Abstract
The INHAND (International Harmonization of Nomenclature and Diagnostic Criteria for Lesions in Rats and Mice) Project (www.toxpath.org/inhand.asp) is a joint initiative of the Societies of Toxicological Pathology from Europe (ESTP), Great Britain (BSTP), Japan (JSTP) and North America (STP) to develop an internationally accepted nomenclature for proliferative and nonproliferative lesions in laboratory animals. The purpose of this publication is to provide a standardized nomenclature for classifying microscopic lesions observed in the female reproductive tract of laboratory rats and mice, with color photomicrographs illustrating examples of some lesions. The standardized nomenclature presented in this document is also available electronically on the internet (http://www.goreni.org/). Sources of material included histopathology databases from government, academia, and industrial laboratories throughout the world. Content includes spontaneous and aging lesions as well as lesions induced by exposure to test materials. There is also a section on normal cyclical changes observed in the ovary, uterus, cervix and vagina to compare normal physiological changes with pathological lesions. A widely accepted and utilized international harmonization of nomenclature for female reproductive tract lesions in laboratory animals will decrease confusion among regulatory and scientific research organizations in different countries and provide a common language to increase and enrich international exchanges of information among toxicologists and pathologists.
Keywords: diagnostic pathology, nomenclature, female reproductive, ovary, uterus, cervix, vagina
Introduction
The INHAND Project (www.toxpath.org/inhand.asp) is collaboration between the Societies of Toxicologic Pathology from Europe (ESTP), Great Britain (BSTP), Japan (JSTP) and North America (STP) to develop internationally accepted nomenclature for proliferative and nonproliferative lesions in laboratory animals. The purpose of this publication is to provide a standardized nomenclature for classifying proliferative and nonproliferative lesions observed in the female reproductive tract of laboratory rats and mice. Standardized nomenclature of proliferative (39Dixon et al. 1999) female reproductive tract lesions in rats was previously published by the STP. The standardized nomenclature of proliferative female reproductive tract lesions presented in this document is also available electronically at the goRENI website on the internet (www.goreni.org).
In this document, the female reproductive tract is divided into the ovary, uterus and oviduct, cervix and vagina and non-proliferative and proliferative lesions are described for each organ. This document contains spontaneous and aging lesions as well as lesions induced by exposure to test materials. Because many lesions are often evaluated in the cycling female rodent, there is a section on normal cyclical changes observed in the ovary, uterus, cervix and vagina to compare normal physiological changes with pathological lesions.
Morphology
I. Normal Cyclical Changes in the Female Reproductive System of Rodents
The duration of the estrous cycle of rodent strains most commonly used in toxicology studies is typically 4 to 5 days (57Goldman et al. 2007). Even within one strain of rat or mouse, though, the length of the estrous cycle may vary between individuals.
The rodent cycle is subdivided in four subsequent phases, proestrus, estrus, metestrus and diestrus (preferred terms). Synonyms for these preferred terms, in the literature, are proestrus, estrus, early diestrus/diestrus 1 and diestrus 2. While the metestrus phase is sometimes referred to as diestrus 1, we recommend the use of the term metestrus. For each phase of the cycle, the ovary, uterus and vagina have a typical morphologic appearance that can be used to determine the stage of the cycle by light microscopy; however, it is important to remember that the phases are on a continuum, and thus the morphology of each organ may vary slightly from the prototypical appearance described herein, particularly in mice. In these cases the later stage of the cycle is assigned. Examination of vaginal smears (cytology) and/or vaginal histology are commonly used methods for identification of the stage of the rodent cycle and can be used as evidence of cyclicity. Importantly, however, microscopic examination of the vagina alone is generally not adequate for assessment of potential perturbations the female reproductive tract in toxicity studies. At a minimum, histopathologic examination of the ovary, uterus and vagina should be conducted not only to determine the stage of the cycle, but also to assess cyclicity and identify and interpret potential perturbations of the cycle. Evaluation of the mammary gland and pituitary gland can also aid in characterizing perturbations of the estrous cycle. Recording of the stage of the cycle for routine screening toxicity studies is not necessary; however, it is important that the pathologist evaluate the female reproductive tract tissues with an awareness of normal cyclicity and understanding of the morphologic features consistent with each phase so that alterations from normal can be detected. If alterations are detected, it is recommended that morphologic diagnoses be used to detail the spectrum of changes present in each of the organs of the female reproductive system, as estrous cycle stages are not suitable standalone morphologic diagnoses. In studies where recording of the stage of the cycle is deemed necessary, it is recommended that the stage of the estrous cycle along with any specific morphologic diagnoses be recorded for all animals being evaluated when possible, recognizing that perturbations of the estrous cycle often make it difficult or inappropriate to assign a ‘stage’ of the cycle to these animals. In these situations, a term such as ‘unable to determine stage’ or ‘indeterminate stage’ can be used, but the morphologic alterations that are apparent in the reproductive organs should be recorded in the individual organs. Interpretation of these changes can be described further in the pathology report.
A. Cyclical changes in the ovary of rodents
The structure and appearance of the ovary changes during the estrous cycle in response to cyclical changes in pituitary and ovarian hormones. In particular, morphological changes in the tertiary follicles and corpora lutea (CL) are synchronized during the cycle (208Westwood 2008; 214Yuan and Foley 2002). Therefore, knowledge of the physiology of the estrous cycle and understanding of each synchronized combination makes it possible to classify the ovary into one of 4 estrous cycle stages based on morphology (208Westwood 2008; 210Yoshida et al. 2009). Follicles can be classified as primordial (oocyte surrounded by a single layer of flattened pregranulosa cells and has an outer basal lamina), growing or atretic. Growing follicles are further described by morphologic appearance as primary (single layer of cuboidal to columnar granulosa cells surrounding the oocyte), secondary (2 or more layers of granulosa cells, a theca cell layer and a zona pellucida between oocyte and granulosa cells), vesicular (secondary follicles with fluid-filled spaces that have not coalesced into a single antrum), and tertiary (antrum and cumulous oophorus [granulosa cells surrounding oocyte] that forms a stalk extending into the antral cavity) (201Vidal et al. 2013; 214Yuan and Foley 2002). This terminology is considered adequate for evaluation of toxicology studies and is preferred. Late tertiary follicles are preovulatory (synonym Graafian) and in these, the cumulus oophorus has broken down and the oocyte and corona radiata are no longer attached to the wall of the follicle. Additional systems for classifying follicles as small, medium, or large using quantitative criteria have been developed for both the mouse and rat (77Hirshfield and Midgley 1978; 153Pedersen and Peters 1968) but because these classifications are quantitative they are less useful for the screening evaluation performed in routine toxicity studies.
Primordial and growing follicles through the vesicular stage are observed in all stages of the estrous cycle and are not useful for determining the stage of the estrous cycle. Healthy (non-atretic) late tertiary follicles are generally present only during proestrus and are useful for staging.
With the exception of late tertiary follicles at proestrus, morphologic changes in the CL are the most useful ovarian morphologic characteristics to help determine the stage of the estrous cycle. In the rodent ovary, the beginning of each cycle occurs with ovulation and the formation of a new generation of CL. CL are classified into new CL resulting from the most recent ovulation, (1-5 days old); recent CL, which are those resulting from ovulations within several cycles prior to the current cycle (approximately 5-20 days old); and old CL, which are older than approximately 4 cycles ago (≥ 21 days old) and have not yet undergone complete regression. New CL resulting from the most recent ovulation are further classified based on hematoxylin and eosin (H&E) staining characteristics as basophilic and eosinophilic. Basophilic CL are observed at estrus, metestrus and early diestrus. Immediately upon ovulation (estrus) basophilic CL are composed of small, ovoid, basophilic luteal cells with sparse cytoplasm. Initially, basophilic CL have a dark central area of non-luteinized granulosa cells, though as luteinization proceeds, a central fluid-filled cavity may develop. During metestrus, the basophilic CL become larger and stain less intensely basophilic. The luteal cells become larger, rounder, and slightly vacuolated; the centers become filled with luteal cells although CL with incomplete centers can still be found. During diestrus, the basophilic CL reaches its largest size; the CL is composed of large, polygonal, lightly basophilic, finely vacuolated luteal cells, usually with completely filled centers. There is no luteolysis. At proestrus, the basophilic CL start to become eosinophilic. In early proestrus, areas of the basophilic CL can be seen turning eosinophilic, but basophilic areas are still present. As proestrus proceeds, the CL formed from the most recent ovulation become completely eosinophilic. In late proestrus, luteolysis (characterized by apoptosis of individual cells) and/or vacuolation can be observed in these eosinophilic CL from the most recent ovulation. Thus, by late proestrus, completely basophilic CL are generally not observed in the ovary of a normally cycling rodent.
Recent CL (those CL within approximately 1-4 cycles of the last or current ovulation) are also eosinophilic. However, they have subtle morphologic differences from the eosinophilic CL of current ovulations. Eosinophilic CL from recent cycles show decreased luteal cell vacuolation and increased fibroblast infiltration compared to those from the current ovulations. These differences are sometimes difficult to discern. Old CL (>4 cycles old) are relatively easy to discern. They have a more central position in the ovary, are small and composed of less intensely staining eosinophilic luteal cells separated by fibrous tissue. They are evidence of previous ovulation.
Proestrus (Figures 1 and 2)
Figure 1.
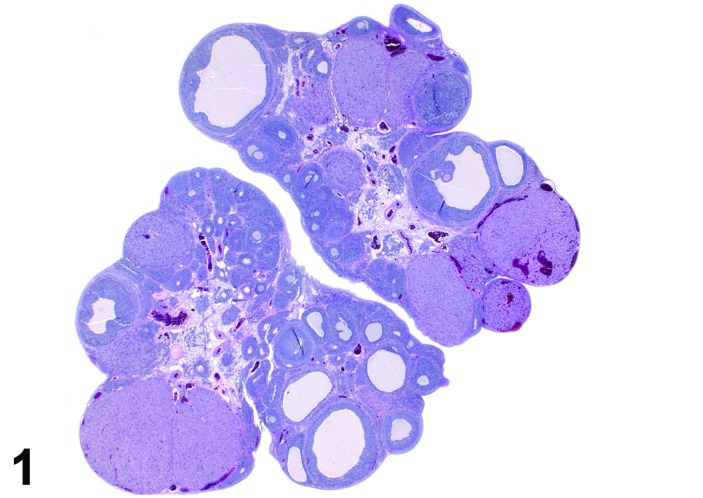
Proestrus Ovary with tertiary (Graafian) Follicles, rat.
Figure 2.
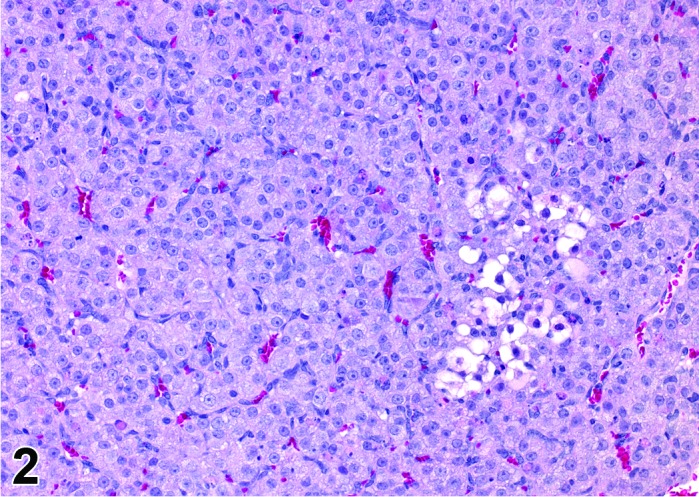
Proestrus CL, rat.
At lower magnification, late tertiary follicles are the easiest component to identify at proestrus.
Follicles: Large tertiary follicles are evident at this stage and are located near the surface of the ovary. Their granulosa cells are cuboidal and/or polygonal. Many of the antral tertiary follicles may be atretic. Although discrimination of healthy tertiary follicles from those undergoing early atresia is sometimes difficult at lower magnification, apoptotic granulosa cells are recognized in the atretic follicles. Following the peak in estrogen, the luteinizing hormone (LH) surge occurs and estrogen levels subsequently decline as the late tertiary follicle begins to produce progesterone in preparation for ovulation.
CL: CL from the last ovulation are large and the staining characteristics start changing from basophilic to eosinophilic; by the end of proestrus, the CL are completely eosinophilic. Degenerative processes in these CL are characterized by cytoplasmic vacuolation or apoptosis in the luteal cells. Occasionally large areas of necrosis and/or mononuclear cell infiltrates are present in these CL. Fibrous tissue proliferation is noted in the eosinophilic CL from previous cycles.
Estrus (Figures 3 and 4)
Figure 3.
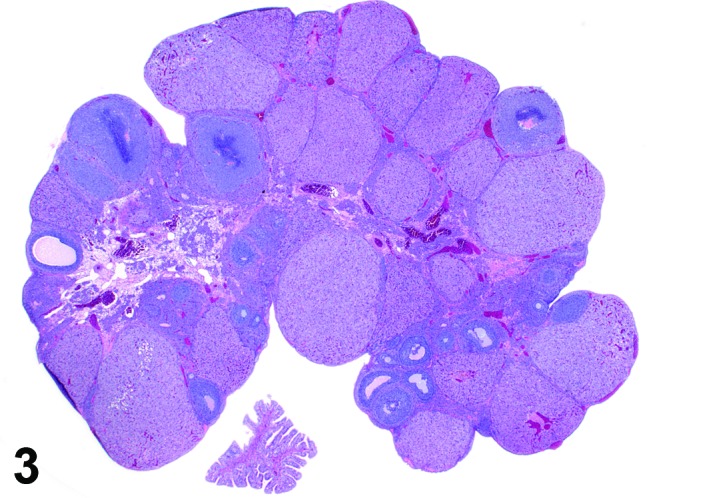
Estrus ovary, rat.
Figure 4.
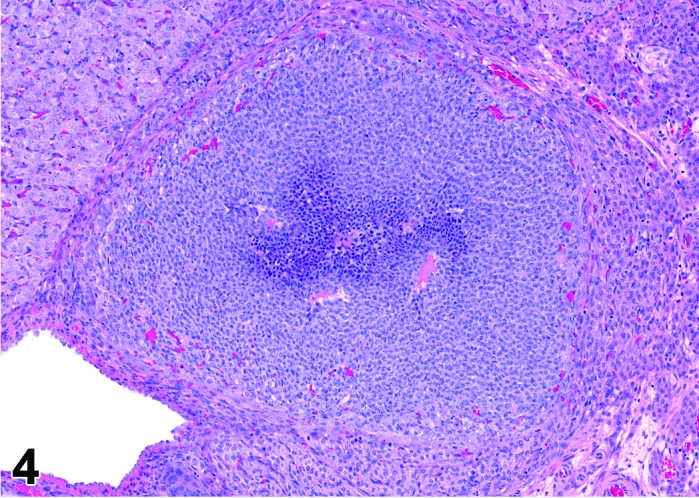
Estrus new CL, rat.
At lower magnification, newly formed basophilic CL are a feature of this stage.
Follicles: There is an absence of healthy (non-atretic) tertiary follicles. A number of smaller vesicular follicles start to grow.
CL: New basophilic CL, formed after the current ovulation, are characteristically observed at this stage. They are composed of basophilic, small, spindle-shaped luteal cells that closely resemble granulosa cells. The presence of newly forming blood vessels (angiogenesis) makes the newly formed basophilic CL easily and clearly distinguishable from the large follicles or atretic follicles, in which, blood vessels are absent in the granulosa cell layer. Sometimes the newly formed basophilic CL have central cavities which may or may not completely fill in as the cycle progresses. Eosinophilic CL from the immediately previous cycle (i.e., from the immediately preceding proestrus) are still large but degenerative processes including apoptosis and fibrosis are more advanced.
Metestrus (Figures 5 and 6)
Figure 5.
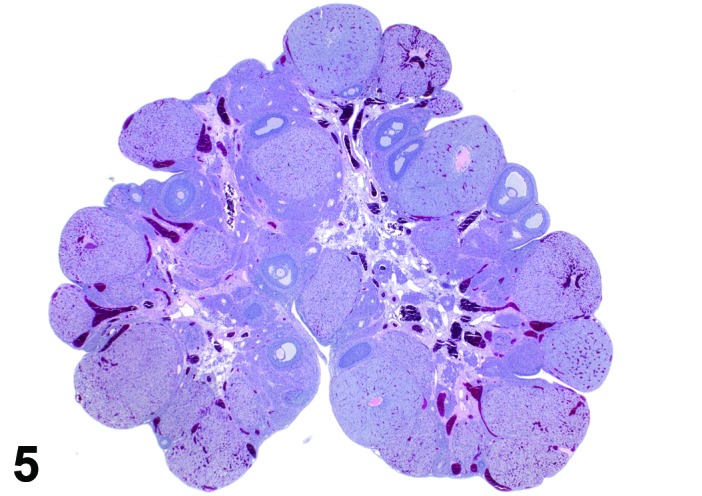
Metestrus ovary, rat.
Figure 6.
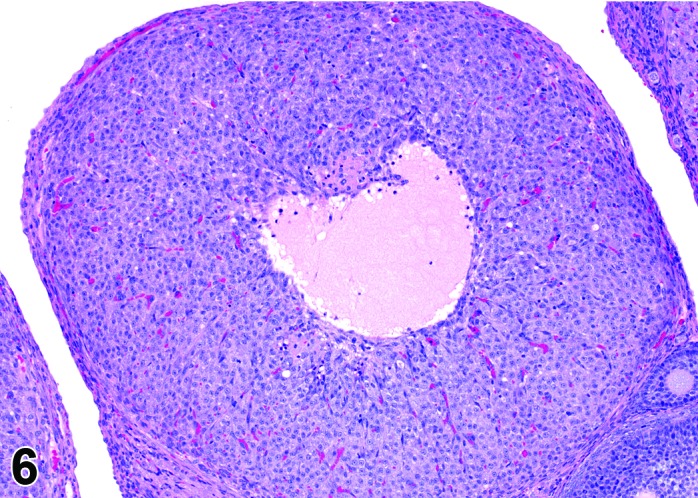
Metestrus CL, rat.
At lower magnification, basophilic CL are easily recognized at metestrus.
Follicles: There are no healthy large late tertiary follicles, but many growing follicles of various types are present.
CL: CL of the current ovulation are characteristically increased in size compared to those at estrus, but still smaller than those at diestrus. The luteal cells still have basophilic cytoplasm with large nuclei. Their nucleoli are prominent. The CL sometimes contain fluid-filled central cavities of various sizes though their incidence is decreased compared to that seen at estrus. CL from the immediately previous cycle demonstrate advanced fibrosis, but their size is still similar to those CL formed during the last ovulation. In rodents, CL produce progesterone at metestrus. Progesterone levels rise briefly during this phase but fall again, in the absence of cervical stimulation, as the CL begin to preferentially produce 20alpha-OH-progesterone and the levels of progesterone decline.
Diestrus (Figures 7 and 8)
Figure 7.
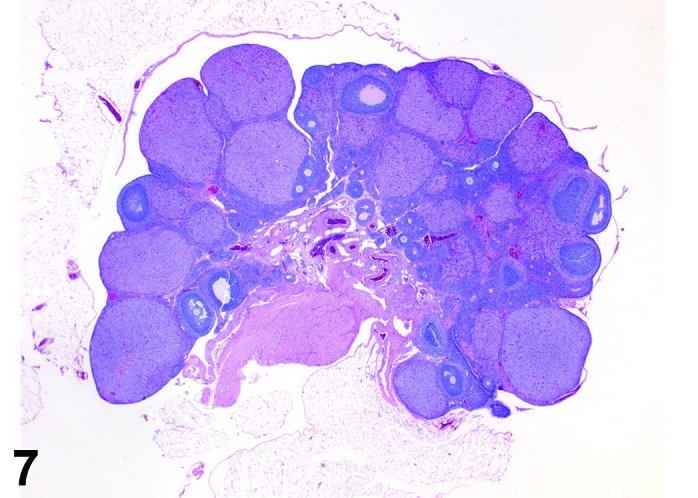
Diestrus Ovary, rat.
Figure 8.
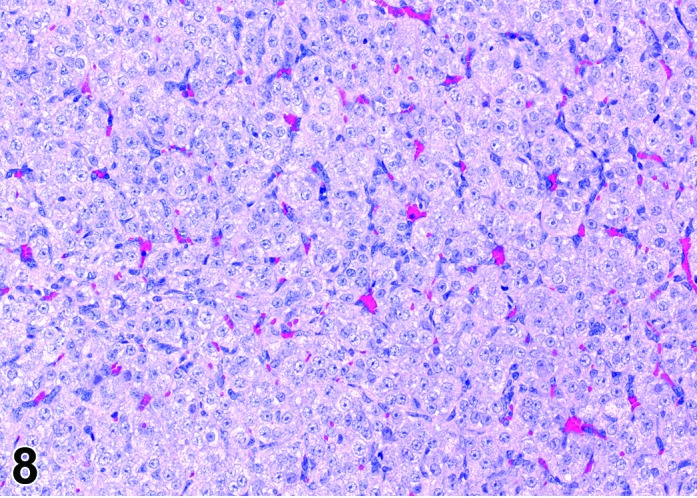
Diestrus CL, rat.
At lower magnification, the CL from the current ovulation are lightly basophilic and have reached maximum size. Tertiary follicles preparing for next ovulation are increased in size.
Follicles: Tertiary follicles are increased in number, but are smaller than those observed at proestrus.
CL: CL of the current ovulation have attained the maximum size. The luteal cells have foamy, slightly basophilic cytoplasm. There is no evidence of luteolysis in these CL. CL from the immediately prior cycle are eosinophilic and vacuolated and fibrous tissue infiltration is advanced.
B. Cyclical changes in the uterus of rodents
Proestrus (Figures 9 and 10)
Figure 9.
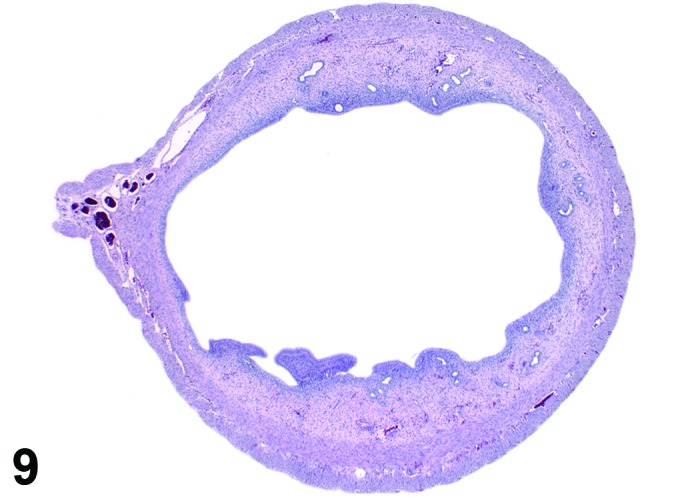
Proestrus Uterus, rat.
Figure 10.
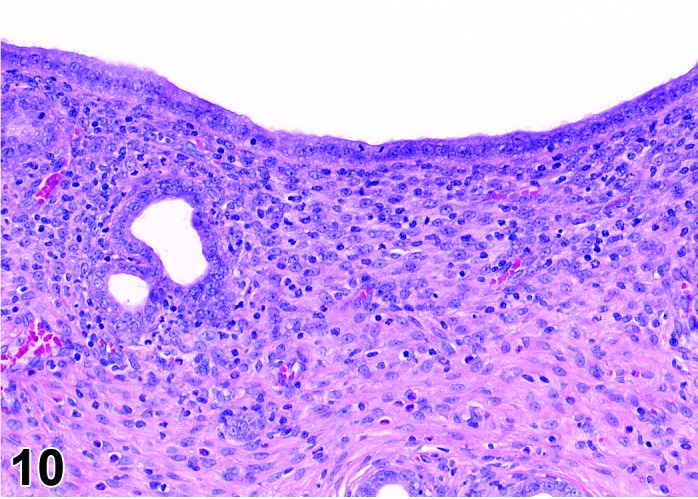
Proestrus Uterus, rat.
During the proestrus phase of the cycle, estradiol from rapidly growing teritiary follicles rises and peaks resulting in significant changes in the rodent uterus. Under the influence of estradiol, the luminal and to a lesser extent the glandular epithelium undergoes hypertrophy. The epithelial cells lining the lumen and glands increase in height from low to more tall columnar cells. Also mitotic activity increases within the epithelium and mitotic figures can be numerous. The stroma can show a more prominent vasculature and early edema. Inflammatory cells start to increase in number and peak at estrus. The lumen becomes markedly dilated and filled with clear fluid towards the end of this phase.
Estrus (Figures 11 and 12)
Figure 11.
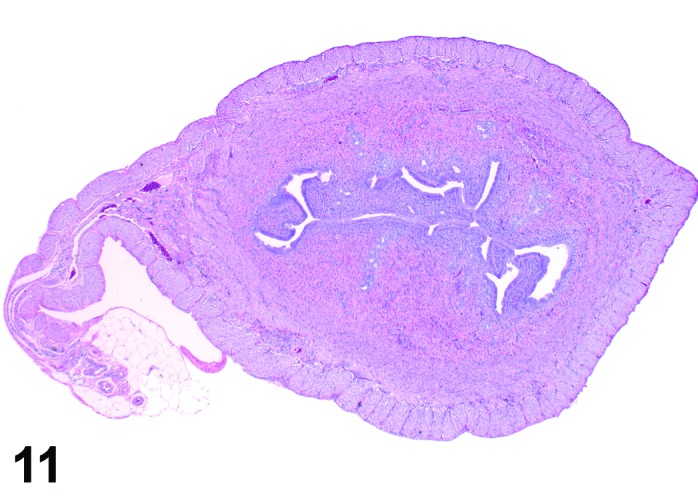
Estrus Uterus, rat.
Figure 12.
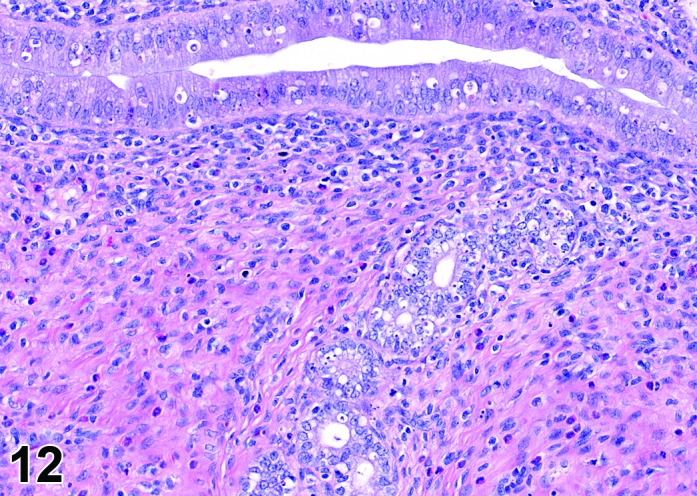
Estrus Uterus, rat.
During the estrus phase (beginning with ovulation), the uterus is morphologically characterized by the appearance of apoptotic epithelial cells. This epithelial cell death starts within the glands but soon also involves the luminal epithelium. Although mitotic figures still can be detected between the apoptotic cells, their number decreases rapidly. In the beginning of this phase, the uterine lumen is dilated, but in late estrus the lumen of the uterine horns returns to its normal shape and volume. The number of inflammatory cells is high during this phase. Circulating progesterone levels (produced by the follicle) fall during estrus.
Metestrus (Figures 13 and 14)
Figure 13.
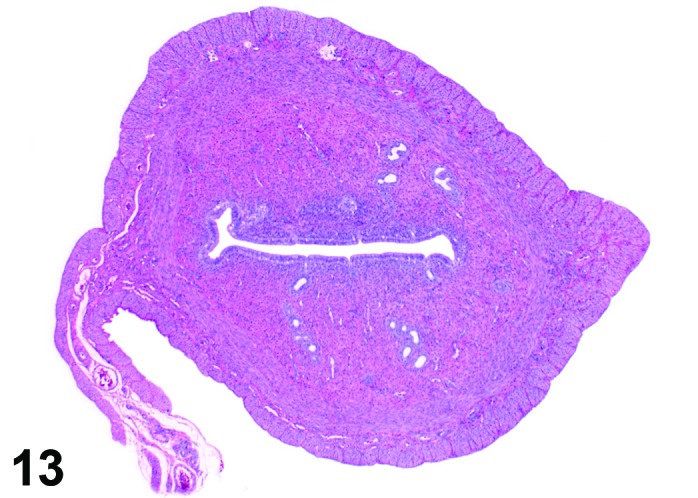
Metestrus Uterus, rat.
Figure 14.
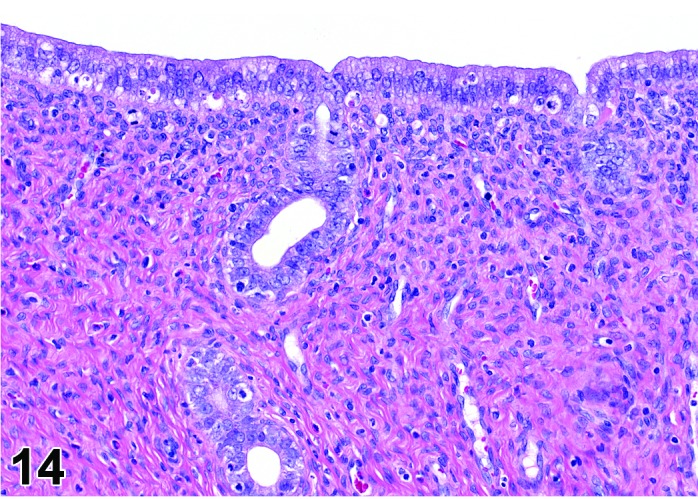
Metestrus Uterus, rat.
As the new corpora lutea develop following ovulation, the metestrus phase of the cycle begins. This phase is characterized by a declining number of apoptotic cells during the first part of this phase with a return to mitotic activity. In general, the epithelial cells that survived or were newly formed are low columnar. The stromal cells in the region underneath the luminal epithelium become slightly more prominent at the end of the metestrus phase with transition to the diestrus phase and a few eosinophils may be present.
Diestrus (Figures 15 and 16)
Figure 15.
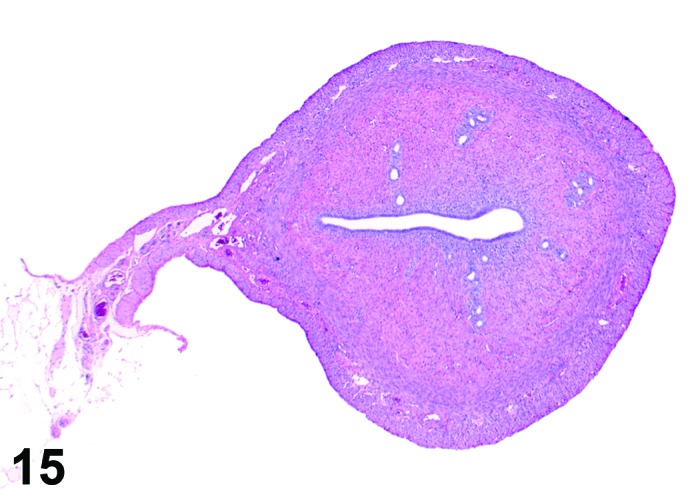
Diestrus Uterus, rat.
Figure 16.
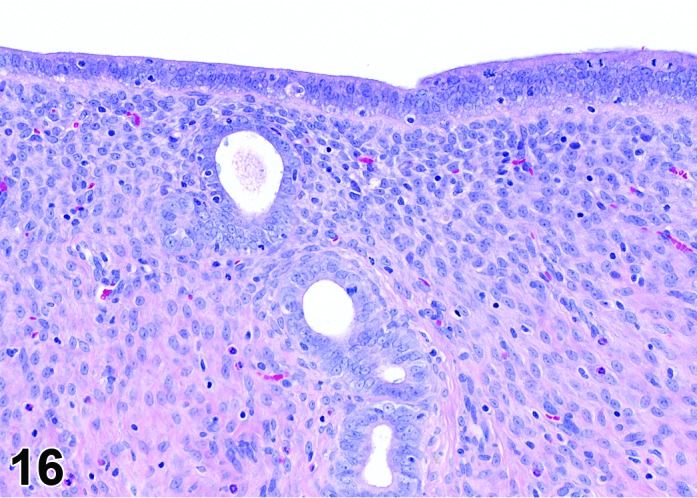
Diestrus Uterus, rat.
During the diestrus phase, in which the elevated progesterone levels return to baseline, the uterus is small and has quite inactive glands that are lined with cuboidal to low columnar epithelial cells. The lumen of the horns is slit-like and can show a saw-tooth appearance. The stroma is compact. Mitotic activity is low because of the low estrogen levels during this phase and a few eosinophils may be present.
C. Cyclical changes in the vagina and cervix of rodents
Proestrus (Figures 17,18,19)
Figure 17.
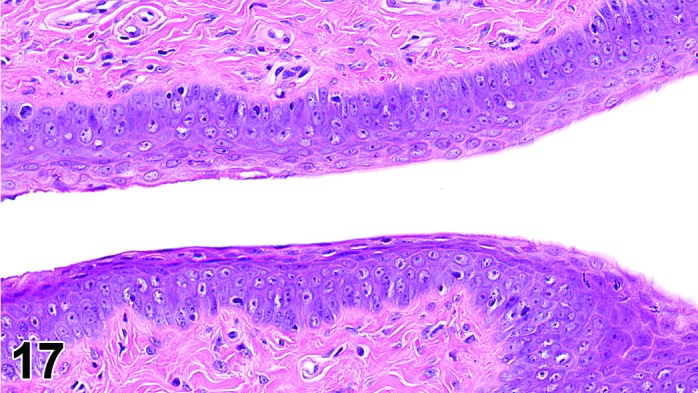
Vagina Early Proestrus, rat.
Figure 18.
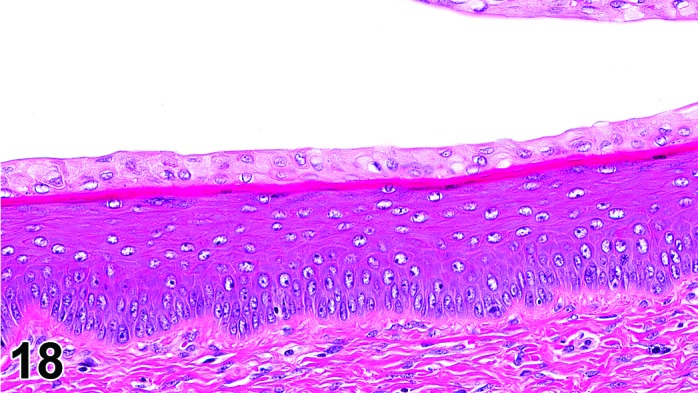
Vagina Mid-Proestrus, rat.
Figure 19.
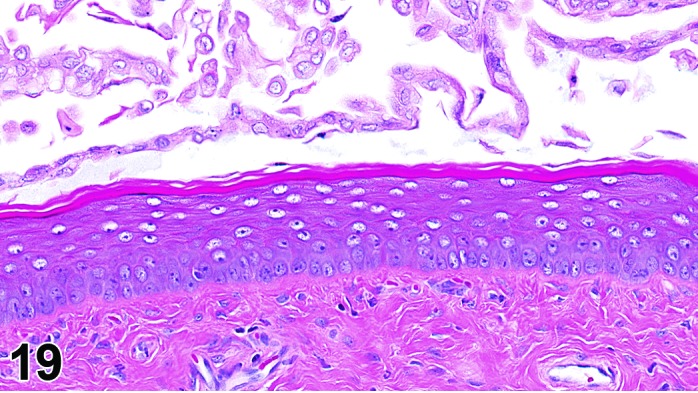
Vagina Late Proestrus, rat.
The beginning of proestrus is defined by the formation of a layer of flattened, keratohyaline rich epithelial cells called the stratum granulosum which overlays the basal epithelium (stratum germinativum). During early proestrus, mitotic figures are present and the superficial mucoid layer (stratum mucification) begins to develop giving the vaginal epithelium 3 distinct layers (basal epithelium, stratum granulosum, and the superficial mucoid layer). As proestrus progresses, a 4th layer begins to form, the stratum corneum, between the stratum granulosum and the mucoid layer. By late proestrus, the fully keratinized stratum corneum results in an intensely eosinophilic band underlying the prominent superficial mucoid layer, which may show signs of desquamation. Only occasional granulocytes are observed during this time.
Estrus (Figures 20)
Figure 20.
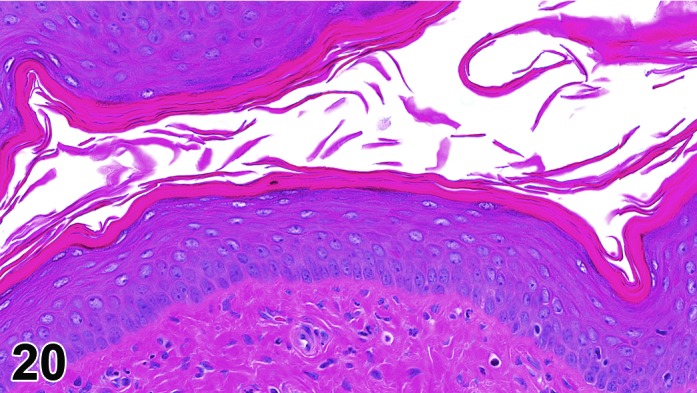
Vagina Estrus, rat.
By early estrus, there is a decrease in the number of mitotic figures and the mucoid layer has sloughed, revealing the now superficial stratum corneum. During estrus there is progressive shedding of the cornified layer with sloughed cornified cells and debris present within the vaginal lumen. There is an increase in neutrophil and possibly eosinophil infiltrations, but numbers may be variable. During late estrus, detachment of the stratum corneum begins.
Metestrus (Figures 21)
Figure 21.
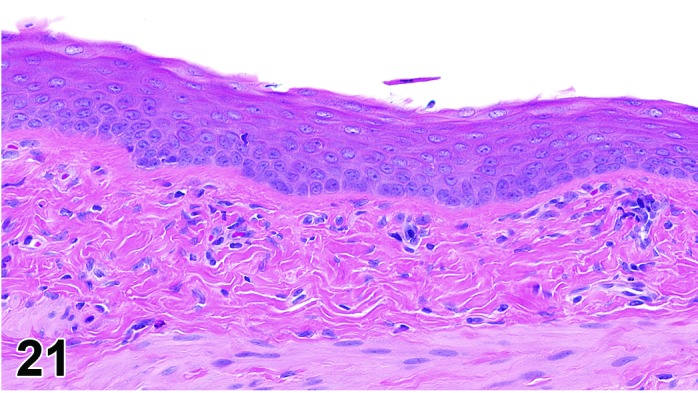
Vagina Metestrus, rat.
The beginning of metestrus is marked by the complete dehiscence of the stratum corneum. Residual squames and debris may be present in the lumen and some cornified epithelium may persist with continued desquamation throughout metestrus. There is progressive loss of the stratum granulosum and the superficial layers of the basal epithelium. There is an increase in the number of granulocytes present.
Diestrus (Figures 22)
Figure 22.
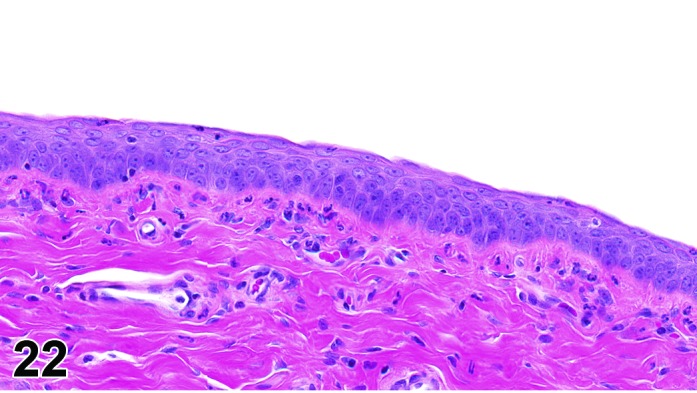
Vagina Diestrus, rat.
At the beginning of diestrus, the vaginal epithelium is at its thinnest point in the cycle and may only be 3-5 cells thick. During diestrus, the vaginal epithelium gradually increases in thickness to 8-10 cells thick and proliferation increases, but without a clear stratum granulosum. There are variable numbers of neutrophils with numbers decreasing as diestrus progresses.
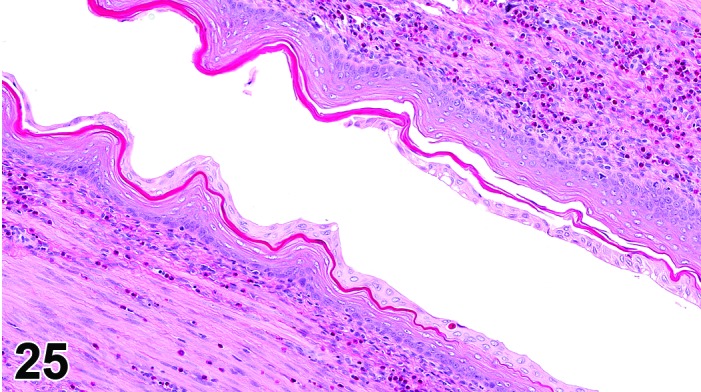
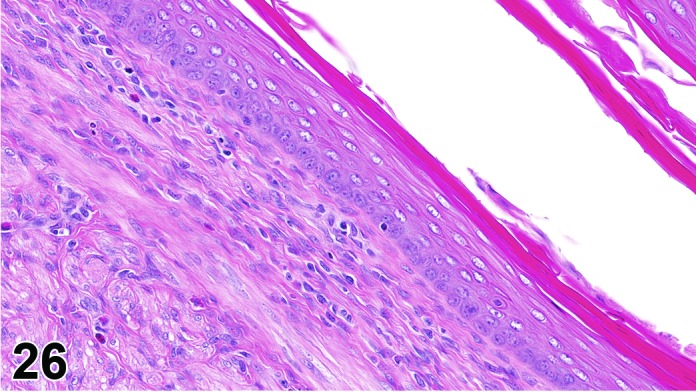
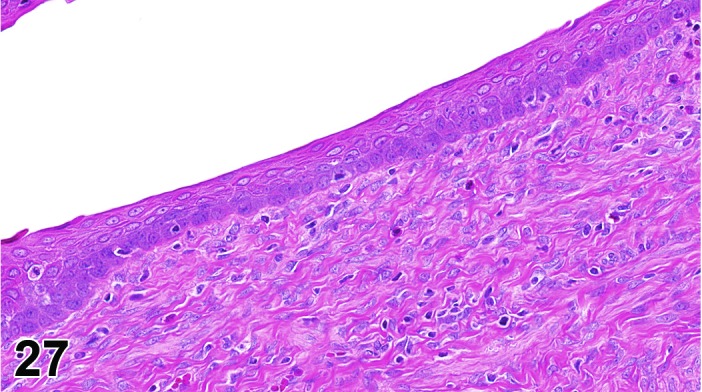
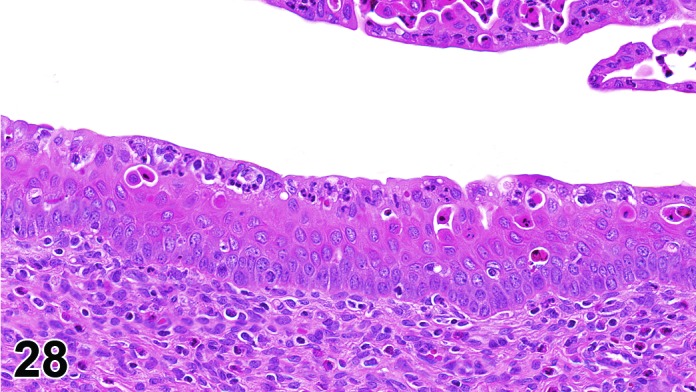
The cervical epithelium (Figures 23,24,25,26,27,28) responds in a similar fashion as the vaginal epithelium, although the magnitude of the response and thickness of the cervical epithelium is typically less than that observed in the vagina. In addition, the changes in the cervix may appear to have a slight time lag when compared to the vagina (i.e., some mucified cells may still be present in the cervix during early estrus, see Fig. 25). The histologic changes in the vagina and cervix of rats and mice are similar; however, the leukocytic infiltration is more prominent in the mouse and during metestrus intraepithelial microabscesses and extension into the lumen can be observed.
Figure 23.
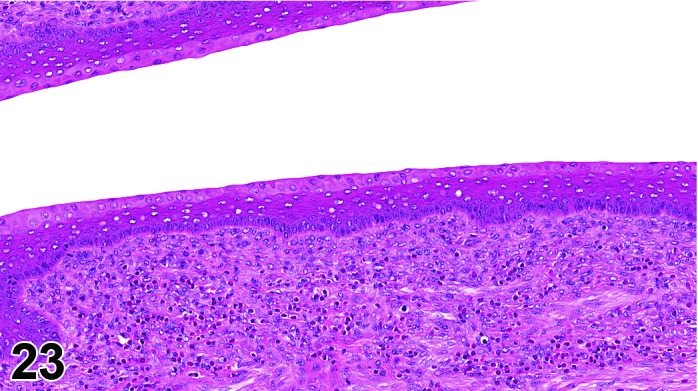
Cervix Proestrus, rat.
Figure 24.
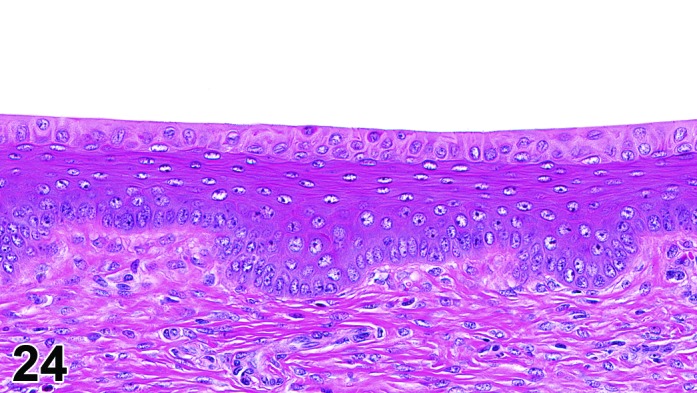
Cervix Proestrus, rat.
Figure 25.
Cervix Early Estrus, rat.
Figure 26.
Cervix Estrus, rat.
Figure 27.
Cervix Metestrus, rat.
Figure 28.
Cervix Diestrus, rat.
D. Postmortem changes in the ovary, uterus, cervix and vagina of rodents
In toxicity studies, the early or unscheduled death of animals may superimpose artifactual changes that hamper histopathologic evaluation. It is important that the pathologist be aware of such artifacts so that they are not misinterpreted as lesions. According to 173Seaman, 1987, the formation of empty spaces around the granulosa cell layer of the follicles is the earliest sign of autolysis in the ovaries. At a room temperature of 72 ± 2° F, the onset of this change is between 30-60 minutes. This is followed by autolytic changes in the corpora lutea and after approximately 4 hours all corpora lutea show separation of the individual luteal cells.
In the uterus, the first postmortem signs become evident after approximately 30 minutes. The first postmortem change is separation of the glandular epithelium from its basement membrane. This is followed by sloughing of the surface epithelium approximately 8 hours later. Postmortem changes in the smooth muscle cells of the myometrium consist of the formation of spaces between the bundles and pyknosis of the nuclei and appear after 4 hours at room temperature (173Seaman 1987). The cervical and vaginal epithelia only show minor autolytic changes over a period of 16 hours (173Seaman 1987).
References
26Corbeil et al. (1985), 39Dixon et al. (1999), 57Goldman et al. (2007), 65Graham (1966), 77Hirshfield and Midgley (1978), 93Kaushic et al. (1998), 113Li and Davis (2007), 153Pedersen and Peters (1968), 160Putti and Varano (1979), 173Seaman (1987), 201Vidal et al. (2013), 203Vrcić et al. (1991), 208Westwood (2008), 210Yoshida et al. (2009), 213Yuan (1987), 214Yuan and Foley (2002)
II. Ovary
A. Nonproliferative Lesions
Amyloid (N) Ovary (Figure 29)
Figure 29.
