1000 in 2000 to 1.6 per 1000 in 2009 (adjusted OR, 0.6; 95% CI, 0.4–0.9). The regional differences in 2000 and 2009 were not statistically significant (2000, P = 0.35; 2009, P = 0.16; Wald test). Table4 also shows the numbers and risk of maternal morbidity on national and regional levels. Nationally, the maternal morbidity risk increased from 1.7% to 2.2% from 2000 to 2009 (adjusted OR, 1.4; 95% CI, 1.2–1.5). The maternal morbidity risk also increased in three health regions: Northern region (adjusted OR, 1.5; 95% CI, 1.1–1.9), Southern region (adjusted OR, 1.5; 95% CI, 1.2–1.8) and Eastern region (adjusted OR, 1.3; 95% CI, 1.1–1.5). The Western region had the lowest risk of maternal morbidity in both 2000 and 2009, and was used as reference for regional comparisons. In 2000, there were no significant regional differences when adjusting for confounding variables (P = 0.3, Wald test), whereas, in 2009, the maternal morbidity risk was significantly higher than the reference in three regions: Northern region (adjusted OR, 1.8; 95% CI, 1.4–2.2), Southern region (adjusted OR, 1.8; 95% CI, 1.5–2.1) and Eastern region (adjusted OR, 1.3; 95% CI, 1.05–1.5).
Table 4.
The World Health Organization (WHO) Emergency Obstetric Care (EmOC) indicators as applied to national and regional levels, Norway, 2000 and 2009. Data from the Medical Birth Registry of Norway on deliveries ≥22 weeks of gestation or birth weight ≥500 g. Population data from Statistics Norway
| Regions | Population | EmOC* estimated need** | EmOC number (coverage) (%) | Basic OC*** | Deliveries (n) | Outside EmOC**** n (%) | Caesarean sections n (%) | Maternal morbidity***** n (%) | Perinatal mortality****** n (%) |
|---|---|---|---|---|---|---|---|---|---|
| 2000 | |||||||||
| Norway | 4 478 497 | 45 | 47 (100) | 12 | 58 632 | 1068 (1.8) | 7653 (13.1) | 988 (1.7) | 124 (2.1) |
| Eastern | 1 592 540 | 16 | 11 (69) | 1 | 20 786 | 229 (1.1) | 3032 (14.6) | 341 (1.6) | 47 (2.2) |
| Southern | 872 493 | 9 | 8 (89) | 2 | 10 480 | 130 (1.2) | 1354 (12.9) | 189 (1.8) | 22 (2.1) |
| Western | 916 018 | 9 | 9 (100) | 0 | 13 078 | 70 (0.5) | 1381 (10.6) | 194 (1.5) | 29 (2.2) |
| Central | 633 118 | 6 | 8 (100) | 2 | 8172 | 144 (1.8) | 1050 (12.8) | 143 (1.8) | 9 (1.1) |
| Northern | 464 328 | 5 | 11 (100) | 7 | 6013 | 495 (8.2) | 825 (14.6) | 119 (2.0) | 16 (2.6) |
| 2009 | |||||||||
| Norway | 4 852 197 | 49 | 41 (83) | 10 | 61 895 | 1289 (2.1) | 10 154 (16.4) | 1331 (2.2) | 99 (1.6) |
| Eastern | 1 770 946 | 18 | 9 (50) | 2 | 23 642 | 299 (1.3) | 4286 (18.1) | 507 (2.1) | 37 (1.5) |
| Southern | 936 066 | 10 | 8 (80) | 0 | 10 682 | 84 (0.8) | 1863 (17.4) | 299 (2.8) | 21 (1.9) |
| Western | 1 006 202 | 10 | 7 (70) | 2 | 13 822 | 254 (1.8) | 1760 (12.7) | 225 (1.6) | 20 (1.4) |
| Central | 673 364 | 7 | 8 (100) | 0 | 8272 | 78 (0.9) | 1376 (16.6) | 152 (1.8) | 11 (1.3) |
| Northern | 465 619 | 5 | 9 (100) | 6 | 5443 | 574 (10.6) | 862 (15.8) | 147 (2.7) | 7 (1.3) |
Emergency obstetric care defined by the provision of all nine WHO signal functions.
Five institutions per 500 000.
Basic obstetric care defined as care for normal, uncomplicated deliveries.