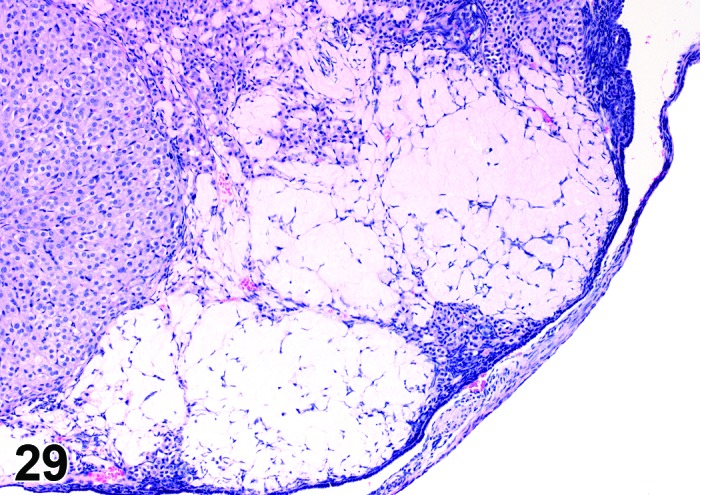
Amyloid, Ovary, mouse.
Specied
Mouse
Synonym(s)
Amyloidosis.
Pathogenesis/cell of origin
Extracellular deposition of polypeptide fragments of serum glycoproteins; the proteins are in β pleated sheet conformation. Can be a result of B cell proliferative disorders or secondary to an inflammatory process.
Diagnostic Features
Accumulation of extracellular, amorphous, acellular pale eosinophilic to gray material in the perivascular spaces or interstices of the ovary, within corpora lutea, and within atretic follicles.
・May be in thin bands or dense sheets.
・May replace areas of the organ.
・May cause gross enlargement or tan discoloration of the ovary.
・Often found in more than one organ (systemic disease).
Special Techniques for Diagnostics
Congo Red stain will cause amyloid to show green birefringence in polarized light.
Differential Diagnoses
・Fibrin deposition:
・Fibrinous exudates appear fibrillar.
・Generally not systemic.
Fibrinoid change:
・Deposition of intensely eosinophilic plasma proteins within vessel walls.
・Cellular debris sometimes present.
・May be accompanied by hemorrhage or thrombosis.
Comment
Amyloidosis is typically a naturally occurring disease in mice and involves the deposition primarily of immunoglobulin light chains; it is common in aging mice and has been induced systemically in mice chronically fed oxazepam. Incidence varies by strain-SJL, C57Bl and CD-1 strains are susceptible and C3H and A/J are relatively resistant. Rats are typically very resistant. Secondary amyloidosis is associated with chronic inflammatory lesions that result in SAA synthesis in the liver.
References
50Frith and Chandra (1991), 66Greaves (2012), 120Maekawa et al. (1996), 136Myers and McGavin (2007), 139National Toxicology Program (1993)
Angiectasis (N) Ovary (Figures 30 and 31)
Figure 30.
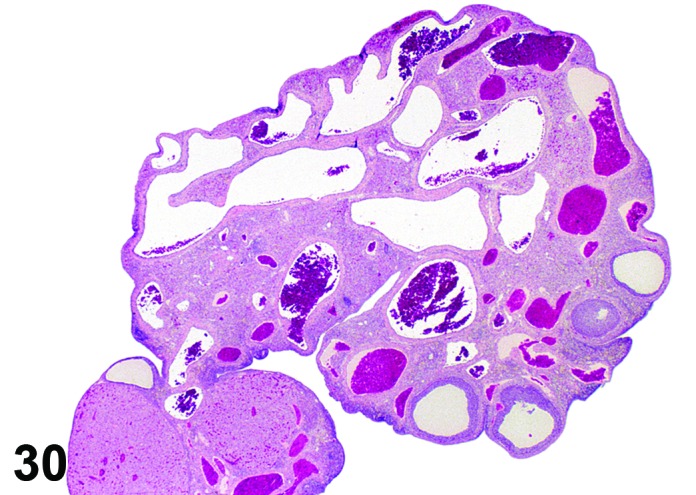
Angiectasis, Ovary, rat.
Figure 31.
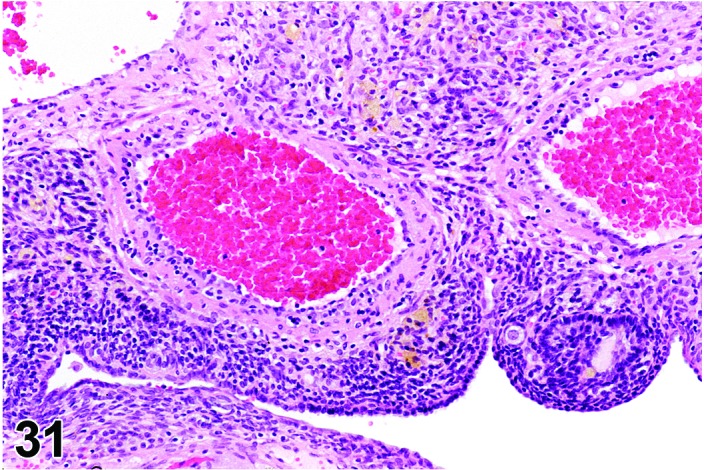
Angiectasis, Ovary, rat.
Species
Mouse; Rat.
Synonym(s)
Vascular ectasia; Telangiectasis.
Pathogenesis/cell of origin
Dilation of pre-existing blood vessels.
Diagnostic Fratures
・Local cystic dilation of pre-existing vessels.
・Affected vessels are cystic and blood-filled and are present within the interstitium especially near the hilus or within follicles or corpora lutea.
・May distort the normal architecture.
・Number of vessels is not increased.
・May be associated with thrombosis, hemorrhage or inflammation.
・Endothelial cell nuclei are flattened and cells are spindloid as in normal vasculature. No evidence of cellular pleomorphism.
・Endothelial cells form a single layer.
Differential Diagnoses
Hemangioma:
・Increased number of endothelial-lined, blood-filled spaces.
・Endothelial cells have hypertrophied nuclei.
・Mitoses may be present.
・Slight cellular pleomorphism or nuclear atypia may be present.
Hyperplasia, angiomatous:
・Increased number of closely spaced small blood vessels.
・Endothelial cell nuclei are predominantly flattened.
・Endothelial cells form a single layer.
・Little supporting stroma.
Hemorrhage:
・Blood is extravascular.
・Vessels are not cystic.
Congestion:
・Number of blood vessels is not increased.
・Other tissues/organs may be affected.
Comment
More common in mice than rats. May be associated with ovarian cysts. Differentiation of angiectasis from angiomatous hyperplasia and hemangioma is sometimes difficult.
References
5Alison et al. (1990), 31Davis et al. (1999), 120Maekawa et al. (1996)
Cyst, bursal (N) Ovary (Figure 32)
Figure 32.
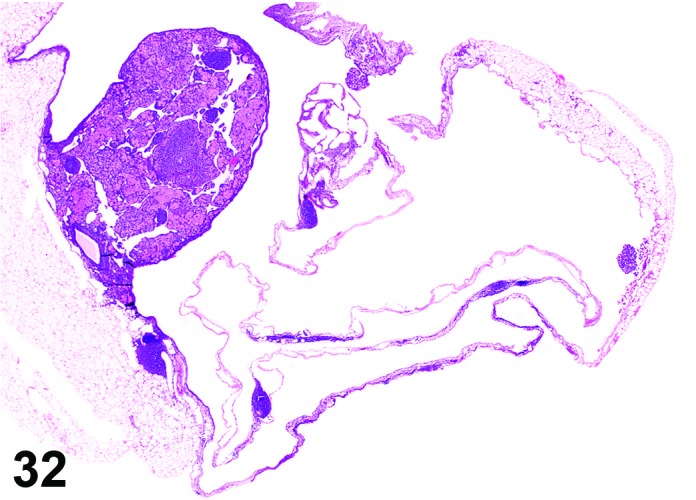
Bursal Cyst, Ovary, mouse.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Distention of the ovarian bursa with fluid.
Diagnostic Features
・Lined by simple squamous epithelium.
・Cyst envelops the ovary and may cause compression, but is not present within the ovary.
Differential Diagnoses
Cyst, follicular:
・Present within the ovary.
・Lined by one to several layers of cuboidal or flattened granulosa cells resting on a thin wall and often encircled by theca cells.
Cyst, rete ovarii:
・Present within hilus/medulla and/or adjacent to the ovary.
・Connection to ovarian hilus is present
・Lined by flattened, cuboidal or columnar epithelium, frequently with apical cytoplasm.
・Epithelium may be ciliated.
・Connection to paraovarian structures in the mesovarium may be seen.
Cyst, paraovarian
・Lined by flattened, cuboidal or columnar epithelium; often have apical nuclei.
・Epithelium may be ciliated.
・No connection to intra-ovarian or hilar structures is apparent.
Cyst, luteal:
・Present within the ovary.
・Lined at least partially by luteinized granulosa cells.
Cyst, NOS:
・If the origin/type of ovarian cyst is not apparent, the term Cyst, NOS may be a more appropriate diagnosis.
Follicle, luteinized, cystic:
・Present within the ovary.
・Lined by one to several layers of cuboidal or flattened granulosa cells resting on a thin wall and often encircled by theca cells.
・Partial luteinization of the granulosa cells lining the wall.
・Luteinization is often asymmetric in the cyst wall.
・Degenerate ovum can sometimes be observed within the cyst cavity and confirms diagnosis.
Cyst, epithelial:
・Present within the ovary.
・Lined by flattened cuboidal to low columnar epithelium.
・Connection to surface epithelium of the ovary is apparent.
Comment
More common in mice than rats. The bursa is an extension of the mesosalpinx and is lined by simple squamous epithelium (mesothelium). Both rats and mice have a complete bursa, which completely envelops the ovary. Bursal cysts are often observed grossly but may not be identified histologically because they often rupture during collection or processing.
References
5Alison et al. (1990), 31Davis et al. (1999), 66Greaves (2012), 133Montgomery and Alison (1987)
Cyst, epithelial (N) Ovary (Figures 33 and 34)
Figure 33.
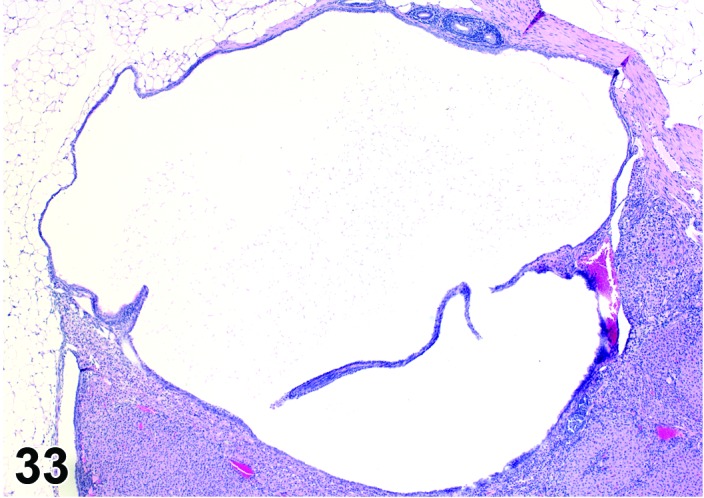
Epithelial Cyst, Ovary, mouse.
Figure 34.
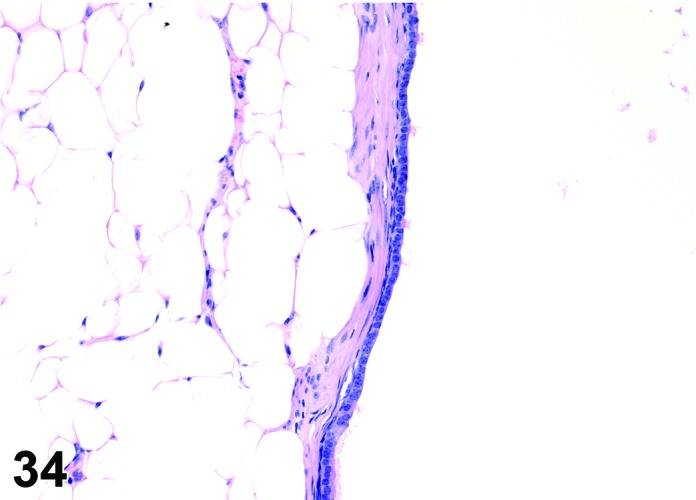
Epithelial Cyst, Ovary, mouse.
Species
Mouse; Rat.
Synonym(s)
Cyst, simple; Cyst, epidermoid; Cyst epithelial inclusion.
Pathogenesis/cell of origin
Epithelial cysts are thought to arise from downgrowth of the surface epithelium of the ovary.
Diagnostic Features
・The cysts are lined by a flattened cuboidal to low columnar epithelium. Some may resemble the epithelium of the ovarian surface.
・Intraovarian cysts lined by flattened, cuboidal or columnar epithelium. Cuboidal and columnar cells may be ciliated.
・Some cysts may contain papillary infoldings of epithelium without complex branching (papillary hyperplasia).
・Majority of cysts tend to be located in cortical region.
・Presence and number of cysts increases with age.
・Connection to surface epithelium is present.
・No apparent connection to paraovarian or rete structures.
Differential Diagnoses
Cyst, follicular:
・Lined by one to several layers of cuboidal or flattened granulosa cells resting on a thin wall and often encircled by theca cells.
・May distort the ovarian architecture.
・Larger than a normal late tertiary follicle.
・May contain degenerating oocyte.
Follicle, luteinized, cystic:
・Present within the ovary.
・Lined by one to several layers of cuboidal or flattened granulosa cells resting on a thin wall and often encircled by theca cells.
・Partial luteinization of the granulosa cells lining the wall.
・Luteinization is often asymmetric in the cyst wall.
・Degenerate ovum can sometimes be observed within the cyst cavity and confirms diagnosis.
・Larger than a normal late tertiary follicle.
Cyst, rete ovarii:
・Present adjacent to or within the ovary, often at the hilus.
・If present in structures adjacent to the ovary, a connection to the ovarian hilus must be apparent.
・Lined by flattened, cuboidal or columnar epithelium; often have apical nuclei.
・Epithelium may be ciliated.
・Connection to paraovarian structures in the mesovarium may be seen.
・Usually distorts the ovarian architecture.
Cyst, paraovarian:
・Lined by flattened, cuboidal or columnar epithelium.
・Epithelium may be ciliated.
・May be associated with smooth muscle
・No connection to intra-ovarian or hilar structures is seen.
Cyst, bursal:
・Envelops ovary but not present within ovary proper.
・Lined by simple squamous epithelium.
Cystadenoma:
・Papillae have focal origin with complex branching; may show slight atypia and are larger than the size of a corpus luteum.
Cyst, NOS:
・If the origin/type of ovarian cyst is not apparent, the term Cyst, NOS may be a more appropriate diagnosis.
Cyst, luteal:
・Present within the ovary.
・Lined by luteinized granulosa cells.
・Larger than a normal corpus luteum.
Comment
Epithelial cysts occur more frequently in mice than in rats. Cytokeratin 8 expression in epithelial cysts may help differentiate from follicular cysts. Superovulation of mice induces an increased number of cortical and/or central ovarian epithelial cysts. In mice, cyst epithelium expresses estrogen and progesterone receptors. Without obvious connection to surface epithelium, it is difficult to differentiate from other epithelial-lined ovarian cysts (those of rete origin). If the connection to overlying surface epithelium of the ovary is not apparent, then the term ‘Cyst, NOS’ is more appropriate.
References
5Alison et al. (1990), 31Davis et al. (1999), 48Fleming et al. (2007), 69Greaves et al. (1992), 114Long (2002), 190Tan et al. (2005), 108Lee et al. (2011), 24Clow et al. (2002), 207Wenzel and Odend’hal (1985)
Cyst, follicular (N) Ovary (Figure 35 and 36)
Figure 35.
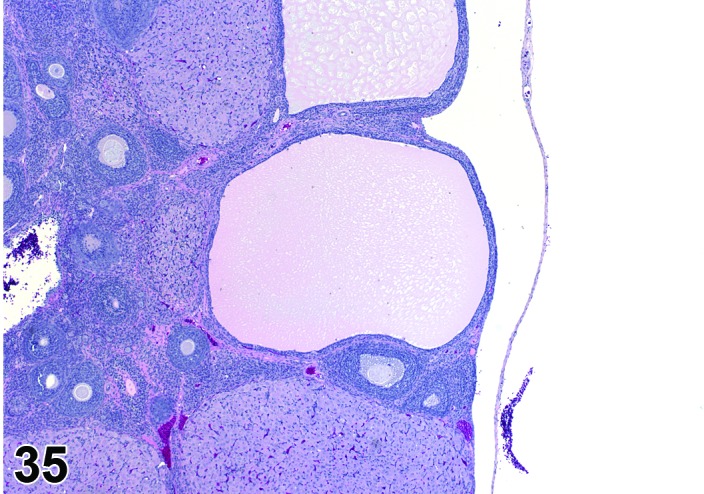
Follicular Cyst, Ovary, rat.
Figure 36.
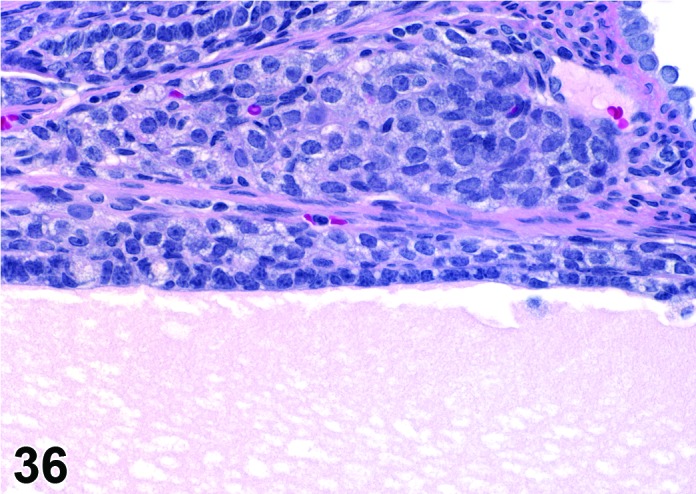
Follicular Cyst, Ovary, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
In general, follicular cysts develop as a result of hormonal imbalance or dysregulation of the hypothalamic-pituitary-gonadal axis and resulting in failure of ovulation.
Diagnostic Features
・Thin walled, filled with pale acidophilic to amphophilic residue or blood; may also contain cell debris, degenerating oocytes, or foamy, vacuolated or hemosiderin-laden macrophages.
・Usually lined by one to four layers of cuboidal granulosa cells; no luteinization.
・Larger cysts may be lined by a single layer of flattened cells resting on a thin fibrous capsule.
・Degeneration of individual granulosa cells is often present.
・Single or multiple cysts may be present.
・Degenerate ovum can sometimes be observed within the cyst cavity.
・Larger than a normal late tertiary (preovulatory) follicle.
Differential Diagnoses
Late tertiary (preovulatory) follicle:
・Contains an oocyte and a well developed theca cell layer;.
・No evidence of granulosa cell apoptosis/atresia.
Atretic late follicle:
・Similar in appearance to follicular cysts but smaller (not larger than a normal late tertiary follicle).
・Contains a degenerating oocyte (but the oocyte may not be visible in the plane of section).
・Granulosa cells show apoptosis.
Follicle, luteinized (+/- modifier cystic):
・Wall comprised of granulosa cells with irregular clumps or groups of large, round to polygonal luteinized cells.
・Degenerate oocyte can sometimes be observed within the follicle.
・Luteinized follicle is not larger than a normal late tertiary follicle.
・The cystic luteinized follicle is larger than a normal late tertiary follicle and has cystic center.
Cyst, luteal:
・Lined by one to several layers of large polygonal luteinized cells.
・Larger than a normal corpus luteum.
Cyst, epithelial:
・Present within the ovary.
・Lined by flattened cuboidal to low columnar epithelium.
・Connection to surface epithelium of the ovary is apparent.
Comment
Follicular cysts are a common finding in untreated rats and mice, particularly with increasing age, and their incidence is also related to strain. In older rats, as the ovarian cycle becomes prolonged, some pre-ovulatory follicles appear to lose the capacity to ovulate during periods of persistent estrus. In young rats, follicular cysts may develop in association with imbalances in thyroid hormone, prolactin, LH, or androgens. Follicular cysts may also result from factors that alter the secretion of, or ovarian responses to, gonadotropins. Some follicles may produce estradiol and can be associated with persistent estrus.
References
31Davis et al. (1999), 66Greaves (2012), 176Shirai et al. (2009)
Cyst, luteal (N) Ovary (Figures 37 and 38)
Figure 37.
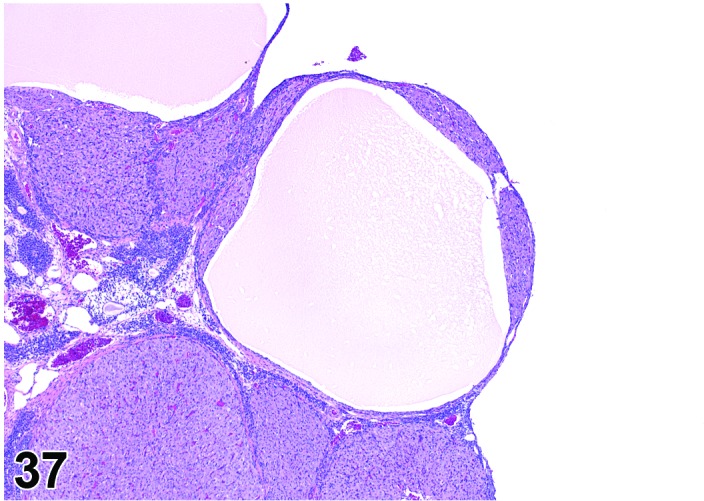
Luteal Cyst, Ovary, rat.
Figure 38.
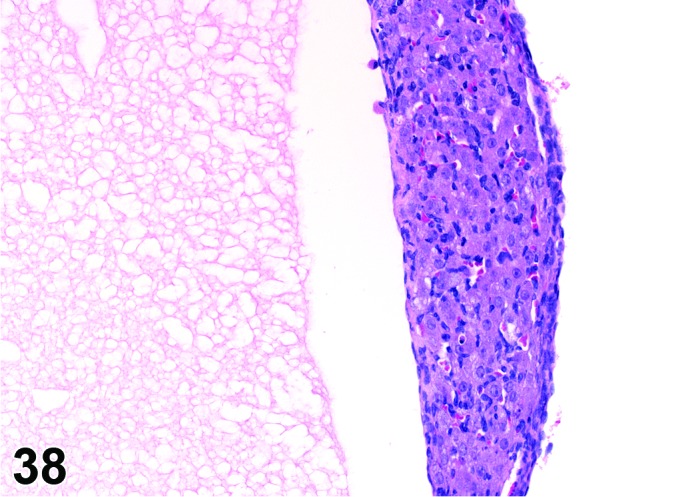
Luteal Cyst, Ovary, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
In general, a luteal cyst develops after the follicle has ovulated and fluid or blood accumulates within the follicle, causing the follicle to expand and be transformed into a luteal cyst.
Diagnostic Features
・Lined completely by several layers of polygonal cells with abundant eosinophilic, finely vacuolated cytoplasm (luteal cells); luteinization is diffuse and symmetric, not in clumps or groups.
・Essentially few non-luteinized granulosa cells.
・Larger than a normal corpus luteum.
・May distort the ovarian architecture.
・Cyst formation occurs in a true CL- no evidence of degenerate ova within the cystic cavity.
Differential Diagnoses
Cyst, follicular:
・Present within the ovary.
・Lined by one to several layers of cuboidal or flattened granulosa cells resting on a thin wall and often encircled by theca cells.
・May distort the ovarian architecture.
Follicle, luteinized (+/- modifier cystic):
・Present within the ovary.
・Lined by one to several layers of cuboidal or flattened granulosa cells resting on a thin wall and often encircled by theca cells.
・Partial luteinization of the granulosa cells lining the wall.
・Luteinization is often asymmetric in the wall.
・Degenerate ovum can sometimes be observed within the central cavity and confirms diagnosis.
・Luteinized follicle is no larger than a normal late tertiary follicle.
・Cystic luteinized follicle is larger than a normal tertiary follicle.
Cyst, rete ovarii:
・Present adjacent to or within the ovary, often at the hilus.
・Lined by flattened, cuboidal or columnar epithelium; often have apical nuclei.
・Epithelium may be ciliated.
・May be associated with smooth muscle.
・Connection to paraovarian structures in the mesovarium may be seen.
・Usually distorts the ovarian architecture.
Cyst, paraovarian:
・Lined by flattened, cuboidal or columnar epithelium.
・Epithelium may be ciliated.
・May be associated with smooth muscle
・No connection to intra-ovarian or hilar structures is seen.
Cyst, bursal:
・Envelops ovary but not present within ovary proper.
・Lined by simple squamous epithelium.
Cyst, epithelial:
・Present within the ovary.
・Lined by flattened cuboidal to low columnar epithelium.
・Connection to surface epithelium of the ovary is apparent.
Cyst, NOS:
・If the origin/type of ovarian cyst is not apparent, the term Cyst, NOS may be a more appropriate diagnosis.
Comment
Luteal cysts are unusual lesions in both mice and rats. These cysts may be associated with increased progesterone, and progesterone receptor inhibitors induce typical luteal cysts in rats.
References
5Alison et al. (1990), 31Davis et al. (1999), 66Greaves (2012), 189Tamura et al. (2009)
Cyst, rete ovarii (N) Ovary (Figures 39 and 40)
Figure 39.
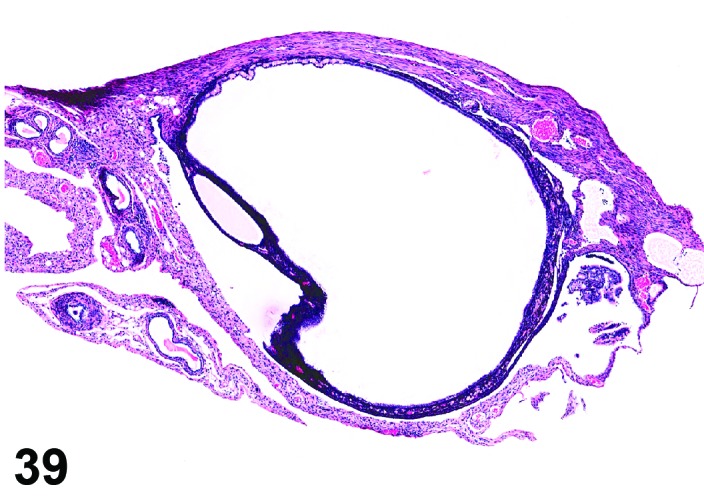
Rete Ovarii Cyst, Ovary, mouse.
Figure 40.
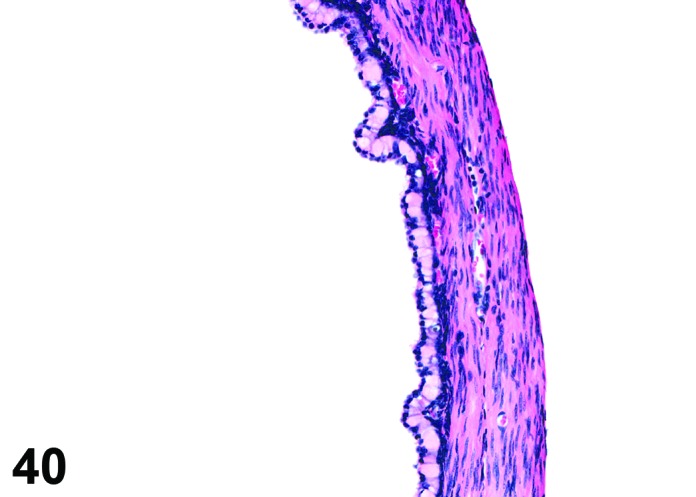
Rete Ovarii Cyst, Ovary, mouse.
Species
Mouse; Rat.
Synonym(s)
Pathogenesis/cell of origin
Rete ovarii cysts arise from remnants of the rete anlage.
Diagnostic Features
・Arise from intraovarian, extraovarian or connecting rete; thus usually present at or connected to the ovarian hilus
・Connection to paraovarian structures in the mesovarium may be seen.
・The cysts are lined by cuboidal or columnar epithelial cells which become flattened if the structure is dilated.
・May have smooth muscle tissue within the cyst wall.
・Epithelium may be ciliated.
・Particularly in mice, frequently distort the ovarian architecture.
Differential Diagnoses
Cyst, paraovarian:
・Present adjacent to the ovary, but have no apparent connection to ovarian or hilar structures.
・Lined by flattened, cuboidal or columnar epithelium; often have apical nuclei.
・Epithelium may be ciliated.
・May be associated with smooth muscle.
・Connection to paraovarian structures in the mesovarium may be seen.
Cyst, bursal:
・Envelops ovary but not present within ovary proper.
・Lined by simple squamous epithelium.
Cyst, epithelial:
・Present within the ovary.
・Lined by flattened cuboidal to low columnar epithelium.
・Connection to surface epithelium is apparent.
Follicle, luteinized (+/- modifier cystic):
・Wall comprised of granulosa cells with irregular clumps or groups of large, round to polygonal luteinized cells.
・Degenerate oocyte can sometimes be observed within the follicle.
・Luteinized follicle is not larger than a normal late tertiary follicle.
・The cystic luteinized follicle is larger than a normal late tertiary follicle and has cystic center.
Cyst, NOS:
・If the origin/type of ovarian cyst is not apparent, the term Cyst, NOS may be a more appropriate diagnosis.
Comment
This is a common lesion in both mice and rats. Cysts that are present at the hilus or extend into the ovary at the hilus or connect to intraovarian structures at the hilus are part of the rete system and should be termed ‘cyst, rete ovarii’. The term ‘paraovarian cyst’ should be used only for those epithelial cysts present in tissues adjacent to the ovary that do not have an apparent connection to the ovary to allow determination of the origin.
References
31Davis et al. (1999), 66Greaves (2012), 69Greaves et al. (1992), 114Long (2002), 133Montgomery and Alison (1987), 55Genadry et al. (1977), 108Lee et al. (2011), 24Clow et al. (2002), 207Wenzel and Odend’hal (1985)
Cyst, paraovarian (N) Ovary (Figures 41 and 42)
Figure 41.
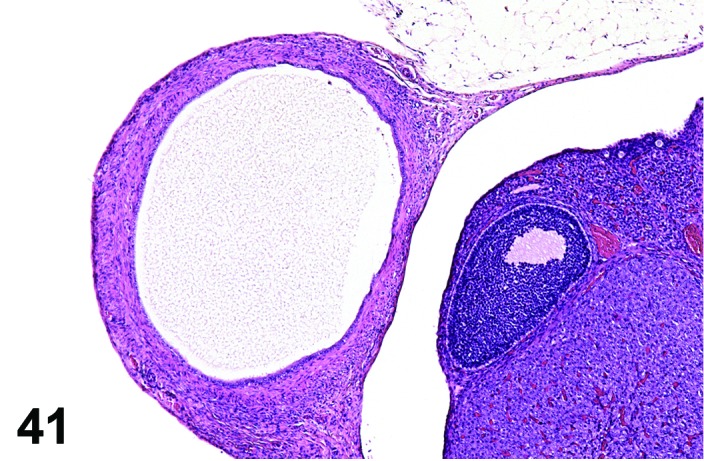
Paraovarian Cyst, Ovary, rat.
Figure 42.
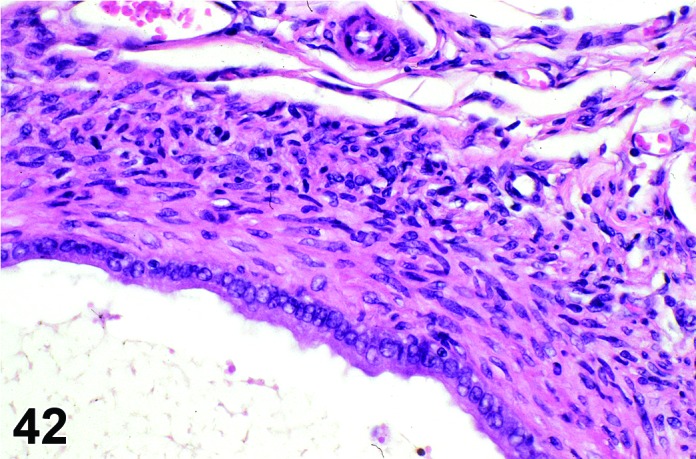
Paraovarian Cyst, Ovary, rat.
Species
Mouse; Rat.
Synonym(s)
Pathogenesis/cell of origin
Paraovarian cysts are thought to arise from remnants of mesonephric or paramesonephric ducts and the rete anlage.
Diagnostic Features
・Paraovarian cysts are located in the mesovarium or mesosalpinx with no apparent connection to hilar or intra-ovarian structures.
・The cysts are lined by cuboidal or columnar epithelial cells which become flattened if the structure is dilated.
・May have smooth muscle tissue within the cyst wall.
・Epithelium may be ciliated.
・Particularly in mice, frequently compress and/or distort the ovarian architecture.
Differential Diagnoses
Cyst, rete ovarii:
・Present adjacent to or within the ovary, often at the hilus.
・Lined by flattened, cuboidal or columnar epithelium; often have apical nuclei.
・Epithelium may be ciliated.
・May be associated with smooth muscle.
・Connection to paraovarian structures in the mesovarium may be seen.
・Usually distorts the ovarian architecture.
Cyst, bursal:
・Envelops ovary but not present within ovary proper.
・Lined by simple squamous epithelium.
Cyst, epithelial:
・Present within the ovary.
・Lined by flattened cuboidal to low columnar epithelium.
・Connection to surface epithelium is apparent.
Follicle, luteinized (+/- modifier cystic):
・Wall comprised of granulosa cells with irregular clumps or groups of large, round to polygonal luteinized cells.
・Degenerate oocyte can sometimes be observed within the follicle.
・Luteinized follicle is not larger than a normal late tertiary follicle.
・The cystic luteinized follicle is larger than a normal late tertiary follicle and has cystic center.
Cyst, NOS:
・If the origin/type of ovarian cyst is not apparent, the term Cyst, NOS may be a more appropriate diagnosis.
Comment
This is a common lesion in both mice and rats. Cysts that are present at the hilus or extend into the ovary at the hilus or connect to intraovarian structures at the hilus are part of the rete system and should be termed ‘cyst, rete ovarii’. The term ‘paraovarian cyst’ should be used only for those epithelial cysts present in tissues adjacent to the ovary that do not have an apparent connection to the ovary to allow determination of the origin.
References
31Davis et al. (1999), 66Greaves (2012), 69Greaves et al. (1992), 114Long (2002), 133Montgomery and Alison (1987), 55Genadry et al. (1977), 108Lee et al. (2011), 24Clow et al. (2002), 207Wenzel and Odend’hal (1985)
Cyst, NOS (N) Ovary; Uterus; Uterine Cervix; Vagina
Species
Mouse; Rat.
Pathogenesis/cell of origin
Mesonephric tubules/ducts, endometrial epithelium, proximal cervical epithelium, and/or cutaneous adnexal structures.
Diagnostic Features
・Dilated, fluid- or keratin-filled structure.
・Epithelial lining often obvious with variable amounts of compressive atrophy.
・May have a smooth muscle wall (mesonephric duct remnant).
・No apparent connection to extra-ovarian or ovarian hilar structures, or to ovarian surface epithelium.
・May have squamous metaplasia and/or keratinization.
Differential Diagnoses
Cystadenoma:
・Cystadenomas often contain multiple cystic spaces, are more densely cellular, and contain mitotic figures.
Ovarian cysts:
・The origin of the cyst is apparent (follicle, corpus luteum, rete ovarii, surface epithelium). See descriptions of individual types of ovarian cysts.
Paraovarian cysts:
・Paraovarian cysts are located in the mesovarium or mesosalpinx with no apparent connection to hilar or intra-ovarian structures.
・The cysts are lined by cuboidal or columnar epithelial cells which become flattened if the structure is dilated.
・May have smooth muscle tissue within the cyst wall.
・Epithelium may be ciliated.
・Particularly in mice, frequently distort the ovarian architecture.
Comment
Cystic structures may be encountered in multiple sites in the female reproductive tract. Depending on the location, size, and chronicity of the cyst it may be difficult to accurately identify the origin; in these cases, the term Cyst, NOS is recommended.
References
109Leininger and Jokinen (1990)
Follicle, luteinized (N) Ovary (Figures 43 and 44)
Figure 43.
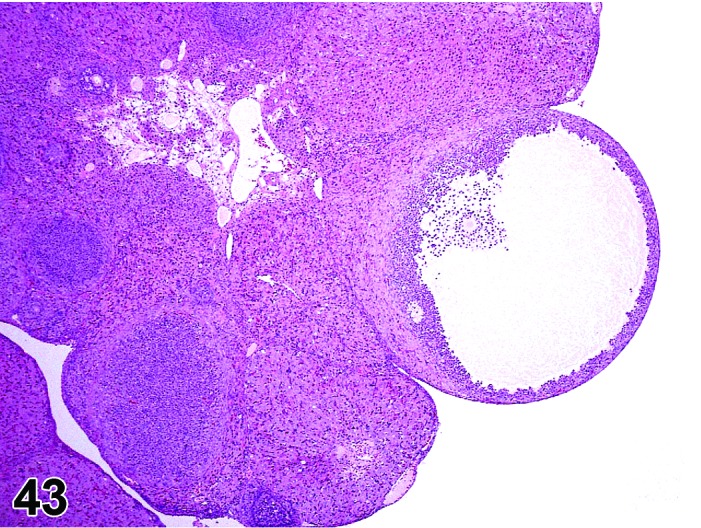
Cystic Luteinized Follicle, Ovary, rat.
Figure 44.
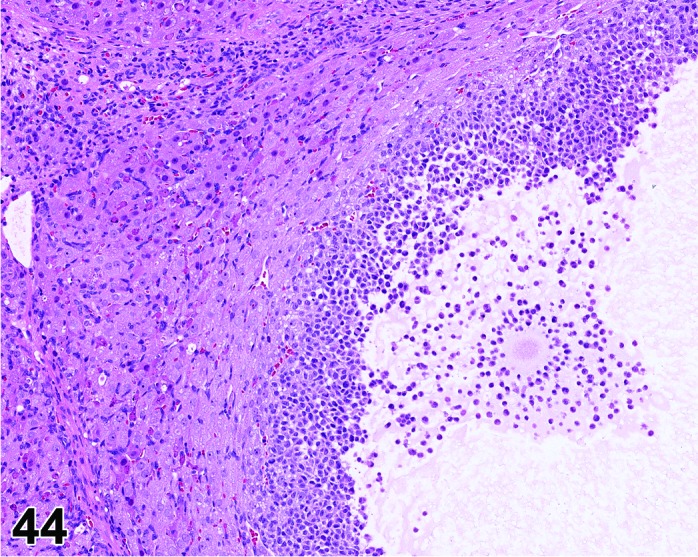
Cystic Luteinized Follicle. Note entrapped Cumulus Oophorus Complex, Ovary, rat.
Species
Mouse; Rat.
Synonym(s)
Luteinized, nonovulatory follicle; Luteinized unruptured follicle.
Modifier
Cystic.
Pathogenesis/cell of origin
In general, a luteinized follicle (LF) develops as a result of insufficient LH secretion in the period before ovulation, or from inhibition of cyclooxygenase 2. The tertiary follicle develops normally but fails to ovulate, and under the influence of the low LH levels the granulosa cells are transformed into luteal cells.
Diagnostic Features
・The hallmark of a LF is the presence of a retained (degenerating) oocyte within a luteinzed, corpus luteum-like structure.
・Lined by one to several layers of cuboidal or flattened granulosa cells resting on a thin wall and often encircled by theca cells.
・Partial luteinization of the granulosa cells lining the wall is evident.
・Luteinization is often asymmetric in the follicular wall.
・No larger than a normal late tertiary follicle.
・Cystic luteinized follicle is larger than a normal tertiary follicle and has a central cavity that contains the unovulated oocyte.
Differential Diagnoses
For Follicle, luteinized:
Late tertiary (preovulatory) follicle:
・Contains an oocyte and a well-developed theca layer.
・Lacks luteinization.
Atretic late tertiary follicle:
・Contains a degenerating oocyte.
・Granulosa cell layers vary from few to many.
・Granulosa cells show apoptosis.
・No larger than a normal late tertiary follicle.
For Follicle, luteinized, cystic:
Cyst, follicular:
・Thin-walled, filled with pale acidophilic to amphophilic residue or blood and epithelium is uniformly cuboidal.
・Lacks evidence of luteinization.
・May contain a degenerate oocyte.
Cyst, luteal:
・Lined by one to several layers of large polygonal luteinized cells.
・Complete luteinization; essentially no areas of non-luteinized granulosa cells.
・Larger than a normal corpus luteum.
・Central cavity does not contain a degenerate oocyte.
Cyst, epithelial:
・Present within the ovary.
・Lined by flattened cuboidal to low columnar epithelium.
Cyst, bursal:
・Envelops ovary but not present within ovary proper.
・Lined by simple squamous epithelium.
Cyst, NOS:
・If the origin/type of ovarian cyst is not apparent, the term Cyst, NOS may be a more appropriate diagnosis.
Comment
With Exemestane, an oral steroidal aromatase inhibitor, presence of oocytes within luteinized structures were encountered in addition to absence of recent basophilic corpora lutea, increased atresia of antral follicles, and interstitial cell hyperplasia. Drug-related increased levels of cAMP were thought to be responsible for this phenomenon. COX2 inhibitors or PPARγ agonists prevent ovulation (entrapped Cumulus Oophorus Complex, COC) by inhibiting follicular rupture and this also results in luteinized follicles in rodents. Entrapped COCs can be found within follicles, but also within the ovarian interstitium. It has been reported that mucification of the oocyte-associated granulosa cells in entrapped COC begins too early due to extracellular matrix breakdown. Cumulus oophorus mucification normally takes place periovulatory and mainly outside the ovary, after ovulation.
References
31Davis et al. (1999), 53Gaytan et al. (2006), 66Greaves (2012), 125Mattheij and Swarts (1995), 129Mirsky et al. (2011), 172Sato et al. (2009b), 194Tsubota et al. (2009)
Vacuolation, theca cell (N) Ovary (Figure 45)
Figure 45.
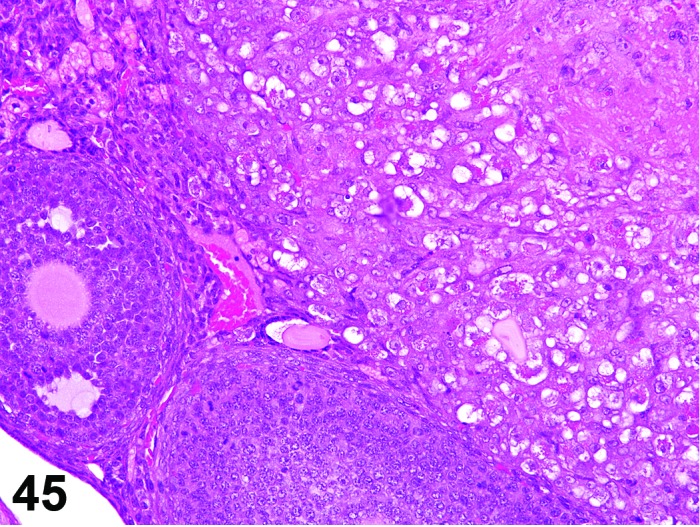
Vacuolation, Theca Cell, Ovary, mouse.
Species
Mouse; Rat.
Modifier
Increased; Decreased.
Pathogenesis/cell of origin
Vacuolation of thecal cells can occur due to inhibition of steroid synthesis leading to lipid accumulation within the cells. Other steroid producing cells, such as the adrenal gland, may also be affected.
Diagnostic Features
・Thecal cells have a normal fine vacuolation related to steroid synthesis. Increased and decreased vacuolation should be more or less, respectively, than that normally present.
・Cells with increased vacuolation may appear larger than normal.
Differential Diagnoses
Vacuolation, interstitial cell:
・Interstitial cells have a normal fine vacuolation related to steroid synthesis. Increased vacuolation should be more than that normally present.
・Cells with increased vacuolation may appear larger than normal.
Phospholipidosis:
・Vacuoles present in cytoplasm of cells.
・Ultrastructurally, vacuoles have abnormal lamellated inclusions.
Comment
Increased or decreased vacuolation may be associated with alterations of steroid synthesis or phospholipidosis. Cationic amphiphilic compounds can induce phospholipidosis. On ultrastructural examination, the vacuoles contain abnormal lamellated inclusions.
References
106Lúllmann-Rauch and Reil (1974)
Vacuolation, granulosa cell (N) Ovary (Figure 46)
Figure 46.
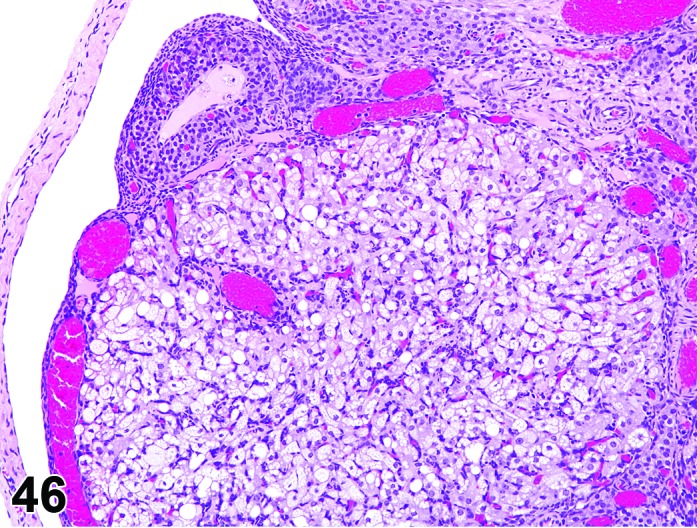
Vacuolation, Granulosa Cell, Ovary, rat.
Species
Mouse; Rat.
Synonym(s)
Fatty change.
Modifier
Increased; Decreased.
Pathogenesis/cell of origin
Vacuolation of granulosa cells can occur due to inhibition of steroid synthesis leading to lipid accumulation within the cells. Other steroid producing cells, such as the adrenal gland, may also be affected.
Diagnostic Features
・Granulosa cells have a normal fine vacuolation related to steroid synthesis. Increased and decreased vacuolation should be more or less, respectively, than that normally present.
・Cells with increased vacuolation may appear larger than normal.
Differential Diagnoses
None.
Comment
Increased or decreased vacuolation may be associated with alterations of steroid synthesis or phospholipidosis. Cationic amphiphilic compounds can induce phospholipidosis. On ultrastructural examination, the vacuoles contain abnormal lamellated inclusions.
References
106Lúllmann-Rauch and Reil (1974)
Vacuolation, interstitial cell (N) Ovary (Figure 47)
Figure 47.
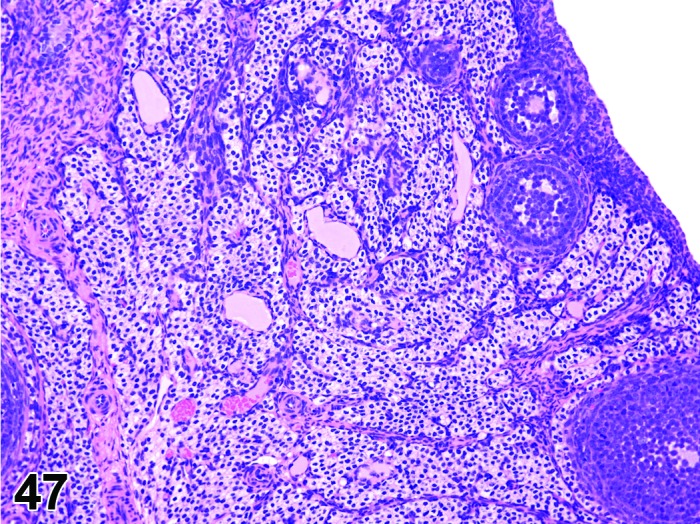
Vacuolation of Interstitial Cells, Ovary, rat.
Species
Mouse; Rat.
Synonym(s)
Fatty change.
Modifier
Increased; Decreased.