Deliveries at basic obstetric care institutions, unplanned deliveries outside institution and planned home deliveries.
Maternal morbidity included the following: maternal intensive care, eclampsia, puerperal sepsis and sepsis during delivery, thromboembolism and haemorrhage ≥1500 ml or blood transfusion.
Intrapartum death and neonatal death before 24 hours per 1000 births (both live and stillborn).
Discussion
Main findings
The risk of unplanned delivery outside an institution has doubled in Norway over the last 30 years and the risk of maternal morbidity increased from 2000 to 2009. These changes coincided with an increasing proportion of women of fertile age living further away from obstetric institutions, and with a reduction in the number of emergency obstetric care institutions to a level below the estimated need.
Strengths and weaknesses
We used population-based registry and census data, and combined various methods and data sources in order to provide a more comprehensive description of the health system during the study period. The MBRN database permitted a long observation period and the large samples necessary to study rare events. We show that the addition of geographical tools to traditional epidemiology can be useful for service evaluation as well as planning.
However, our study had some limitations. Travel zone calculations were based on standardised conditions and may underestimate actual travel time. Further, planned home deliveries were not registered separately in the MBRN before 1999, and the risk increase for unplanned delivery outside institutions may be underestimated. Planned home deliveries were rare in the reference period (1979–83) and constituted 0.037% (20/54492) of the deliveries in 1975–6.18 Finally, our definition of maternal morbidity included the main causes of potentially life-threatening complications.15 The increase in maternal morbidity over time may have several explanations, and we could not separate improved diagnosis and reporting from other contributing factors. National guidelines for diagnosis, monitoring and treatment of maternal and fetal complications have been updated regularly since 1995, but lack of adherence has been reported.19–22 Caesarean section also increases the risk of maternal complications both in the actual and subsequent pregnancies.23,24 Within-country variation of caesarean section rates may have an impact on maternal morbidity. The increase in maternal morbidity may also be related to changes in maternal risk factors, rather than reduced timeliness and adequacy of the provided care. Adjustment for maternal diabetes did not change the estimates and was not included in the final regression models. Adjustment for maternal smoking increased the estimates slightly, probably as a result of decreasing frequency of daily smoking. Daily smoking was reported by 24% of pregnant women in 2000, compared with 17% in 2009 (MBRN, http://mfr-nesstar.uib.no/mfr/). We could not adjust for maternal obesity, and ethnicity must be included among the risk factors in future studies.2,23,25,26
Interpretation
Although travel distances in Norway may be longer than in many high-income countries, we complied with international standard definitions and indicator frameworks to aid comparison over time and across settings.14 When analysing the availability of institutions, we considered hourly time categories to be a realistic approach to the Norwegian demographics. In the Netherlands, an estimated travel time exceeding 20 minutes was associated with increased risk of adverse neonatal outcome in home deliveries with subsequent hospital transfer.11 Compared with a recent study from the USA, the proportion of women who lived outside the 1-hour travel zone was three times higher in Norway when including all obstetric institutions, and almost twice as high when including only emergency obstetric care institutions.27 A higher proportion of Native American women (18.8%) lived outside the 1-hour drive to a perinatal centre.27 Similarly, we found higher proportions of women (35–72%) who lived outside the 1-hour zone to emergency obstetric care institutions in the Northern region. This region covers the main Sami cultural and economic areas in Norway.28 Neither Statistics Norway nor the MBRN register the indigenous identity of Sami women. Consequently, it was not possible to assess the availability for this group in particular.
The risk of unplanned delivery outside institutions increased in both urban and rural counties in our study. The risk in Norway during the period 2004–09 was higher than the previously reported 0.1% of births in national data from Finland.29 In our study, the risk was 0.3–0.5% in the three most urban counties; this was lower than the 0.6% reported from an urban area in Scotland.30 However, the risk more than doubled in all three counties from 1979–83 to 2004–09. Mechanisms may differ between locations and involve factors such as geographical distance and traffic constraints, as well as admission criteria in large, busy obstetric departments.