Pathogenesis/cell of origin
Vacuolation of interstitial cells can occur due to inhibition of steroid synthesis leading to lipid accumulation within the cells. Other steroid producing cells, such as the adrenal gland, may also be affected.
Diagnostic Features
・Interstitial cells have a normal fine vacuolation related to steroid synthesis. Increased and decreased vacuolation should be more or less than that normally present.
・Cells with increased vacuolation may appear larger than normal.
Differential Diagnoses
Vacuolation, theca cell:
・Fine vacuolation related to steroid synthesis. Increased vacuolation should be more than that normally present.
・Cells with increased vacuolation may appear larger than normal.
Hypertrophy, interstitial cell:
・Interstitial cells, arranged in cords or nests, are enlarged and polyhedral with ample clear to pale-eosinophilic, sometimes vacuolated cytoplasm.
・Decreased nuclear: cytoplasmic ratio.
Comment
Increased or decreased vacuolation may be associated with alterations of steroid synthesis or phospholipidosis. Cationic amphiphilic compounds can induce phospholipidosis. On ultrastructural examination, the vacuoles contain abnormal lamellated inclusions.
References
66Greaves (2012), 106Lúllmann-Rauch and Reil (1974), 115Long et al. (2001)
Vacuolation, corpora lutea (N) Ovary (Figures 48 and 49)
Figure 48.
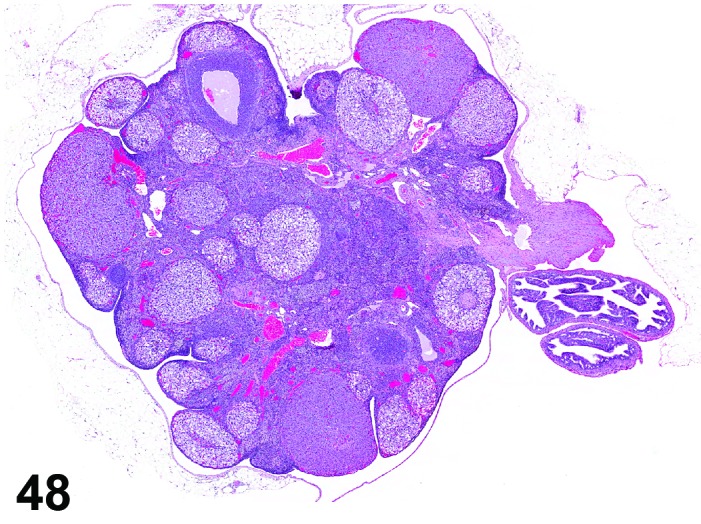
Vacuolation of CL, Ovary, rat.
Figure 49.
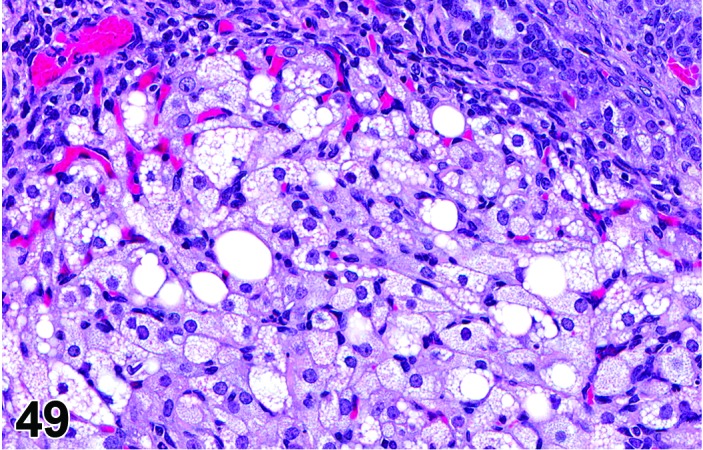
Vacuolation of CL Ovary, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Vacuolation of luteal cells can occur due to inhibition of steroid synthesis leading to lipid accumulation within the cells. Luteal cells can also be affected in cases of phospholipidosis.
Diagnostic Features
・Microvesicular or macrovesicular cytoplasmic vacuolation of luteal cells in CL other than the CL of the most recent ovulation at diestrus/proestrus.
・Luteal cells may appear enlarged.
・Lack of significant luteolysis in the affected CL.
・Overall size of the CL may be increased.
Differential Diagnoses
Hypertrophy, corpora lutea
Normal vacuolation:
・Microvesicular vacuolation is normal in CL of the most recent ovulation during diestrus and early proestrus; macrovesicular vacuolation with luteolysis is normal in the CL of the most recent ovulation during mid to late proestrus.
Comment
Vacuolation is typically observed as part of the degeneration seen at proestrus; this should not be diagnosed. Increased numbers of vacuolated corpora lutea or vacuolation observed in CL of the most recent ovulation other than at proestrus should be diagnosed. Vacuolated CL have been described with anthracycline compounds. Foamy cytoplasmic vacuolation can be indicative of phospholipidosis. Phospholipidosis can be induced by cationic amphiphilic compounds. On ultrastructural examination, the vacuoles contain abnormal lamellated inclusions. Vacuolation may cause apparent enlargement of the luteal cells, and this can be difficult to discern from luteal hypertrophy.
References
5Alison et al. (1990), 25Comereski et al. (1994), 106Lullmann-Rauch and Reil (1974)
Mineralization (N) Ovary (Figures 50 and 51)
Figure 50.
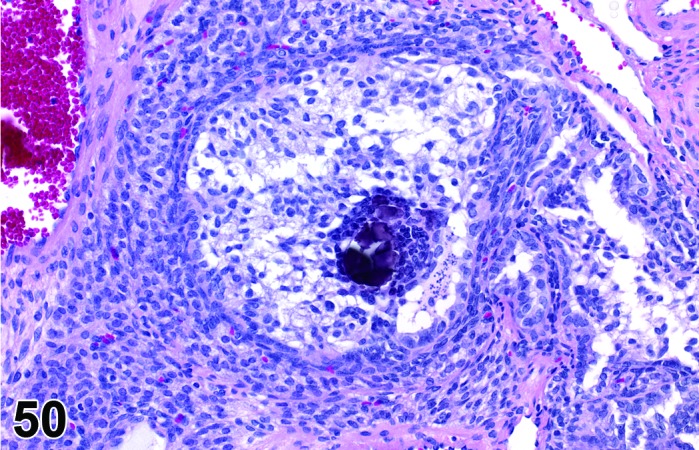
Mineralization of Oocyte, Ovary, rat.
Figure 51.
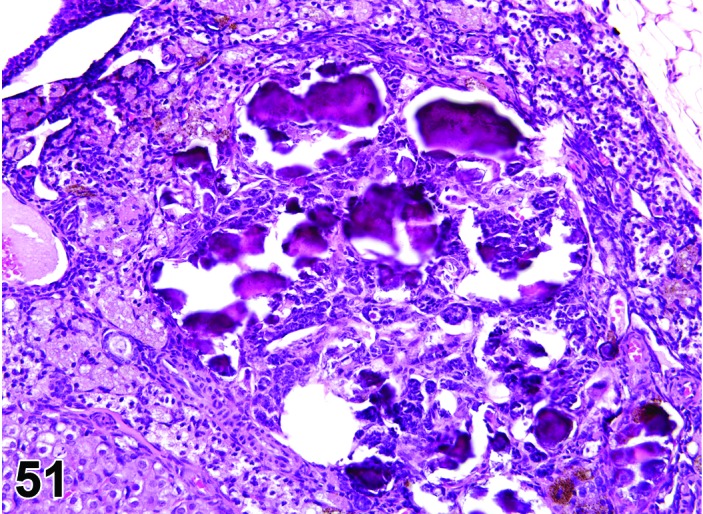
Mineralization of Interstitial Cells, Ovary, rat.
Species
Mouse; Rat.
Modifier
Oocyte; Corpus luteum; Interstitial cell;
Pathogenesis/cell of origin
Uncertain etiology.
Diagnostic Features
・Mineralization of oocytes, corpora lutea, and interstitial cells is characterized by granular basophilic material partially or completely replacing resident structures.
Differential Diagnoses
None.
Comment
Mineralization of oocytes and interstitial cells of the ovary may become more prominent with advanced age.
References
66Greaves (2012)
Infiltrate, inflammatory cell (N) Ovary (Figure 52)
Figure 52.
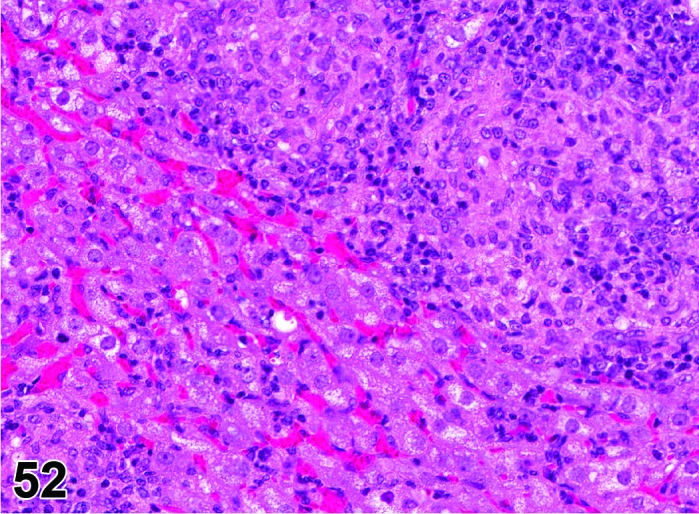
Inflammatory Cell Infiltrate, mononuclear, Ovary, rat.
Species
Mouse; Rat.
Modifier
Eosinophilic; Histiocytic; Neutrophilic; Lymphocytic; Mononuclear; Mixed.
Pathogenesis/cell of origin
Movement of inflammatory cells from the blood, bone marrow or hemo-lymphatic organs into tissue as a result of increased secretion of interleukins and/or specific cell chemoattractants.
Diagnostic Features
・Infiltration of inflammatory cells in the ovarian parenchyma.
・Major cell type(s) comprising the infiltrate depends on the specific interleukins and chemoattractants produced.
・The inflammatory infiltrate is modified with the major cell type present (comprising >50% of the cells); if no one cell type comprises >50% of the infiltrate, ‘mixed cell’ may be used.
・Infiltrate, inflammatory cell, mononuclear is generally used for mixed infiltrates comprised predominantly (>50%) of lymphocytes, plasma cells, monocytes and/or histiocytes, or when the specific non-segmented cell type cannot be discerned but comprises at least 50% of the cell population.
Differential Diagnoses
Sarcoma, histiocytic:
・Uniform population of rounded or oval cells with abundant foamy, eosinophilic cytoplasm and elongated or folded nuclei; palisading tumor cells surrounding necrotic foci; mitotic figures may be numerous. Tumor cells are also detected in other organs.
Lymphoma, malignant:
・Monomorphic mononuclear cells with cellular atypia or mitotic figures invading throughout the parenchyma. Usually no giant cells are seen and spleen and lymph nodes are frequently involved. Tumor cells can also be detected in other organs.
Inflammation, ovary (any type):
・Infiltration of inflammatory cells (granulocytes, macrophages, lymphocytes, plasma cells, mixed), accompanied by other inflammatory changes (congestion, edema, hemorrhage, exudate, necrosis, fibrosis, etc.) in the tissue.
References
5Alison et al. (1990), 31Davis et al. (1999), 133Montgomery and Alison (1987)
Inflammation, ovary (N) Ovary (Figure 53)
Figure 53.
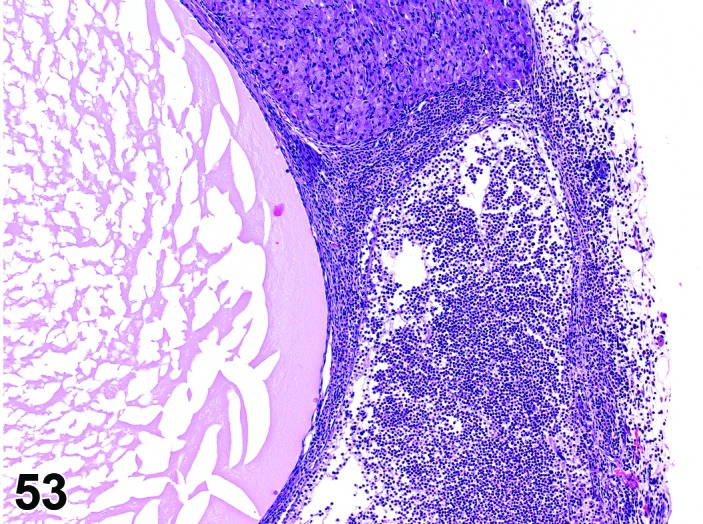
Inflammation, Neutrophilic, Ovary, rat.
Species
Mouse; Rat.
Synonym(s)
Oophoritis.
Modifier
Neutrophilic; Lymphocytic; Mononuclear; Mixed. Other modifiers include suppurative, granulomatous.
Pathogenesis/cell of origin
Breakdown of the normal muco-cutaneous barrier or direct introduction of pathogenic organisms into the body, and secondary involvement of the ovary via septicemia. Additionally, bacteria can exist in the body as latent infections, and under appropriate conditions such as severe stress, toxicity or neoplasia and chemically induced immunosuppression, these may produce severe disease with bacteremia or septicemia and rarely involvement of the ovary.
Diagnostic Features
・Infiltration of inflammatory cells in the ovary parenchyma, and sometimes progression to the oviduct or periovarian fat.
・Evidence of tissue degeneration/necrosis/regeneration is present, along with other evidence of an inflammatory response: hemorrhage, congestion, edema, exudate, fibroplasia, angiectasis, fibrosis.
・Causative organisms may be present.
・Major composing cells are different dependent on the inciting agent and inflammatory process; inflammation should be modified with the major cell type (comprising at least 50% of the cells) present.
・Granulomatous inflammation has epithelioid macrophages as the predominant cell type; fibrosis and giant cell formation are sometimes seen.
・Suppurative inflammation is a specific diagnosis that has predominantly neutrophilic inflammation and large areas of necrotic tissue and abscess formation.
Differential Diagnoses
Sarcoma, histiocytic:
・Uniform population of rounded or oval cells with abundant foamy, eosinophilic cytoplasm and elongated or folded nuclei; palisading tumor cells surrounding necrotic foci; mitotic figures may be numerous. Tumor cells are also detected in other organs.
Lymphoma, malignant:
・Monomorphic mononuclear cells with cellular atypia or mitotic figures invading throughout the parenchyma. Usually no giant cells are seen and spleen and lymph nodes are frequently involved. Tumor cells can also be detected in other organs.
Infiltrate, inflammatory cell:
・Infiltration of inflammatory cells (granulocytes, macrophages, lymphocytes, plasma cells, mixed), but other inflammatory changes (congestion, edema, hemorrhage, exudate, necrosis, fibrosis, etc.) are absent or of limited severity.
Comment
Rare in rats and mice. May be seen with systemic infections caused by Mycoplasma pulmonis, Streptococcus pneumoniae, Pasteurella pneumotropica, Pseudomonas aeruginosa and Corynebacterium kutscheri.
References
5Alison et al. (1990), 31Davis et al. (1999), 133Montgomery and Alison (1987)
Age-related Atrophy (N) Ovary (Figure 54)
Figure 54.
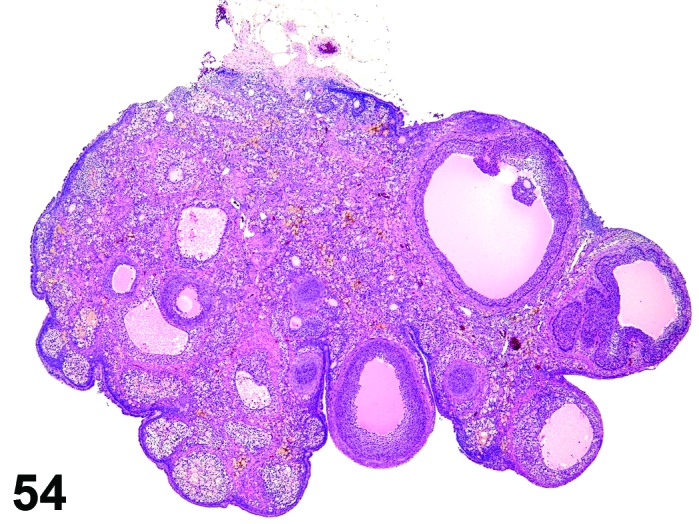
Age-related Atrophy, Ovary, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Cessation of the normal estrous cycle caused by age-related depletion of primordial follicles and changes in endocrine responsiveness of the hypothalamic-pituitary-ovarian axis. An outcome of reproductive senescence in the female.
Diagnostic Features
* Smaller than cycling ovary.
・Decrease in the number of oocytes, follicles and corpora lutea.
・Few or no primordial follicles.
・Follicles and corpora lutea that are present are not typical of a normal estrous cycle stage.
・Mainly three patterns:
・Few or no growing or antral follicles; corpora lutea are prominent.
・No corpora lutea or no recent corpora lutea; large atretic follicles prominent; may have cystic follicles.
・No corpora lutea or no recent corpora lutea; few or no growing follicles.
・Abundant interstitial cells are seen in the stroma.
・In aged mice, cords of epithelial cells and tubules are prominent and dissect through the interstitial gland tissue.
・In aged rats, age-related ovarian atrophy is often accompanied by sex cord stromal hyperplasia and lipofuscin.
Differential Diagnoses
Atrophy induced by xenobiotics or radiation:
・Xenobiotic-induced ovarian atrophy may occur as a result of exposure to radiation or chemicals targeting primordial or primary follicles or those affecting sex hormone synthesis/release. Morphological differentiation of spontaneous age-related atrophy from chemically-induced atrophy in aged animals is difficult.
Comment
The morphological patterns of age-related ovarian atrophy are varied and influenced by many factors. The vaginal or uterine morphology is often influenced by the ovarian changes. If the ovary has prominent atretic or cystic follicles and lacks corpora lutea, the vagina may show cornification indicating an increase in the 17 beta-estradiol/progesterone ratio (persistent estrus). Alternatively, animals with prominent CL sometimes have vaginal mucification, indicating a decrease in the 17 beta-estradiol/progesterone ratio (i.e., persistent diestrus). Age-related atrophy of the ovary is also heavily influenced by multiple factors including species, strain and housing conditions. Age-related ovarian atrophy is often not diagnosed in carcinogenicity studies and its incidence in aged animals is likely underappreciated.
References
5Alison et al. (1990), 31Davis et al. (1999), 120Maekawa et al. (1996), 155Peluso and Gordon (1992).
Atrophy (N) Ovary (Figure 55)
Figure 55.
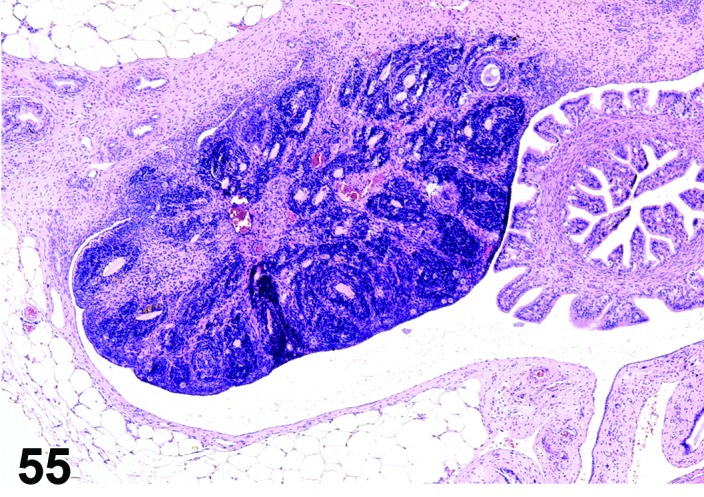
Atrophy, Ovary, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Cessation of the normal estrous cycle caused by toxicant-induced reduction of oocytes or sex cord/stromal cells, or alteration of hypothalamic-pituitary-ovarian axis that ultimately results in decreased gonadotropin-releasing hormone (GnRH), luteinizing hormone (LH) and/or follicle stimulating hormone (FSH).
Diagnostic Features
・Small ovary.
・Decreased number or absence of oocytes, follicles and corpora lutea.
・No patterns indicating normal estrous cycling.
・The morphologic appearance of the ovary depends on the length of time the toxicant has been administered and the target of the toxic agent; the earliest change is often a decrease in healthy antral follicles, but corpora lutea may be normal or only slightly reduced in number. In later stages, no new corpora lutea are observed, but early follicular development may still be seen if primordial follicles are present.
・Interstitial cells may be small and spindle-shaped or enlarged and vacuolated.
Differential Diagnoses
Age-related atrophy:
・Difficult to distinguish morphologically from a test article-related change, particularly at the end of longer term studies (>90 days); short-term studies are often needed to determine if there is a test article-related effect. Careful comparison with controls is essential.
Immaturity:
・Key histomorphologic features in ovarian development as described during PND 22-32 can be used to distinguish the normal developing ovary, such as numerous primordial and primary follicles that can be readily visualized at PND 20 to PND 25 in the immature rat ovary, that are typically found in dense clusters scattered along the cortical periphery at the ovarian hilus, which are less commonly observed in the mature or senescent ovary.
Comment
Destruction of oocytes in primordial follicles by radiation or ovotoxic agents such as 4-vinylcyclohexene diepoxide or maternal treatment with busulfan results in ovarian atrophy within a short period of time. Lesions in the pituitary gland causing a decrease in FSH release (i.e., space occupying neoplasia) also can cause ovarian atrophy. The immature ovary may resemble atrophy.
References
5Alison et al. (1990), 31Davis et al. (1999), 79Hoyer (2004), 157Picut et al. (2014), 212Yoshida et al. (2005)
Atrophy, corpora lutea (N) Ovary (Figure 56)
Figure 56.
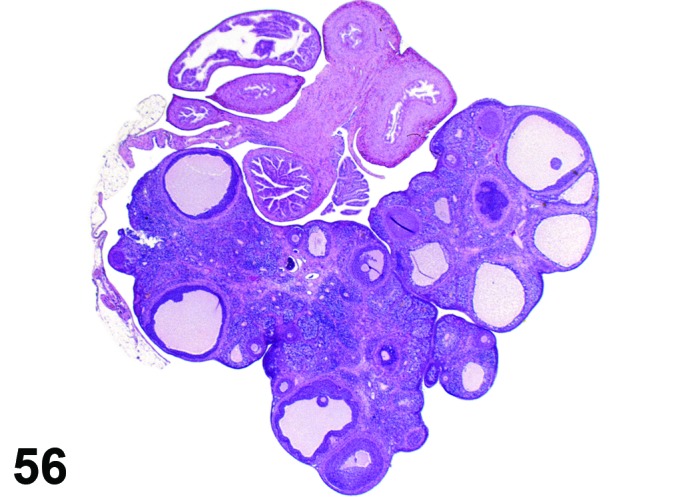
Atrophy, CL, Ovary, rat.
Species
Mouse; Rat.
Synonym(s)
Small corpora lutea.
Diagnostic Features
・Decreased size of new or recently formed corpora lutea.
Differential Diagnoses
Abnormal estrous cycling/anovulation:
・The ovary has old corpora lutea (CL) but lacks new or recently formed CL. The CL are smaller than similar types of corpora lutea in normal cycling rats.
Comment
The sizes of the CL are smaller but the number of CL is normal if the estrous cycle is normal. This change might be induced by chemicals that inhibit angiogenesis in CL.
References
171Sato et al. (2009a)
Increased number, corpora lutea (H) Ovary (Figures 57 and 58)
Figure 57.
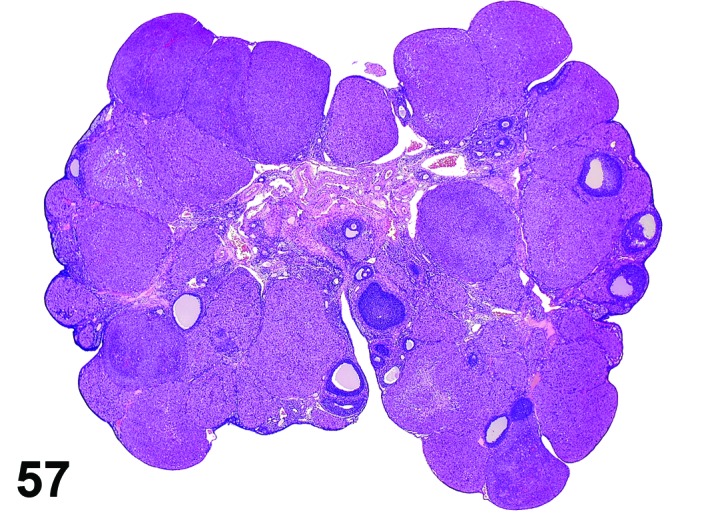
Increased number of CL, Ovary, rat.
Figure 58.
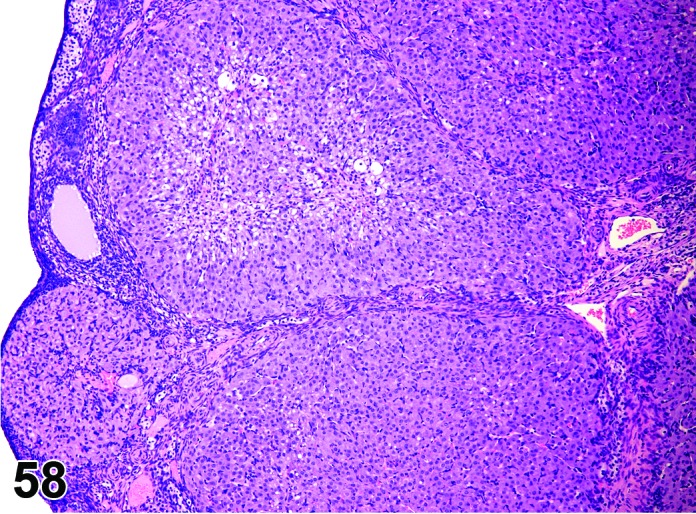
Increased number of CL, Ovary, rat.
Species
Mouse; Rat.
Synonym(s)
Retained corpora lutea.
Pathogenesis/cell of origin
Decreased prolactin resulting in decreased luteolysis of the CL during late proestrus, thus the number of nondegenerating CLs is increased with each successive cycle. Superovulation may also cause this change resulting from increased ovulations per cycle.
Diagnostic Features
・Increased number of corpora lutea, but size is normal.
・Ovary weight may be increased.
Features of increased number, corpora lutea induced by decreased prolactin:
・CL appear morphologically similar to each other (i.e., old, basophilic and eosinophilic CLs normally observed in cycling animals are not readily identified).
・CL have no to minimal luteolysis.
・May be functional (i.e., secrete progesterone) or non-functional; if functional, may see effects of increased progesterone in other parts of the reproductive tract such as vaginal mucification.
・Ovary weight may be increased.
Features of increased number, corpora lutea induced by superovulation:
・The corpora lutea are numerous but show normal morphologic stage-specific changes (i.e., basophilia, eosinophilia, luteolysis).
・Generally estrous cyclicity is maintained.
Differential Diagnoses
Hypertrophy, corpora lutea:
・CL are larger but not increased in number.
Comment
Agents that increase dopamine/decrease prolactin and agents that cause superovulation (PMSG or hCG) can induce this change. If retained corpora lutea from decreased prolactin are hormonally inactive, the estrous cycle may not be disturbed.
References
104Kumazawa et al. (2009), 105Löseke and Spanel-Borowski (1996), 164Rehm et al. (2007)
Decreased number/absent corpora lutea (N) Ovary (Figure 59)
Figure 59.
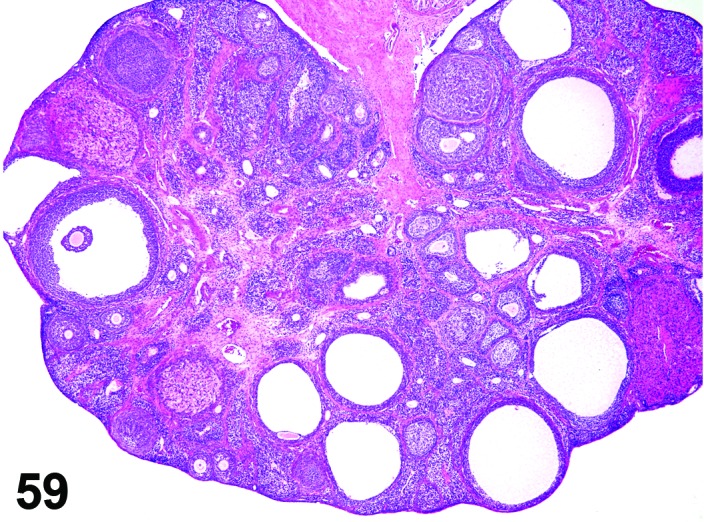
Decreased Number CL, Ovary, rat.
Species
Mouse; Rat.
Modifier
Old; New; Recent.
Pathogenesis/cell of origin
Block or premature cessation of ovulation.
Diagnostic Features
・Decreased number or complete lack of recent, new and/or old corpora lutea (CL).
・Concomitant changes in ovarian morphology vary depending on the cause of the ovulation block and the length of time that ovulation has not occurred:
・A decrease in all follicle types, or in subsets of follicle types (i.e., tertiary follicles) may be present.
・Increased atretic follicles or cystic follicles may be present.
・Luteinized follicles may be present.
・Decreased old corpora lutea indicates lack of normal estrous cycling/ovulation in the previous 3-4 weeks.
・Decreased new and/or recent corpora lutea but presence of old corpora lutea indicates ovulation/estrous cycling has been interrupted within the last 1-3 cycles.
Differential Diagnoses
Atrophy, corpora lutea:
・Decreased size of newly or recently formed corpora lutea.
Comment
A common lesion when estrous cyclicity is disrupted. This morphologic lesion is part of the change observed in senescent ovaries but in short term studies, decreased number/absent corpora lutea should be used in conjunction with other terms to assist in characterizing the observed features.
References
Decreased number/absent follicles (N) Ovary (Figure 60)
Figure 60.
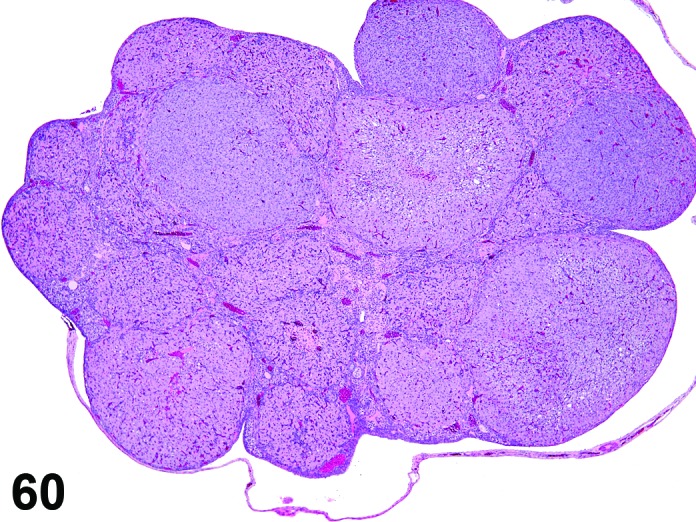
Absent Follicles, Ovary, rat.
Species
Mouse; Rat.
Modifier
Primordial; Primary; Secondary; Vesicular; Tertiary; Atretic.
Diagnostic Features
・Decreased numbers of follicles relative to ovaries from control animals.
・The type(s) of follicles that are decreased should be specified if possible.
Differential Diagnoses
Senescence:
・Spontaneous age-related decline in cyclicity with decreased follicles and CL; this change should also be present in control animals. More common in studies > 3 months duration. For studies of shorter duration, terms that describe the specific morphology such as decreased follicles are preferred.
Atrophy:
・Decreased follicles can be seen as part of the change observed in atrophic ovaries but in short term studies, the term decreased follicles in conjunction with other terms specifically describing the morphology is preferred to a diagnosis of atrophy.
Comment
A decrease in large follicles as a result of increased follicular atresia is observed with a number of cytotoxic drugs, for example cisplatin. The sensitivity of primordial, primary, secondary vesicular or tertiary follicles to this toxicity can vary according to the particular xenobiotic. Degeneration of oogonia in utero or in the immediate postnatal period may cause significant depletion of primordial follicles. CYP1B1 or PCNA immunostaining can be used to highlight oocytes within primordial follicles in rats for the purposes of evaluation and/or counting. The Society of Toxicologic Pathology recommends that follicle counting can be used to further characterize suspected or demonstrated ovarian toxicants and therefore should be considered a second tier technique in rodent toxicology studies. If the loss of primordial follicles is complete or nearly complete, ovarian atrophy (characterized by an absence of follicles in all phases of maturation) as well as secondary atrophy of the uterus and vagina and changes in mammary tissue will be observed in the qualitative assessment.
References
12Bolon et al. (1997), 79Hoyer (2004), 83Ito et al. (2009), 90Kao et al. (1999), 100Kodama et al. (2009), 149Nozaki et al. (2009),162 Regan et al. (2005), 166Sakurada et al. (2009)
Degeneration, corpora lutea (N) Ovary (Figures 61 and 62)
Figure 61.
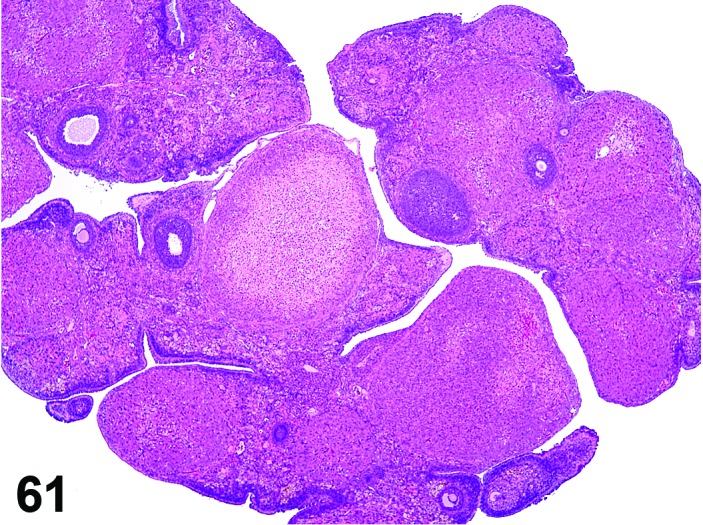
Degeneration, CL, Ovary, rat.
Figure 62.
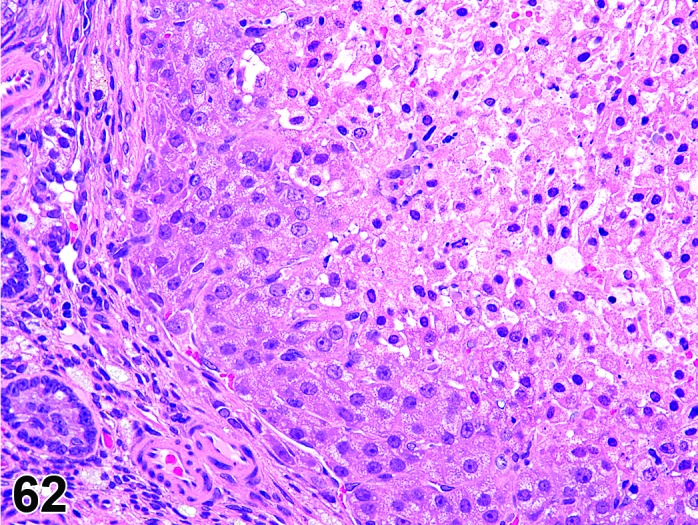
Degeneration, CL, Ovary, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Interruption of circulation such as thrombus formation or disturbance of normal angiogenesis.
Diagnostic Features
・Degeneration/coagulation necrosis of luteal cells in corpora lutea of the most recent ovulation other than at proestrus.
・Hyaline change (eosinophilic homogeneous material) may be observed.
・Mineralization may be seen.
Differential Diagnoses
Normal regression:
・Process observed in CL of the most recent ovulation during proestrus.
・Primarily inflammation and necrosis.
Hyaline change, Mineralization and Fibrosis:
・Can be observed in old, involuted corpora lutea (those from ≥4 cycles ago) in control animals.
Comment
Degeneration is observed normally in CLs of the most recent ovulation during proestrus, and degeneration in excess of that normally observed may be difficult to ascertain.
References
5Alison et al. (1990)
Atretic follicles, increased number (N) Ovary (Figure 63
Figure 63.
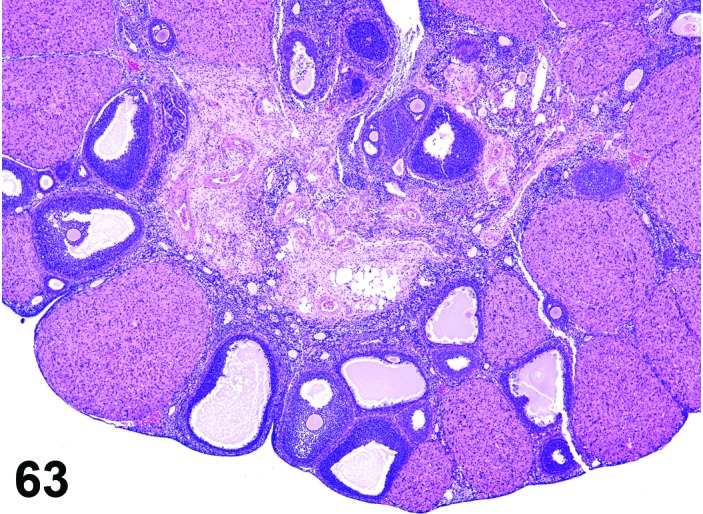
Increased Number of Atretic Follicles, Ovary, rat.
Species
Mouse; Rat.
Synonym(s)
Follicular degeneration.
Modifier
Primordial; Primary; Secondary; Vesicular; Tertiary; Atretic.
Diagnostic Features
・Any or all of the following features may be present and indicate follicular atresia:
・Pyknotic granulosa and/or theca cell nuclei.
・Apoptotic bodies at the periphery of the antrum.
・Cell debris in the antrum.
・Detachment of granulosa cells from follicular basement membrane.
・In addition, the following features may be present in atretic follicles:
・Reduced thickness of the granulosa cell layer.
・Macrophages present in the antrum in late stage.
・Hypertrophy of the theca cell layer.
・Dissolution of the corona radiata.
・Degeneration of the ovum.
・Careful comparison to controls is essential for diagnosis.
・The type(s) of atretic follicles (primordial, primary, secondary, vesicular, tertiary) that are increased should be specified if possible.
Differential Diagnoses
Necrosis, ovarian:
・Necrosis of other structures is present.
Physiologic atresia:
・Under normal physiological conditions, the major peak of atresia occurs in the vesicular and tertiary follicles, but atresia can be observed in follicles at all stages in control animals.
Comment
Atresia is a physiological, degenerative process through which many follicles are removed from the growing pool and involves apoptosis of follicular granulosa cells. The thickness of the granulosa layer reduces as atresia progresses from early to late stage. Increased numbers of atretic follicles can be produced by blocking the pre-ovulatory luteinizing hormone surge with a gonadotropin-releasing hormone (GnRH) antagonist. This prevents ovulation of the pre-ovulatory follicles, which then become atretic. Androgens, IL-6, TNF-α and tamoxifen can also induce atresia.
References
41Durlinger et al. (2000), 89Kaipia and Hsueh (1997), 149Nozaki et al. (2009), 195Tsujioka et al. (2009)
Degeneration, oocyte (N) Ovary (Figure 64)
Figure 64.
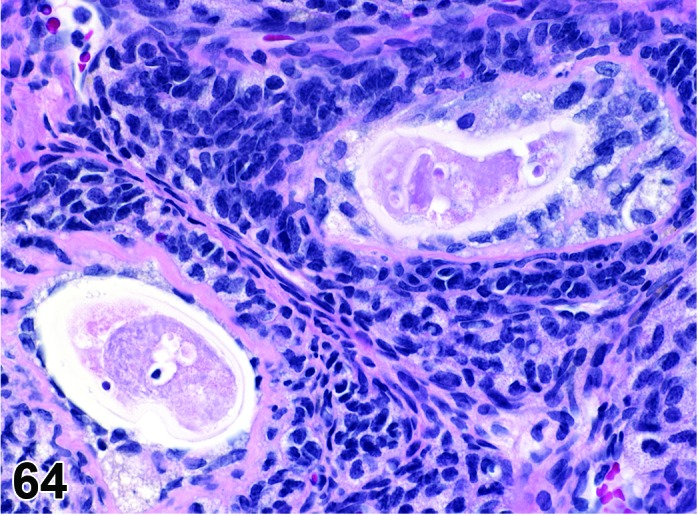
Degeneration, Oocyte, Ovary, rat.
Species
Mouse; Rat.
Diagnostic Features
・Nuclear changes (chromatin condensation, pyknosis, fragmentation).
・Disorganization of corona radiata.
・Disruption and thinning of zona pellucida.
・No evidence of degeneration/necrosis of granulosa or theca cells.
Differential Diagnoses
Granulosa cell apoptosis:
・Pyknosis and/or karyorrhexis of nuclei of individual cells.
・Individual cell shrinkage with dense eosinophilic cytoplasm.
Comment
Oocyte degeneration and loss have been shown to occur following exposure of rodent ovaries to ionizing radiation or cytotoxic drugs. Oocyte degeneration can be observed in the absence of changes to the granulosa cells. Atresia of primordial, primary and secondary follicles begins with degeneration of the oocyte and is then accompanied by granulosa cell degeneration.
References
66Greaves (2012), 73Harada et al. (2003), 192Toaff et al. (1979)
Pigment (N) Ovary (Figures 65 and 66)
Figure 65.
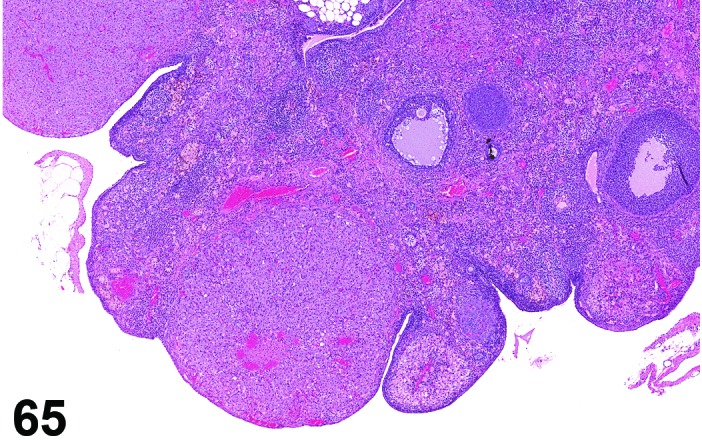
Ovary pigment, rat.
Figure 66.
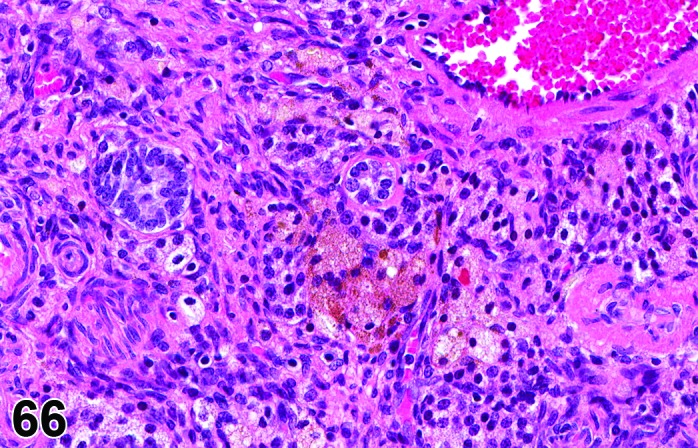
Ovary pigment, rat.
Species
Mouse; Rat.
Modifier
Hemosiderin; Lipofuscin; Ceroid. Alternatively, if pigment isn’t definitively identified, colors can be used as modifiers.
Pathogenesis/cell of origin
Pigmentation in the ovary most commonly consists of lipofuscin and/or hemosiderin and is present in interstitial cells and ovarian macrophages.
Diagnostic Features
Hemosiderin:
・Consists of a golden brown, granular pigment.
・Stains used to identify the iron content include Prussian blue reaction or Perl’s stain.
Lipofuscin/ceroid:
・Consists of breakdown products of cell membrane lipids.
・Associated with cell turn over, degeneration and/or necrosis.
・Pigment is golden brown and granular.
・Special stains include Sudan black, Schmorl’s stain, Oil red O, carbol lipofuscin stain, Periodic Acid Schiff’s (PAS) reaction, lysosomal acid phosphatase, esterase and Ziehl-Neelsen acid fast stains.
Ceroid:
・Variant of lipofuscin with similar staining properties.
・Golden yellow autofluorescence under ultraviolet light.
・Stains include Sudan Black B, Schmorl’s reaction and Oil red O, PAS and Ziehl-Neelsen acid fast stains.
Differential Diagnoses
None.
Comment
Previous follicular hemorrhage can result in focal areas containing hemosiderin-laden macrophages. Lipofuscin (includes ceroid) accumulates with age in rats and mice and is the most common pigment. It is found mainly in the interstitial cells. A particularly high incidence has been reported for C57BL/6 mice. Lipofuscin is a complex of alcohol-insoluble, oxidized polyunsaturated lipid pigment resulting from the peroxidation of unsaturated lipids. Melanin is reported occasionally in the pigmented B6C3F1 mouse.
References
Edema (N) Ovary (Figures 67 and 68)
Figure 67.
Edema, Ovary, rat.
Figure 68.
Edema, Ovary, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
In the ovary, potential mechanisms underlying the development of edema are the same as for other tissues and might include increased vascular permeability, increased hydrostatic pressure, and reduced intravascular oncotic pressure.
Diagnostic Features
・Clefts or clear empty spaces within the stroma surrounding rather than replacing or displacing resident cells and structures.
・In massive edema, resident cells and structures may be suspended in a clear field.
・Clusters of stromal cells around clear empty spaces may impart a microcystic appearance.
Differential Diagnoses
None.
Comment
Edema is an infrequently used term in toxicologic pathology with respect to the ovary. The term has mainly been applied in experimental models of vascular compromise due to ligation or torsion.
References
Ovotestis (N) Ovary (Figures 69 and 70)
Figure 69.
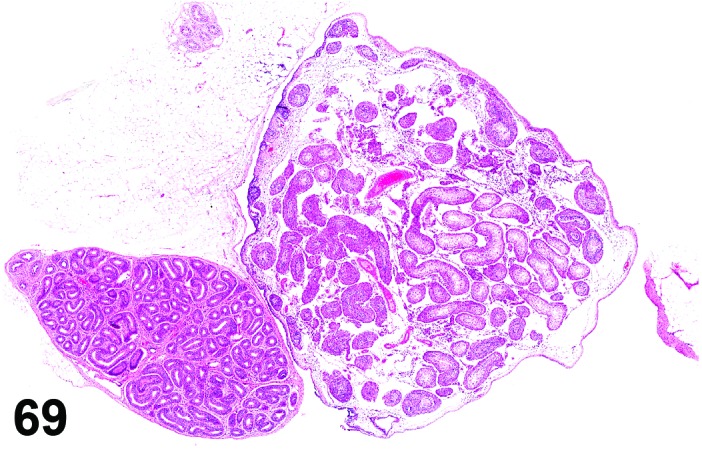
Ovotestis, Ovary.
Figure 70.
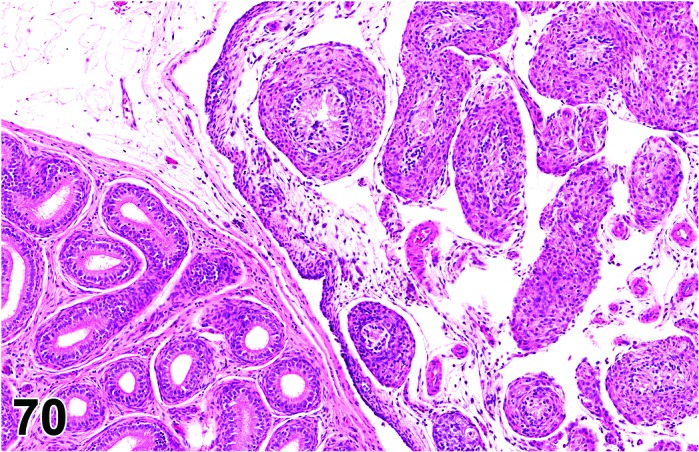
Ovotestis, Ovary.
Species
Mouse; Rat.
Synonym(s)
Intersex; Hermaphroditism.
Pathogenesis/cell of origin
Development of ovotestis is a poorly understood malformation. Normal testicular differentiation depends on the SRY gene on the Y chromosome. Hermaphroditism is the overall term for animals with an ovotestis (unilateral or bilateral) or having an ovary on one side and a testis on the contralateral side.
Diagnostic Features
・Gonadal tissue containing both ovarian and testicular tissue.
・Seminiferous tubules are lined by Sertoli cells and may also contain spermatogonia.
・Follicular development and corpora lutea have been reported in the ovarian component.
Differential Diagnoses
Tumor, Sertoli cell, benign:
・Compression is present.
・Lack of significant cellular pleomorphism.
・Lack of areas of necrosis/hemorrhage.
・Spermatogonia are not present.
Hyperplasia, Sertoli cell:
・Diameter of lesion is smaller than or equal to the size of a corpus luteum and
・Minimal or no compression.
・Spermatogonia are not present.
Tumor, Sertoli cell, malignant:
・Cellular pleomorphism is present.
・Areas of hemorrhage and necrosis are present.
・Infiltrative growth pattern or disruption of ovarian capsule is present.
・Metastases are present.
・Spermatogonia are not present.
Comment
This condition is very rare in rats and mice. It is more common in chimeric mice.
References
37Diters et al. (2007), 69Greaves et al. (1992), 88Kai et al. (2003), 126McIntyre and La Perle (2007)
Follicle, polyovular (N) Ovary (Figure 71)
Figure 71.
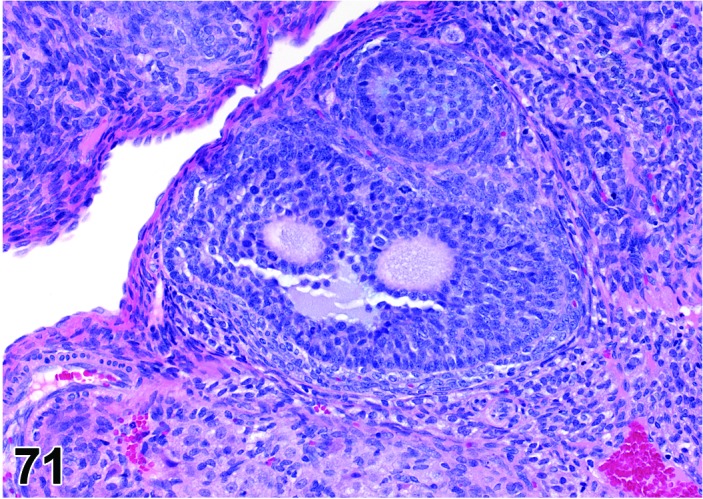
Polyovular Follicle, Ovary, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Polyovular follicles appear to occur due to a failure of the normal mechanisms separating oocytes during the formation of primordial follicles in the neonatal rodent.
Diagnostic Features
・Multiple (two or more) oocytes surrounded by granulosa cells within a common follicle.
Differential Diagnoses
None.
Comment
Polyovular follicles occur with a low frequency in mice and rats though there may be some slight differences among the various strains. The administration of compounds with estrogenic activity to neonatal mice (before Day 5 post-partum) results in an increase in the incidence of polyovular follicles. This has been attributed to estrogenic dysregulation of genes involved in breakdown of germ cell cysts during the formation of primordial follicles. Work in a number of mutant mouse models has also implicated a number of specific gene products as potential participants in the formation of polyovular follicles.
References
21Chen et al. (2007), 95Kent (1960), 96Kent (1962), 98Kim et al. (2009), 191Telfer and Gosden (1987)
Ectopic Tissue (N) Ovary
Species
Mouse; Rat.
Diagnostic Features
・Presence of histologically normal tissue from a non-ovarian organ/site within the ovary.
Differential Diagnoses
Ovotestis:
・Gonad containing both ovarian follicular and testicular tubular structures.
Teratoma, benign; Teratoma, malignant:
・Encapsulated, usually expansile mass composed of an amalgam of tissues derived from all three germ layers (endoderm, mesoderm, ectoderm).
Metastases
Comment
Ectopic tissue within the ovary is rarely reported in rat and mouse. In humans, rare congenital lesions consistent with ectopic tissue in the ovary include splenic-ovarian fusion (ovarian splenosis), adrenal cortical rests, and uterus-like ovarian masses.
References
23Clement (2002)
Immaturity (N) Ovary (Figure 72)
Figure 72.
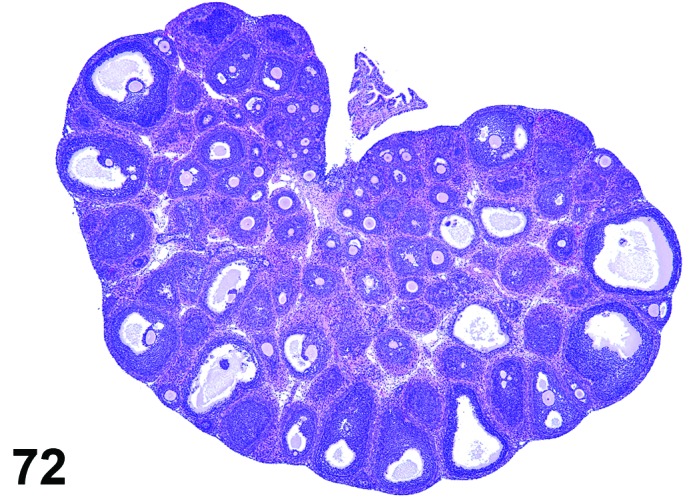
Immature Ovary (PND22), rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Age related development of ovary.
Diagnostic Features
・Morphologic features of ovarian development prior to the onset of estrous cycling and/or ovulation..
Comment
The developing ovary is important in reproductive toxicity studies. There are key histomorphologic features in ovarian development as described during PND 22-32 that can be used to distinguish the normal developing ovary, such as numerous primordial and primary follicles that can be readily visualized at PND 20 to PND 25 in the immature rat ovary, that are typically found in dense clusters scattered along the cortical periphery at the ovarian hilus. Clusters of primordial and primary follicles are less commonly observed in the mature ovary. Histologically, the presence of one or more corpora lutea would be consistent with a mature ovary. Immaturity of the ovary relative to animal age may occasionally be encountered secondary to test article administration as demonstrated in immature mice administered chlorpromazine or perphenazine.
References
Hypertrophy, interstitial cell (N) Ovary (Figures 73 and 74)
Figure 73.
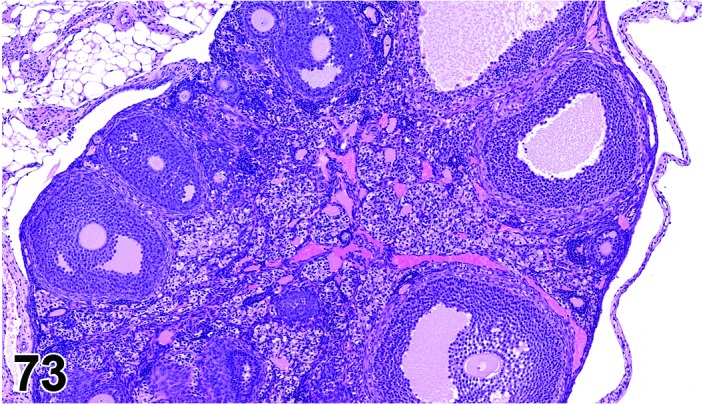
Interstitial Cell Hypertrophy, Ovary, mouse.
Figure 74.
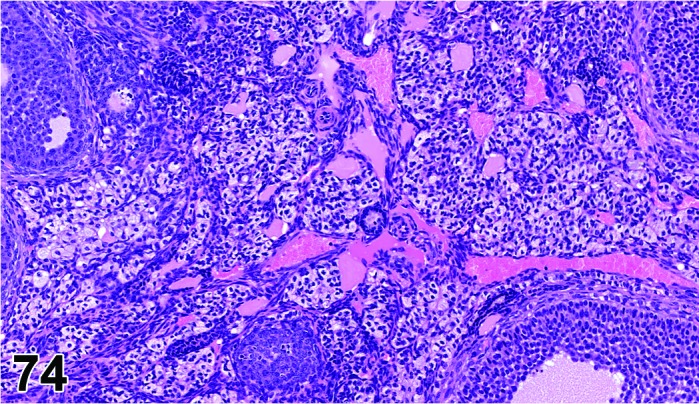
Interstitial Cell Hypertrophy, Ovary, mouse.
Species
Mouse; Rat.
Pathogenesis/cell of origin
May occur as a physiological response to increases in luteinizing hormone secretion or in response to xenobiotic administration.
Diagnostic Features
・Interstitial cells, arranged in cords or nests, are enlarged and polyhedral with ample clear to pale-eosinophilic, sometimes vacuolated cytoplasm.
・Decreased nuclear: cytoplasmic ratio.
Differential Diagnoses
Hyperplasia, interstitial cell:
・Hyperplastic cells are increased in proportion to other ovarian structures.
Vacuolation, interstitial cell:
・Vacuolated cells may appear to be enlarged due to accumulation of cytoplasmic vacuoles.
・May be difficult to discern from true hypertrophy.
Comment
Interstitial cell hypertrophy is a common change observed in ovarian aging and atrophy, and often occurs in combination with interstitial cell hyperplasia. Hypertrophy in non-atrophied ovaries of adult rodents has been reported in association with the administration of gonadotropins as well as some organophosphate and thiocarbamate compounds. Interstitial cells are steroidogenically active and, in some cases, hypertrophied cells contain oil-red-O-positive-staining neutral lipid.
References
5Alison et al. (1990), 31Davis et al. (1999), 214Yuan and Foley (2002)
Hypertrophy, corpora lutea (N) Ovary (Figures 75 and 76)
Figure 75.
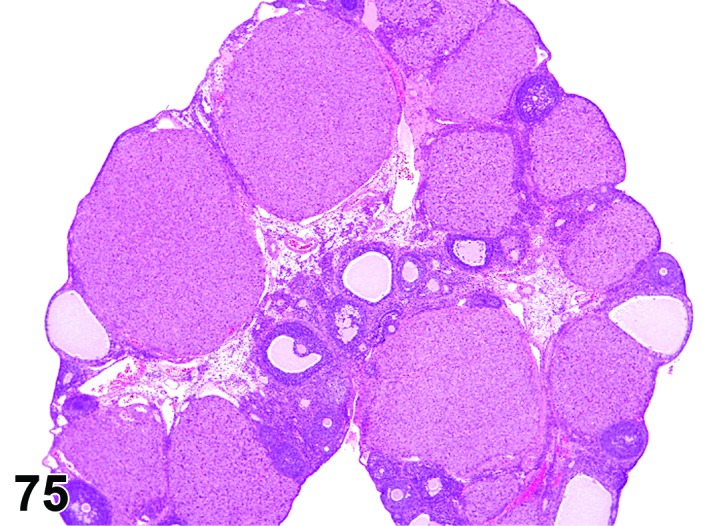
CL Hypertrophy, Ovary, rat.
Figure 76.
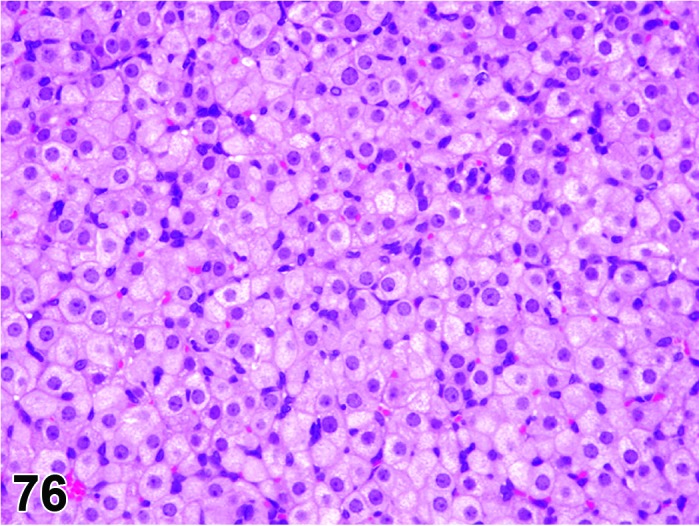
CL Hypertrophy, Ovary, rat.
Species
Mouse; Rat.
Synonym(s)
Enlarged corpora lutea; Activated corpora lutea; Pseudopregnancy.
Pathogenesis/cell of origin
Increased activity of steroidogenesis in corpora lutea.
Increased prolactin secretion with preservation of functional corpora lutea.
Diagnostic Features
・Large corpora lutea compared with the CL of the most recent diestrus.
・Enlarged luteal cells with lightly basophilic or eosinophilic cytoplasm.
・Few degenerative luteal cells present in affected CL.
・Not all CL are affected.
・May be accompanied by changes in other reproductive organs such as mammary gland lobuloalveolar hyperplasia and lactogenic secretion due to the increased prolactin, and mucification of the vagina from increased progesterone.
・Ovary weight may be increased.
Differential Diagnoses
Corpora lutea, increased number:
・The CLs are of normal size.
Vacuolation, corpora lutea, increased:
・The CLs are of normal size or larger than normal.
・Increased cytoplasmic vacuolation is present.
Comment
This change can be seen spontaneously, as a result of increased prolactin, or with agents that directly or indirectly activate steroidogenesis of corpora lutea. Hypertrophied luteal cells often produce progesterone.
References
30Davis et al. (1997), 40Dodo et al. (2009), 82Ishii et al. (2009), 164Rehm et al. (2007), 175Shibayama et al. (2009), 188Taketa et al. (2011), 214Yuan and Foley (2002)
B. Nonneoplastic Proliferative Lesions
Hyperplasia, interstitial cell (H) Ovary (Figures 77 and 78)
Figure 77.
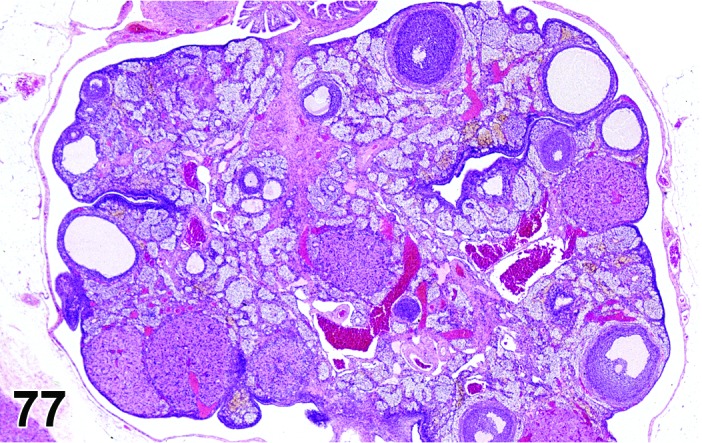
Interstitial Cell Hyperplasia, Ovary, rat.
Figure 78.
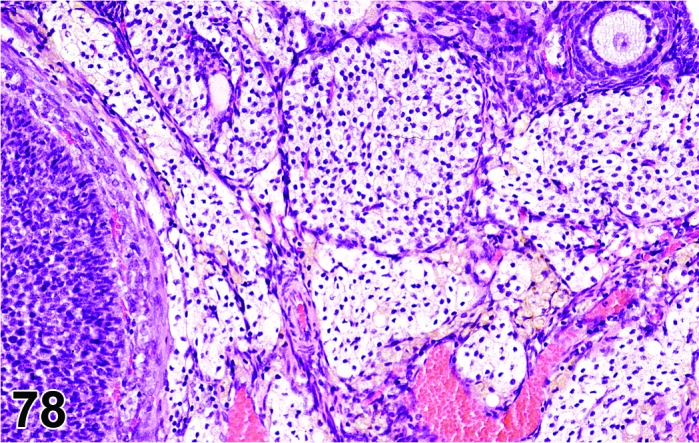
Interstitial Cell Hyperplasia, Ovary, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
May occur as a physiological response to increases in luteinizing hormone secretion or in response to xenobiotic administration.
Diagnostic Features
・Interstitial cells are increased in proportion to other ovarian structures.
・Interstitial cells, arranged in cords or nests, they may be enlarged and polyhedral with ample clear to pale-eosinophilic, sometimes vacuolated cytoplasm.
Differential Diagnoses
Vacuolation, interstitial cell:
・Vacuolated cells may appear to be enlarged due to accumulation of cytoplasmic vacuoles.
・May be difficult to discern from true hypertrophy.
Hypertrophy, interstitial cell:
・Interstitial cells, arranged in cords or nests, are enlarged and polyhedral with ample clear to pale-eosinophilic, sometimes vacuolated cytoplasm.
・Decreased nuclear: cytoplasmic ratio.
Hyperplasia, tubulostromal (H) Ovary (Figures 79 and 80)
Figure 79.
Tubulostromal Hyperplasia, Ovary, mouse.
Figure 80.
Tubulostromal Hyperplasia, Ovary, mouse.
Species
Mouse; Rat.
Synonym(s)
Hyperplasia, epithelial.
Pathogenesis/cell of origin
Surface epithelium and stromal cells of the ovary.
Diagnostic Features
・Infiltration of surface epithelial cells into the ovary accompanied by a variable proliferation of stromal cells.
・Generally a diffuse lesion, but can be focal/nodular.
・May form a ring of hyperplasia around the ovary, especially in mice.
・Minimal extension onto the bursal surface may occur.
・No atypia.
・Focal lesions are less than or equal to the size of a corpus luteum.
・No distinct compression.
Differential Diagnoses
Adenoma, tubulostromal:
・Focal lesion larger in diameter than a corpus luteum or
・Compression is evident and
・Slight pleomorphism/atypia may be present and
・Invasion, if present, limited to ovarian bursa.
Hyperplasia, cystic/papillary:
・Absence of interstitial cell component and
・Epithelial cells generally taller than in tubulostromal proliferative lesions and/or
・Presence of cystic and/or papillary structures.
Comment
Diffuse hyperplastic lesions are very common in old mice and in some rat strains.
References
5Alison et al. (1990), 31Davis et al. (1999), 32Davis et al. (2001), 39Dixon et al. (1999), 133Montgomery and Alison (1987), 155Peluso and Gordon (1992)
Hyperplasia, cystic/papillary (H) Ovary (Figure 81)
Figure 81.
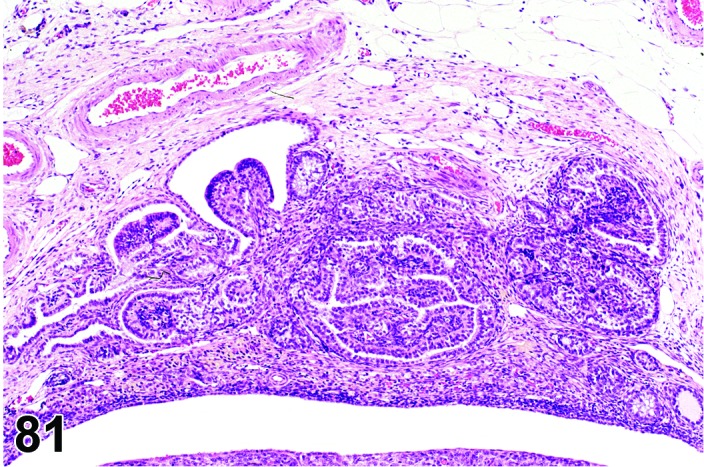
Cystic/Papillary Hyperplasia, Ovary rat.
Species
Mouse; Rat.
Synonym(s)
Hyperplasia, papillary; Hyperplasia, cystic.
Pathogenesis/cell of origin
Surface epithelium of the ovary.
Diagnostic Features
・Small, focal, often cystic lesions arising from the surface epithelium.
・Often present at hilus of ovary, but no evidence of intratubular (rete ovarii) origin
・Cystic lesions lined by single layer of cuboidal to columnar epithelium that is sometimes ciliated.
・Cystic lesions may show papillary projections into the cyst lumen with up to 3 layers of well-differentiated epithelial cells lining the papilla.
・Non-cystic lesions consist of papillary projections of the surface epithelium of the ovary.
・No cellular atypia.
・Proliferative lesion is smaller than or equal in size to a corpus luteum.
Differential Diagnoses
Hyperplasia, tubulostromal:
・Lesion is generally within the ovary and not predominantly on surface and
・Minimal if any extension onto ovarian surface and
・Often are diffuse lesions and
・Presence of stromal hyperplasia and
・Lack of cystic or papillary structures and/or
・Epithelium often shorter than cystic/papillary hyperplasia.
Cystadenoma:
・Minimal cellular atypia may be present and/or
・Lesion larger than a corpus luteum.
Adenoma, tubulostromal:
・Focal lesion larger in diameter than a corpus luteum or
・Compression is evident and
・Slight pleomorphism/atypia may be present and
・Invasion, if present, limited to ovarian bursa.
Hyperplasia, rete ovarii:
・Evidence of origin within rete ovarii.
・May have focal polypoid in-growths.
・Nuclei often present in apical cytoplasm.
・May contain areas of ciliated cells.
・May contain smooth muscle.
Comment
Common lesion particularly in the hilus of some rat and mouse strains. The main differential diagnosis is hyperplasia of the rete ovarii, however, distinguishing cystic/papillary hyperplasia from rete ovarii hyperplasia is difficult and arbitrary if evidence of intratubular origin (for the rete lesion) isn’t observed.
References
32Davis et al. (2001), 39Dixon et al. (1999), 133Montgomery and Alison (1987), 155Peluso and Gordon (1992), 114Long (2002)
Hyperplasia, granulosa cell (H) Ovary (Figure 82)
Figure 82.
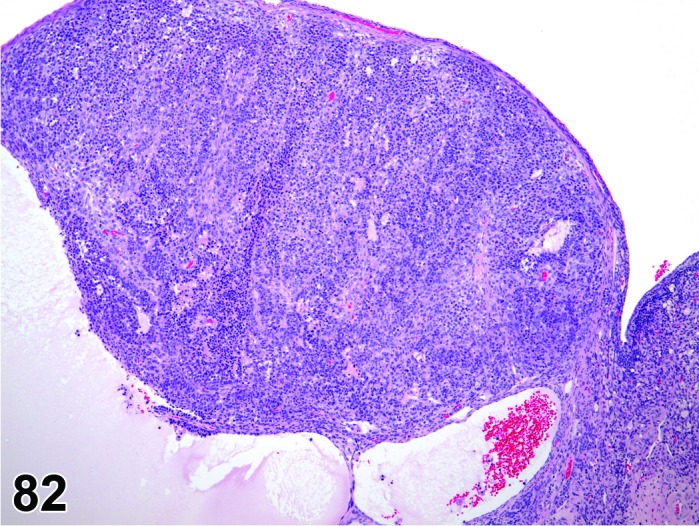
Hyperplasia of Granulosa Cells, Ovary, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Sex cord/stromal cells.
Diagnostic Features
・Cellular morphology resembles that of normal granulosa cells.
・Focal group or groups of disorganized granulosa cells.
・Lesions may be cystic.
・Solid lesion is smaller than or equal to the size of a corpus luteum.
・Nuclei round to oval with coarsely stippled chromatin.
・Cytoplasm varies from scant to moderate depending upon degree of luteinization and is faintly eosinophilic and vacuolated.
・Mitotic figures may be seen.
・Atypia minimal or not present.
・No distinct compression of surrounding tissue.
Differential Diagnoses
Tumor, granulosa cell, benign:
・Distinct compression of surrounding tissue.
・Variable degrees of luteinization may be present.
・Diameter of non-cystic, solid proliferative lesion is larger than size of a corpus luteum.
Luteoma, benign; Thecoma, benign; or Tumor, Sertoli cell, benign:
・One cell type predominates (>70%).
・Discrete nodule.
・Compression is present.
・Diameter of proliferative lesion is larger than the size of a corpus luteum (thecoma and Serotoli cell tumors) or 3 corpora lutea in the case of a luteoma.
Comment
Granulosa cell hyperplasia is typically focal/multifocal and must be differentiated from tangential or parasagittal sections through thick layers of granulosa cells commonly present around large follicles. The biology of granulosa cell hyperplasia is unknown and may represent the precursor lesion to granulosa cell neoplasia. Selective estrogen receptor modulator (SERM) treatment of rats results in increased plasma concentrations of luteinizing hormone and estradiol-17β (E2) and failure of ovulation manifested by retained anovulatory (luteinized) follicles, lack of corpora lutea, and hyperplasia of granulosa cells. In this study, cystic lesions with total diameter greater than a corpus luteum were found to be reversible and were thus termed granulosa cell hyperplasia rather than adenoma. Therefore this guideline suggests using the diameter of solid tissue growth (i.e. non-cystic) rather than the total diameter as a diagnostic feature.
References
5Alison et al. (1990), 31Davis et al. (1999), 32Davis et al. (2001), 39Dixon et al. (1999), 112Lewis (1987), 115Long et al. (2001), 133Montgomery and Alison (1987)
Hyperplasia, theca cell (H) Ovary
Species
Mouse; Rat.
Pathogenesis/cell of origin
Sex cord/stromal cells.
Diagnostic Features
・Composed of densely packed fusiform cells, usually arranged in interlacing bundles and whorls giving a nodular appearance.
・Variable amount of lipid vacuoles are present in cytoplasm.
・Focal discrete lesion that is well demarcated from surrounding tissue with no compression.
・Luteinization may be present.
・No cellular atypia.
・Size is smaller than or equal to the size of a corpus luteum.
Differential Diagnoses
Thecoma, benign:
・Distinct compression of surrounding tissue.
・Minimal cellular atypia may be present.
・Variable degrees of luteinization may be present.
・Size of proliferative lesion is larger than size of a corpus luteum.
・Collagen, if present, is sparse and is arranged around bundles of cells.
Fibrosis/Fibroplasia:
・Lacks lipid-laden cells and luteinization.
・Collagen is present and is arranged around individual cells.
・Fibroplasia may be associated with inflammatory cell infiltrates and angiogenesis.
Fibroma:
・Lacks lipid-laden cells and luteinization.
・Compression is present.
・Collagen is present and is arranged around individual cells rather than around bundles of cells.
Luteoma, benign; Tumor, granulosa cell, benign; or Tumor, Sertoli cell, benign:
・One cell type predominates (>70%).
・Discrete nodule.
・Cellular atypia may be present.
・Size of proliferative lesion is larger than the size of a corpus luteum (granulosa and Serotoli cell tumors) or 3 corpora lutea in the case of a luteoma.
Comment
In a rat model of polycystic ovary syndrome (PCOS) it was found that theca cells were in excess and the ovaries had histopathological features similar to those in women with PCOS.
References
5Alison et al. (1990), 31Davis et al. (1999), 32Davis et al. (2001), 39Dixon et al. (1999), 205Wang et al. (2012)
Hyperplasia, Sertoli cell (H) Ovary (Figure 83)
Figure 83.
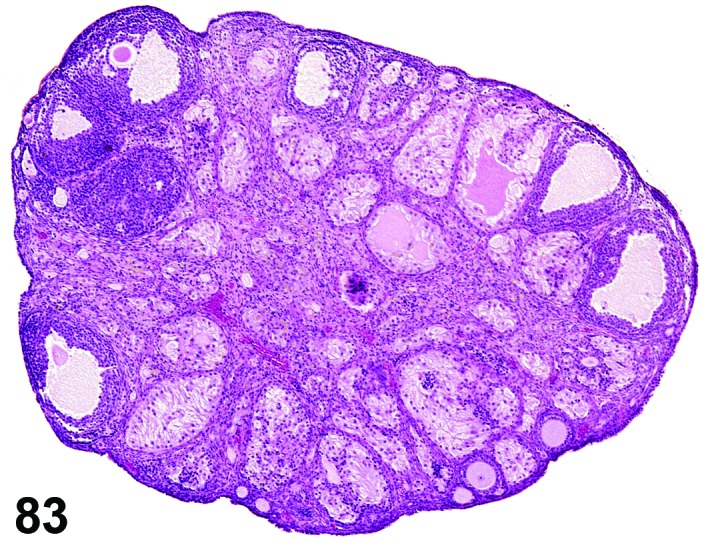
Hyperplasia of Sertoli Cells, Ovary, mouse.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Sex cord/stromal cells.
Diagnostic Features
・Sertoli cell tumors resemble their testicular counterpart histologically.
・Often arise in the hilus.
・Characterized by seminiferous-like tubules lined by cells with basally located nuclei and abundant faintly eosinophilic, vacuolated cytoplasm extending into the lumen.
・Frequently have areas with focal nests of Sertoli cells without obvious tubular structures.
・No cellular atypia.
・Focal lesion is smaller than or equal to the size of a corpus luteum.
・Compression or capsule is not present.
Differential Diagnoses
Tumor, Sertoli cell, benign:
・Distinct compression of surrounding tissue.
・Minimal cellular atypia may be present.
・Size of proliferative lesion is larger than size of a corpus luteum.
・Fibrous capsule usually present.
Luteoma, benign; Thecoma, benign or Tumor, granulosa cell, benign:
・One cell type predominates (>70%).
・Discrete nodule.
・Compression is present.
・Cellular atypia may be present.
・Size of proliferative lesion is larger than the size of a corpus luteum (thecoma and granulosa cell tumors) or 3 corpora lutea in the case of a luteoma.
Comment
The formation of testis-like tubules is most often seen in senile ovaries and has been observed in rats hypophysectomized at 26 days of age with tubules appearing at 5 months, but most common at 11 months of age. Spontaneous Sertoli cell hyperplasia has been described in the Sprague-Dawley rat.
References
5Alison et al. (1990), 31Davis et al. (1999), 32Davis et al. (2001), 39Dixon et al. (1999), 43Engle (1946), 71Gregson et al. (1984), 133Montgomery and Alison (1987)
Hyperplasia, sex cord stromal, mixed (H) Ovary (Figures 84 and 85)
Figure 84.
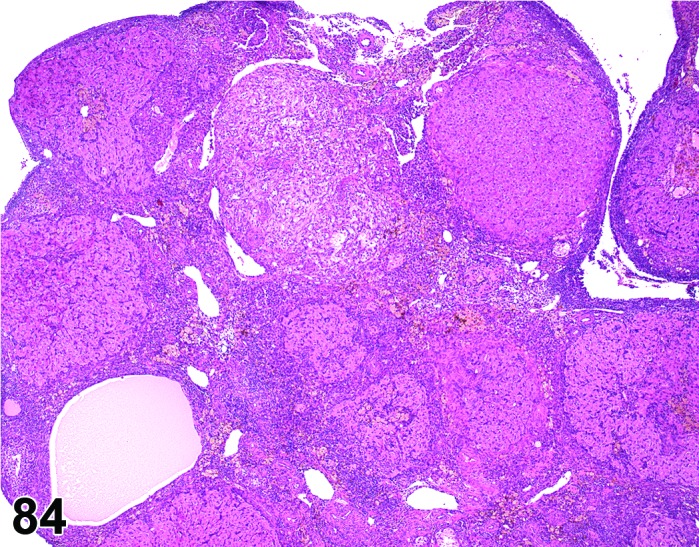
Sex Cord Stromal Hyperplasia, Multifocal, Mixed, Ovary, rat.
Figure 85.
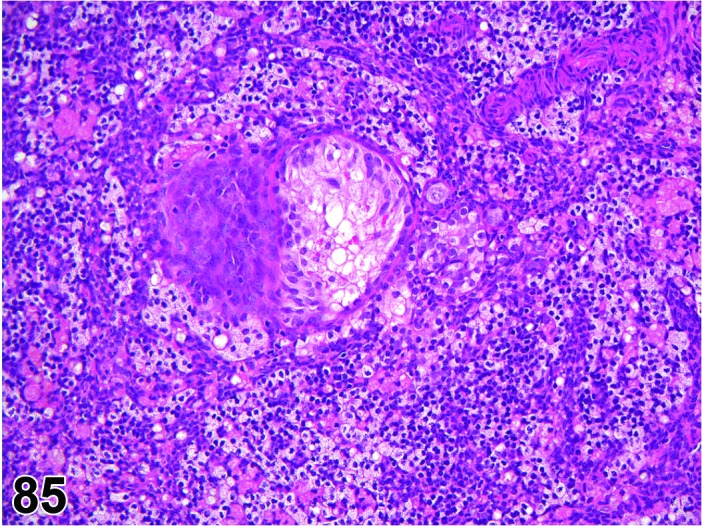
Sex Cord Stromal Hyperplasia, Diffuse, Mixed, Ovary, rat.
Species
Mouse; Rat.
Modifier
Diffuse; Focal.
Pathogenesis/cell of origin
Sex cord/stromal cells.
Diagnostic Features
・Lesion may show variable morphological spectrum including granulosa cells, theca cells, Sertoli cells and luteal cells, often in varying quantities, but no one cell type is predominant (>70%).
・Common lesion.
・Cellular atypia minimal or not present.
・In old rats, two distinct lesions can be distinguished - focal and diffuse mixed type.
・In mice, generally only the focal type is seen.
Focal:
・Focal lesions are discrete and well demarcated from the adjacent tissue.
・Size of solid tissue growth (e.g. non-cystic area) does not exceed the size of a large corpus luteum.
・Significant compression or effacement of ovarian tissue not present.
Diffuse mixed type (old age type):
・Lesion which may be present multifocally or diffusely throughout the ovary and involve the entire ovary.
・Poorly demarcated; has gradual transition into the adjacent tissues.
・No significant atypia or invasion.
・Comprised of a mixture of stromal and sex cord cells. Cell types most frequently associated with this type of lesion are Sertoli type cells and stromal cells. The Sertoli type cells have a clear cytoplasm and are arranged in cords or strands, occasionally in tubules.
・May cause an overall increase in the size of the ovary, but the diameter of the lesion itself is smaller than or equal to the diameter of a normal ovary.
Differential Diagnoses
Tumor, sex cord stromal, mixed, benign:
・Differentiation between hyperplasia and sex cord stromal tumors is arbitrary and difficult.
・Distinct mass with compression of adjacent tissue.
・Focal atypia may be present.
・Focal lesions with a non-cystic area larger than a corpus luteum is a tumor
・Diffuse mixed lesion larger than size of normal ovary is a tumor
Tumor, granulosa cell, benign or Tumor, Sertoli cell, benign or Luteoma, benign or Thecoma, benign:
・One cell type predominates (>70%).
・Discrete nodule.
・Compression is present.
・Diameter of proliferative lesion is larger than the size of a corpus luteum (granulosa cell, thecoma and Sertoli cell tumors) or 3 corpora lutea in the case of a luteoma.
Comment
The diffuse mixed type (old age type) hyperplasia is common in old rats.
References
5Alison et al. (1990), 112Lewis (1987), 133Montgomery and Alison (1987)
Hyperplasia, smooth muscle, mesovarial (H) Ovary (Figure 86)
Figure 86.
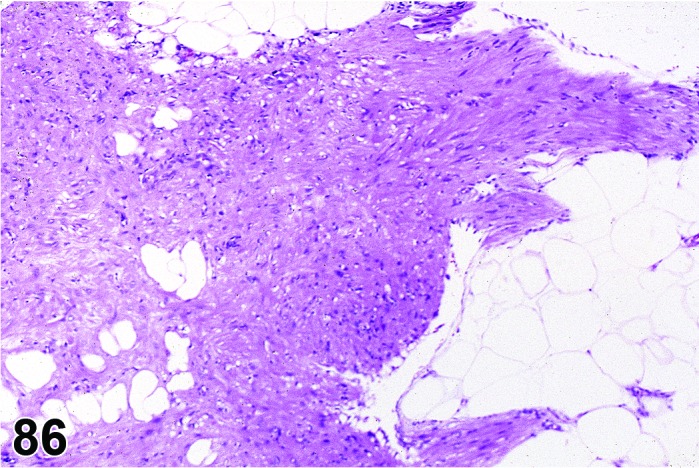
Hyperplasia, Smooth Muscle, Mesovarium, rat.
Species
Rat.
Pathogenesis/cell of origin
Smooth muscle.
Diagnostic Features
・Irregular areas of excessive smooth muscle within the mesovarium, suspensory ligaments or ovarian hilus.
・Smooth muscle fibers arranged in parallel or irregularly.
Differential Diagnoses
Leiomyoma, mesovarial:
・Cells of leiomyomas are arranged in interlacing and criss-crossing bundles or sometimes whorls.
Comment
Hyperplasia of the mesovarial smooth muscle has been reported in association with the development of leiomyomas induced experimentally in rats after administration of β-adrenergic agonists. There have been no reports of this finding in mice.
References
63Gopinath and Gibson (1987), 94Kelly et al. (1993), 140Nelson et al. (1972)
Hyperplasia, rete ovarii (H) Ovary (Figure 87)
Figure 87.
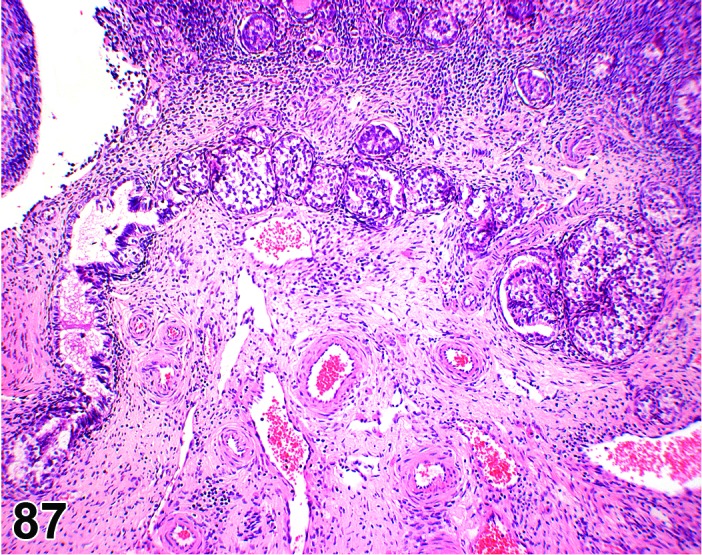
Hyperplasia, Rete Ovarii, Ovary, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Epithelium of the rete ovarii.
Diagnostic Features
・Diffuse thickening of rete tubular epithelium; cells may be hypertrophied and vacuolated or densely packed.
・Evidence of origin in rete ovarii (present at hilus; connection to intra- or extra-ovarian rete).
・May have focal polypoid in-growths.
・More extensive lesions may have slight papillary pattern.
・Nuclei often present in apical cytoplasm.
・May contain areas of ciliated cells.
・May contain smooth muscle.
・May occur in cystically dilated tubules.
Differential Diagnoses
Adenoma, rete ovarii:
・Distention of affected rete tubule by intratubular mass.
Cyst, rete ovarii:
・Absence of hyperplasia and thickening of rete epithelium.
Cyst, paraovarian:
・Occurs in paraovarian structures with no apparent connection to ovarian hilus or intra-ovarian structures.
Hyperplasia, cystic/papillary:
・Arises from surface epithelium; no evidence of origin in rete ovarii.
Comment
This age-related change may be underreported because of its inconspicuous appearance, location at the periphery of the ovary and inconsistent presence of the rete in randomly orientated sections. Hyperplasia of the rete ovarii is reported in the CD-1 mouse and in the rat occurs at a higher incidence in the Wistar Han. Distinction from cystic/papillary hyperplasia which is thought to arise from ovarian surface epithelium is arbitrary and difficult.
References
C. Neoplastic Proliferative Lesions
Adenoma, rete ovarii (B) Ovary (Figure 88)
Figure 88.
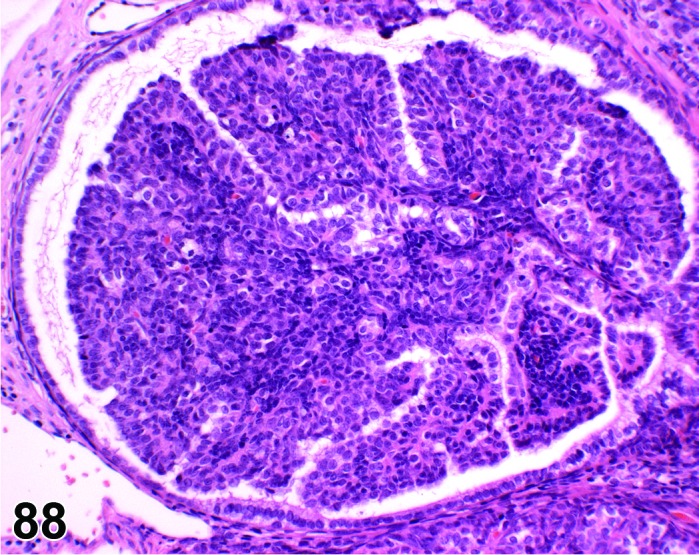
Rete Ovarii Adenoma, Ovary, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Epithelium of the rete ovarii.
Diagnostic Features
・Distention of affected rete tubule by intratubular mass.
・Usually has papillary structures with central stalk and branching fibrovascular stroma.
・Cells are cuboidal to columnar with scant cytoplasm and frequently central to apical nuclei.
・Generally no evidence of secretory material.
・Occasionally, foci of ciliated cells and foci of densely packed basophilic cells with hyperchromatic nuclei can be seen.
・No evidence of extratubular invasive growth or cellular atypia.
Differential Diagnoses
Hyperplasia, rete ovarii:
・Absence of distention of affected rete tubule by intratubular mass.
Cystadenoma:
・Does not arise from within rete ovarii.
Comment
This age-related change may be underreported because of its inconspicuous appearance, location at the hilus of the ovary and inconsistent presence of the rete in randomly orientated sections. Adenoma of the rete ovarii is reported in the CD-1 mouse; however, in the rat it occurs at a higher incidence in the Wistar Han. Distinction from cystadenoma (arising from ovarian surface epithelium) is difficult and arbitrary. The defining criterion for adenoma of rete ovarii is obvious origin within the extraovarian or intraovarian rete. To the authors’ knowledge, carcinoma of the rete ovarii has not been reported in mice and rats.
References
114Long (2002)
Cystadenoma (B) Ovary (Figure 89 and 90)
Figure 89.
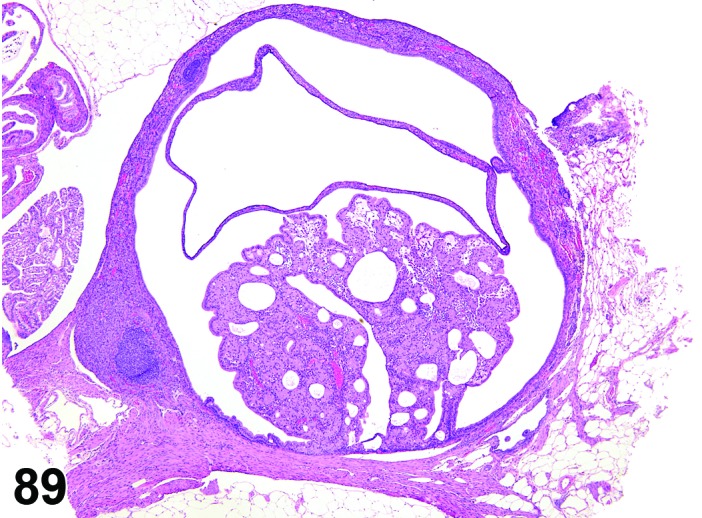
Cystadenoma, Ovary, mouse.
Figure 90.
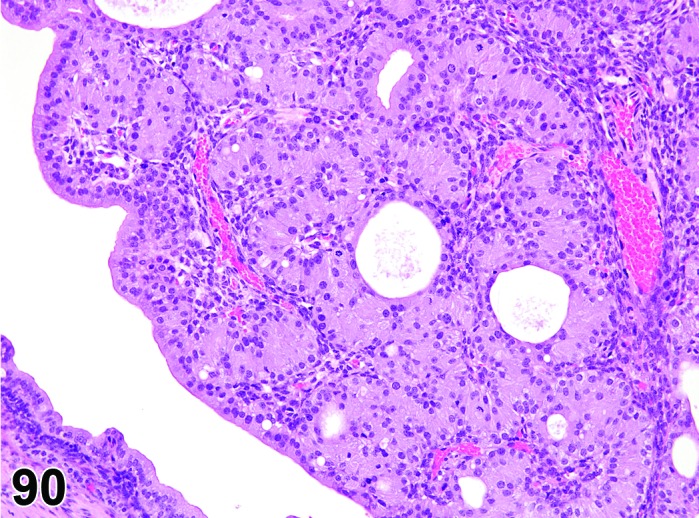
Cystadenoma, Ovary, mouse.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Surface epithelium of the ovary.
Diagnostic Features
・Single or multiple cystic and/or papillary structures lined by cuboidal or low columnar epithelium that may be ciliated.
・Generally present on surface of ovary with little or no invasion of the ovary proper.
・Non-cystic lesions consist of nodules or papillary structures projecting from the surface epithelium of the ovary.
・Cysts may contain serous fluid or blood.
・Generally little or no cellular atypia present.
・Cysts and papillary structures separated by delicate stroma.
・Compression of adjacent ovarian stroma frequently present, but no invasion.
・Proliferative lesion is larger than a corpus luteum.
Differential Diagnoses
Hyperplasia, cystic/papillary:
・Single layer of well-differentiated epithelial cells.
・No atypia.
・Lesion smaller than or equal to size of a corpus luteum.
Cystadenocarcinoma:
・Cellular atypia and pleomorphism are frequent.
・Mitotic figures are frequent.
・Invasion of periovarian tissue or metastases is present.
Adenoma, rete ovarii:
・Intratubular lesion that arises in the rete ovarii.
Adenoma, tubulostromal:
・Intra-ovarian lesion.
・Frequently has stromal component containing variably luteinized cells.
Comment
Common lesion in some mouse strains; uncommon in rats. Differentiating cystadenoma from adenoma of the rete ovarii is difficult and arbitrary if evidence of intratubular origin isn’t observed.
References
2Alison and Morgan (1987a), 4Alison et al. (1987b), 5Alison et al. (1990), 71Gregson et al. (1984), 112Lewis (1987), 114Long (2002)
Cystadenocarcinoma (M) Ovary (Figures 91 and 92)
Figure 91.
Cystadenocarcinoma, Ovary, rat.
Figure 92.
Cystadenocarcinoma, Ovary, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Surface epithelium of the ovary.
Diagnostic Features
・Solid or cystic mass lined by cuboidal or low columnar pleomorphic epithelium that may be ciliated.
・Mitotic figures are frequent.
・Folds or papillary projections may be present.
・Stromal compartment is delicate and not an inherent part of the tumor
・Infiltration of adjacent tissue is present.
・Optional modifiers are papillary, cystic, serous and mucinous; however these are not generally used in toxicology studies.
Differential Diagnoses
Carcinoma, yolk sac:
・Visceral and parietal patterns present.
・Contains PAS-positive, eosinophilic matrix.
Cystadenoma:
・Cellular atypia is minimal or absent.
・Mitotic figures are infrequent.
・Lacks invasive growth pattern.
Carcinoma, tubulostromal:
・Frequently has a stromal component containing variably luteinized cells.
Choriocarcinoma:
・Contains both visceral and parietal patterns.
・Hematocysts, hemorrhage and necrosis are frequent.
Mesothelioma, malignant:
・Extra-ovarian location; often in ovarian bursa.
・May be present on peritoneal surface of other tissues.
・Stroma may be prominent and have hyaline appearance.
・Cells may have a ‘hobnail’ appearance.
・Distinction from cystadenocarcinoma difficult.
Carcinoma, embryonal:
・Composed of poorly differentiated fusiform cells with large nuclei.
・May have areas of yolk sac carcinoma and/or areas of well differentiated tissues of ectodermal, mesodermal or endodermal origin.
Comment
Common lesion in some mouse strains; uncommon in rats. Difficult to distinguish from mesothelioma, malignant.
References
2Alison and Morgan (1987a), 3Alison and Morgan (1987b), 31Davis et al. (1999), 39Dixon et al. (1999), 66Greaves (2012), 71Gregson et al. (1984)
Adenoma, tubulostromal (B) Ovary (Figure 93)
Figure 93.