The 2.2% incidence of maternal morbidity in our study was higher than previous reports from Norway and Europe. The Mothers Mortality and Severe Morbidity Survey B (MOMS-B) reported a Norwegian incidence rate of 0.86% for severe maternal morbidity based on data collected from the capital county, Oslo, during 1995. European rates ranged from 0.6 to 1.5%, and the MOMS-B studies did not include thromboembolism.31 The incidence of severe maternal morbidity was 0.71% in a prospective Dutch study which applied a stricter definition of severe maternal morbidity.2,15 The wider case definition in our study was also reflected by a morbidity/mortality ratio of 266 : 1; other studies have reported ratios of 118 : 1 and 49 : 1.32,33 Although the wider definition influenced the reported rates, the definitions and report form were similar throughout the period, thus allowing for the evaluation of change over time as well as regional differences.
Our study focused on institution numbers and not on institution size. However, we observed a reduction in the number of small institutions and an increasing proportion of the deliveries took place in the largest institutions. In France, small institutions had a higher frequency of inadequate/inappropriate management of severe post-partum haemorrhage.34 A recent study from the USA reported increased risk of maternal complications in the institutions with the lowest volumes, which apparently also included non-obstetric institutions.35
Conclusions
The findings in the current study indicated reduced quality from the health system perspective, as demonstrated by a reduced availability of institutions and an increased risk of unplanned delivery outside institutions. The WHO indicators were secondary outcomes in our study. However, they were useful in the Norwegian high-income context and the indicator assessment pointed to the emerging inequalities described in the cross-sectional and cohort analyses. Availability and access must be considered to a larger extent in service planning and evaluation, and structural issues, such as the risk factors for unplanned delivery outside institutions in urban and rural areas, need to be addressed.
We would expect the risk of morbidity to be unchanged or reduced following centralisation. The maternal mortality and delivery-related perinatal mortality were low and indicated good quality of clinical care in the institutions. Nevertheless, we reported an increase in the risk of maternal morbidity and increasing regional differences in such risk. We do not believe that our findings can be fully explained by differences in diagnoses, reporting practices or increases in risk factors where information was lacking. More knowledge is needed to understand the interaction between structural factors and clinical outcomes. A comprehensive analysis of neonatal mortality and morbidity was beyond the scope of this study, but must be included when drawing the final conclusions on quality in obstetric care. Further research should aim to inform the debate concerning the distribution of benefits and burden in the centralisation of obstetric care. Whether mothers pay the price for efforts to improve neonatal outcome remains to be answered.
Disclosure of interests
None.
Contribution to authorship
HME and OFN outlined the initial idea. HME, N-HM, OFN and KK contributed to the definition of the research question and to the development of the final study design. HME and KK performed the statistical analyses. HME drafted the manuscript, tables and figures. HME, NHM, OFN and KK participated in the interpretation of the results, draft revision and approved the final version of the manuscript.
Details of ethics approval
The regional ethical council, REK-Vest, approved the study and granted exemption from the principle of individual consent (ID 2010/3243/R).
Funding
The cost of the cross-sectional analyses and the initial planning was funded by a Western Regional Health Authority research grant for the project, ‘Priorities across clinical specialties’ (project nr 240046), led by OFN. The funders had no role in the study design, data collection, data interpretation or writing of the report.
Acknowledgments
We thank the reviewers for valuable comments and advice. Bjørn Thorsdalen (Statistics Norway and subsequently the Norwegian Institute of Public Health) introduced the GIS method for the study group and participated in the planning of the cross-sectional studies. Truc Ngyen Trung (Department of Global Public Health and Primary Care) assisted us with the development of the figures.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Travel time to all obstetric institutions.
Travel time to emergency obstetric care institutions.
References
- 1.BiEC Group. Perinatal and maternal outcomes by planned place of birth for healthy women with low risk pregnancies: the Birthplace in England national prospective cohort study. BMJ. 2011;343:d7400. doi: 10.1136/bmj.d7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zwart JJ, Richters JM, Ory F, de Vries JI, Bloemenkamp KW, van Roosmalen J. Severe maternal morbidity during pregnancy, delivery and puerperium in the Netherlands: a nationwide population-based study of 371,000 pregnancies. BJOG. 2008;115:842–50. doi: 10.1111/j.1471-0528.2008.01713.x. [DOI] [PubMed] [Google Scholar]
- 3.Christiaens W, Bracke P. Place of birth and satisfaction with childbirth in Belgium and the Netherlands. Midwifery. 2009;25:e11–9. doi: 10.1016/j.midw.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Schroeder E, Petrou S, Patel N, Hollowell J, Puddicombe D, Redshaw M, et al. Cost effectiveness of alternative planned places of birth in women at low risk of complications: evidence from the Birthplace in England national prospective cohort study. BMJ. 2012;344:e2292. doi: 10.1136/bmj.e2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benoit C, Zadoroznyj M, Hallgrimsdottir H, Treloar A, Taylor K. Medical dominance and neoliberalisation in maternal care provision: the evidence from Canada and Australia. Soc Sci Med. 2010;71:475–81. doi: 10.1016/j.socscimed.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137:511–20. doi: 10.7326/0003-4819-137-6-200209170-00012. [DOI] [PubMed] [Google Scholar]
- 7.Urbach DR, Baxter NN. Does it matter what a hospital is “high volume” for? Specificity of hospital volume–outcome associations for surgical procedures: analysis of administrative data. BMJ. 2004;328:737–40. doi: 10.1136/bmj.38030.642963.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Billingsley KG, Morris AM, Dominitz JA, Matthews B, Dobie S, Barlow W, et al. Surgeon and hospital characteristics as predictors of major adverse outcomes following colon cancer surgery: understanding the volume–outcome relationship. Arch Surg. 2007;142:23–31. doi: 10.1001/archsurg.142.1.23. discussion 2. [DOI] [PubMed] [Google Scholar]
- 9.Tracy SK, Sullivan E, Dahlen H, Black D, Wang YA, Tracy MB. Does size matter? A population-based study of birth in lower volume maternity hospitals for low risk women. BJOG. 2006;113:86–96. doi: 10.1111/j.1471-0528.2005.00794.x. [DOI] [PubMed] [Google Scholar]
- 10.Moster D, Lie RT, Markestad T. Neonatal mortality rates in communities with small maternity units compared with those having larger maternity units. BJOG. 2001;108:904–9. doi: 10.1111/j.1471-0528.2001.00207.x. [DOI] [PubMed] [Google Scholar]
- 11.Ravelli AC, Jager KJ, de Groot MH, Erwich JJ, Rijninks-van Driel GC, Tromp M, et al. Travel time from home to hospital and adverse perinatal outcomes in women at term in the Netherlands. BJOG. 2011;118:457–65. doi: 10.1111/j.1471-0528.2010.02816.x. [DOI] [PubMed] [Google Scholar]
- 12.Dummer TJ, Parker L. Hospital accessibility and infant death risk. Arch Dis Child. 2004;89:232–4. doi: 10.1136/adc.2003.030924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paxton A, Bailey P, Lobis S, Fry D. Global patterns in availability of emergency obstetric care. Int J Gynaecol Obstet. 2006;93:300–7. doi: 10.1016/j.ijgo.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 14.WHO, UNFPA, UNICEF, AMDD. Monitoring Emergency Obstetric Care – A Handbook. Geneva: WHO; 2009. [Google Scholar]
- 15.Say L, Souza JP, Pattinson RC. Maternal near miss – towards a standard tool for monitoring quality of maternal health care. Best Pract Res Clin Obstet Gynaecol. 2009;23:287–96. doi: 10.1016/j.bpobgyn.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 16.White paper nr 12. En gledelig begivenhet. Oslo Ministry of Health and Care Services. 2008.
- 17.Elveg – the electronic road database. 2009. www.statkart.no/nor/Land/Fagomrader/Vegdatasamarbeidet/Elektronisk_vegnett/ ]. Accessed 9 July 2012.