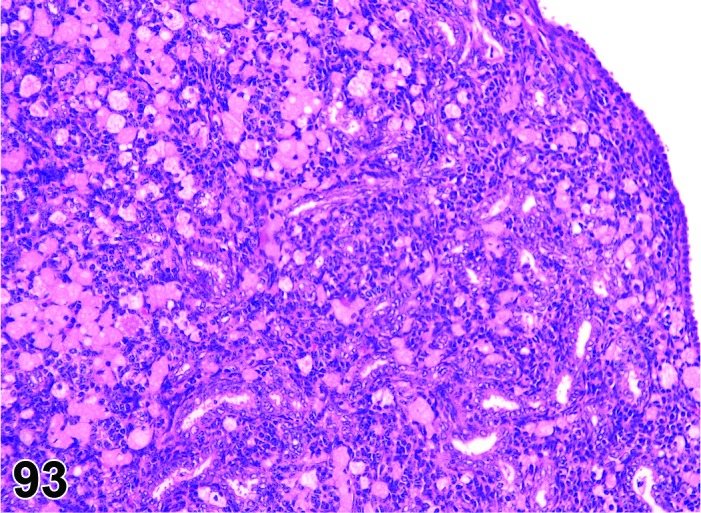
Tubulostromal Adenoma, Ovary, mouse.
Species
Mouse; Rat.
Synonym(s)
Tubular adenoma.
Pathogenesis/cell of origin
Surface epithelium of the ovary.
Diagnostic Features
・Nodular structure which shows compression or replacement of tissue.
・Delicate tubules lined by cuboidal epithelium that resemble or are continuous with surface epithelium of the ovary.
・Tubules are separated by packets of cells of probable sex cord stromal origin, which may show varying degrees of luteinization.
・Sertoliform tubules and/or glomerular-like structures may be present but do not predominate.
・Ratio of tubular to non-tubular components, degree of tubular dilation and amount of vacuolation/luteinization highly variable, but tubular structures with cuboidal epithelium predominate.
・Diameter of proliferative lesion is larger than that of a corpus luteum.
・May have areas with cystic dilation of the tubular structures.
・Slight cellular pleomorphism/atypia may be seen.
・Some extension into the ovarian bursa, especially near the hilus, may be present.
Differential Diagnoses
Hyperplasia, tubulostromal:
・No distinct compression or displacement of adjacent tissue.
・Size is smaller than or equal to a corpus luteum.
・Lack of cellular atypia.
Tumor, Sertoli cell, benign:
・Sertoli cells and tubules predominate.
・Cells can vary from tall columnar with basal nuclei and vertically oriented cytoplasm lining well-differentiated tubules to nests of vacuolated cells without basal nuclear orientation (most frequently observed in Sprague-Dawley rats).
・Fibrovascular stroma present.
・No evidence of connection to ovarian surface epithelium.
Tumor, sex cord stromal, benign (Tumor, granulosa cell, benign, Thecoma, benign, Luteoma, benign, or Tumor, sex cord stromal, mixed, benign):
・Sex cord-stromal cells predominate (differentiation between luteoma, thecoma, granulosa cell, or mixed tumor is made upon the predominant cell type in the tumor).
・No evidence of connection to ovarian surface epithelium.
Carcinoma, tubulostromal:
・Cellular atypia present.
・Increased mitotic figures are observed.
・Local invasion beyond the ovarian bursa may be present.
・Metastases may be observed.
Cystadenoma / Cystadenocarcinoma:
・Absence of interstitial cell component.
・Neoplastic epithelial cells generally taller than in tubulostromal tumors.
・Frequently forms cystic spaces and/or papillary structures.
Comment
Alcian blue stain highlights acid mucopolysaccharides sometimes present in tubular epithelium. Common tumors in some strains of mice; rare in rats.
References
5Alison et al. (1990), 31Davis et al. (1999), 39Dixon et al. (1999), 66Greaves (2012), 71Gregson et al. (1984), 112Lewis (1987), 116Maekawa (1990), 135Morgan and Alison (1987)
Carcinoma, tubulostromal (M) Ovary (Figure 94)
Figure 94.
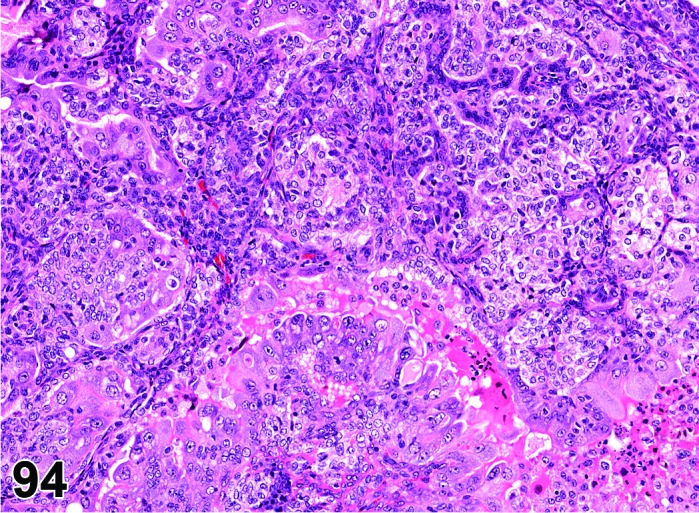
Tubulostromal Carcinoma, Ovary, mouse.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Surface epithelium of the ovary.
Diagnostic Features
・Delicate tubules lines by cuboidal epithelium that resemble or are continuous with surface epithelium of the ovary.
・Tubules are separated by packets of cells of probable sex cord stromal origin which may show varying degrees of luteinization.
・Ratio of tubular to non-tubular components, degree of tubular dilation and amount of vacuolation/luteinization highly variable, but tubular structures predominate.
・High degree of pleomorphism and atypia.
・Diameter of proliferative lesion is larger than that of a corpus luteum.
・Mitotic figures may be numerous.
・Infiltration of adjacent tissues beyond the ovarian bursa is present.
・Metastases may be present.
Differential Diagnoses
Adenoma, tubulostromal:
・Slight or no pleomorphism/atypia.
・Invasion, if present, limited to ovarian bursa.
Cystadenocarcinoma:
・Absence of interstitial cell component.
・Neoplastic epithelial cells generally taller than those in tubulostromal tumors.
・Presence of cystic spaces and papillary structures.
Comment
Rare tumor in rats and mice.
References
2Alison and Morgan (1987a), 4Alison et al. (1987b), 5Alison et al. (1990), 31Davis et al. (1999), 39Dixon et al. (1999), 66Greaves (2012), 71Gregson et al. (1984), 112Lewis (1987), 116Maekawa (1990), 120Maekawa et al. (1996), 135Morgan and Alison (1987)
Carcinoma, yolk sac (M) Ovary; Uterus (Figures 95 and 96)
Figure 95.
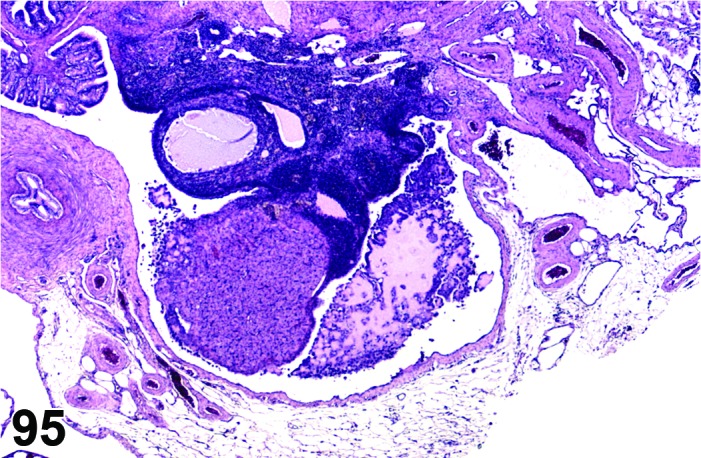
Yolk Sac Carcinoma, Ovary, rat.
Figure 96.
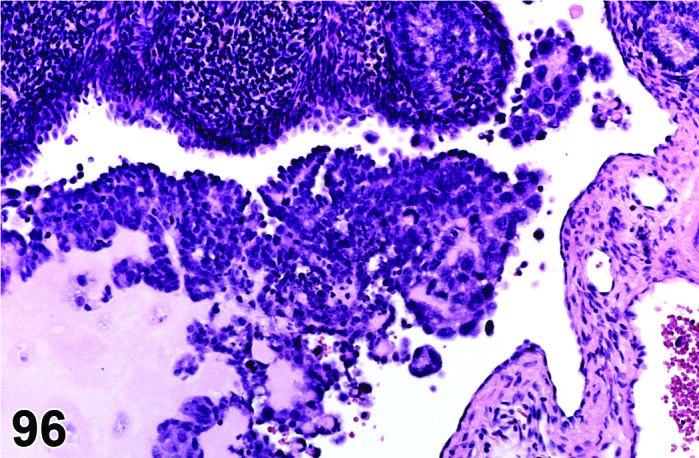
Yolk Sac Carcinoma, Ovary, rat.
Species
Mouse; Rat.
Synonym(s)
Tumor, endodermal sinus, malignant; Tumor, yolk sac, malignant.
Pathogenesis/cell of origin
Probable variant of germ cell tumor
Diagnostic Features
・Usually has patterns mimicking two layers of fetal membranes- the parietal and the visceral yolk sac.
・Parietal yolk sac tumor cells.
・Produce an abundant, eosinophilic, PAS-positive matrix in which nests and cords of neoplastic cells are embedded.
・Are polygonal or cuboidal endodermal cells with amphophilic cytoplasm, pleomorphic nuclei and often multiple nucleoli.
・Contain PAS-positive intracytoplasmic droplets or granules.
・Often form rosettes, cords or papillary structures; less frequently form cysts or glomerular bodies.
・Visceral yolk sac endodermal cells.
・Are cylindrical.
・Do not contain PAS-positive droplets.
・May form papillary pattern composed of a central capillary embedded in mesenchymal tissue and surrounded by visceral endoderm.
・Large cells with weakly staining cytoplasm and large nuclei, or giant cells surrounding blood-filled spaces may be present.
・Metastases often composed of both parietal and visceral components; lymph nodes, kidneys, liver and spleen commonly involved.
Differential Diagnoses
Cystadenocarcinoma:
・Composed of pleomorphic often cuboidal epithelium.
・Epithelium may be ciliated.
・Lack of PAS-positive matrix.
・Lack of intracytoplasmic PAS-positive droplets/granules.
Adenocarcinoma, endometrial (uterus, primary site; ovary, invasive from uterus):
・Cells can form glandular structures.
・Lack of PAS-positive matrix.
・Lack of intracytoplasmic PAS-positive droplets/granules.
Choriocarcinoma:
・Areas of necrosis and hemorrhage are frequent.
・Lack of PAS-positive matrix.
・Lack of intracytoplasmic PAS-positive droplets/granules and
・Composed of 2 cell types- small basophilic cells (placental cytotrophoblasts) and large giant cells (syncytiotrophoblasts).
Carcinoma, embryonal:
・Differentiation of yolk sac carcinoma from embryonal carcinoma is sometimes difficult.
・Embryonal carcinoma composed of fusiform, poorly differentiated cells with large nuclei and prominent nucleoli.
・May have areas of yolk sac carcinoma and/or areas of well-differentiated tissues from 1 or 2 but not all 3 germ cell layers.
Comment
Rare tumor in rats and mice. Spontaneous yolk sac carcinomas may be more commonly seen in BDII/Han rats. Yolk sac carcinoma with trophoblastic differentiation develops spontaneously after pregnancy. Tumor stains positive for laminin by immunohistochemistry.
References
4Alison et al. (1987b), 5Alison et al. (1990), 31Davis et al. (1999), 39Dixon et al. (1999), 66Greaves (2012),109 Leininger and Jokinen (1990), 112Lewis (1987), 116Maekawa (1990), 120Maekawa et al. (1996), 122Majeed et al. (1986), 130Mitsuhashi et al. (1993), 177Sobis (1987)
Choriocarcinoma (M) Ovary; Uterus (Figures 97 and 98)
Figure 97.
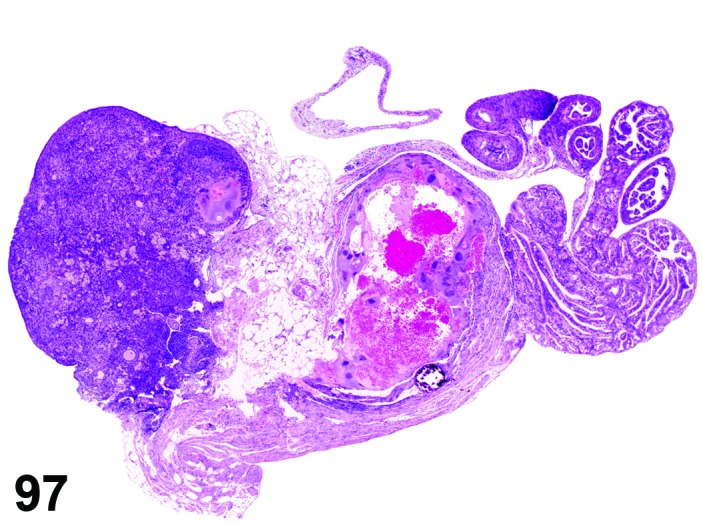
Choriocarcinoma, Ovary, mouse.
Figure 98.
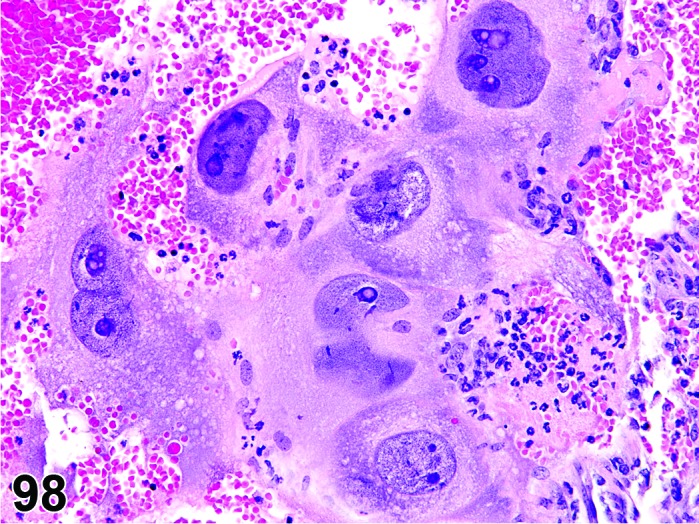
Choriocarcinoma, Ovary, mouse.
Species
Mouse; Rat.
Synonym(s)
Chorioepithelioma, malignant.
Pathogenesis/cell of origin
Trophoblasts.
Diagnostic Features
・Composed of 2 cell types: small, round amphophilic to basophilic cells similar to placental cytotrophoblasts, and giant cells with single large nuclei (trophoblastic giant cells) or multiple pleomorphic nuclei (syncytiotrophoblasts) and prominent nucleoli.
・Erythrophagocytosis and/or intracellular PAS-positive material sometimes present in giant or syncytial cells.
・Highly infiltrative growth by both types of cells.
・Hemorrhage and necrosis often present.
Differential Diagnoses
Carcinoma, yolk sac:
・Contains PAS-positive eosinophilic matrix.
・Visceral and parietal patterns present.
Cystadenoma:
・Cysts and papillary structures often present.
・Composed of pleomorphic often cuboidal epithelium.
・Epithelium may be ciliated.
・Lack of intracytoplasmic PAS-positive droplets/granules.
Carcinoma, embryonal:
・Composed of poorly differentiated fusiform cells with large nuclei.
・May have areas of yolk sac carcinoma and/or areas of well differentiated tissues from 1 or 2 but not all 3 germ cell layers.
Hemangiosarcoma:
・Composed of 1 cell type.
・Tumor forms true vascular spaces lined by malignant endothelial cells.
Comment
Rare tumor in mice and rats. Choriocarcinoma is usually associated with pregnancy or induced by displacement of the visceral yolk sac after fetectomy.
References
1Alison et al. (1987a), 2Alison and Morgan (1987a), 4Alison et al. (1987b), 5Alison et al. (1990), 31Davis et al. (1999), 39Dixon et al. (1999), 109Leininger and Jokinen (1990), 112Lewis (1987), 116Maekawa (1990), 120Maekawa et al. (1996), 178Sobis (1987a) , 211Yoshida et al. (1997)
Carcinoma, embryonal (M) Ovary; Uterus (Figures 99 and 100)
Figure 99.
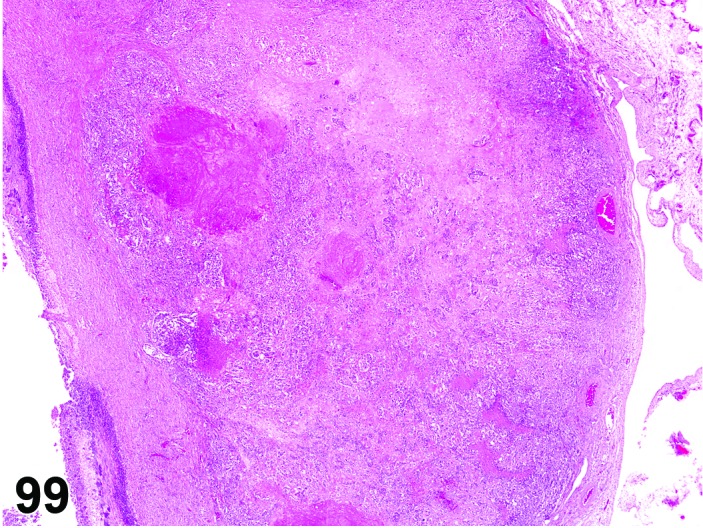
Embryonal Carcinoma, Ovary, rat.
Figure 100.
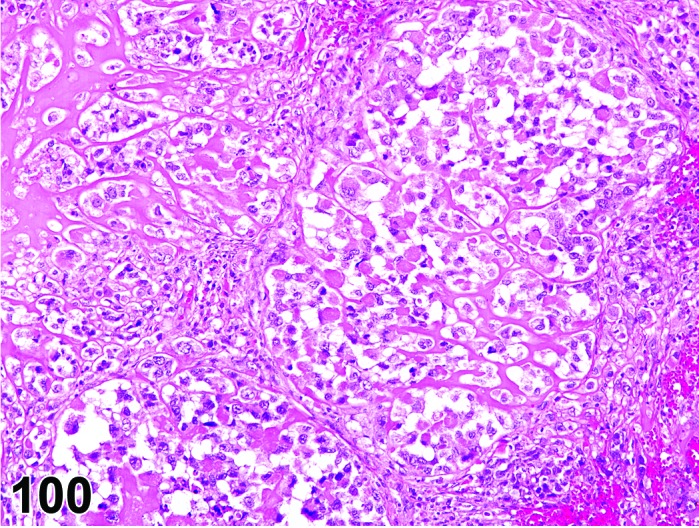
Embryonal Carcinoma, Ovary, rat.
Species
Rat.
Pathogenesis/cell of origin
Probable germ cell origin.
Diagnostic Features
・Composed of round or fusiform, poorly differentiated cells.
・Cells have large nuclei and prominent nucleoli.
・Mitotic figures are numerous.
・Highly infiltrative growth.
・Areas of yolk sac carcinoma may be present.
・Well-differentiated tissues such as bone, cartilage and/or skin may be present.
・Metastases usually composed of undifferentiated cells but may contain areas of yolk sac carcinoma.
Differential Diagnoses
Carcinoma, yolk sac:
・Contains PAS-positive eosinophilic matrix.
・Visceral and parietal patterns present.
・Does not contain other types of neoplastic tissue (well differentiated or poorly differentiated).
Cystadenocarcinoma:
・Composed of 1 cell type, although may be highly pleomorphic.
・Cysts and papillary structures often present.
・Epithelium may be ciliated.
Choriocarcinoma:
・Areas of necrosis and hemorrhage are frequent.
・Composed of 2 cell types- small basophilic cells (placental cytotrophoblasts) and large giant cells (syncytiotrophoblasts).
Teratoma, malignant:
・Contains tissues from all 3 germ cell layers.
Comment
Spontaneous embryonal carcinoma has not been described in the mouse but can be induced in the rat by injection of mouse sarcoma virus.
References
4Alison et al. (1987b), 39Dixon et al. (1999), 47Faccini et al. (1990), 51Frith and Ward (1988), 110Lemon and Gubareva (1979), 147Nielsen et al. (1976), 163Rehm et al. (1984), 170Sass and Rehm (1994), 174Serov et al. (1973), 179Sobis (1987b), 181Squire et al. (1978)
Dysgerminoma (M) Ovary (Figures 101 and 102)
Figure 101.
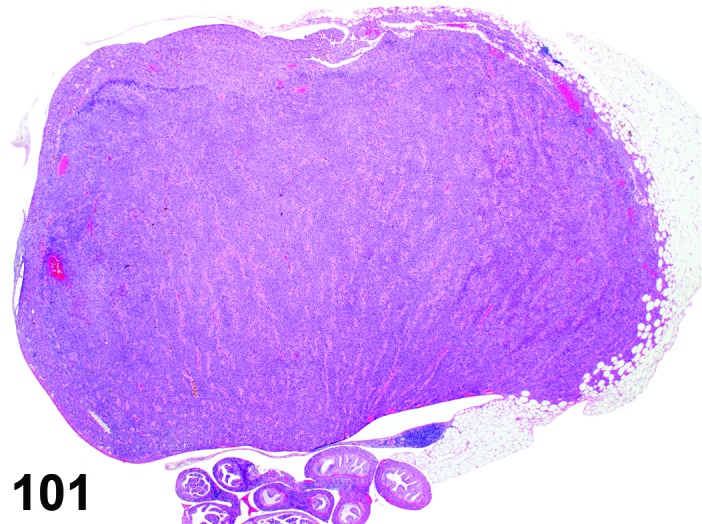
Dysgerminoma, Ovary, mouse.
Figure 102.
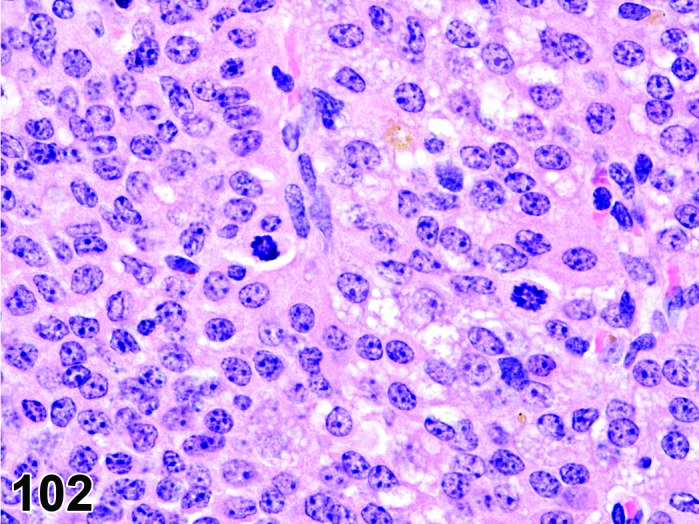
Dysgerminoma, Ovary, mouse.
Species
Mouse.
Synonym(s)
Ovarian seminoma.
Pathogenesis/cell of origin
Probable germ cell origin.
Diagnostic Features
・Composed of sheets of large round undifferentiated cells with pale or clear cytoplasm.
Differential Diagnoses
Carcinoma, yolk sac:
・Contains PAS-positive eosinophilic matrix.
・Visceral and parietal patterns present.
Cystadenocarcinoma:
・Cysts and/or papillary structures may be present.
・Composed of pleomorphic often cuboidal epithelium.
・Epithelium may be ciliated.
Choriocarcinoma:
・Composed of 2 cell types- small basophilic cells (placental cytotrophoblasts) and large giant cells (syncytiotrophoblasts).
・Hematocysts, hemorrhage and necrosis are frequent.
Comment
Rare tumor in mice; not reported in the rat.
References
2Alison and Morgan (1987a), 5Alison et al. (1990), 31Davis et al. (1999)
Leiomyoma, mesovarial (M) Ovary (Figures 103,104,105)
Figure 103.
Leiomyoma, Mesovarium, rat (Courtesy C. Gopinath).
Figure 104.
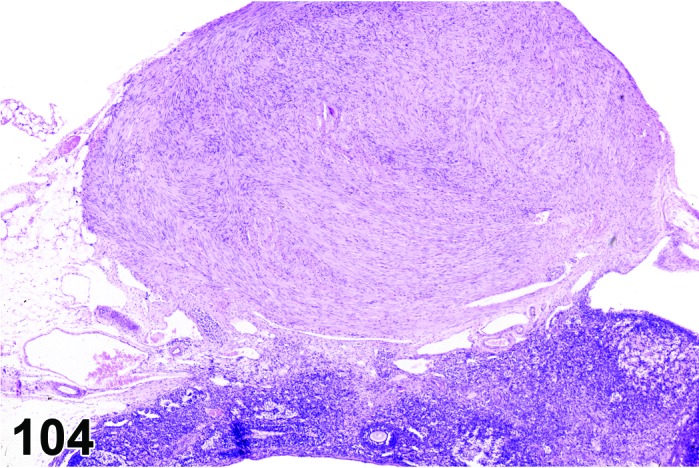
Leiomyoma, Mesovarium, rat (Courtesy C. Gopinath).
Figure 105.
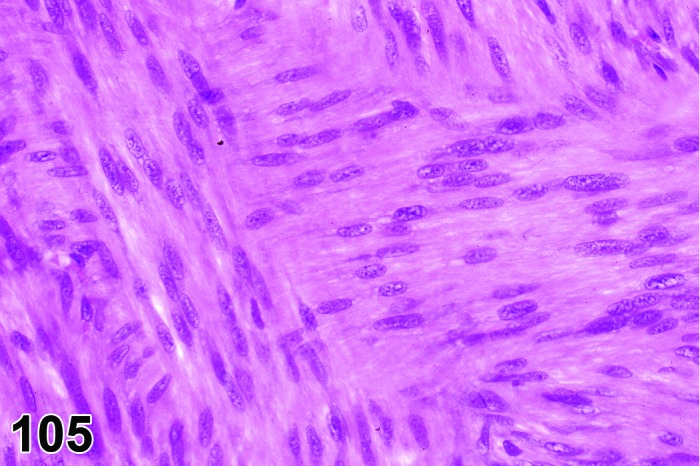
Leiomyoma, Mesovarium, rat (Courtesy C. Gopinath).
Species
Rat.
Pathogenesis/cell of origin
Mesenchymal origin; smooth muscle cell.
Diagnostic Features
・Well circumscribed growths of smooth muscle fibers arranged in interlacing or perpendicular bundles and sometimes whorls.
・Elongate muscle cells with abundant eosinophilic cytoplasm and cylindrical, cigar-shaped nuclei with blunt ends.
・Varying proportions of sparse collagen between bundles.
Differential Diagnoses
Hyperplasia, smooth muscle, mesovarial:
・Hyperplasia lacks characteristic interlacing bundles or whorls of cells.
Comment
Leiomyomas of the mesovarium are rare spontaneous tumors in rats. They have been induced experimentally in rats treated with β-adrenergic agonists. This tumor is reported to occur more frequently on the right side. Leiomyomas of the mesovarium have not been reported in mice.
References
Tumor, granulosa cell, benign (B) Ovary (Figures 106 and 107)
Figure 106.
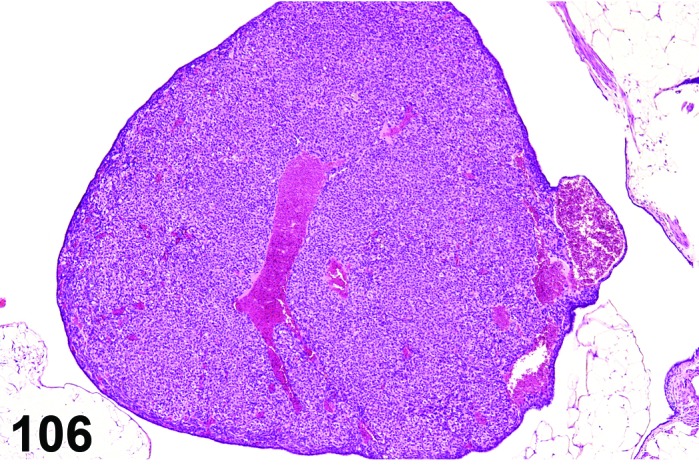
Granulosa Cell Tumor, Benign, mouse.
Figure 107.
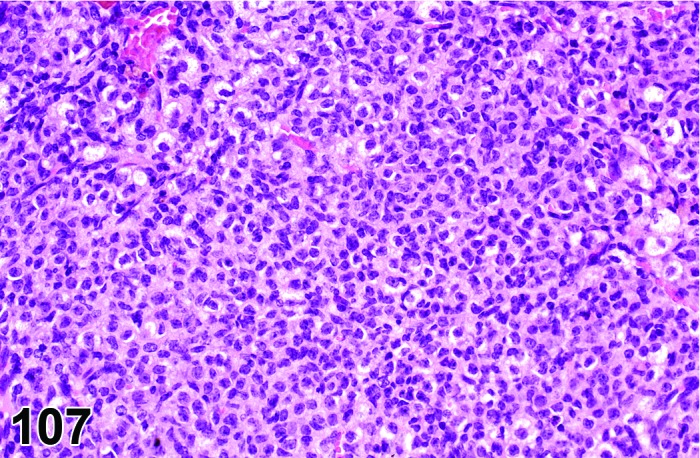
Granulosa Cell Tumor, Benign, mouse.
Species
Mouse; Rat.
Synonym(s)
Tumor, sex cord stromal, benign, granulosa cell type.
Pathogenesis/cell of origin
Sex cord/stromal cells.
Diagnostic Features
・Cellular morphology resembles that of normal granulosa cells.
・Composed of predominantly (>70%) granulosa cells.
・Other types of sex cord/stromal cells may be present in varying numbers; however, the predominant cell type is the granulosa cell.
・Nuclei round to oval with coarsely stippled chromatin.
・Variable degrees of luteinization are present.
・Cytoplasm varies from scant to moderate depending upon degree of luteinization and is faintly eosinophilic and vacuolated.
・Minimal cellular atypia may be present.
・Low number of mitotic figures may be present.
・Call-Exner bodies uncommon.
・Several patterns such as cystic, microfollicular, solid and trabecular identified but the tumors are generally not subclassified according to pattern.
・Large tumors may contain areas of hemorrhage, necrosis and/or lipofuscin granules.
・Distinct compression or tissue displacement is evident.
・Diameter of non-cystic, solid proliferative lesion is larger than size of a corpus luteum.
Differential Diagnoses
Hyperplasia, granulosa cell:
・No distinct compression of surrounding tissue and
・Atypia minimal or not present and
・Solid lesion is smaller than or equal to the size of a corpus luteum.
Luteoma, benign; Thecoma, benign; or Tumor, Sertoli cell, benign
・>70% of the tumor cells are luteal cells, theca cells or Sertoli cells.
Luteoma, benign:
・Predominant cell type (>70%) is a polyhedral cell with abundant eosinophilic, vacuolated cytoplasm and nuclei without significant chromatin stippling.
Thecoma, benign:
・Predominant cell type (>70%) is a fusiform theca cell with whorled pattern.
・Cells sometimes contain lipid droplets.
・Collagen may be present between bundles of cells.
Tumor, granulosa cell, malignant:
・Predominant cell type (>70%) is a granulosa cell with coarsely stippled chromatin,.
・Areas of hemorrhage/necrosis may be present.
・Infiltrative growth pattern may be present.
・Atypia is commonly present.
・Metastases may be present.
Comment
Granulosa cell tumors are the most common ovarian tumors in Fischer F344 and Sprague-Dawley rats. In mice, the incidence of granulosa cell tumors varies markedly between the various strains. Granulosa cell tumors occur with a high frequency in the SWR strain. In most other strains granulosa cell tumors are relatively uncommon. Granulosa cell tumors may secrete estrogen.
References
2Alison and Morgan (1987a), 3Alison and Morgan (1987b), 4Alison et al. (1987b), 5Alison et al. (1990), 71Gregson et al. (1984), 118Maekawa and Hayashi (1987)
Tumor, granulosa cell, malignant (M) Ovary (Figures 108 and 109)
Figure 108.
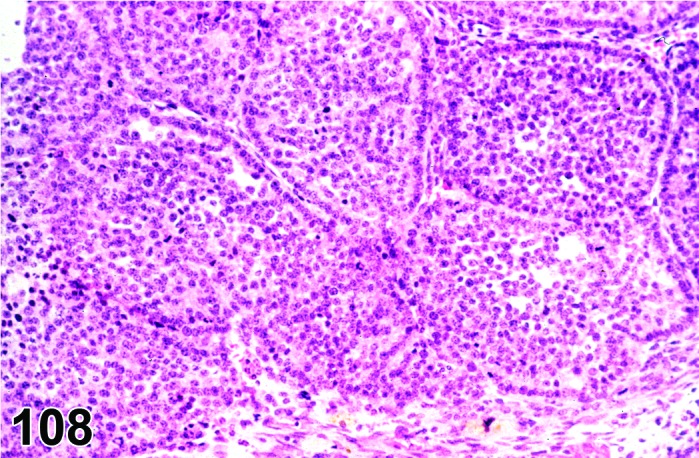
Granulosa Cell Tumor, Malignant, Ovary, mouse.
Figure 109.
Granulosa Cell Tumor, Malignant, Ovary, mouse.
Species
Mouse; Rat.
Synonym(s)
Gynoblastoma; Tumor, sex cord stromal, malignant, granulosa cell type.
Pathogenesis/cell of origin
Sex cord/stromal cells.
Diagnostic Features
・Predominant cell type is the granulosa cell (>70%).
・Cellular morphology resembles that of normal granulosa cells, but moderate cellular atypia and pleomorphism are often present.
・Nuclei round to oval with coarsely stippled chromatin.
・Call-Exner bodies are uncommon.
・Cytoplasm varies from scanty to moderate depending upon degree of luteinization and is faintly eosinophilic and vacuolated.
・Several patterns such as follicular, solid and trabecular identified, but are not used to subclassify the neoplasms in toxicology studies.
・Occasionally granulosa cell tumors show areas or are partially composed of fusiform theca-like cells.
・Mitotic figures may be numerous.
・Focal areas of necrosis and hemorrhage are frequently present.
・Local invasion is present.
・Tumor may show distant metastases to kidneys, lungs and lymph nodes.
Differential Diagnoses
Tumor, granulosa cell, benign:
・No significant atypia.
・No invasion or infiltrative growth pattern.
・Minimal areas of necrosis or hemorrhage may occasionally be present, but only in very large tumors.
・Occasional mitotic figures may be present.
・No metastases.
Tumor, Sertoli cell, malignant:
・Predominant cell type (>70%) is Sertoli cell.
・Composed of tubular structures and/or areas with nests of vacuolated cells without obvious tubular structures.
・Metastases to peritoneal cavity may be present.
Thecoma, malignant:
・Composed of >70% theca cells.
Comment
The distinction between benign and malignant granulosa cell tumor is based on the degree of atypia, infiltrative growth pattern, presence of metastasis, and areas of necrosis and hemorrhage indicative of a high growth rate.
References
2Alison and Morgan (1987a), 3Alison and Morgan (1987b), 4Alison et al. (1987b), 5Alison et al. (1990), 71Gregson et al. (1984), 112Lewis (1987), 118Maekawa and Hayashi (1987)
Tumor, Sertoli cell, benign (B) Ovary (Figures 110 and 111)
Figure 110.
Sertoli Cell Tumor, Benign, Ovary, rat.
Figure 111.
Sertoli Cell Tumor, Benign, Ovary, rat.
Species
Mouse; Rat.
Synonym(s)
Tumor, gonadal stromal, benign; Tumor, sex cord stromal, benign; Tumor, sex cord stromal, benign, Sertoli type; Tumor, sustentacular, benign; Sertoliform tubular adenoma.
Pathogenesis/cell of origin
Sex cord/stromal cells.
Diagnostic Features
・Tumor composed predominantly (>70%) Sertoli cells, although other types of sex cord/stromal cells may be present.
・Sertoli cell tumors resemble their testicular counterpart histologically.
・Often arise in the hilus.
・Compression is present.
・Fibrous capsule usually present.
・Characterized by seminiferous-like tubules separated by fibrovascular stroma and lined by cells with basally located nuclei and abundant faintly eosinophilic, vacuolated cytoplasm extending into the lumen.
・Frequently have areas with nests of vacuolated cells/Sertoli cells without obvious tubular structures.
・A variation of the Sertoli cell tumor is composed of irregular tubules lined by vacuolated cells without basal nuclei (see comment).
・If differentiation between focal hyperplasia and benign Sertoli cell tumor cannot be made on the basis of criteria listed above, a proliferative lesion with a diameter larger than the size of a corpus luteum is interpreted to be a tumor.
Differential Diagnoses
Hyperplasia, Sertoli cell:
・Diameter of lesion is smaller than or equal to the size of a corpus luteum and
・Minimal or no compression.
Tumor, Sertoli cell, malignant:
・Cellular pleomorphism is present.
・Areas of hemorrhage and necrosis are present.
・Infiltrative growth pattern or disruption of ovarian capsule is present.
・Metastases are present.
Tumor, granulosa cell, benign:
・Cells comprising tumor are predominantly granulosa cells (>70%), with coarsely stippled chromatin, variable luteinization and lack of tubular growth pattern.
・Cells have stippled chromatin.
Thecoma, benign:
・Cells comprising tumor are predominantly theca cells.
Comment
In rats these tumors are lobulated, solid, white-yellow masses with occasional cysts. This category includes sertoliform tubular adenomas which have been previously described as occurring primarily in Sprague-Dawley rats. These tumors are composed of irregular tubules of pale vacuolated cells with indistinct cell boundaries which may give a syncytial appearance. These cells often have intracytoplasmic hyaline-like PAS-positive inclusions. This variant differs from the other Sertoli cell type tumors in that the tubular cells lack basal nuclei and vertically oriented cytoplasm.
References
4Alison et al. (1987b), 5Alison et al. (1990), 71Gregson et al. (1984), 184Stoica et al. (1987)
Tumor, Sertoli cell, malignant (M) Ovary (Figures 112 and 113)
Figure 112.
Sertoli Cell Tumor, Malignant, Ovary, rat.
Figure 113.
Sertoli Cell Tumor, Malignant, Ovary, rat.
Species
Mouse; Rat.
Synonym(s)
Androblastoma; Arrhenoblastoma; Tumor, gonadal stromal, malignant; Tumor, sex cord stromal, malignant; Tumor, sex cord stromal, malignant, Sertoli type; Tumor, sustentacular, malignant.
Pathogenesis/cell of origin
Sex cord/stromal cells.
Diagnostic Features
・Sertoli cell tumors resemble their testicular counterpart.
・Composed predominantly (>70%) of Sertoli cells; areas of other sex cord/stromal cell types especially granulosa cells may be present but are not the predominant cell type.
・Characterized by seminiferous-like tubules separated by fibrovascular stroma and lined by cells with basally located nuclei and abundant faintly eosinophilic cytoplasm extending into the lumen.
・Nests of rounded pleomorphic cells without tubule formation often observed.
・Focal necrosis and hemorrhage may be present.
・Local invasion is present.
・Metastases (often to peritoneal surfaces) may be present.
Differential Diagnoses
Tumor, Sertoli cell, benign:
・Lack of infiltrative growth pattern.
・Lack of significant cellular pleomorphism.
・Lack of areas of necrosis/hemorrhage.
・Lack of metastases.
Tumor, granulosa cell, malignant:
・Predominant cell type is granulosa cell (>70%).
・Lack of tubular growth pattern.
References
2Alison and Morgan (1987a), 4Alison et al. (1987b), 5Alison et al. (1990), 71Gregson et al. (1984), 112Lewis (1987), 174Serov et al. (1973), 184Stoica et al. (1987), 214Yuan and Foley (2002)
Tumor, sex cord stromal, mixed, benign (B) Ovary (Figures 114 and 115)
Figure 114.
Sex Cord Stromal Tumor, Mixed, Benign, Ovary, rat.
Figure 115.
Sex Cord Stromal Tumor, Mixed, Benign, Ovary, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Sex cord/stromal cells.
Diagnostic Features
・Tumor consists of a mixture of granulosa, luteal, theca, Sertoli and stromal cells, which may show various degrees of differentiation. No cell type predominates (>70%).
・Discrete, well-demarcated, focal lesions which are bigger than one large corpus luteum.
・Compression is present.
・Cellular atypia is minimal.
・Included in this category are also extremely large diffuse mixed-type lesions in rats which encompass the whole ovary and are in size/diameter markedly larger than a normal ovary.
Differential Diagnoses
Thecoma, benign:
・Predominant cell type (>70%) is fusiform theca cell arranged in whorls. Collagen may be present around bundles of cells and cells may contain lipid vacuoles.
Tumor, granulosa cell, benign:
・Predominant cell type (>70%) is the granulosa cell.
Tumor, Sertoli cell, benign:
・Predominant cell type (>70%) is the Sertoli cell arranged in tubular structures or nests of vacuolated cells.
Hyperplasia, sex cord stromal, mixed, focal:
・Composed of a mixture of sex cord stromal cells (theca, luteal, granulosa and/or Sertoli) in which no one cell type predominates (present in >70% of lesion).
・Focal lesion smaller in size than a large corpus luteum.
・Minimal or no compression.
Hyperplasia, sex cord stromal, mixed, diffuse - rat:
・Composed of a mixture of sex cord stromal cells (theca, luteal, granulosa and/or Sertoli) in which no one cell type predominates (present in >70% of lesion).
・Nondiscrete lesion that displaces much of ovarian tissue.
・Minimal or no compression.
・Overall lesion is smaller than size of normal ovary.
Comment
These tumors have been previously classified as undifferentiated gonadal stromal neoplasms characterized by having a complex profile of a mixture of sex cord stromal cells.
References
2Alison and Morgan (1987a), 5Alison et al. (1990), 32Davis et al. (2001)
Tumor, sex cord stromal, mixed, malignant (M) Ovary
Species
Mouse; Rat.
Synonym(s)
Gonadal stromal tumor, malignant; Granulosa-thecal cell tumor, malignant; Mixed tumor, malignant.
Pathogenesis/cell of origin
Sex cord/stromal cells.
Diagnostic Features
・Tumor consists of a mixture of granulosa, luteal, theca, Sertoli and stromal cells, which may show various degrees of differentiation. No cell type predominates (>70%).
・Discrete, well-demarcated focal lesions, which are bigger than one large corpus luteum.
・Included in this category are also extremely large diffuse mixed-type lesions, which encompass the whole ovary and are in size/diameter markedly larger than a normal ovary (old age type sex cord stromal hyperplasia).
・Necrosis, high mitotic rate and invasion.
Differential Diagnoses
Tumor, granulosa cell, malignant:
・Predominant cell type (>70%) is the granulosa cell.
Tumor, Sertoli cell, malignant:
・Predominant cell type (>70%) is the sertoli cell.
Thecoma, malignant:
・Predominant cell type (>70%) is fusiform theca cells.
Comment
This is a rare tumor in rats and a very rare tumor in mice.
References
Thecoma, benign (B) Ovary (Figure 116)
Figure 116.
Thecoma, Benign, Ovary, mouse.
Species
Mouse; Rat.
Synonym(s)
Tumor, sex cord stromal, benign, thecoma type; Tumor, theca cell, benign.
Pathogenesis/cell of origin
Sex cord/stromal cells.
Diagnostic Features
・Composed of densely packed fusiform cells, usually arranged in interlacing bundles and whorls giving a nodular appearance.
・Variable amount of lipid vacuoles in cytoplasm. Collagen if present is mainly between bundles of cells.
・Extensive necrosis in large tumors can occur, with only perivascular persistence of viable tissue.
・Focal areas of mineralization and hyalinization may be present.
・Generally a capsule is not present.
・Compression is present.
・Size of proliferative lesion is larger than the size of a corpus luteum.
Differential Diagnoses
Fibroma:
・Lacks lipid-laden cells.
・Collagen is present and is arranged around individual cells rather than around bundles of cells.
Fibrosarcoma:
・Lacks lipid-laden cells.
・Collagen is present and is arranged around individual cells rather than around bundles of cells.
・Cellular pleomorphism often present and may include giant cells and multinucleated cells.
・Mitotic figures may be numerous.
Hyperplasia, sex cord stromal, mixed:
・Composed of a mixture of sex cord stromal cells (theca, luteal, granulosa, Sertoli and/or stromal) in which no one cell type predominates (present in >70% of lesion).
・Focal lesion smaller in size than a large corpus luteum.
・Diffuse lesion replaces much of ovarian tissue-rat.
・Minimal or no compression.
Tumor, granulosa cell, benign
Tumor, Sertoli cell, benign
Luteoma, benign:
・Differentiation between thecomas and the other sex cord stromal tumors is made on the predominant cell type present. In thecoma the major cell type is spindle-shaped theca cell.
Thecoma, malignant:
・Cellular pleomorphism/atypia is present.
・Prominent mitotic figures are present.
・Areas of necrosis may be present.
・Invasion of periovarian tissue is present.
・Metastases are present.
Comment
Reticulin stain may help determine the arrangement of collagen to differentiate between thecoma and fibroma.
References
2Alison and Morgan (1987a), 5Alison et al. (1990), 39Dixon et al. (1999)
Thecoma, malignant (M) Ovary (Figure 117)
Figure 117.
Thecoma, Malignant, Ovary, rat.
Species
Mouse; Rat.
Synonym(s)
Tumor, sex cord stromal, malignant, thecoma type; Tumor, theca cell, malignant.
Pathogenesis/cell of origin
Sex cord/stromal cells.
Diagnostic Features
・Composed of densely packed fusiform theca cells usually arranged in whorls giving a nodular appearance.
・Spindle-shaped cells arranged in interlacing bundles and whorled patterns.
・Variable amount of lipid and collagen present. Collagen when present is arranged between bundles of cells.
・Focal areas of mineralization and hyalinization may occur.
・Mitotic figures may be numerous.
・Cellular pleomorphism is present.
・Multiple areas of necrosis suggesting rapid growth are present.
・Infiltration of adjacent tissue is present.
Differential Diagnoses
Fibroma:
・Lacks lipid-laden cells.
・Collagen is present and is arranged around individual cells rather than around bundles of cells.
・Cellular pleomorphism is not present.
Fibrosarcoma:
・Lacks lipid-laden cells.
・Collagen is present and is arranged around individual cells rather than around bundles of cells.
・Cellular pleomorphism often present and may include giant cells and multinucleated cells.
Tumor, granulosa cell, malignant
Tumor, Sertoli cell, malignant:
・Differentiation between thecomas and the other sex cord stromal tumors is made on the predominant cell type present. In the case of thecoma the major cell type is spindle-shaped theca cell.
Thecoma, benign:
・Lack of infiltrative growth.
・Minimal or no cellular atypia.
・No metastases.
References
Luteoma, benign (B) Ovary (Figures 118,119,120)
Figure 118.
Luteoma. Ovary, rat.
Figure 119.
Luteoma, Ovary, rat.
Figure 120.
Luteoma, Ovary, rat.
Species
Mouse; Rat.
Synonym(s)
Gonadal stromal sex cord tumor, benign, luteoma type; Luteinized granulosa cell tumor, benign.
Pathogenesis/cell of origin
Sex cord/stromal cells.
Diagnostic Features
・Composed of highly luteinized cells with abundant pale granular cytoplasm and distinct cell boundaries.
・Nuclei are round to oval without significant stippling of chromatin.
・Intranuclear cytoplasmic invaginations and mast cells occasionally present.
・Tumor generally shows a slight degree of cellular pleomorphism.
・May be divided into small lobules separated by connective tissue.
・Size of proliferative lesion is larger than that of three corpora lutea.
Differential Diagnoses
Differentiation between luteomas and other sex cord stromal tumors is made upon the predominant cell type present. Granulosa cell tumors and thecomas do not have such a uniform high degree of luteinization.
Tumor, granulosa cell, benign:
・Predominant cell type (>70%) is the granulosa cell and
・Nuclei have characteristically stippled chromatin and
・May have areas of luteinization but these areas do not predominate and/or
・May have Call-Exner bodies.
Thecoma, benign:
・Predominant cell type (>70%) is the spindle-shaped theca cell arranged in interlacing bundles and whorls.
・Luteinized areas, if present, do not predominate.
・Hyalinized or mineralized stroma may be present.
Tumor, sex cord stromal, mixed, benign:
・Composed of a mixture of sex cord/stromal cell types (theca, granulosa, Sertoli, luteal, and stromal); no one cell type comprises >70% of the lesion.
Hypertrophy, corpora lutea:
・No cellular pleomorphism/atypia.