- 18.Bakketeig LS, Bergsjo P. Birth during transportation in Norway. An analysis of births outside an institution from 1 February 1975 to 31 January 1976. Tidsskr Nor Laegeforen. 1977;97:923–70. [PubMed] [Google Scholar]
- 19.Bjørge L, Henriksen T, Øian P, Hordnes K, Sand S. Guidelines for obstetric care. In: Bjørge L, Henriksen T, Øian P, Hordnes K, Sand S, editors. The Norwegian Society for Obstetrics and Gynecology. Oslo: The Norwegian Medical Association; 2008. [Google Scholar]
- 20.Andersgaard AB, Herbst A, Johansen M, Ivarsson A, Ingemarsson I, Langhoff-Roos J, et al. Eclampsia in Scandinavia: incidence, substandard care, and potentially preventable cases. Acta Obstet Gynecol Scand. 2006;85:929–36. doi: 10.1080/00016340600607149. [DOI] [PubMed] [Google Scholar]
- 21.Winter C, Macfarlane A, Deneux-Tharaux C, Zhang WH, Alexander S, Brocklehurst P, et al. Variations in policies for management of the third stage of labour and the immediate management of postpartum haemorrhage in Europe. BJOG. 2007;114:845–54. doi: 10.1111/j.1471-0528.2007.01377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bjornerem A, Acharya G, Oian P, Maltau JM. Postpartum hemorrhage – prophylaxis and treatment in Norway. Tidsskr Nor Laegeforen. 2002;122:2536–7. [PubMed] [Google Scholar]
- 23.Al-Zirqi I, Vangen S, Forsen L, Stray-Pedersen B. Prevalence and risk factors of severe obstetric haemorrhage. BJOG. 2008;115:1265–72. doi: 10.1111/j.1471-0528.2008.01859.x. [DOI] [PubMed] [Google Scholar]
- 24.Al-Zirqi I, Stray-Pedersen B, Forsen L, Vangen S. Uterine rupture after previous caesarean section. BJOG. 2010;117:809–20. doi: 10.1111/j.1471-0528.2010.02533.x. [DOI] [PubMed] [Google Scholar]
- 25.Philibert M, Deneux-Tharaux C, Bouvier-Colle MH. Can excess maternal mortality among women of foreign nationality be explained by suboptimal obstetric care? BJOG. 2008;115:1411–8. doi: 10.1111/j.1471-0528.2008.01860.x. [DOI] [PubMed] [Google Scholar]
- 26.Knight M, Kurinczuk JJ, Spark P, Brocklehurst P. Inequalities in maternal health: national cohort study of ethnic variation in severe maternal morbidities. BMJ. 2009;338:b542. doi: 10.1136/bmj.b542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rayburn WF, Richards ME, Elwell EC. Drive times to hospitals with perinatal care in the United States. Obstet Gynecol. 2012;119:611–6. doi: 10.1097/AOG.0b013e318242b4cb. [DOI] [PubMed] [Google Scholar]
- 28.Sami statistics Oslo, Kongsvinger Statistics Norway. 2010. Report No.: ISBN 978-82-537-7742-9 Elektronisk versjon.
- 29.Viisainen K, Gissler M, Hartikainen AL, Hemminki E. Accidental out-of-hospital births in Finland: incidence and geographical distribution 1963–1995. Acta Obstet Gynecol Scand. 1999;78:372–8. [PubMed] [Google Scholar]
- 30.Rodie VA, Thomson AJ, Norman JE. Accidental out-of-hospital deliveries: an obstetric and neonatal case control study. Acta Obstet Gynecol Scand. 2002;81:50–4. doi: 10.1046/j.0001-6349.2001.00420.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang WH, Alexander S, Bouvier-Colle MH, Macfarlane A. Incidence of severe pre-eclampsia, postpartum haemorrhage and sepsis as a surrogate marker for severe maternal morbidity in a European population-based study: the MOMS-B survey. BJOG. 2005;112:89–96. doi: 10.1111/j.1471-0528.2004.00303.x. [DOI] [PubMed] [Google Scholar]
- 32.Waterstone M, Bewley S, Wolfe C. Incidence and predictors of severe obstetric morbidity: case–control study. BMJ. 2001;322:1089–93. doi: 10.1136/bmj.322.7294.1089. discussion 93–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brace V, Penney G, Hall M. Quantifying severe maternal morbidity: a Scottish population study. BJOG. 2004;111:481–4. doi: 10.1111/j.1471-0528.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- 34.Bouvier-Colle MH, Ould El Joud D, Varnoux N, Goffinet F, Alexander S, Bayoumeu F, et al. Evaluation of the quality of care for severe obstetrical haemorrhage in three French regions. BJOG. 2001;108:898–903. doi: 10.1111/j.1471-0528.2001.00224.x. [DOI] [PubMed] [Google Scholar]
- 35.Kyser KL, Lu X, Santillan DA, Santillan MK, Hunter SK, Cahill AG, et al. The association between hospital obstetrical volume and maternal postpartum complications. Am J Obstet Gynecol. 2012;207:42e1–17. doi: 10.1016/j.ajog.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Travel time to all obstetric institutions.
Travel time to emergency obstetric care institutions.