・Size is less than than 3 normal corpora lutea.
Comment
Luteoma, malignant has not been reported. Nuclear atypia or pleomorphism does not imply malignancy. Luteinized cells sometimes occur in malignant and benign granulosa cell tumors. If a luteoma is present in each ovary, then it is normal practice to record two separate tumors for that animal. A bilateral distribution must also be distinguished from hyperplasia, even if the size of the lesion is large.
References
5Alison et al. (1990), 71Gregson et al. (1984), 112Lewis (1987), 118Maekawa and Hayashi (1987)
Teratoma, benign (B) Ovary (Figures 121 and 122)
Figure 121.
Teratoma, Benign, Ovary, mouse.
Figure 122.
Teratoma, Benign, Ovary, mouse.
Uterus Species
Mouse; Rat.
Synonym(s)
Benign cystic teratoma; Dermoid cyst; Mature teratoma.
Pathogenesis/cell of origin
Multipotential embryonic tissue.
Diagnostic Features
・Must contain tissue derived from all three primary embryonic germ layers: ectoderm, mesoderm, and endoderm.
・Tissue components are generally well-differentiated.
・May be cystic or solid.
・Cysts lined by epithelium that may be cuboidal, enteric, respiratory, or keratinized/squamous.
・Mature nervous tissue, gastrointestinal elements, skeletal muscle, thyroid tissue, hair follicles, cartilage and bone frequently observed.
・No local invasion or metastases.
Differential Diagnoses
Teratoma, malignant:
・Contains poorly differentiated tissue resembling embryonic tissue.
・Invasion is present.
・Necrosis and hemorrhage may be present.
・Metastases are present.
Comment
Teratomas of the ovary are usually benign in rats and mice. Spontaneous teratomas of the uterus are uncommon. These tumors can be experimentally induced in the uterus by methods such as displacement of visceral yolk sac after fetectomy in rats.
References
32Davis et al. (2001), 39Dixon et al. (1999), 116Maekawa (1990), 131Miwa et al. (1987), 147Nielsen et al. (1976), 174Serov et al. (1973), 180Sobis (1987c)
Teratoma, malignant (M) Ovary; Uterus (Figures 123 and 124)
Figure 123.
Teratoma, Malignant, Ovary, mouse.
Figure 124.
Teratoma, Malignant, Ovary, mouse.
Species
Mouse; Rat.
Synonym(s)
Immature teratoma; Cystic teratoma.
Pathogenesis/cell of origin
Multipotential embryonic tissue.
Diagnostic Features
・Typically contains tissue derived from all three primary embryonic germ layers: ectoderm, mesoderm, and endoderm.
・May be cystic or solid.
・Cysts lined by epithelium that may be cuboidal, enteric, respiratory, or keratinized/squamous in nature.
・Tissues are poorly differentiated and resemble embryonic tissue.
・Local invasion is present.
・Areas of necrosis and hemorrhage may be present.
・Metastases may be present.
Differential Diagnoses
Teratoma, benign:
・Tissues are well-differentiated.
・No significant areas of hemorrhage or necrosis.
・Lack of local invasion.
・No metastases.
Comment
Spontaneous malignant teratomas of the uterus are rare.
References
32Davis et al. (2001), 39Dixon et al. (1999), 116Maekawa (1990), 131Miwa et al. (1987), 147Nielsen et al. (1976), 163Rehm et al. (1984), 174Serov et al. (1973), 180Sobis (1987c)
III. Uterus and Oviduct (Uterine Tube)
A. Nonproliferative Lesions
Aplasia, segmental (N) Uterus
Species
Mouse; Rat.
Synonym(s)
Agenesis, partial; Dysgenesis.
Pathogenesis/cell of origin
Paramesonephric ducts – a developmental absence of a portion of the uterus/uterine horn. Diagnostic Features
・Absence of one or more portions of the corpus uteri (usually observed grossly).
・Defective or abnormal development of corpus uteri resulting in abnormal anatomy.
Differential Diagnoses
Atrophy:
・Uterine atrophy is an age-related change or can be induced by xenobiotics, antiestrogens, or ovariectomy comprising a reduction in uterine size/volume. All components are present, albeit diminished.
Comment
This is not a histopathological lesion, but rather an anatomic abnormality. It is a congenital, developmental absence of tissue.
References
31Davis et al. (1999), 47Faccini et al. (1990), 69Greaves et al. (1992), 109Leininger and Jokinen (1990)
Atrophy (N) Uterus; Uterine Cervix; Vagina (Figure 125 and 126)
Figure 125.
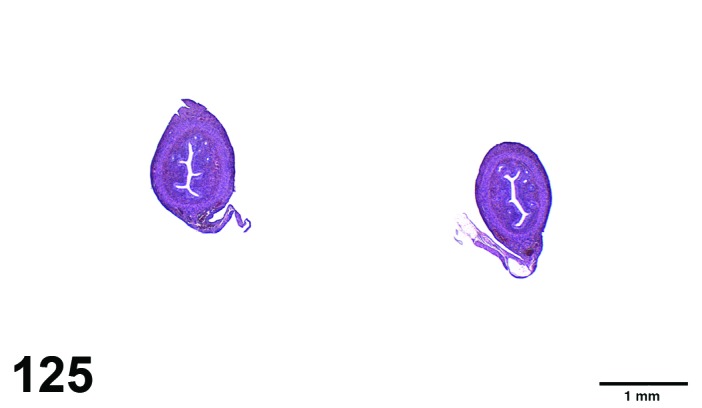
Atrophy, Uterus, rat.
Figure 126.
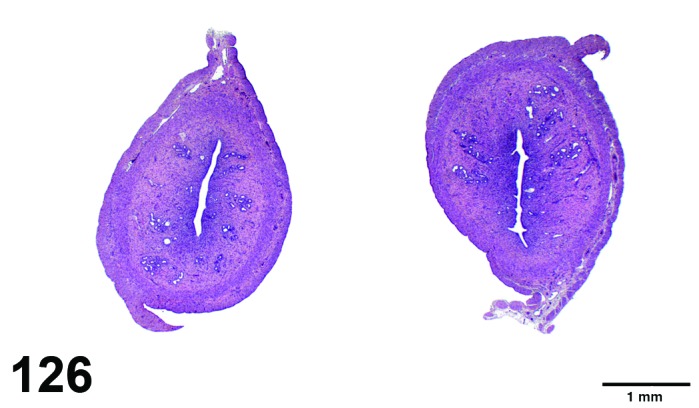
Normal, Uterus, aged-rat.
Species
Mouse; Rat.
Modifier
Epithelial; Endometrial; Myometrial; Stromal.
Pathogenesis/cell of origin
Epithelium, smooth muscle cells, stromal cells or combinations.
Diagnostic Features
・Generally, atrophy affects the whole uterus.
・The uterus is decreased in weight and size, with thin uterine horns.
・The number of endometrial glands is reduced.
・The luminal and glandular epithelial cells are reduced in height (cuboidal to flat) without signs of hormonal influences.
・The nuclei in atrophic epithelium are closely packed together with reduced cytoplasm.
・Reduced stromal cellularity with (especially in older rodents) increase in stromal collagen (stromal hyalinization).
・The nuclei of stromal cells have more rounded hyperchromatic nuclei.
・Thinning of the myometrium with smooth muscle cells that are reduced in size.
Differential Diagnoses
Immaturity:
・The absence of significant hormonal influence on the uterus in juvenile animals can mimic those present in aged animals, causing a morphology more or less similar to those observed in true atrophy.
Hypoplasia:
・Hypoplasia points to a developmental retardation.
Comment
Uterine atrophy related to ovariectomy is a classical example. Uterine atrophy is commonly observed with advancing of age of rats and mice and related to declining estrogen (and progesterone) levels. Suppression of gonadotropins probably precedes the decline of the ovarian steroid and ovarian atrophy therefore is often related. Certain compounds that have an effect on the release of gonadotropic hormones or that interfere with the ovarian steroid production can cause atrophy of the uterus. Also long-term progestogen treatment can cause this phenomenon. Cyclophosphamide, an alkylating agent that interferes with the follicular development in the ovary can cause suppression of plasma estrogen levels and consequently cause uterine atrophy. Also a primary decline in the number of nuclear estrogen receptors changes the sensitivity of the tissue for estrogens. Downregulation of the estrogen receptor causes atrophy because of a reduced cellular responsiveness to estrogen or related compound. In some cases compounds induced selective atrophy of certain components of the uterus: continuous administration of tamoxifen to mice for 24 months produced hyperplasia of the uterine endometrial epithelium accompanied by atrophy of the myometrium for the first 3 months, followed by atrophy of both the endometrium and myometrium for the remaining 21 months of the study.
References
Hypoplasia (N) Uterus (Figure 127)
Figure 127.
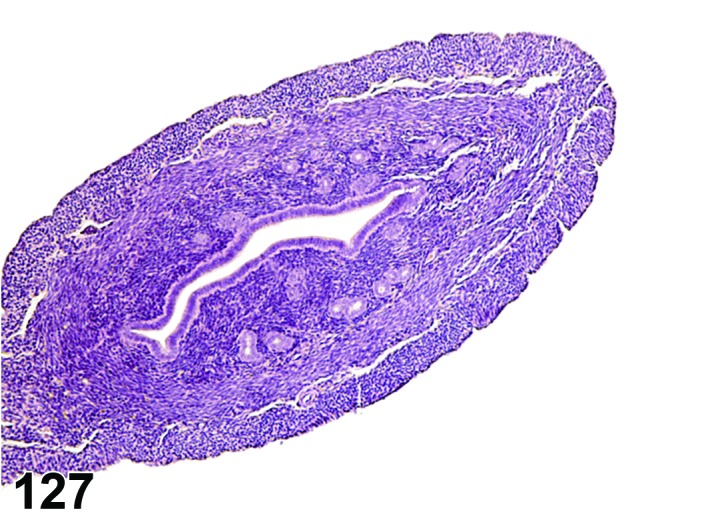
Hypoplasia, Uterus, mouse.
Species
Mouse; Rat.
Modifier
Endometrial; Epithelial.
Pathogenesis/cell of origin
Paramesonephric ducts.
Diagnostic Features
・Small uterus with reduced glandular tissue and stromal elements that has never reached full development.
・Diagnosis should be reserved for neonatal or in utero exposures, or transgenic models.
Differential Diagnoses
Aplasia, segmental:
・Complete absence of one or more portions of the corpus uteri.
Atrophy:
・Uterine atrophy is an age-related change or induced by xenobiotics comprising a reduction in uterine size/volume. Atrophy occurs after full, normal development.
Comment
The use of the term hypoplasia implies that the organ has never developed to full maturity, whereas atrophy implies a reduction following full development. Morphologically, it is difficult to differentiate these two. Endometrial hypoplasia has been induced following neonatal exposure to high doses of estrogens while exposure of neonates to antiestrogens results in epithelial hypoplasia in rats. In transgenic mice with a disrupted estrogen receptor gene, uterine endometrial hypoplasia occurs.
References
13Branham et al. (1988), 38Dixon et al. (1997), 128Medlock et al. (1997)
Hypertrophy, epithelial (N) Uterus (Figure 128)
Figure 128.
Hypertrophy of the Luminal Epithelium, Uterus, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Luminal and glandular epithelium.
Diagnostic Features
・The epithelial cells lining the uterine lumen and/or glands are tall columnar in shape.
・The cytoplasm/nuclear ratio is increased, and the cells contain lightly basophilic cytoplasm.
・Mitotic figures are often seen within this hypertrophied epithelium.
Differential Diagnoses
None.
Comment
Hypertrophy of the uterine epithelium is often seen in rats and mice. Physiologic hypertrophy caused by increased levels of estrogens during the proestrus and estrus phase of the cycle is not usually recorded. Since the change is mediated by estrogens an increase in proliferation is often present. Supraphysiologic levels of estrogens or compounds with estrogenic activity can induce hypertrophy in excess of that seen during the normal estrous cycle. In the case of persistent estrus for instance, the uterine epithelium becomes tall columnar. The change is generally mediated via the estrogen receptors present on the epithelial cells. In studies, an increased incidence of epithelial hypertrophy could point to drug-induced alterations of the hormonal status (e.g., relative estrogen dominance).
References
11Biegel et al. (1998)
Hypertrophy, myometrial (N) Uterus (Figure 129)
Figure 129.
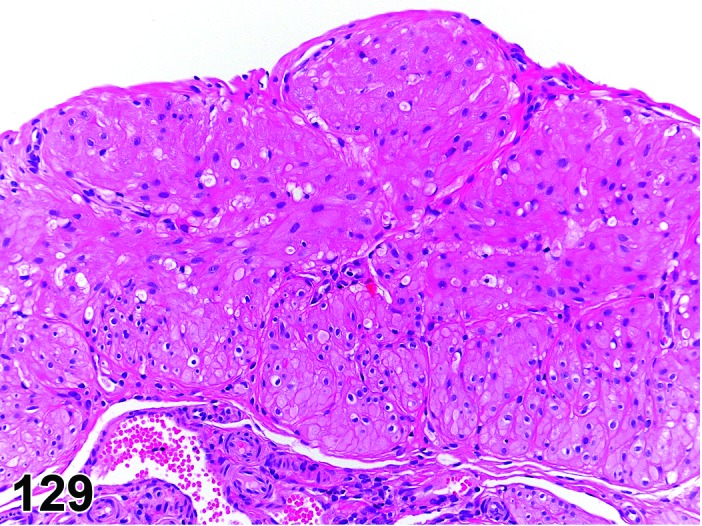
Hypertrophy of the Myometrium, Uterus, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Smooth muscle cells of the myometrium.
Diagnostic Features
・The uterus is enlarged and rigid.
・The cytoplasmic volume of the smooth muscle cells within the myometrium is increased.
Differential Diagnoses
None.
Comment
Myometrial hypertrophy is an unusual change in rat and mice. It sometimes accompanies endometrial hyperplasia. Certain synthetic hormones can induce it. Estradiol benzoate given to immature rats can cause this change. In mature female Wistar rats, the anabolic androgenic steroid nandrolone decanoate caused an increase in myometrium thickness, while the endometrium was significantly thinner. It is hypothesized that androgenic receptors expressed by smooth muscle cells or the estrogenic and progesterone-like effect of this compound could be involved in this hypertrophy.
References
63Gopinath and Gibson (1987), 111Lerner et al. (1966), 132Mobini Far et al. (2007)
Cyst, NOS (N) Uterus; Uterine Cervix; Vagina; Ovary (See Ovary Section)
Dilation, luminal (N) Uterus (Figure 130)
Figure 130.
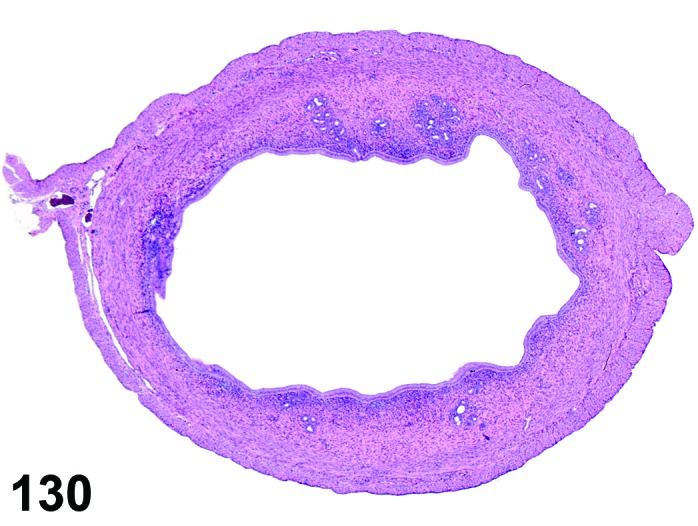
Luminal Dilation, Uterus, mouse.
Species
Mouse; Rat.
Synonym(s)
Hydrometra, dilatation.
Pathogenesis/cell of origin
Luminal epithelium.
Diagnostic Features
・Dilation of the uterine horns by serous, proteinaceous fluid; the change is often observed grossly.
・Increased serous fluid production is part of the proestrus phase of the cycle easily judged on the vaginal epithelium (which shows early keratinization covered by a layer of mucified cells).
・In most cases both horns and the body are involved, but the change also can appear in one horn or focally.
・Usually, the endometrial lining is attenuated or atrophic and the wall of the uterus thinned due to the increasing pressure, but in less severe cases the endometrium can still be normal.
Differential Diagnoses
Hematometra or Pyometra:
・Also can cause luminal dilation, but signs of intraluminal hemorrhage or purulent inflammatory material can be observed, respectively. In the presence of endometrial stromal polyps the lumen also can become dilated.
Comment
Distention of the uterus horns is a common finding in cycling rats. The change is a normal feature during the proestrus and estrus phase of the cycle, when the endometrial cells secrete a watery fluid under the influence of estrogen. Estrogenic compounds also can induce such a distention of the uterine horns. Sometimes the change is accompanied by the presence of cystic dilated glands. In studies, an increased incidence of luminal dilation can point to drug-induced alterations of the hormonal status (e.g., relative estrogen dominance).
Dilation, glandular, cystic (N) Uterus (Figure 131)
Figure 131.
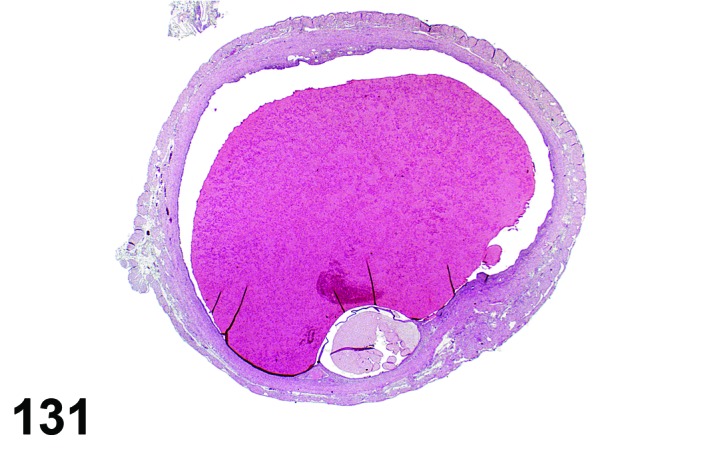
Cystic Glandular Dilation, Uterus, mouse.
Species
Mouse; Rat.
Modifier
Cystic.
Pathogenesis/cell of origin
Glandular epithelium.
Diagnostic Features
・Focal dilation of a limited number of uterine glands by serous or proteinaceous fluid.
・Notable dilation with compression of lining epithelium can be seen (cystic dilation/cyst).
・No increase in mitotic activity or evidence of proliferative changes.
Differential Diagnoses
Hyperplasia, glandular, cystic:
・This lesion can be distinguished from glandular dilation by the absence of increased proliferative activity of the glands involved in glandular dilation. The number of glands in cystic endometrial hyperplasia is often increased and the stromal compartment is rich in collagen.
Comment
Gland necks can become obstructed causing dilation of the more basal parts of the glands. This dilation in most cases is a purely mechanical change, not to be confused with true cystic endometrial hyperplasia, which is a proliferative lesion. The change is found in 6% of rats in 2-year studies. The modifier “cystic” should be used to differentiate the more severe form of the lesion rather than using a separate diagnostic term.
References
15Brown and Leininger (1992), 47Faccini et al. (1990), 69Greaves et al. (1992), 196Tucker (1997)
Adenomyosis (N) Uterus (Figure 132
Figure 132.
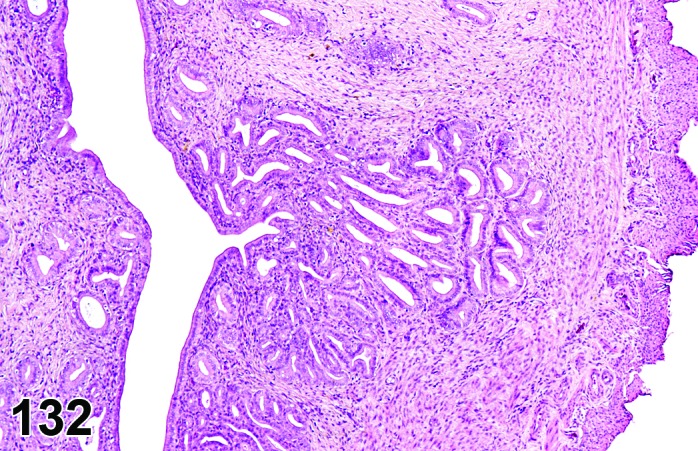
Adenomyosis, Uterus, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Endometrium.
Diagnostic Features
・Presence of well-differentiated endometrial elements (glands and stroma) within the myometrium.
・In advanced cases, foci or nodules may project from the uterus but do not penetrate through the serosa.
・No evidence of atypia in epithelial or stromal elements.
・Lack of scirrhous reaction to epithelial elements.
Differential Diagnoses
Adenoma, endometrial:
・Neoplastic proliferation of epithelial elements, often polypoid and/or projecting into the uterine lumen, showing no evidence of invasion into adjacent structures.
Adenocarcinoma, endometrial:
・Atypical, neoplastic proliferation of epithelial elements with invasion into myometrium. May extend beyond the serosa into the peritoneum. Pleomorphism and atypia are common and mitotic figures can be frequent. In some cases there is a prominent scirrhous response to the proliferating epithelial cells.
Comment
Adenomyosis has been likened to endometriosis in humans and primates but it should not be equated/used as a synonym in rodents since adenomyosis does not spread to the peritoneal cavity (a characteristic of endometriosis), nor do rodents menstruate. The cause of this lesion is not fully understood but is thought to be related to hormonal imbalance. It is commonly found in aging mice. It is not so common, but can still be seen, in aging rats. Adenomyosis has been induced in mice with hormone (estrogen and/or progesterone) modulators and other xenobiotics inducing hyperprolactinemia.
References
47Faccini et al. (1990), 66Greaves (2012), 76Heywood and Wadsworth (1981), 109Leininger and Jokinen (1990), 119Maekawa and Maita (1996)
Angiectasis (N) Uterus (Figure 133)
Figure 133.
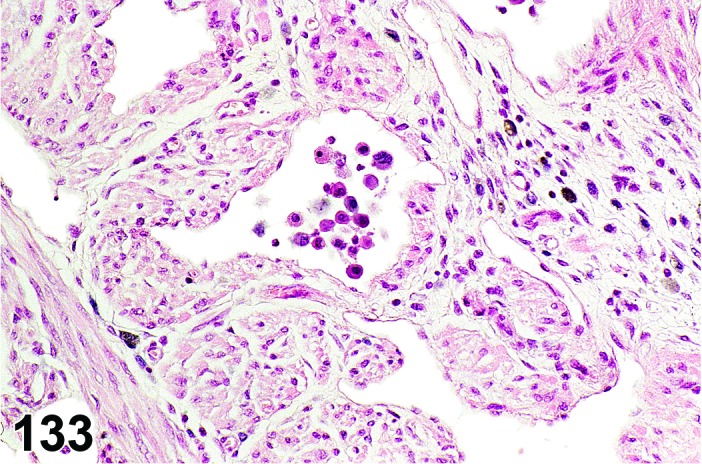
Angiectasis, Uterus, mouse.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Vascular tissue.
Diagnostic Features
・Presence of multiple dilated/cystic small (thin-walled) blood vessels, usually within the myometrium, possibly distorting the normal architecture.
・Enlarged capillary channels lined by normal endothelial cells.
・May be associated with thrombosis, hemorrhage or inflammation.
Differential Diagnoses
Hemangioma:
・Focal areas of increased numbers of blood-filled spaces lined with uniform endothelial cells, distorting the architecture of the affected tissue.
Hemorrhage:
・Extravascular blood present in the endometrium or myometrium.
Congestion:
・Widely dilated, blood-filled vasculature not distorting the affected tissue.
Lymphangiectasis:
・Dilated lymphatics do not contain significant amount of erythrocytes and are generally empty or only filled with pale proteinaceous material.
Comment
More common in mice than rats. The changes are often found in association with cystic endometrial hyperplasia, but can occur independently.
References
15Brown and Leininger (1992), 31Davis et al. (1999), 47Faccini et al. (1990), 120Maekawa and Maita (1996)
Infiltrate, inflammatory cell (N) Uterus; Oviduct; Uterine Cervix; Vagina (Figure 134)
Figure 134.
Inflammatory Cell Infiltrate versus Normal, Uterus, rat.
Species
Mouse; Rat.
Modifier
Eosinophilic; Histiocytic; Neutrophilic; Lymphocytic; Mononuclear; Mixed.
Pathogenesis/cell of origin
Inflammatory reaction.
Diagnostic Features
・Diffuse infiltration of the uterus by granulocytes, macrophages, lymphocytes, or a mixture of the above.
・Other inflammatory changes (vascular congestion, edema, exudates, necrosis, fibrosis) are absent or of limited severity.
Differential Diagnoses
Infiltrate, inflammatory cell, lymphocytic, endometrium:
・Endometrial lymphocytes are common in the endometrium of young cycling rats. In the past, endometrial lymphocytes were erroneously described as endometrial polymorphonuclear granulocytes because of their lobulated nuclei.
Infiltrate, neutrophilic, endometrium:
・Are normally present within the non-inflamed endometrium during phases of the estrus cycle, particularly during metestrus, and in the endometrial epithelium in diestrus. In these cases, it is recommended not to record, if part of the normal cyclic activity.
Inflammation, endometrium:
・Inflammatory reaction of the uterine epithelium and mucosa with involvement of epithelial and stromal components.
Inflammation, endometrium (N) Uterus (Figures 135 and 136)
Figure 135.
Inflammation, Endometrium, Neutrophilic, Uterus, mouse.
Figure 136.
Inflammation, Endometrium, Lymphocytic, Uterus, mouse.
Species
Mouse; Rat.
Synonym(s)
Endometritis.
Modifier
Neutrophilic; Lymphocytic; Mononuclear; Mixed. Other modifiers include suppurative, granulomatous.
Diagnostic Features
・Inflammatory reactions may be limited to the mucosa with little or no exudate into the lumen, and with limited involvement of the myometrium.
・Neutrophils and small numbers of lymphocytes are present in the endometrial glands and supporting stroma in acute inflammation.
・Appearance varies depending upon the severity and duration of the preceding acute inflammation; however, fibrosis and infiltration by lymphocytes, plasma cells and macrophages occur in more chronic inflammation.
・Endometrial glands may be atrophic or cystic due to periglandular fibrosis in chronic inflammation.
・Endometrial lining may be eroded, ulcerated, or show squamous metaplasia depending on the severity and duration of the inflammation.
・Luminal and glandular epithelial changes range from desquamation of epithelial cells to erosion, ulceration and necrosis.
・Stromal changes include edema, vasodilation and necrosis.
The diagnosis of inflammation of the endometrium should be reserved for lesions where significant epithelial and stromal inflammatory changes are present.
Differential Diagnoses
Infiltrate, inflammatory cell
Inflammation, myometrium:
・A more severe lesion than endometritis, which extends to involve the myometrium.
Pyometra:
・A more severe lesion than endometritis with a significant purulent exudate.
Comment
Inflammation of the endometrium is frequently seen as a component of endometrial hyperplasia. Inflammation of the endometrium must be distinguished from the normal inflammatory cell infiltrates present in the uterus. Eosinophil infiltrates are common in the endometrium of young cycling rats, and their presence does not seem to correlate with cyclic activity or with any pathologic process in the uterus. Neutrophil infiltrates are normally present within the non-inflamed endometrium during phases of the estrous cycle, particularly during metestrus, and in the endometrial epithelium in diestrus.
References
15Brown and Leininger (1992), 31Davis et al. (1999), 109Leininger and Jokinen (1990)
Inflammation, myometrium (N) Uterus (Figures 137)
Figure 137.
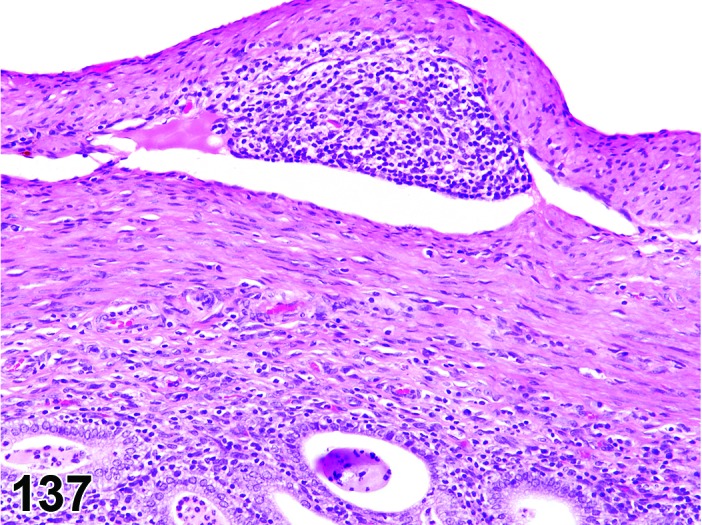
Inflammation, mixed, Myometrium, Uterus, rat.
Species
Mouse; Rat.
Synonym(s)
Myometritis.
Modifier
Neutrophilic; Lymphocytic; Mononuclear; Mixed. Other modifiers include suppurative, granulomatous.
Pathogenesis/cell of origin
Inflammatory reaction.
Diagnostic Features
・Inflammatory reaction extending from the mucosa to involve the myometrium, with limited purulent exudate into the lumen.
・In an acute inflammatory reaction, neutrophils and small numbers of lymphocytes are present in the endometrial glands, supporting stroma, and myometrium and as the changes progress to subchronic the number of neutrophils may be replaced by increasing numbers of lymphocytes, plasma cells and macrophages.
・The diagnosis of myometritis should be reserved for lesions where there is significant involvement of the myometrium. A chronic inflammatory reaction extending from the mucosa to involve the myometrium, with limited purulent exudate into the lumen.
・The appearance varies depending upon the severity and duration of the preceding acute inflammation; however, there is fibrosis and infiltration by lymphocytes; plasma cells and macrophages in chronic inflammation.
・Epithelial changes range from desquamation of epithelial cells to erosion, ulceration and necrosis.
・Stromal and myometrial changes include edema, vasodilation and necrosis.
・Endometrial glands may be atrophic or cystic due to periglandular fibrosis in chronic inflammaton.
・The diagnosis of inflammation of the myometrium should be reserved for lesions where there is significant involvement of the myometrium.
Differential Diagnoses
Inflammation, endometrium:
・Inflammatory lesion confined to the endometrium.
Pyometra:
・Often a sequel to inflammation of the endometrium, with a significant purulent exudate.
References
15Brown and Leininger (1992), 31Davis et al. (1999), 99King et al. (1996), 109Leininger and Jokinen (1990), 132Mobini Far et al. (2007)
Inflammation, uterus (N) Uterus (See Uterine Cervix and Vagina Section)
Abscess(es) (N) Uterus (Figure 138)
Figure 138.
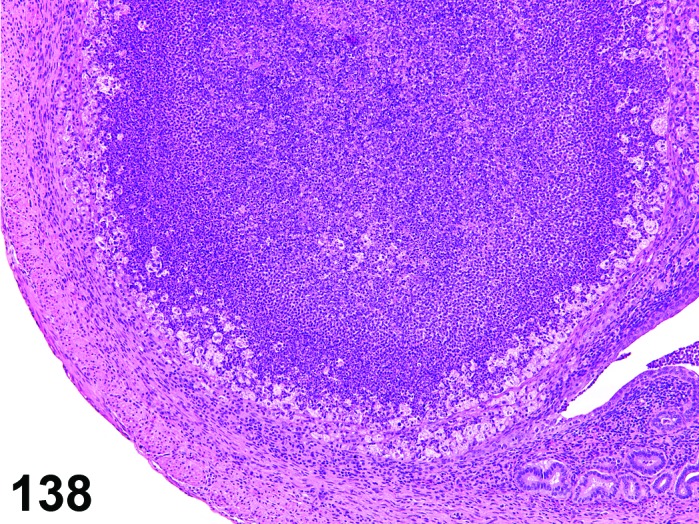
Abscess, Uterus, mouse.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Inflammatory reaction.
Diagnostic Features
・An abscess is characterized by the presence of a central core of necrosis and neutrophils, surrounded by a capsule.
・The capsule is initially composed of neutrophils and fibrinous exudate, which is then organized into granulation tissue, and eventually in a chronic abscess, by fibrous granulation tissue infiltrated by neutrophils, macrophages, lymphocytes and plasma cells.
Differential Diagnoses
Infiltrate, inflammatory cell, mixed:
・Less extensive, lesion composed predominantly of neutrophils, lymphocytes, mononuclear cells or other inflammatory cell types.
Granuloma:
・An aggregate of macrophages (including epithelioid and/or multinucleated) cells with a variable but smaller component of other leukocytes (neutrophils and lymphocytes) and limited fibrosis and capillary proliferation.
Tumor, granular cell, benign; Tumor, granular cell, malignant:
・Lesions are generally localized at the boundary of the cervix and vagina, on the serosal surface, and are composed of large rounded cells with pale granular eosinophilic cytoplasm, which stains faintly positive with PAS. Nuclei are small, dark and centrally located. There is limited connective tissue.
References
70Greaves and Seely (1996)
Pyometra (N) Uterus (Figure 139)
Figure 139.
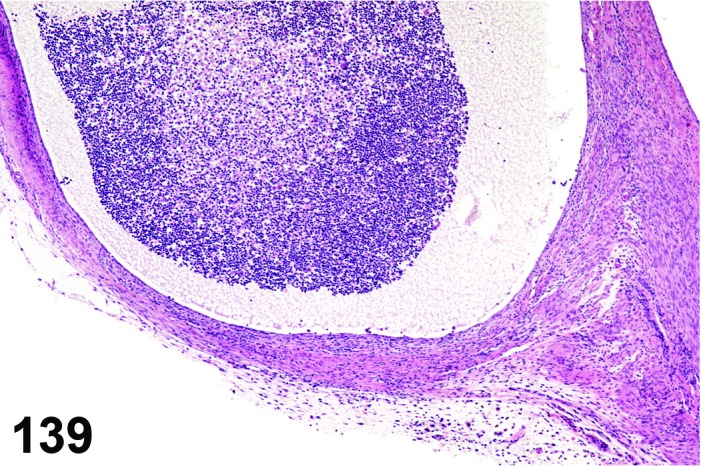
Pyometra, Uterus, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Pyometra is a common sequel to infection of the genital tract by Mycoplasma pulmonis. Klebsiella oxytoca has also been identified as a factor in aged B6C3F1 mice; however, other normal vaginal flora such as Staphylococcus aureus, Proteus mirabilis and Escherichia coli may be contributing factors. Estrogen treatment is generally considered to increase the susceptibility to pyometra; however, this varies with the strain of rat: Sprague Dawley rats are relatively resistant to this effect, while Wistar and Brown Norway rats are more sensitive.
Diagnostic Features
・The characteristic feature of pyometra is moderate or severe dilation of the uterine lumen by the accumulation of viscous, purulent or hemopurulent exudate.
・The uterine endometrium is ulcerated and covered by inflammatory cells and necrotic debris.
・Squamous metaplasia may be present, and may be related to the hormonal state of the animal.
・Inflammatory infiltrates extend deep into the myometrium.
Differential Diagnoses
Inflammation, endometrium:
・There is little accumulation of exudate in the uterine lumen in endometritis.
Mucometra:
・Mucometra is the sterile accumulation of mucous exudate in the uterus as a result of obstruction of normal outflow. It is occasionally seen as a sequel to imperforate vagina in mice.
References
14Brossia et al. (2009), 31Davis et al. (1999), 109Leininger and Jokinen (1990), 185Suckow et al. (2006), 186Sundberg (1990)
Granuloma (N) Uterus (Figure 140)
Figure 140.
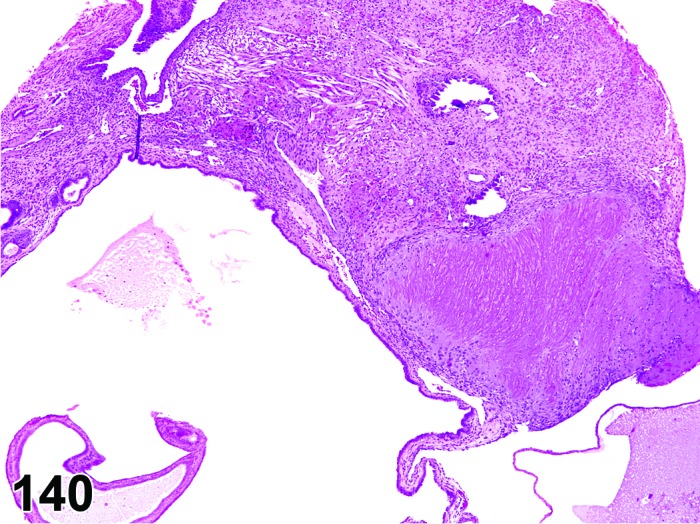
Granuloma, Uterus, mouse.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Chronic inflammatory reaction.
Diagnostic Features
・An aggregate of macrophages (including epithelioid and/or multinucleated cells) with a variable but smaller component of other leukocytes (neutrophils and lymphocytes) with varying amounts of fibrosis and capillary proliferation.
Differential Diagnoses
Abscess(es):
・Firmly attached lesion typically with a central necrotic core and a fibrous capsule.
Infiltrate, inflammatory cell, mixed:
・Less extensive, lesion composed predominantly of neutrophils, lymphocytes, mononuclear cells or other inflammatory cell types.
Tumor, granular cell, benign; Tumor, granular cell, malignant:
・Lesions are generally localized at the boundary of the cervix and vagina, on the serosal surface, and are composed of large rounded cells with pale granular eosinophilic cytoplasm, which stains positively with PAS. Nuclei are small, dark and centrally located. There is limited connective tissue.
References
70Greaves and Seely (1996)
Amyloid (N) Uterus (Figure 141)
Figure 141.
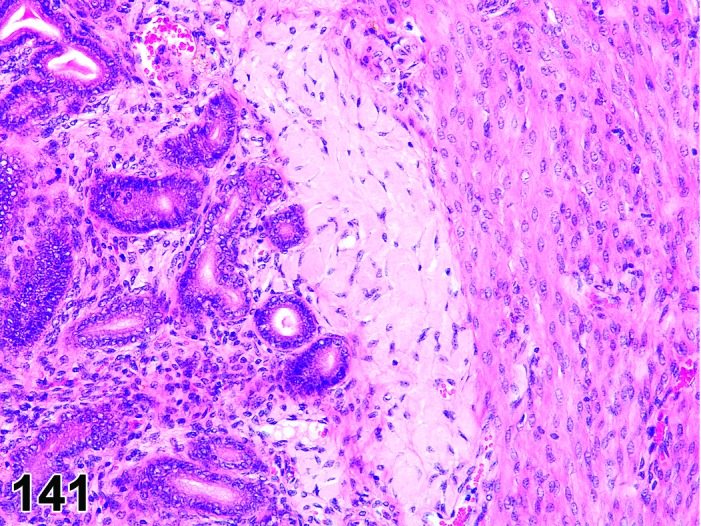
Amyloid, Uterus, mouse.
Species
Mouse
Pathogenesis/cell of origin
Extracellular deposits of polypeptide fragments of a chemically diverse group of glycoproteins in various tissues.
Diagnostic Features
・Amorphous eosinophilic material, often perivascular (particularly in the periadventitium of medium sized arteries), but also in the stroma of the endometrium and myometrium.
・Green birefringence using polarized light with Congo Red stain.
Differential Diagnoses
Necrosis:
・Congo Red negative; Other evidence of damage.
Degeneration:
・Congo Red negative.
Fibrosis:
・Congo Red negative.
Edema:
・Degree of eosinophilia is variable to non-existent; Congo Red negative.
Comment
Amyloid deposits in the uterus are most often seen in aged mice of several strains but have not been reported in B6C3F1, BALB/c or C57BL/6 strains.
References
Pigment (N) Uterus (Figures 142 and 143)
Figure 142.
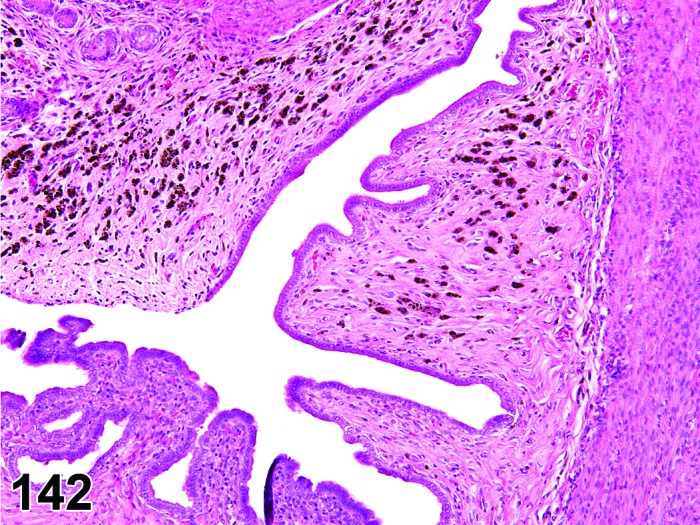
Hemosiderin Pigment, Uterus, rat.
Figure 143.
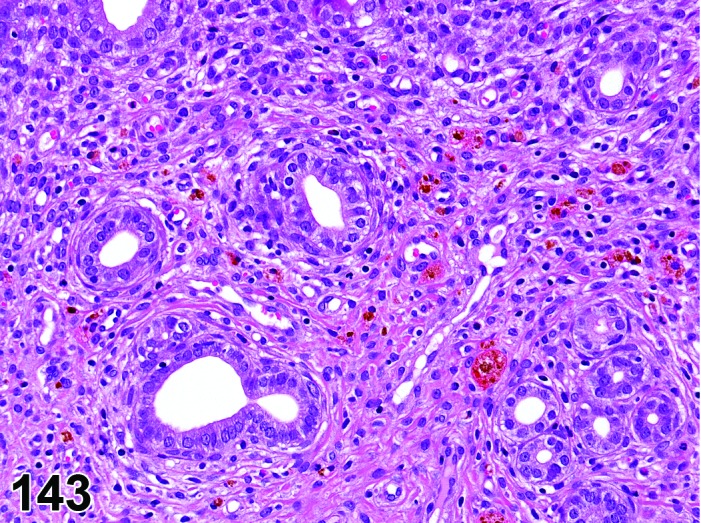
Ceroid Pigment Deposits, Uterus, rat.
Species
Mouse; Rat.
Modifier
Hemosiderin; Ceroid; Lipofuscin/ceroid. Alternatively, if pigment isn’t definitively identified, colors can be used as modifiers.
Pathogenesis/cell of origin
Brown, granular pigment derived from red blood cells (hemosiderin), or cell membrane lipids (lipofuscin/ceroid).
Diagnostic Features
Hemosiderin:
・Golden-brown, granular, iron-positive pigment derived from red blood cells.
・Hemosiderin-laden macrophages can be seen near the endometrial glands and luminal epithelium.
・Hemosiderin can be found in phagocytic perivascular stromal cells in the endometrium of rats.
・Stains include Perl’s iron stain or the Prussian blue reaction.
Lipofuscin/ceroid:
・Consists of breakdown products of cell membrane lipids.
・Associated with cell turn over, degeneration and/or necrosis.
・Pigment is golden brown and granular.
・Special stains include Sudan black, Schmorl’s stain, Oil red O, carbol lipofuscin stain, Periodic Acid Schiff’s (PAS) reaction, lysosomal acid phosphatase, esterase and Ziehl-Neelsen acid fast stains.
Ceroid:
・Variant of lipofuscin with similar staining properties.
・Golden yellow autofluorescence under ultraviolet light.
・Stains include Sudan Black B, Schmorl’s reaction and Oil red O, PAS and Ziehl-Neelsen acid fast stains.
Other Pigments (pigment, formalin):
・Formalin-precipitated hemoglobin is a dark brown pigment that can appear in tissues that are fixed in improperly buffered formalin.
・The pigment is microcrystalline and anisotropic and thus has birefringent properties, which makes it easy to detect using polarized light.
・It does not stain positive in the Prussian blue reaction.
・It is an artifact.
Differential Diagnoses
None.
Comment
Variable numbers of hemosiderin-laden macrophages are often present in the endometrium of aging rats at 10-12 months of age. Hemosiderin pigment is readily stainable when using Perl’s iron stain or the Prussian blue reaction. Ceroid and lipofuscin are difficult to distinguish from each other in a conventional H&E stain and a variety of staining methods can be used to identify ceroid/lipofuscin, such as Sudan Black B, Schmorl’s reaction and Oil red O. Nevertheless, although ceroid and lipofuscin both are lipochromes consisting mainly of lipid residues as result of lysosomal digestion (of among other membranes) and thus closely related, ultrastructural and chemical characteristic differences exist. Ceroid is believed to be at an earlier stage of oxidation compared to lipofuscin. Schmorl’s method, along with the Long Ziehl-Neelsen technique can differentiate between both pigments, since ceroid is negative when using Schmorl’s method.
References
15Brown and Leininger (1992), 27Cornillie and Lauweryns (1985), 120Maekawa and Maita (1996), 152Pears (1985)
Apoptosis (N) Uterus (Figures 144)
Figure 144.
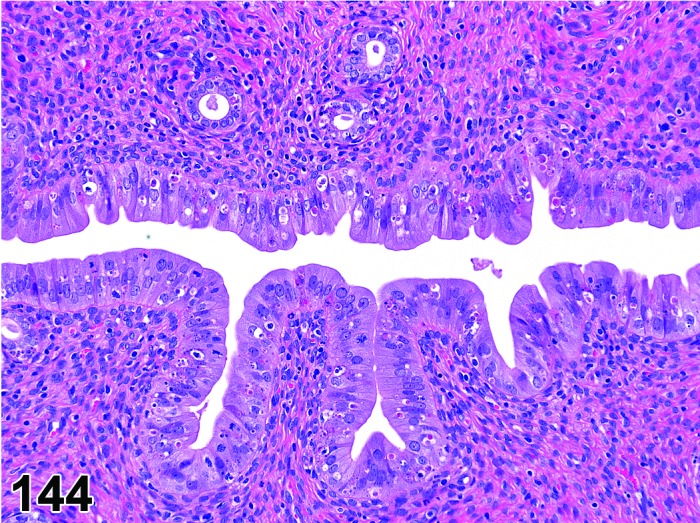
Apoptosis of the Luminal Epithelium, Uterus, rat.
Species
Mouse; Rat.
Modifier
Endometrial; Luminal; Epithelial; Glandular; Stromal.
Pathogenesis/cell of origin
Endometrial cells including luminal epithelial, glandular, stromal cells.
Diagnostic Features
・Pyknosis and/or karyorrhexis of nuclei of individual cells.
・Individual cell shrinkage with dense, eosinophilic cytoplasm.
・Individualized cell debris, with “halo”, within luminal and/or glandular epithelium.
Differential Diagnoses
Estrus/metestrus:
・Apoptosis, single cell death, and degeneration are all normal physiologic responses during the estrous cycle related to rapid decreases in serum E2 levels after ovulation. If the animal is cycling normally, a diagnosis of apoptosis should not be used.
Comment
Epithelial and/or glandular apoptosis has been described in animals with hormonal disruption affecting regular cyclical activity such as estrogenic xenobiotics. The change should only be diagnosed if in excess of that occurring normally in a cycling animal or if observed as a characteristic of hormonal disruption in an animal without features of normal cyclical activity.
References
75Hendry et al. (1997)
Necrosis (N) Uterus (Figure 145)
Figure 145.
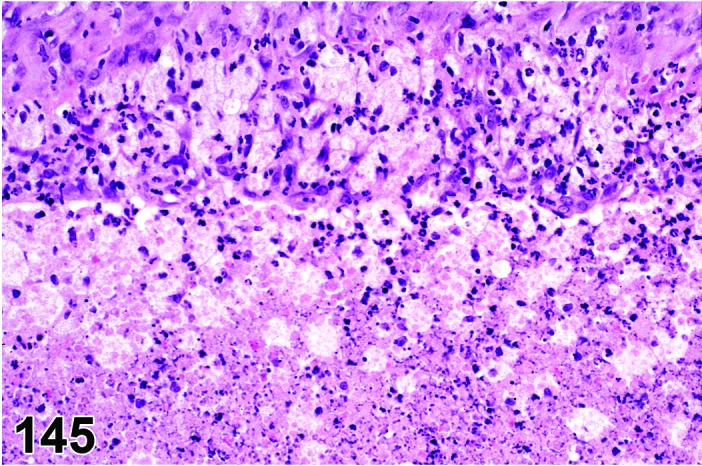
Necrosis, Uterus, rat.
Species
Mouse; Rat.
Modifier
Endometrial; Myometrial.
Pathogenesis/cell of origin
Endometrial and/or glandular epithelial cells, stromal cells and smooth muscle cells of the muscularis.
Diagnostic Features
・Pyknosis and/or karyorrhexis of the nuclei.
・Cytoplasmic eosinophilia.
・Cellular swelling or shrinkage.
・Usually associated with inflammation.
・Sloughing of epithelial cells into the uterine lumen may occur.
・May result in ulceration or erosion.
Differential Diagnoses
Autolysis:
・Uniform dissolution of tissue in section. Smooth muscle and stromal cells more resistant to autolysis than epithelia.
Artifactual damage
Comment
Intravaginal administration of the vaginal spermicide nonoxynol-9 induces mucosal damage and acute necrotizing inflammation in the uterus, cervix and vagina. Intrauterine instillation of quinacrine hydrochloride solution in white mice causes proliferation, followed by disintegration and necrosis of the epithelial tissue.
References
Fibrosis (N) Uterus (Figures 146 and 147)
Figure 146.
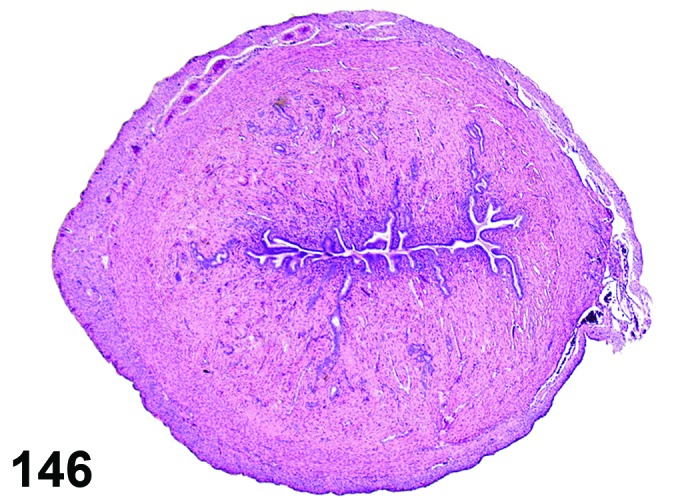
Fibrosis, Stromal, Uterus, rat.
Figure 147.
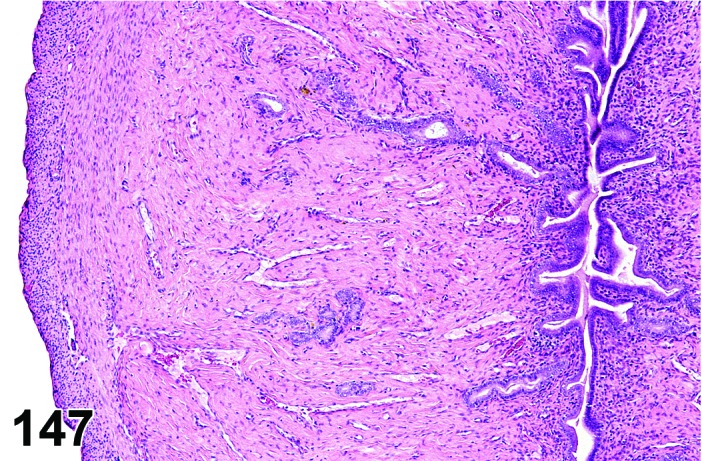
Fibrosis, Stromal, Uterus, rat.
Species
Mouse; Rat.
Synonym(s)
Hyalinization, stromal.
Modifier
Myometrial; Stromal.
Pathogenesis/cell of origin
Mesenchymal/connective tissue within the endometrial stroma or myometrium.
Diagnostic Features
・Increased fibroblasts with/without increased collagen production within the tissues.
・Increased amount of collagen within the endometrial stroma or myometrium.
・Reduced cellularity of the stroma and replacement with mature connective tissue (often referred to as hyalinization rather than fibrosis).
・Special stains are useful to confirm the presence of increased collagen.
Differential Diagnoses
Amyloid:
・Congo Red positive, often perivascular.
Comment
Stromal fibrosis is a common age-related change in rats and mice and accompanies a progressive reduction of the glandular tissues. If the lesion is predominantly acellular, then the term stromal hyalinization is often used. Fibrosis of the uterus has also been reported following chronic treatment with Zearalenone, an estrogenic mycotoxin (NTP, technical report series, No. 235).
References
15Brown and Leininger (1992), 31Davis et al. (1999), 66Greaves (2012), 138National Toxicology Program (1982)
Decidual reaction (H) Uterus (Figure 148)
Figure 148.
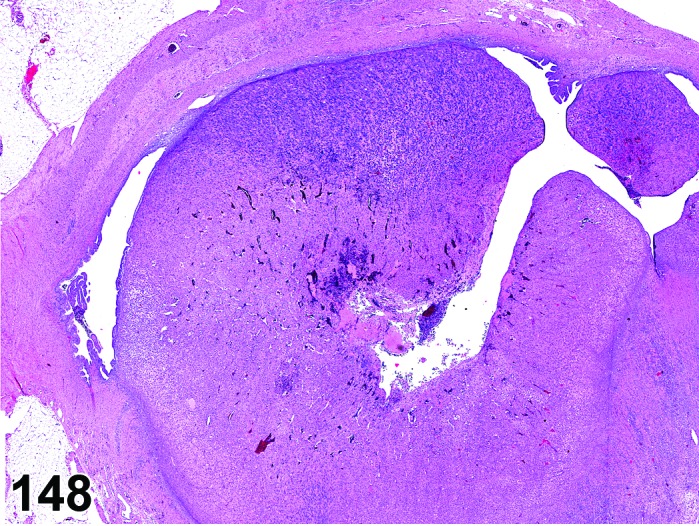
Decidual Reaction, Uterus, rat.
Species
Mouse; Rat.
Synonym(s)
Deciduoma; Decidual nodule, Decidual alteration.
Pathogenesis/cell of origin
Uterine stromal cells and uterine metrial gland cells.
Diagnostic Features
・Primarily occurs in uterus.
・Focal nodular proliferation of large eosinophilic stromal or mesenchymal cells (decidual tissue) with high levels of structural organization and regional variation.
・Fully developed decidual reaction has two distinct areas: antimesometrial region containing closely packed mesenchymal cells with small capillary channels; mesometrial region containing spiny mesometrial cells with long cytoplasmic processes and abundant glycogen, which are commonly binucleated, and granulated metrial gland cells with extensive cytoplasm containing PAS-positive cytoplasmic granules.
・A clear boundary between the decidual reaction and surrounding stroma is often lacking.
・May be primary or associated with another lesion such as endometrial stromal polyp.
・May be associated with inflammation.
Differential Diagnoses
Decidualization, focal:
・Focal lesion consisting of strongly hypertrophied (“decidualized”) stromal cells with PAS positive cytoplasmic granules and prominent nuclei. Often this change is found (secondary) in relation to or as part of other lesions such as tumors or induced hormonal changes.
Deciduosarcoma:
・Rare malignant tumor of hypertrophied stromal cells with abundant PAS positive rarefied cytoplasm intermingled with numerous globular lymphocytes. Strongly hypertrophied blood vessels characterize the lesion.
Sarcoma, endometrial stromal:
・Malignant tumor composed primarily of spindle shaped mesenchymal cells. In general the PAS reaction is negative in the tumor cells.
Mesenchymal proliferative lesion:
・Proliferative lesion of the genitourinary tract of mice, usually occurring in the submucosa of the urinary bladder. Composed of large eosinophilic epithelioid and spindle cells.
Pregnancy:
・Decidual reaction in the pseudopregnant state may resemble the decidual reaction during pregnancy; however, it lacks a fetus, placental labyrinth, spongiotrophoblast layer, giant cells, yolk sac, nucleated fetal erythrocytes, etc.
Comment
Decidual reaction is a rare spontaneous condition in most strains of rats and mice. The change mimics the true “deciduoma of pregnancy” related to early pregnancy. The change is not a neoplastic lesion, but rather a response to implantation or a mechanical stimulus (such as intrauterine contraceptive devices). The reaction occurs on day 3 and 4 of pseudopregnancy and depends on exposure to progesterone for at least 48 hours followed by a minute amount of estrogen at the end of this period. The normal life span of the nodular decidual reaction is from day 4 to 16 of pseudopregnancy. Decidual reactions can be induced by various compounds given at the proper time in the estrous cycle or after progesterone treatment. Also various forms of mechanical irritation or trauma, as well as electric stimulation or instillation of agents into the uterus can induce a decidual reaction. These lesions may occur rarely in young animals.
References
32Davis et al. (2001), 39Dixon et al. (1999), 74Hart-Elcock et al. (1987), 109Leininger and Jokinen (1990)
Decidualization, focal (N) Uterus (Figure 149)
Figure 149.
Focal Decidualization, Uterus, rat.
Species
Mouse; Rat.
Synonym(s)
Stromal pseudodecidualization, focal; Decidual alteration.
Pathogenesis/cell of origin
Uterine decidualized stromal cells.
Diagnostic Features
・In focal stromal decidualization, stromal cells form nodular lesions within the endometrial stroma.
・The stromal cells are strongly hypertrophied with prominent nuclei.
・Within the cytoplasm of these hypertrophied cells, PAS+ material (glycogen) can be demonstrated.
・Mitotic figures can be found within this change.
Differential Diagnoses
Hyperplasia, endometrial stromal:
・Increased stromal component, characterized by proliferation of slender spindle or stellated stromal cells with a variable amount of intercellular collagen dependent on the age of the lesion. In general the spindle cells do not contain abundant intracytoplasmic PAS+ material.
Decidual reaction:
・Nodular proliferation of large eosinophilic stromal or mesenchymal cells (decidual tissue) with a high level of structural organization and regional variation. When fully developed, the decidual reaction has two distinct areas: antimesometrial region containing closely packed mesencymal cells with small capillary channels; mesometrial region containing spiny mesometrial cells with long cytoplasmic processes and abundant glycogen, which are commonly binucleated, and granulated metrial gland cells with extensive cytoplasm containing PAS-positive cytoplasmic granules.
Comment
Focal stromal decidualization is an uncommon stromal change. The hypertrophied stromal cells in the lesion resemble the true decidualized stromal cells in the decidual reaction, but the lesion does not show the structural organization as seen in the “decidual reaction”. Also in polyps and tumors focal areas of decidualized stroma can be observed.
Decidualization of the endometrial stroma as well as deciduomas can be induced by various substances, such as prostaglandins, growth hormone or prolactin given at the proper time in the estrous cycle or after progesterone treatment. Intrauterine contraceptive devices or other intrauterine inserted materials or substances can cause stromal decidualization. It is also believed that uterine intraluminal polyps can cause the induction of stromal decidualization, since areas of decidualized stroma can be found within polyps or other tumors.
References
109Leininger and Jokinen (1990)
Mesonephric duct remnants (N) Uterus
Species
Mouse; Rat.
Pathogenesis/cell of origin
Mesonephric ducts, Wolffian duct remnants
Synonym(s)
Persistent Mesonephric ducts
Diagnostic Features
・Tubular or cystic structure(s) lined by epithelial cells and smooth muscle, resembling poorly developed vas deferens.
・These structures are seen within the mesovarial adipose tissue, laterally along the site of attachment of the broad ligament of the uterine horns or body, and/or within the vaginal submucosa.
・May be unilateral or bilateral.
Differential Diagnoses
Cyst, serosal:
・Fluid-filled cysts lined by epithelium within the serosa of the uterus. No smooth muscle is present.
Comment
In general toxicity studies, mesonephric duct remnants are seldom recorded because such structures are believed to be congenital. However, neonatal CD-1 mice, treated with subcutaneous injections of bisphenol A (BPA) in corn oil on day 1-5 developed a range of lesions in the female reproductive tract, among others cystic mesonephric (Wolffian) duct remnants (Newbold et al. 2007). Since paraovarian cysts of mesonephric (Wolffian) origin were also observed in mice treated with BPA it appears that the mesonephric duct system (Wolffian duct) may be a target of BPA because both cystic structures have the same fetal tissue origin (Newbold et al. 2009).
References
Metaplasia, squamous cell (N) Uterus (Figures 150 and 151)
Figure 150.
Squamous Metaplasia, Uterus, rat.
Figure 151.
Squamous Metaplasia, Uterus, anti-Cytokeratin 14, rat.
Species
Mouse; Rat.
Synonym(s)
Squamous cell metaplasia.
Modifier
Non-keratinizing; Keratinizing.
Pathogenesis/cell of origin
Squamous metaplasia originates from (progenitor) cells located at the basal side of the endometrial lining and/or gland necks.
Diagnostic Features
・Presence of foci/areas of stratified squamous non-keratinizing or keratinizing epithelium, not closely related to the uterocervical junction in one or both uterus horns.
・Squamous epithelium can be found replacing the uterine columnar lining epithelium as flat areas of normal squamous epithelium, or as focal mostly round (cannonball) areas at the neck of the endometrial glands.
・In rare cases the whole uterine epithelium is diffusely replaced by squamous epithelium. In such cases the number of glands often is reduced or absent. In cases of keratinizing squamous metaplasia, the lumen can be filled or dilated with keratin (squames).
Differential Diagnoses
Papilloma, squamous cell:
・Squamous cell papilloma is a papillary growth protruding into the uterine lumen.
Carcinoma, squamous cell:
・Squamous cell carcinomas may be well differentiated but cells have varying degrees of atypia and often infiltrate into submucosa and muscularis, and serosa.
Comment
Squamous metaplasia is commonly observed as a focal lesion. Squamous metaplasia often develops in the rat and mouse uterus under estrogen dominance, e.g. in response to elevated endogenous estradiol level, or when treated with high doses of estrogenic compounds or combination oral contraceptives. In rats and mice the anti-estrogen tamoxifen tends to act as an estrogen when given in high dosages and consequently also induces squamous metaplasia in both species. Keratinization is not usually prominent. In rare cases the whole uterus epithelium can become transformed into squamous epithelium. Squamous metaplasia also can appear as a reactive change in relation to inflammatory changes. Anti-p63 antibodies can be used to demonstrate the basal cells within the squamous epithelium in metaplastic foci.
References
8Anisimov and Nikonov (1990), 17Campbell (1987), 64Gopinath et al. (1987), 66Greaves (2012), 69Greaves et al. (1992), 86Johnson (1987), 109Leininger and Jokinen (1990), 214Yuan and Foley (2002)
Vacuolation, epithelial cell (N) Uterus (Figure 152)
Figure 152.
Vacuolation, Epithelial Cell, Uterus rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Endometrial epithelial cells.
Diagnostic Features
・Fine to coarse cytoplasmic vacuolation.
・Can be found within hyperplastic epithelial lesions.
・Vacuoles are optically empty or contain material that can be stained using PAS or Alcian blue (representing intracellular mucins).
Differential Diagnoses
None.
Comment
Vacuolation of the endometrial epithelial lining or glands is uncommon in rats and mice. Treatment with high doses of certain (synthetic) hormones however can cause vacuolar changes within the epithelial lining. A slightly different type of vacuolation can be seen when amphiphilic cationic drugs are given to rodents. Such drugs can cause intralysosomal storage of polar phospholipids in various organs, including the uterus. The anorectic drug chlorphentermine and the tricyclic antidepressant imipramine, as well as tamoxifen, can cause vacuolar changes within the epithelial and smooth muscle cells of the rat uterus. Electron microscopically, the vacuolated structures proved to be lysosomes filled with typical whorled, lamellated membranous structures typical of phospholipidosis.
References
Inflammation, oviduct (N) Oviduct (Figure 153)
Figure 153.
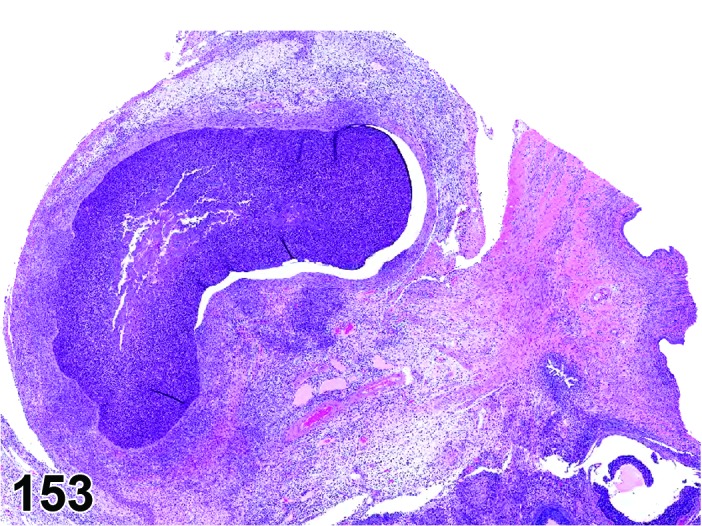
Inflammation, Neutrophilic, Oviduct, rat.
Species
Mouse; Rat.
Modifier
Neutrophilic; Lymphocytic; Mononuclear; Mixed. Other modifiers include suppurative, granulomatous.
Diagnostic Features
・Grossly, the uterine tube may be distended and there may be exudate in the uterine tube.
・There is exudation of neutrophils, lymphocytes and/or macrophages into the uterine tube lumen.
・Necrosis can be present.
・The oviductal epithelium may be hyperplastic.
・Lymphoid proliferation can occur in the submucosa.
Differential Diagnoses
Distension of the uterine tube:
・In most instances of inflammation there is an associated exudate that can be seen grossly and/or microscopically.
Comment
Mild inflammation of the oviduct occurs in rats and mice. The most common cause of salpingitis in natural infections of rats is Mycoplasma pulmonis. Klebsiella oxytoca has been implicated in salpingitis in B6C3F1 mice.
References
16Busch and Naglić (1995), 33Davis et al. (1987), 109Leininger and Jokinen (1990), 161Rao et al. (1987)
Salpingitis isthmica nodosa (N) Oviduct (Figure 154)
Figure 154.
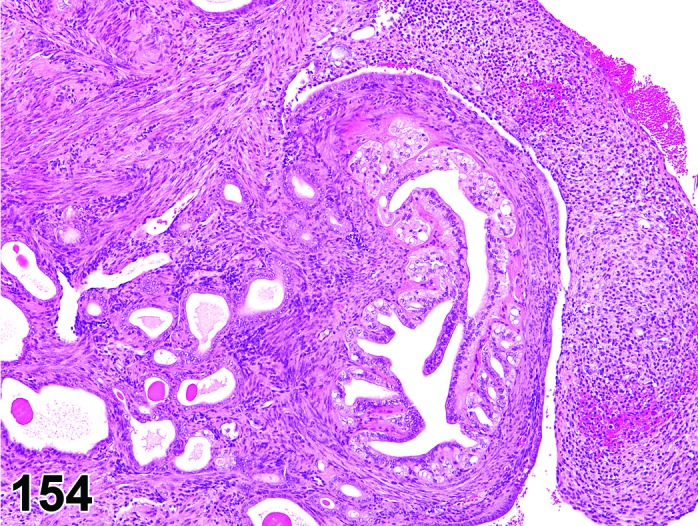
Salpingitis Isthmica Nodosa, Oviduct, mouse.
Species
Mouse.
Synonym(s)
SIN, diverticulosis.
Pathogenesis/cell of origin
Uterine tube (oviductal) epithelium.
Diagnostic Features
・Grossly, serosa of uterine tube is irregular or nodular.
・May involve the entire uterine tube or can be focal.
・The oviductal muscular wall becomes thickened and epithelium form channels that show short projections into the lumen.
・The oviductal epithelium can also form extensive folds that extend into the muscularis dissecting the muscle bundles.
・The oviductal epithelium forms glandular structures that may or may not connect to the oviductal lumen.
・Glandular structures may become cystic diverticulae and lose their connection with the lumen.
・With time there may be involvement of the entire oviductal serosa.
・Inflammation may or may not be present.
Differential Diagnoses
None.
Comment
This lesion is commonly seen in CD-1 mice exposed to the synthetic estrogen, diethylstilbestrol.
References
Atrophy (N) Oviduct (Figure 155)
Figure 155.
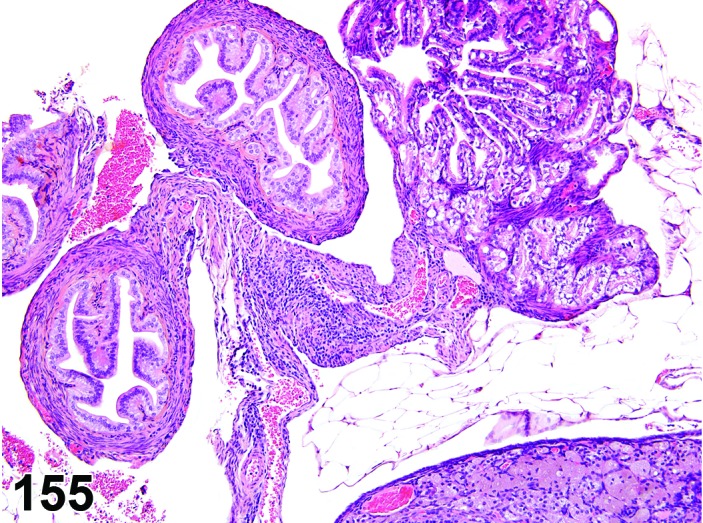
Atrophy, Oviduct, mouse.
Species
Mouse; Rat.
Diagnostic Features
・The smooth muscle cells may be decreased resulting in reduced smooth muscle wall thickness.
・The epithelium is reduced from typically columnar to cuboidal.
・There is flattening of the mucosal folds.
・Flattening of the fimbria may be present.
・Lesion may be focal or diffuse.
Differential Diagnoses
Hypoplasia:
・Congenital hypoplasia of the uterine tube is rare, but must be considered.
Comment
Spontaneous atrophy of the uterine tube is rare in rats and mice although there are reports of this occurring experimentally following intravaginal inoculation of mice with an infectious agent such as Chlamydia trachomatis or manipulation of thyroid hormone in rats.
References
6Amadi et al. (2007),34de la Maza et al. (1994), 109Leininger and Jokinen (1990)
B. Nonneoplastic Proliferative Lesions
Hyperplasia, glandular, cystic (H) Uterus (Figures 156 and 157)
Figure 156.
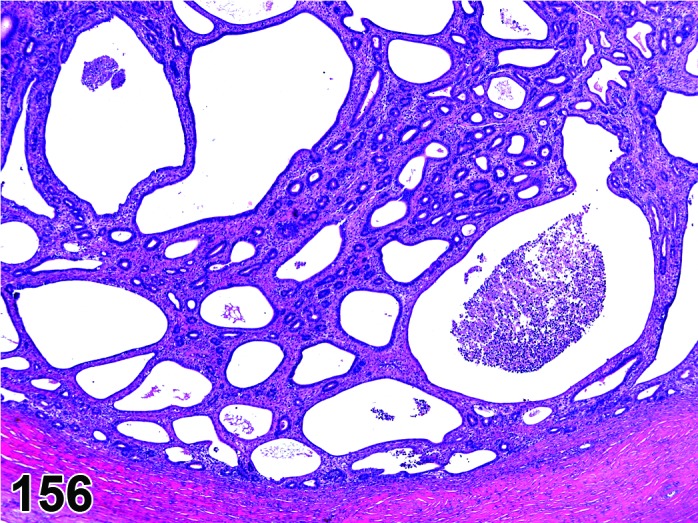
Cystic Glandular Hyperplasia, Uterus, rat.
Figure 157.
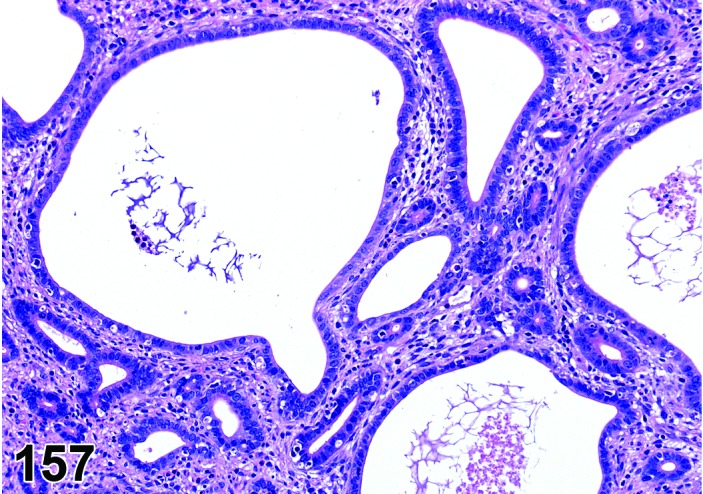
Cystic Glandular Hyperplasia, Uterus, rat.
Species
Mouse; Rat.
Synonym(s)
Cystic endometrial hyperplasia; CEH.
Modifier
Cystic; Endometrial.
Pathogenesis/cell of origin
Uterine glandular epithelium.
Diagnostic Features
・Glandular hyperplasia may be focal or diffuse and characterized by an increased number of active proliferating glands. Proliferation is obvious and reflected in an increased number of mitotic figures.
・Endometrial glands involved become tortuous, dilated and usually cystic.
・Cellular atypia can be observed within the lesion.
・Glandular cysts are lined with a single layer of epithelium and may attain large size.
・The stromal compartment of the lesion is often less then in the surrounding/normal endometrium. Sometimes, however the stroma can be more cellular in the hyperplastic area(s).
・Adenomyosis is regularly seen in severe cases.
Differential Diagnoses
Dilation, glandular, cystic:
・Dilated glands are the result of an obstruction.
Polyp, glandular:
・Polyps have more prominent stroma with fibrovascular component and protrude into the uterine lumen.
Comment
Focal or diffuse glandular hyperplasia is occasionally observed spontaneously in adult rats. The lesion is especially common in older rats, which can enter a stage of persistent estrus due to an inadequate luteinizing hormone (LH) surge causing persistence of estrogen secreting ovarian follicles resulting in an increased E2:P4 ratio and ovarian hormone imbalance. It is also a common ageing lesion in a variety of different mouse strains. The form of hyperplasia in mice is characterized by an increased number of cystic dilated or irregular glands lined by hyperchromatic cuboidal to columnar epithelial cells with round or oval nuclei and small PAS-positive cytoplasmic droplets separated by normal appearing stroma. Prolonged estrogen excess produced by administration of synthetic hormones or other xenobiotics with estrogenic effects similarly can induce endometrial hyperplasia and eventually neoplasia in rats and mice. “Cystic endometrial hyperplasia” is occasionally observed in adult rats and it is a very common lesion in old mice. 206Ward et al. (1979) found an incidence of 35% of cystic endometrial hyperplasia in B6C3F1 mice.
References
66Greaves (2012), 68Greaves and Faccini (1984), 87Jones et al. (1997), 206Ward et al. (1979)
Hyperplasia, glandular, focal (H) Uterus (Figure 158)
Figure 158.
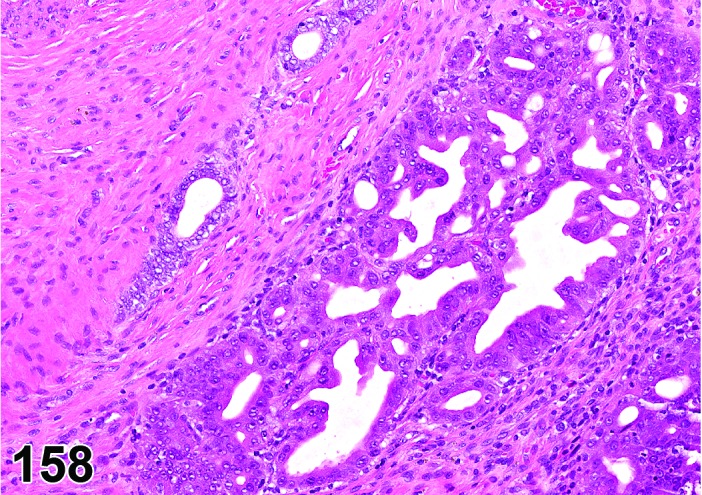
Hyperplasia, Glandular, Focal, Uterus, rat.
Species
Mouse; Rat.
Synonym(s)
Adenomatous hyperplasia; Atypical glandular hyperplasia.
Modifier
Atypical.
Pathogenesis/cell of origin
Uterine glandular epithelium.
Diagnostic Features
・Focal glandular hyperplasia consists of compactly arranged, disorganized glands separated by only a sparse stromal compartment. Glands can be observed lying ‘back-to-back’ with hardly any stroma in between.
・The lesion is found within the normal morphological boundaries of the endometrium.
・Often the enlarged eosinophilic or basophilic cuboidal to columnar epithelial cells lining the glands may show pleomorphism and atypia.
・The large nuclei of the cells in this lesion are hyperchromatic. Nuclei often have large conspicuous nucleoli.
・Proliferation is obvious and reflected in an increased number of mitotic figures.
・Stratification and pilling up of epithelial cells can be a feature.
・Gland lumens may not be obvious.
Differential Diagnoses
Hyperplasia, glandular, cystic:
・Glands are cystic and often lined by low cuboidal to flattened, single layered epithelium. Atypia is mild or absent. Since the lesions is related to hormonal changes, it is observed more diffusely throughout the endometrium and less as a solitary lesion.
Polyp, glandular:
・Polyps have more prominent stroma with fibrovascular component and protrude into the uterine lumen.
Adenoma, endometrial:
・Well-delineated solitary masses that may compress, but do not invade the surrounding endometrium or adjacent myometrium.
・The epithelium is well-differentiated and arranged in papillary, glandular or tubular structures which are lined by cuboidal to columnar cells one to two cell layers thick.
Adenocarcinoma, endometrial:
・Adenocarcinoma shows cytological characteristics of a malignant tumor, i.e. infiltrative growth pattern, atypia, and/or metastasis.
Comment
Spontaneous focal glandular hyperplasia is only rarely observed in rats and mice. In most cases, the lesion is induced. Focal glandular hyperplasia is considered to be a precancerous lesion, in contrast to cystic glandular hyperplasia which is often seen in adult rats and is a very common lesion in old mice with an incidence of 35% in B6C3F1 mice. Focal glandular hyperplasia is often observed following administration of uterine carcinogens. The distinction between hyperplasia and true neoplasia often is difficult because of the gradual transition from hyperplasia into adenoma or carcinoma. In an outbred colony of Wistar Han rats, as well as in an inbred BDII/Han rat from the same colony, a high incidence of hyperplasia and neoplastic lesions was described. The Donryu rat is an experimental model to study this transition. These and other rodent models of endometrial cancer are the subject of an extensive review by 202Vollmer (2003).
References
35Deerberg et al. (1995), 36Deerberg et al. (1981), 39Dixon et al. (1999), 137Nagaoka et al. (1994), 202Vollmer (2003)
Hyperplasia, endometrial, diffuse (H) Uterus (Figures 159 and 160)
Figure 159.
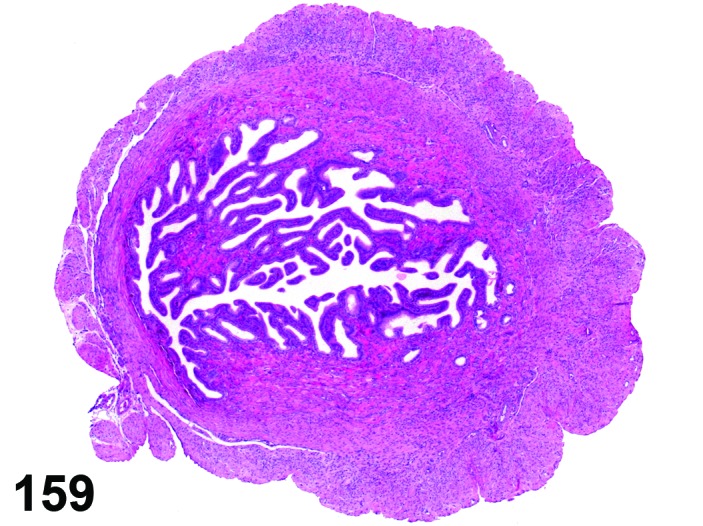
Diffuse Endometrial Hyperplasia, Uterus, rat.
Figure 160.
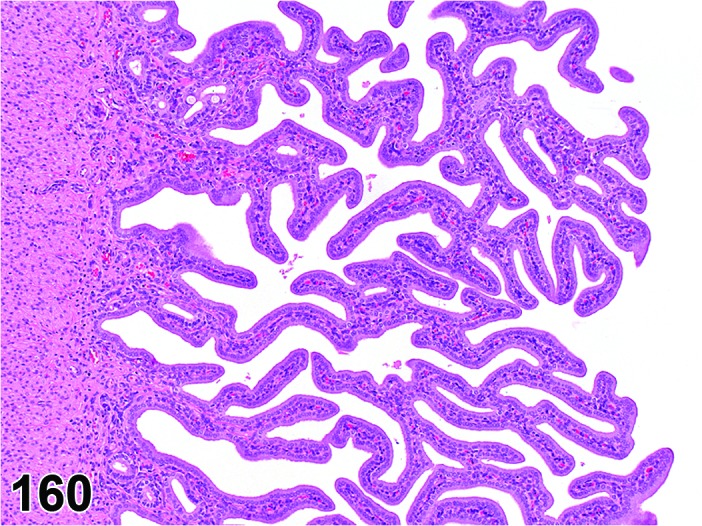
Diffuse Endometrial Hyperplasia, Uterus, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Luminal endometrial epithelium.
Diagnostic Features
・Because of the proliferation of the luminal epithelium, the total luminal surface increases. The endometrial glands seem to become part of the luminal surface that is transformed to a more papillary surface.
・The number of glands consequently decreases.
・In general, cellular atypia is not observed.
・Causes an increase in tortuosity of the whole uterus.
Differential Diagnoses
Hyperplasia, glandular, cystic:
・In glandular hyperplasia, the number of gland profiles increases focally or diffusely. The glands often become cystic and the stromal component is often decreased compared to normal endometrium.
Comment
Diffuse endometrial hyperplasia is a rare lesion, usually involving both horns, which can be induced by high doses of medroxyprogesterone and other synthetic progestagens. Also, hyperplasia of the luminal epithelium may occur in conjunction with focal glandular hyperplasia and may show signs of atypia or papillary formation in response to chemical exposures. In these cases we recommend the diagnosis of hyperplasia, endometrial, focal with the use of modifiers such as atypical or papillary.
Hyperplasia, endometrial stromal (H) Uterus (Figure 161)
Figure 161.
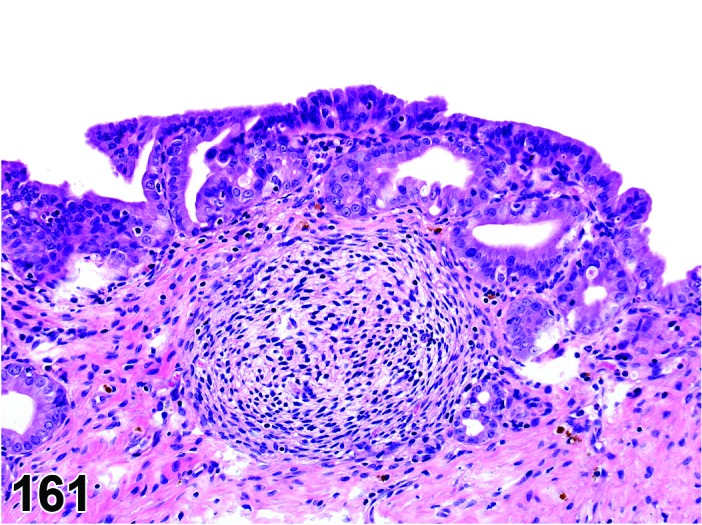
Endometrial Stromal Nodular Hyperplasia, rat, Uterus.
Species
Mouse; Rat.
Synonym(s)
Endometrial stromal hyperplasia.
Pathogenesis/cell of origin
Endometrial mesenchymal cell.
Diagnostic Features
・Increased stromal component, characterized by proliferation of spindle or stellated stromal cells with a variable amount of intercellular collagen dependent on the age of the lesion.
・Lesion generally oriented along the normal anatomical structures, e.g. circular around cervix, and does not cause gross anatomical abnormalities or distortions.
・The growth is non-invasive.
・Endometrial glands are absent or sparse within the lesions.
・Mitotic index and vascularization may be prominent especially in early lesions.
・Older lesions are characterized by dense collagen stroma or fibrosis.
Differential Diagnoses
Polyp, endometrial stromal:
・Nodular proliferation of endometrial stromal cells often protruding into the lumen covered by cuboidal to columnar epithelium.
Decidualization, focal:
・Focal decidualization is characterized by proliferation of strongly hypertrophied stromal cells, containing variable amounts of PAS positive cytoplasmic material (glycogen).
Sarcoma, endometrial stromal:
・Malignant lesion characterized by infiltrative growth and high mitotic index. Infiltrative growth pattern does not follow existing anatomical structures, leading to gross distortions. The cells within stromal sarcoma are poorly differentiated spindle cells and cellular pleomorphism is obvious. Further areas of hemorrhage and necrosis often are present within stromal sarcoma.
Comment
Hyperplasia, endometrial stromal (H) is a common finding in the uterus and cervix of aged mice of most strains. A diffuse stromal hyperplasia can be seen in the uterus of old rats, although this change is more common in the cervix. Stromal hyperplasia has been observed in F344 rats. The lesion may result macroscopically in a dense white firm slightly enlarged cervix.
References
109Leininger and Jokinen (1990)
Hyperplasia, angiomatous (H) Uterus (Figure 162)
Figure 162.
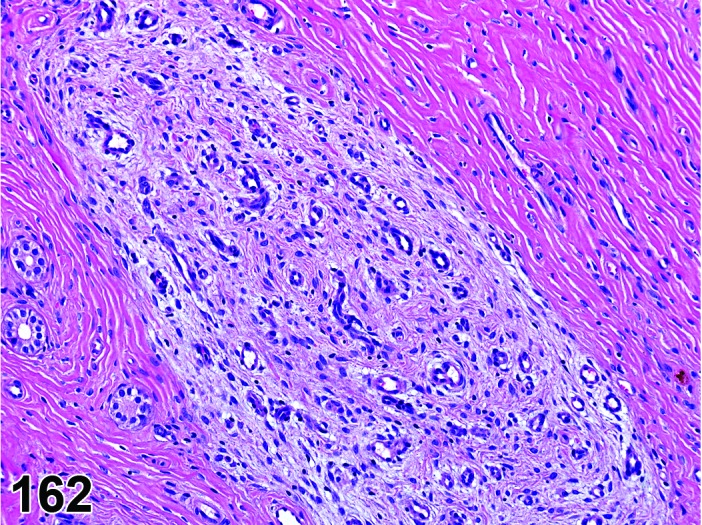
Focal stromal angiomatous hyperplasia, Uterus, rat.
Species
Mouse; Rat.
Synonym(s)
Angiomatous hyperplasia.
Pathogenesis/cell of origin
Vascular cells.
Diagnostic Features
・Focal, well-demarcated lesion within the endometrium or myometrium consisting of an increased number of closely packed vascular structures, not distorting the surrounding tissue.
・The vascular spaces usually are blood-filled.
・The supporting tissue in the lesion can range from abundant to sparse.
・Mitotic figures are virtually absent and there is no nuclear atypia.
Hemangioma:
・Focal blood-filled spaces lined with uniform endothelial cells, usually compressing the surrounding tissues.
Differential Diagnoses
Hemangiosarcoma:
・Irregular expansive and infiltrative lesions, consisting of vascular structures or solid areas with collapsed vascular channels. The endothelial cells may be multilayered. Endothelial cells within hemangiosarcoma are usually more prominent and have pleomorphic enlarged hyperchromatic nuclei. Mitotic figures are common. Metastases are common.
References
Hyperplasia, myometrial (H) Uterus (Figure 163)
Figure 163.
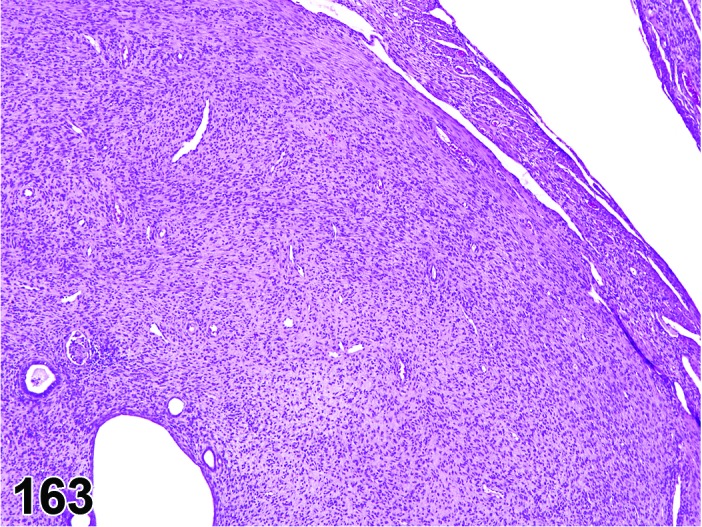
Myometrial Hyperplasia, Uterus, mouse.
Species
Mouse; Rat.
Synonym(s)
Myometrial hyperplasia.
Pathogenesis/cell of origin
Myometrial cell.
Diagnostic Features
・Focal or diffuse lesion within the myometrium consisting of an increased number of well-differentiated smooth muscle cells arranged in fascicles, and not distorting the surrounding tissue.
・Some collagen may be interspersed between smooth muscle cells.
・Mitotic figures are virtually absent and there is no nuclear atypia.
Differential Diagnoses
Leiomyoma:
・Typically, well-circumscribed masses that may be solitary or multiple and compress adjacent structures.
Hyperplasia, epithelial cell (H) Oviduct (Figure 164)
Figure 164.
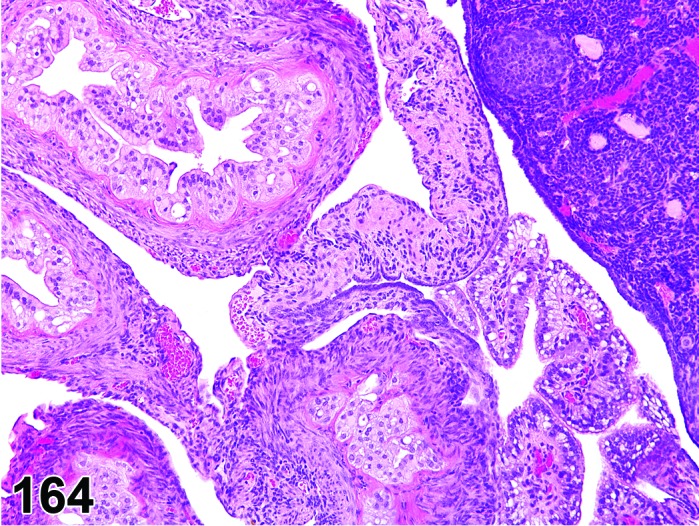
Epithelial Hyperplasia, Oviduct, mouse.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Oviductal epithelium.
Diagnostic Features
・Oviductal epithelium becomes tortuous and forms papillary extensions of columnar cells that protrude into the oviductal lumen.
Differential Diagnoses
None.
Comment
This lesion can be seen in response to administration of synthetic estrogens in mice. Hyperplasia of the oviductal epithelium or stroma has not been identified in the F344 rat.
References
86Johnson (1987), 109Leininger and Jokinen (1990), 144Newbold et al. (2009)
C. Neoplastic Proliferative Lesions
Polyp, glandular (B) Uterus; Uterine Cervix (Figures 165 and 166)
Figure 165.
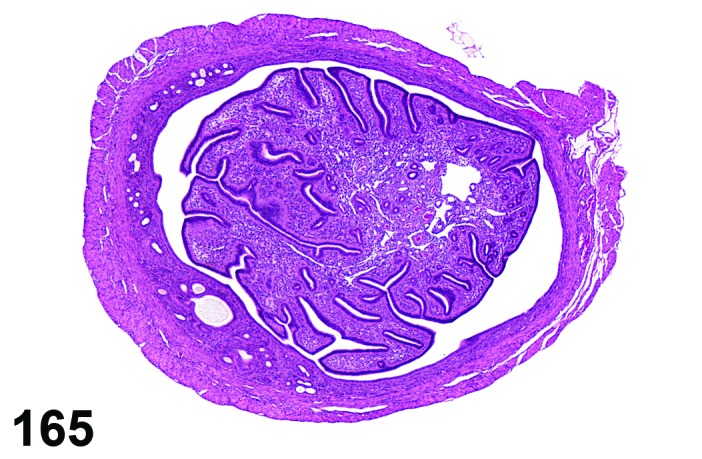
Glandular Polyp, Uterus, mouse.
Figure 166.
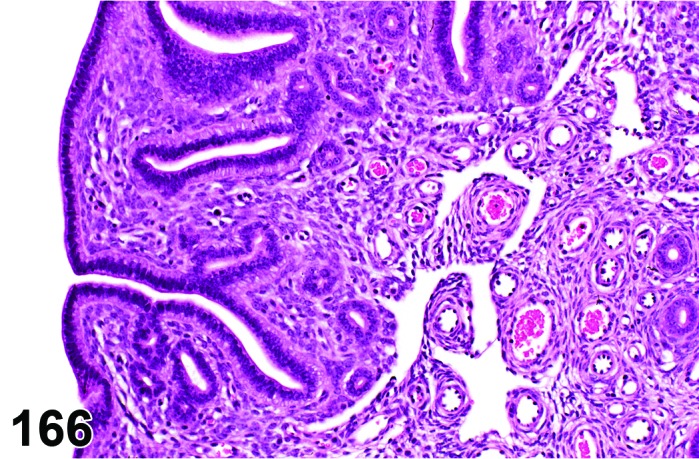
Glandular Polyp, Uterus, mouse.
Species
Mouse; Rat.
Synonym(s)
Polyp, adenomatous.
Pathogenesis/cell of origin
Uterine glandular epithelium and fibrovascular stroma.
Diagnostic Features
・Primarily a polypoid mass protruding into the uterine lumen, but may arise from the uterine cervix or extend into the lumen of the vagina.
・Lesions extending into the vaginal lumen may be edematous, ulcerated, inflamed, and/or infarcted.
・Prominent often hyperplastic glandular component consisting of cuboidal to columnar epithelium, which is continuous with the endometrial lining epithelium, and similar in appearance.
・Stroma composed of spindle-shaped or stellate cells with variable amounts of collagen and endothelial-lined vascular spaces.
・Endometrial glands, often cystic and hyperplastic are present throughout the stroma.
Differential Diagnoses
Polyp, endometrial stromal:
・No or very few glandular elements entrapped.
Adenoma, endometrial:
・Papillary adenomas have little or no proliferation of endometrial stroma, which is delicate.
Polyp, vaginal:
・Vaginal polyps are covered by squamous epithelium; connective tissue core tends to be denser.
References
32Davis et al. (2001), 66Greaves (2012), 68Greaves and Faccini (1984)
Polyp, endometrial stromal (B) Uterus; Uterine Cervix (Figures 167 and 168)
Figure 167.
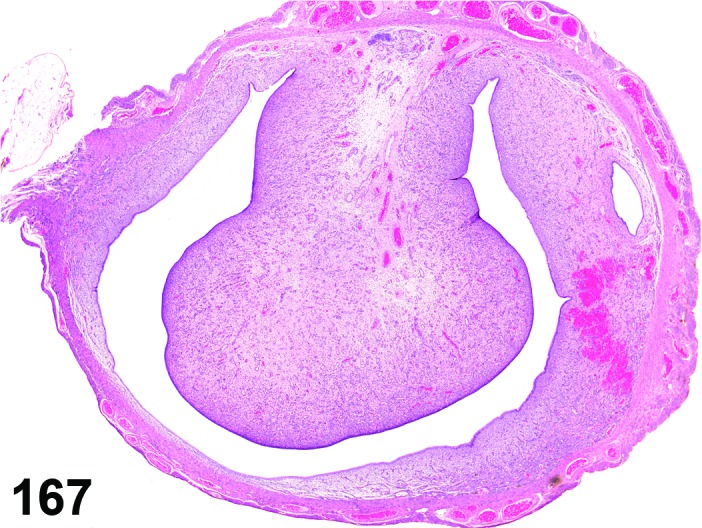
Endometrial Stromal Polyp, Uterus, rat.
Figure 168.
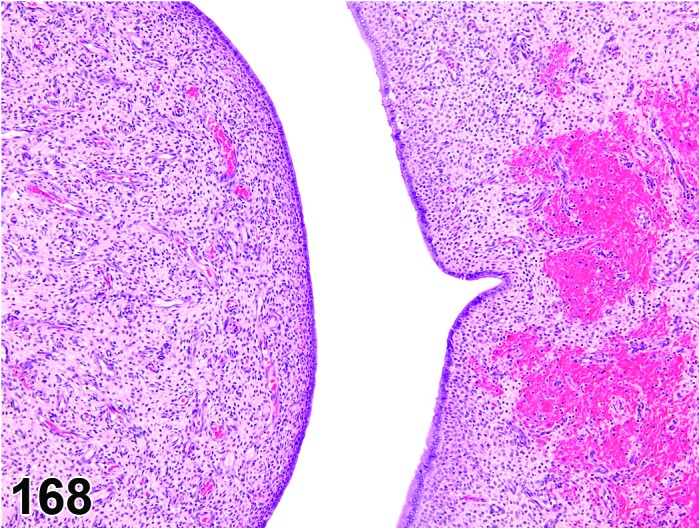
Endometrial Stromal Polyp, Uterus, rat.
Species
Mouse; Rat.
Synonym(s)
Tumor, endometrial stromal, benign; Tumor, stromal, benign.
Pathogenesis/cell of origin
Uterine endometrial stroma.
Diagnostic Features
・Primarily a polypoid mass protruding into the uterine lumen, but may arise from the uterine cervix or extend into the lumen of the vagina.
・Lesions extending into the vaginal lumen may be edematous, ulcerated, inflamed, and/or infarcted.
・Normally covered by cuboidal to columnar epithelium, which is continuous with endometrial lining epithelium.
・The predominant bulk of the lesion is composed of stromal spindle-shaped or stellate cells with variable amounts of collagen and blood vessels.
・Growth is characteristically expansive without much invasion.
・A few endometrial glands may be entrapped in this lesion.
・Lesions may be solitary or multiple.
Differential Diagnoses
Polyp, glandular:
・Presence of prominent and often hyperplastic glandular structures throughout a major part of the polyp.
Polyp, vaginal:
・Presence of vaginal epithelium (stratified squamous) on the surface.
Sarcoma, endometrial stromal:
・If the endometrial stromal cells are not well differentiated and well demarcated, and have an infiltrative growth pattern, lesions are classified as early endometrial stromal sarcomas. This lesion may also originate within a stromal polyp.
Comment
Occasionally endometrial stromal sarcomas arise from polyps and generally show malignant features such as rapid growth and invasiveness.
References
32Davis et al. (2001), 39Dixon et al. (1999), 59Goodman and Hildebrandt (1987b), 66Greaves (2012), 68Greaves and Faccini (1984), 109Leininger and Jokinen (1990)
Sarcoma, endometrial stromal (M) Uterus (Figures 169 and 170)
Figure 169.
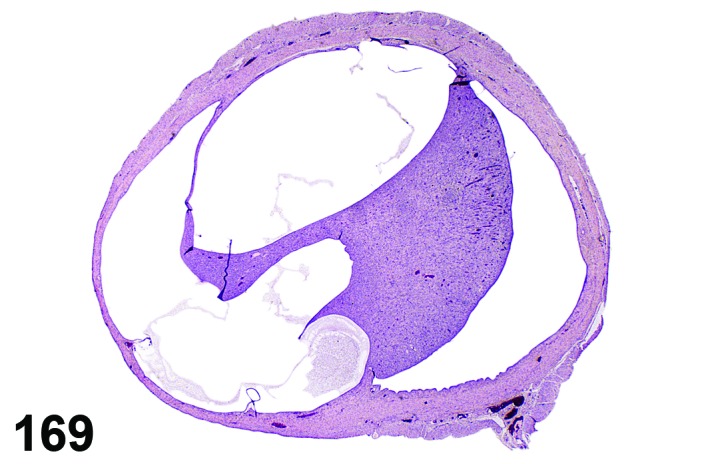
Endometrial Stromal Sarcoma, Uterus, rat.
Figure 170.
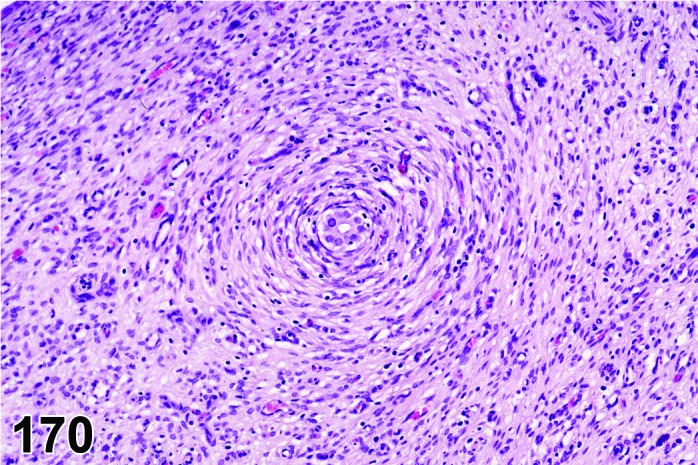
Endometrial Stromal Sarcoma, Uterus, rat.
Species
Mouse; Rat.
Synonym(s)
Sarcoma, mesenchymal; Sarcoma, uterine.
Pathogenesis/cell of origin
Uterine endometrial stroma.
Diagnostic Features
・May be found in the uterine wall or as polypoid masses projecting into the lumen.
・The predominant bulk of the mass is composed of stromal spindle-shaped cells with variable amounts of collagen and endothelial-lined vascular spaces.
・Invasion of adjacent tissue is present.
・May be present within polypoid mass.
・Cells are poorly differentiated spindle cells.
・Cellular pleomorphism may be present.
・Cell borders are indistinct.
・Cytoplasm is scant to moderate, pale and eosinophilic.
・Nuclei are elliptical, elongated and hyperchromatic. They may appear oval or round when cut in cross-section.
・Mitotic figures are numerous.
・Areas of hemorrhage and necrosis may be present.
・Metastasis is rare.
・May be positive for S-100 and vimentin, negative for desmin and actin.
Differential Diagnoses
Leiomyosarcoma or Fibrosarcoma or Schwannoma, malignant:
・Diagnosis should be made by process of elimination of the different morphological characteristics and growth pattern in which special stains may be useful. Should be distinguished from other mesenchymal tumors by means of special stains. Diagnosis can be easily made if the malignant lesion originates from within an endometrial stromal polyp.
Polyp, endometrial stromal:
・Endometrial stromal polyp distinguished by polypoid projection into the lumen and by maturity of supporting stroma and lack of infiltration.
References
62Goodman and Hildebrandt (1987e), 66Greaves (2012), 68Greaves and Faccini (1984), 109Leininger and Jokinen (1990)
Sarcoma, histiocytic (M) Uterus (Figures 171 and 172)
Figure 171.
Histiocytic Sarcoma, Uterus, mouse.
Figure 172.
Histiocytic Sarcoma, Uterus, mouse.
Species
Mouse; Rat.
Synonym(s)
Reticulum cell sarcoma.
Pathogenesis/cell of origin
Cells of the mononuclear phagocyte system.
Diagnostic Features
・Uniform population of histiocytic cells with abundant eosinophilic cytoplasm.
・Nuclei are dark, pleomorphic, slightly elongated, and curved or folded.
・Focal necrosis surrounded by palisaded cells is characteristic.
・Multinucleate giant cells and phagocytosis may be present.
・Liver is almost always also involved.
Differential Diagnoses
Schwannoma, malignant:
・Poorly differentiated malignant schwannomas may resemble histiocytic sarcoma; however, they lack giant cells and abundant eosinophilic cytoplasm, and they stain positively with S-100.
Leiomyosarcoma:
・Poorly differentiated leiomyosarcomas may also resemble histiocytic sarcoma; however, they are not multicentric lesions and the liver is not involved.
Lymphoma, malignant, pleomorphic:
・Cells may be similar; however, the cytoplasm is less eosinophilic and giant cells are absent. Lymph nodes are also involved.
Decidual reaction:
・The large round or polygonal cells with eosinophilic cytoplasm in the decidual alteration of old rats mimic histiocytic sarcoma, and may form a polypoid mass; however, the nuclei are large and pale. These are not multicentric lesions and the liver is not involved.
Comment
Lysozyme, MAC-2, and F4/80 are reliable and specific markers in mice.
References
31Davis et al. (1999), 52Frith et al. (2001), 72Hao et al. (2010)
Papilloma, squamous cell (B) Uterus; Uterine Cervix; Vagina (See Uterine Cervix and Vagina Section)
Adenoma, endometrial (B) Uterus (Figures 173 and 174)
Figure 173.
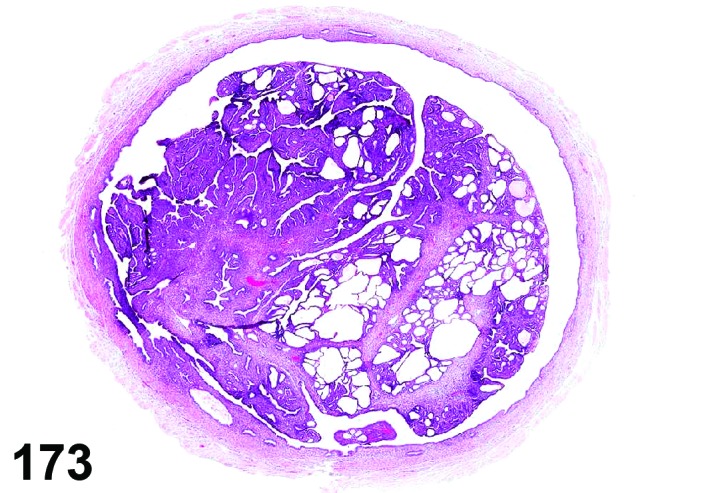
Endometrial Adenoma, Uterus, mouse.
Figure 174.
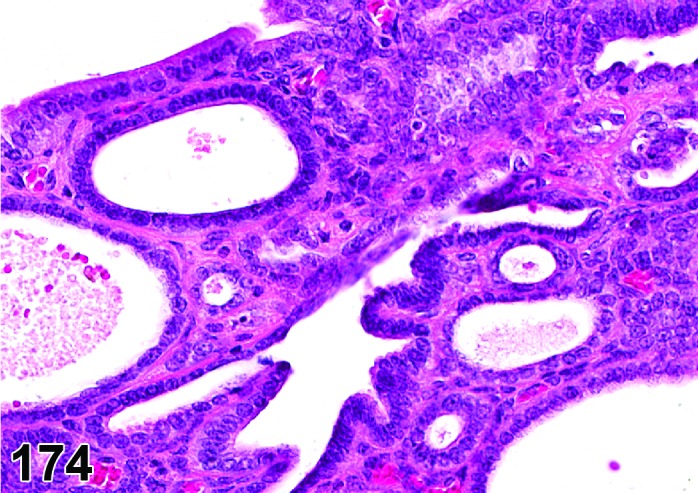
Endometrial Adenoma, Uterus, mouse.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Endometrial epithelium.
Diagnostic Features
・Primarily uterine tumor, but may arise from uterine cervix or appear in vagina by extension.
・Arises from surface epithelium and may have either a broad base or a delicate stalk.
・Epithelium is well-differentiated and arranged in papillary, glandular or tubular structures which are lined by cuboidal to columnar cells one to two cell layers thick.
・Some glandular structures may contain cysts filled with exudate.
・Focal squamous metaplasia may be present especially near cervix.
・Form well-delineated solitary masses that may compress, but not invade the surrounding endometrium or adjacent myometrium, or protrude into the uterine lumen.
・The modifier papillary is used if the predominant growth pattern of the tumor is papillary.
Differential Diagnoses
Polyp, glandular:
・May be confused with endometrial glandular polyp. Well differentiated endometrial stroma is generally a constant feature of the endometrial glandular polyp, but in papillary adenoma, there is little or no stromal proliferation.
Hyperplasia, glandular, focal:
・This type of hyperplasia is characterized by an increase of the epithelial component within the normal morphological boundaries.
Hyperplasia, endometrial stromal:
・Endometrial stromal hyperplasia is generally a diffuse condition involving endometrium, whereas, adenomas are focal proliferative lesions.
Adenocarcinoma, endometrial:
・Adenocarcinoma shows cytological characteristics of a malignant tumor, i.e. infiltrative growth pattern, atypia, and/or metastasis.
Comment
Rare tumor, but can be produced experimentally with certain chemicals and hormones.
References
8Anisimov and Nikonov (1990), 31Davis et al. (1999), 32Davis et al. (2001), 51Frith and Ward (1988), 60Goodman and Hildebrandt (1987c), 68Greaves and Faccini (1984), 109Leininger and Jokinen (1990), 181Squire et al. (1978), 197Turusov et al. (1994)
Adenocarcinoma, endometrial (M) Uterus; Uterine Cervix (Figures 175 and 176)
Figure 175.
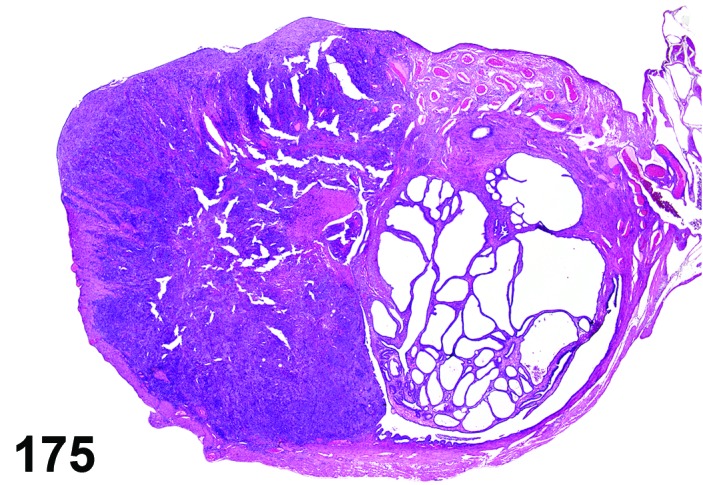
Endometrial Adenocarcinoma, Uterus, rat.
Figure 176.
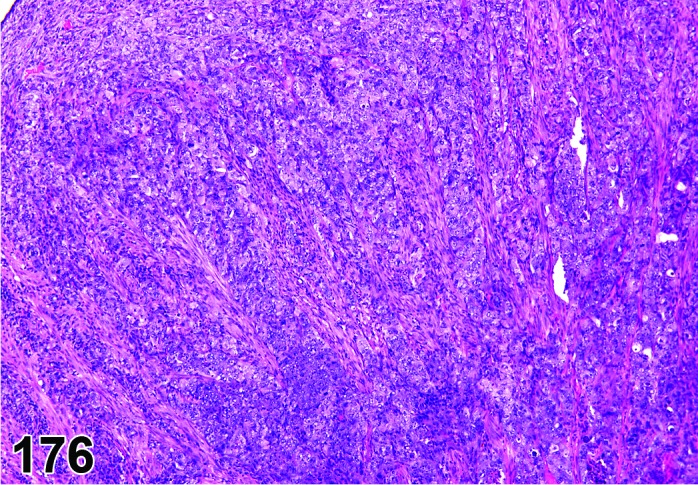
Endometrial Adenocarcinoma, Uterus, rat.
Species
Mouse; Rat.
Synonym(s)
Endometrial Carcinoma; Endometrial Adenocarcinoma.
Pathogenesis/cell of origin
Endometrial epithelium.
Diagnostic Features
・Primarily a uterine tumor but may appear in the uterine cervix or vagina by metastasis.
・Tumors are typically poorly circumscribed and invade the myometrium, may extend into and occlude the uterine lumen, can involve the uterine cervix, or metastasize to distant sites.
・Epithelial cells form solid nests, cords, papillary or acinar structures that are within or supported by a stroma.
・Epithelium may be well-differentiated, anaplastic or show cellular and nuclear atypia, pleomorphism, and mitosis.
・Tumor cells are typically cuboidal to columnar and are usually one or two cell layers thick. In some instances multiple cell layers may give a piling or crowding effect.
・Lumens of tumor acini may be cystic and contain accumulations of cellular debris, mixed inflammatory cells and secretory material.
・Areas of necrosis and hemorrhage may also be present in tumors.
Differential Diagnoses
Adenoma, endometrial:
・Characterized as a solitary and circumscribed mass composed of well-differentiated epithelial cells without atypia or a high growth rate.
Carcinoma, adenosquamous:
・These tumors have at least 10% or more squamous differentiation.
Comment
Uncommon tumor in many strains of rats and mice, but can be induced experimentally in rodents with certain chemicals such as bromoethane, chloroethane and ethylene oxide, estrogenic compounds and hormones. Perinatal treatment with estrogenic compounds induced endometrial adenocarcinoma in rats and mice. Also, rat strains such as Donryu, BDII/Han and Wistar Han are prone to spontaneously develop endometrial adenocarcinomas. Squamous differentiation has been observed in adenocarcinomas.
References
31Davis et al. (1999), 36Deerberg et al. (1981), 35Deerberg et al. (1995), 39Dixon et al. (1999), 58Goodman and Hildebrandt (1987a), 109Leininger and Jokinen (1990), 117Maekawa (1994), 137Nagaoka et al. (1994), 141Newbold et al. (2001), 143Newbold et al. (2007), 156Picut et al. (2003), 209Yoshida et al. (2002)
Carcinoma, adenosquamous (M) Uterus; Uterine Cervix (Figure 177)
Figure 177.
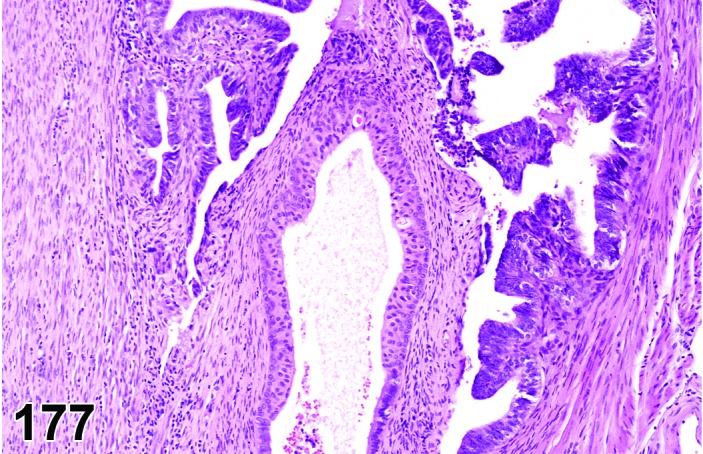
Adenosquamous Carcinoma, Uterus, mouse.
Species
Mouse; Rat.
Synonym(s)
Adenoacanthoma, malignant.
Pathogenesis/cell of origin
Endometrial epithelium.
Diagnostic Features
・Primarily uterine tumor, but may arise from uterine cervix or appear in vagina by extension.
・Adenocarcinoma with foci or zones of squamous epithelial differentiation in which at least or >10% of the lesion should be squamous.
Differential Diagnoses
Adenocarcinoma, endometrial:
・The differentiation between adenocarcinoma and adenosquamous carcinoma is made upon the degree of squamous differentiation (>10% in adenosquamous carcinoma).
Carcinoma, squamous cell:
・These tumors lack significant glandular components.
References
Carcinoma, squamous cell (M) Uterus; Uterine Cervix; Vagina (Figures 178 and 179)
Figure 178.
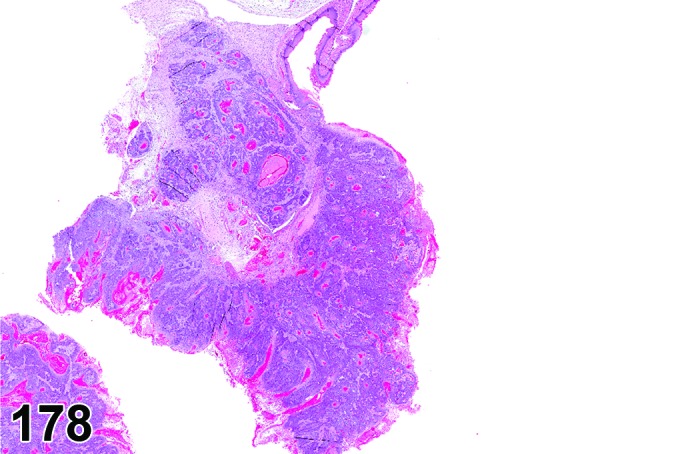
Squamous Cell Carcinoma, Uterus, rat.
Figure 179.
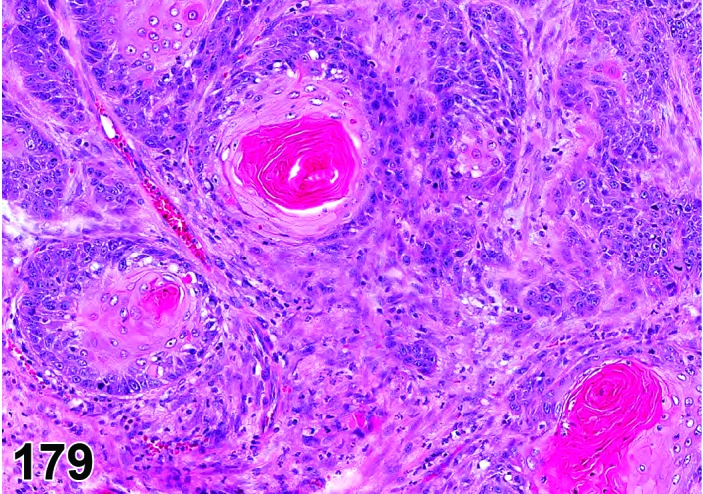
Squamous Cell Carcinoma, Uterus, rat.
Species
Mouse; Rat.
Synonym(s)
Carcinoma, epidermoid.
Modifier
Keratinizing; Non-keratinizing.
Pathogenesis/cell of origin
Endometrial epithelial origin or surface epithelium of vagina. May be epithelium of cervix with squamous differentiation.
Diagnostic Features
・Primary squamous cell carcinoma of endometrial epithelial origin must be distinguished from squamous cell carcinomas arising from the vaginal or cervical epithelium.
・Cells arranged in cords and nests that may have an exophytic growth pattern or may invade deep into submusoca, muscularis, serosa and contiguous organs.
・Tumor cells large and polygonal with prominent vesicular nuclei containing one or more nucleoli.
・Epithelium on the luminal surface markedly thickened, dysplastic and keratinized (“keratin pearls”). Keratin pearls may also be present in deeper tissues.
・Usually well differentiated and heavily infiltrated with leukocytes.
・Stroma may be scant or abundant giving a scirrhous structure.
Differential Diagnoses
Papilloma, squamous cell:
・Differentiation between squamous cell papilloma and squamous cell carcinoma is made on the growth pattern of the tumor and the absence of marked atypia and invasion into the adjacent tissue in the papilloma.
Carcinoma, adenosquamous:
・Differentiation between carcinoma squamous cell and adenosquamous carcinoma is made upon the presence of a primarily glandular tubular structure in the adenosquamous carcinoma. The squamous cell carcinoma, on the other hand, has a more solid growth pattern and does not show glandular structure.
Keratoacanthoma:
・Differentiation between keratoacanthoma and squamous cell carcinoma is based on its low mitotic rate, lack of invasion and the degree of differentiation of the cells in the keratoacanthoma.
Metastases
References
8Anisimov and Nikonov (1990), 9Ashley (1990), 32Davis et al. (2001), 39Dixon et al. (1999), 61Goodman and Hildebrandt (1987d), 64Gopinath et al. (1987), 66Greaves (2012), 68Greaves and Faccini (1984), 109Leininger and Jokinen (1990)
Carcinoma, embryonal (M) Uterus; Uterine Cervix; Ovary (See Ovary Section)
Carcinoma, yolk sac (M) Uterus; Uterine Cervix; Ovary (See Ovary Section)
Choriocarcinoma (M) Uterus; Uterine Cervix; Ovary (See Ovary Section)
Tumor, mixed Mullerian, benign (B) Uterus; Uterine Cervix
Species
Mouse; Rat.
Synonym(s)
Benign mixed mesodermal tumor
Modifier
Homologous; Heterologous.
Pathogenesis/cell of origin
Pluripotent mesodermal cells of the Mullerian ducts.
Diagnostic Features
・Tumor primarily can occur in ovary, uterus (cervix, vagina).
・Well circumscribed polyp-like lesion protruding into the uterine lumen.
・Tumor is composed of an admixture of well differentiated benign epithelial and benign mesenchymal elements.
・Two types can be differentiated:
・Homologous type: The benign mesenchymal elements are derived from cell-types that are a normal constituent of the tissue involved and can be differentiated towards fibrous tissue, smooth muscles and/or endometrial stroma-like tissue.
・Heterologous type: The heterologous type contains benign mesenchymal elements that normally are not found in the uterus such as striated muscle, cartilage, bone and/or adipose tissue.
Differential Diagnoses
Tumor, mixed Mullerian, malignant:
・The (homologous or heterologous) tumor is composed of an admixture of malignant epithelial and mesenchymal elements.
Teratoma, benign or Teratoma, malignant:
・The teratoma contains tissues derived from three germ cell layers, i.e., mesenchymal, epithelial, and neuronal tissues.
Adenoma, endometrial:
・Tumor only composed of epithelial elements often forming glandular structures.
Comment
Tumors derived from the anlage of the Mullerian ducts show a biphasic pattern and can exhibit both epithelial and mesenchymal characteristics. Immunohistochemical characteristics, such as the expression of epithelial markers in tumorous mesenchymal structures, point to a common progenitor cell origin.
References
91Kaspareit-Rittinghausen and Deerberg (1990),199van den Brink-Knol and van Esch (2010)
Tumor, mixed Mullerian, malignant (M) Uterus; Uterine Cervix (Figure 180)
Figure 180.
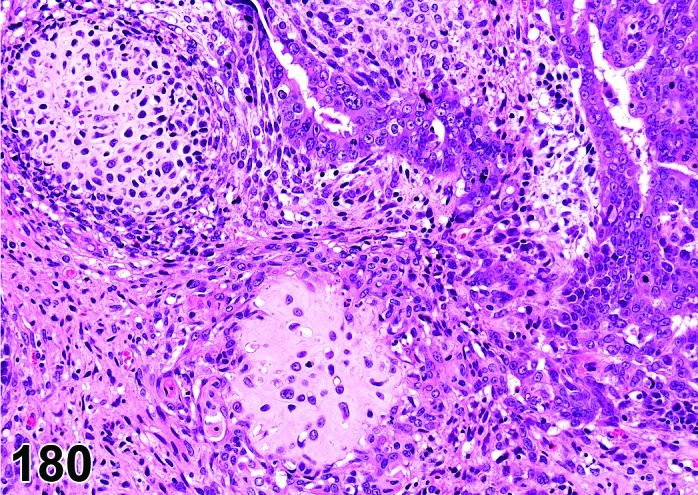
Malignant Mixed Mullerian Tumor (MMMT), Uterus, rat.
Species
Mouse; Rat.
Synonym(s)
Malignant mixed mesodermal tumor; Carcinosarcoma.
Modifier
Homologous; Heterologous.
Pathogenesis/cell of origin
Pluripotent mesodermal cells of the Mullerian ducts.
Diagnostic Features
・Tumor primarily can occur in ovary, uterus (cervix, vagina).
・The tumor often is polypoid and protrudes into the lumen of the tissue involved. Local invasion of surrounding tissues can be seen.
・Infiltratively growing, highly malignant tumor composed of epithelial and mesenchymal elements.
・Both the epithelial and mesenchymal components of the tumor can range from benign, well differentiated to poorly differentiated or anaplastic cellular elements.
・In the less differentiated areas, nuclei can be very pleomorphic and bizarre.
・Mitotic figures are frequent in all malignant elements.
・Two types can be differentiated:
・Homologous: The mesenchymal elements are derived from cell-types that are a normal constituent of the tissue involved and can be differentiated towards fibrous tissue, smooth muscle and/or endometrial stroma-like tissue.
・Heterologous: The heterologous type contains mesenchymal elements that normally are not found in the uterus such as striated muscle, cartilage, bone and/or adipose tissue. Areas with cartilaginous and rhabdomyosarcomatous differentiation are often present.
Differential Diagnoses
Tumor, mixed Mullerian, benign:
・In the benign variant of this tumor, both the epithelial and mesenchymal compartments of the tumor are well differentiated, do not have malignant characteristics and are well circumscribed.
Teratoma, benign or Teratoma, malignant:
・Teratoma contains tissues derived from three germ layers, i.e., mesenchymal, epithelial, and neuronal tissues.
Rhabdomyosarcoma:
・Tumor only composed of malignant rhabdomyoblasts, often showing cross-striation.
Comment
Tumors derived from the anlage of the Mullerian ducts show a biphasic pattern and can exhibit both epithelial and mesenchymal characteristics. The sarcomatous part may differentiate into recognizable smooth or striated muscle, cartilage, and adipose tissue (heterologous mixed tumor). The term homologous mixed tumor is used if the tumor consists simply of glands and malignant mesenchyme. Immunohistochemical characteristics, such as the expression of epithelial markers in tumorous mesenchymal structures, point to a common progenitor cell origin. Metastases in general are epithelial rather than mesenchymal or combinations.
References
91Kaspareit-Rittinghausen and Deerberg (1990),199van den Brink-Knol and van Esch (2010)
Teratoma, benign (B) Uterus; Ovary (See Ovary Section)
Teratoma, malignant (M) Uterus; Ovary (See Ovary Section)
Leiomyoma (B) Uterus; Uterine Cervix; Vagina (Figures 181 and 182)
Figure 181.
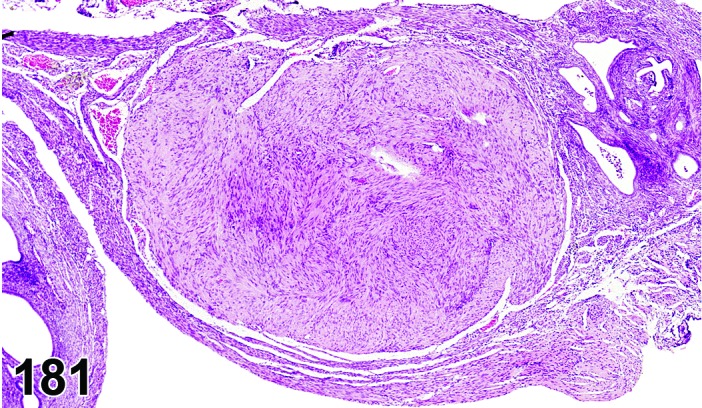
Leiomyoma, Uterus, mouse.
Figure 182.
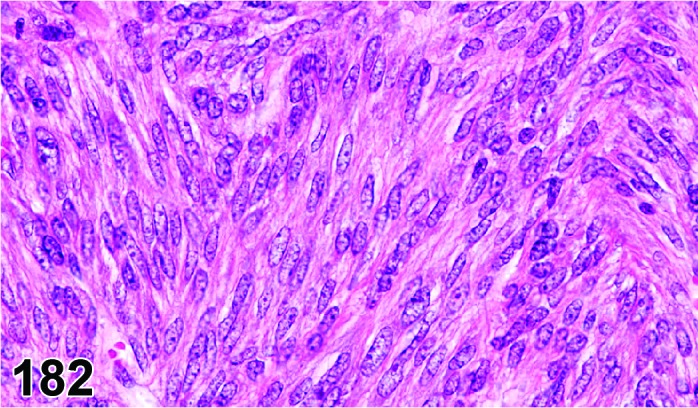
Leiomyoma, Uterus, mouse.
Species
Mouse; Rat.
Modifier
Myometrium; Mesovarium; Uterus.
Pathogenesis/cell of origin
Smooth muscle cell.
Diagnostic Features
・Tumor primarily occurs in uterus and mesovarium, but may occur in uterine cervix and vagina.
・Typically, well-circumscribed masses that may be solitary or multiple and compress adjacent structures.
・Tumor cells form interlacing bundles and whorls reminiscent of normal smooth muscle cells.
・Varying amounts of collagenous tissue and vascularity.
・Tumor cells are spindle-shaped, have abundant eosinophilic cytoplasm, distinct borders and “cigar-shaped” or blunt-ended nuclei.
・There is minimal nuclear pleomorphism and mitotic figures are rare.
Differential Diagnoses
Leiomyosarcoma:
・Cellular and nuclear pleomorphism, poorly circumscribed and invasive tumor with spindled tumor cells having prominent nucleoli and mitotic activity.
Fibroma:
・Has extensive collagen formation that can be shown by special stains, such as van Gieson’s or Masson’s Trichrome stain.
Comment
Longitudinal myofibrils have been reported in leiomyoma cells using the phosphotungstic acid-hematoxylin (PTAH) stain and can be used to differentiate these cells from fibroblasts or fibrocytes. Most leiomyoma cells are immunoreactive for desmin and alpha smooth muscle actin. Diethylstilbestrol (DES) has been shown to induce uterine leiomyomas in CD-1 mice following developmental exposures. Uterine smooth muscle cells express estrogen receptors and appear to be hormonally responsive.
References
31Davis et al. (1999), 39Dixon et al. (1999), 45Ernst et al. (2001a), 146Newbold et al. (2002)
Leiomyosarcoma (M) Uterus; Uterine Cervix; Vagina (Figures 183 and 184)
Figure 183.
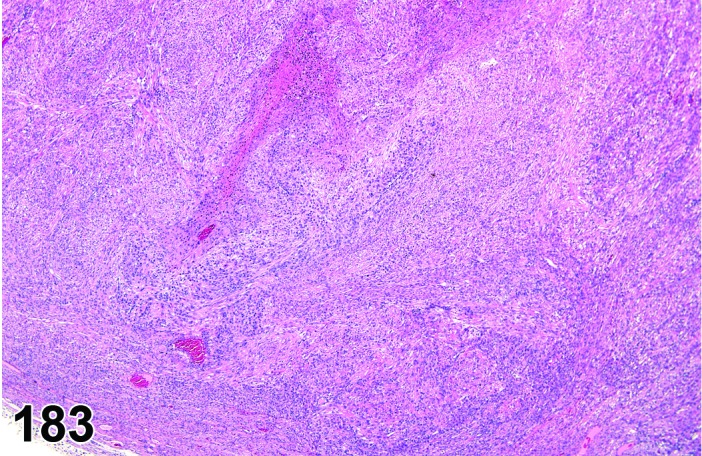
Leiomyosarcoma, Uterus, rat.
Figure 184.
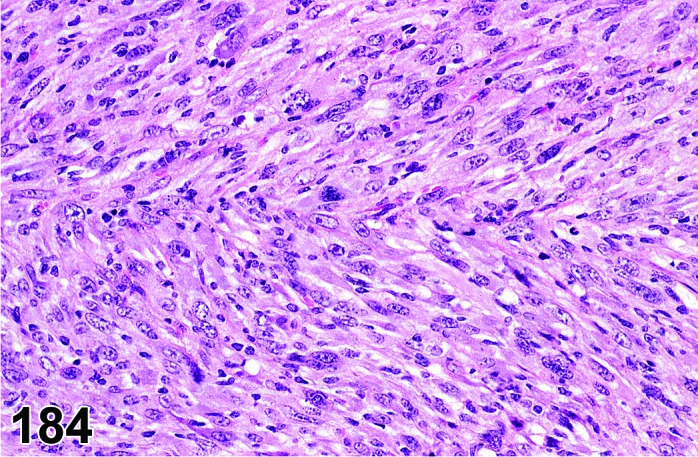
Leiomyosarcoma, Uterus, mouse.
Species
Mouse; Rat.
Modifier
Uterus.
Pathogenesis/cell of origin
Pluripotential mesenchymal stem cells, smooth muscle cells.
Diagnostic Features
・Tumor primarily occurs in uterus and mesovarium, but may occur in uterine cervix and vagina.
・Poorly delineated mass with disorganized and invasive growth patterns, although metastasis is not common.
・Appear hypercellular due to decrease in amount of cytoplasm of anaplastic cells.
・Tumor cells are spindled to pleomorphic and have blunt ended to oval nuclei.
・Nuclei may be pleomorphic and have high mitotic activity, prominent nucleoli and hyperchromasia.
・Multinucleated tumor cells may be present.
・Some collagenous stroma and varying degrees of vascularity may be present.
・Focal areas of hemorrhage, necrosis or cysts may be present.
Differential Diagnoses
Leiomyoma:
・Typically lacks cellular pleomorphism and atypia, mitosis and local invasion.
Fibrosarcoma:
・Usually has increased amounts of collagen and is negative for alpha smooth muscle actin and desmin immunoreactivity. Lack characteristic “cigar shaped or blunt ended nuclei of smooth muscle cells.
Fibrosarcoma, pleomorphic:
・They show several patterns of growth characterized as storiform, fascicular, myxoid, and pleomorphic with pleomorphic and myxoid being the most common. Giant multinucleated cells, erythrophagocytic cells, and foamy cells have all been described to occur within this tumor.
Sarcoma, histiocytic:
・Composed of spindle to rounded cells with dark nuclei and adequate amounts of eosinophilic cytoplasm. The tumor cells may be haphazardly arranged or may take on a whorling pattern. Tumor cells may take on a fusiform shape resembling connective tissue cells or fibroblasts and giant cells may be present throughout the tumors.
Comment
Longitudinal myofibrils have been reported in leiomyoma cells using the phosphotungstic acid-hematoxylin (PTAH) stain and can be used to differentiate these cells from fibroblasts or fibrocytes. Most leiomyosarcoma cells are immunoreactive for desmin and alpha smooth muscle actin, TGF-alpha and EGF receptor. Uterine smooth muscle cells express estrogen receptors and appear to be hormonally responsive.
References
31Davis et al. (1999), 39Dixon et al. (1999), 45Ernst et al. (2001a), 134Moore et al. (2000)
Schwannoma, benign (B) Uterus; Uterine Cervix; Vagina (Figures 185 and 186)
Figure 185.
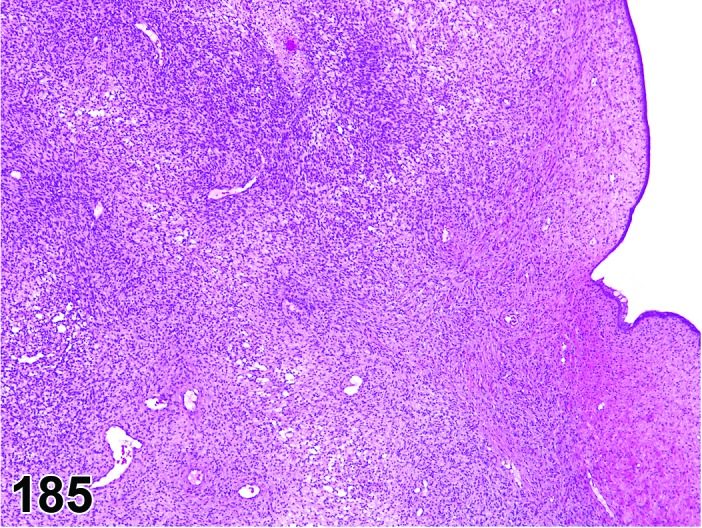
Schwannoma, Benign, Uterus rat.
Figure 186.
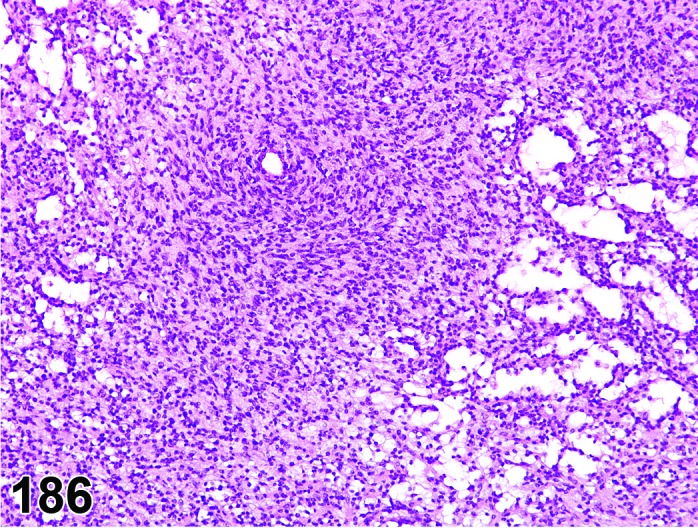
Schwannoma, Benign, Uterus, rat.
Species
Mouse; Rat.
Synonym(s)
Neurilemmoma, benign; Neurinoma, benign.
Pathogenesis/cell of origin
Schwann cell, considered to be neuroectodermal with facultative mesenchymal features.
Diagnostic Features
・Expansile, compressive and usually encapsulated.
・Elongated cells with indistinct borders arranged in an interlacing or whorling pattern, or more loosely arranged cells within a clear matrix.
・Two different growth-patterns can be recognized:
・Antoni type A pattern shows nuclear palisading, sometimes forming “Verocay bodies” (palisading nuclei surrounding homogeneous, eosinophilic intercellular material).
・Antoni type B pattern shows a more loose arrangement of cells, often containing cystic spaces containing eosinophilic/proteinaceous fluid and blood cells and lined by more cuboidal cells.
・Both patterns may or may not be present within the same neoplasm.
・S-100 protein immunoreactivity and the presence of basement membrane in electron micrographs support the diagnosis of Schwannoma.
Differential Diagnoses
Schwannoma, malignant:
・Cellular atypia, invasion or distant metastases are present and/or increased mitotic activity.
Polyp, endometrial stromal:
・Polypoid mass protruding into lumen covered by endometrial epithelium composed of spindle or stellate cells, variable amounts of collagen, blood vessels and/or glandular tissue.
Fibroma:
・Prominent bundles of collagen with low cellularity; negative S-100 immunoreactivity.
Leiomyoma:
・Eosinophilic, spindle-shaped cells with blunt-ended nuclei; negative S-100 immunoreactivity but positive for desmin and smooth muscle actin. Often bundles of cells align perpendicular to one another (“herringbone” pattern).
References
18Cardesa et al. (1990), 45Ernst et al. (2001a), 46Ernst et al. (2001b), 67Greaves et al. (2004), 69Greaves et al. (1992), 107Landes et al. (1990), 121Maekawa and Mitsumori (1990), 183Stewart et al. (1974), 204Walker et al. (1994)
Schwannoma, malignant (M) Uterus; Uterine Cervix; Vagina (Figures 187 and 188)
Figure 187.
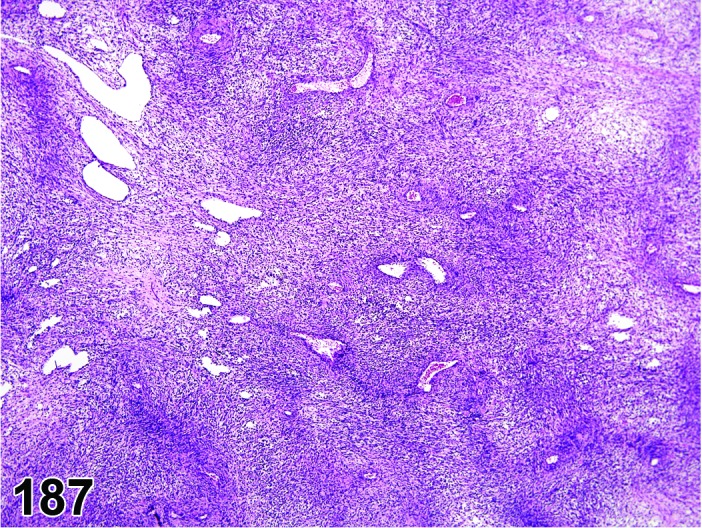
Schwannoma, Malignant, Uterus, rat.
Figure 188.
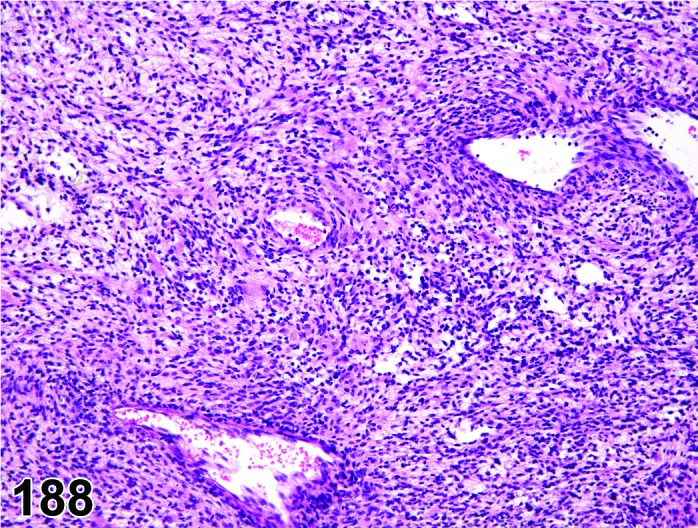
Schwannoma, Malignant, Uterus, rat.
Species
Mouse; Rat.
Synonym(s)
Neurilemmoma, malignant; Neurinoma, malignant.
Pathogenesis/cell of origin
Schwann cell, considered to be neuroectodermal with facultative mesenchymal features.
Diagnostic Features
・Expansile, poorly delineated mass with invasive growth pattern.
・Elongated cells with indistinct borders arranged in an interlacing or whorling pattern or more loosely arranged cells within a clear matrix.
・Two growth-patterns can be recognized:
・Antoni type A pattern shows nuclear palisading, sometimes forming “Verocay bodies” (palisading nuclei surrounding homogeneous, eosinophilic intercellular material).
・Antoni type B pattern shows a more loose arrangement of cells, often containing cystic spaces containing eosinophilic/proteinaceous fluid and blood cells and lined by more cuboidal cells. Large, dilated blood vessels may also be seen.
・Both patterns may or may not be present within the same neoplasm.
・Generally infiltrative growth pattern into the adjacent tissues.
・High mitotic activity, mitotic or cellular atypia, necrosis and/or distant metastases are all indicative of malignancy.
・S-100 protein immunoreactivity and the presence of basement membrane in electron micrographs support the diagnosis of Schwannoma.
Differential Diagnoses
Schwannoma, benign:
・No cellular atypia, invasion or distant metastases are present and very low mitotic rate.
Sarcoma, endometrial stromal:
・Absence of cystic cavities or “Verocay bodies”; negative S-100 immunoreactivity.
Fibrosarcoma:
・No basal lamina present; negative S-100 protein immunoreactivity. Variable amounts of collagen.
Leiomyosarcoma:
・Eosinophilic, spindle-shaped cells with blunt-ended nuclei; negative S-100 immunoreactivity but positive for desmin and smooth muscle actin.
Sarcoma, histiocytic:
・Composed of spindle to rounded cells with dark nuclei and adequate amounts of eosinophilic cytoplasm. The tumor cells may be haphazardly arranged or may take on a whorling pattern. Tumor cells may take on a fusiform shape resembling connective tissue cells or fibroblasts and giant cells may be present throughout the tumors.
Comment
Malignant schwannoma is occasionally observed in the uterus/cervix of rats. Rare tumor in most strains of mice; however, in the NHO strain schwannoma is seen in the uterus and other organs. Malignant schwannoma has been induced by direct-acting alkylating agents such as N-nitrosoethylurea or methylmethane-sulfonate acting as transplacental carcinogens in rats. Schwannomas were also induced following postnatal exposure of rats with 7,12-dimethylbenz[α]anthracene, or N-nitrosomethylurea. Malignant Schwannomas were induced in double transgenic mice expressing simian virus 40 large tumor antigen and prokaryotic LacZ under the control of the myelin basic protein (MBP) promoter. Genetically engineered mouse models of neurofibromatosis, with manipulation of genes NF1 or NF2, develop peripheral nerve sheaths tumors, including schwannomas.
References
18Cardesa et al. (1990), 45Ernst et al. (2001a), 67Greaves et al. (2004), 69Greaves et al. (1992), 107Landes et al. (1990), 121Maekawa and Mitsumori (1990), 183Stewart et al. (1974), 204Walker et al. (1994)
IV. Uterine Cervix and Vagina
A. Nonproliferative Lesions
Aggregate, granular cell (N) Uterine Cervix; Vagina (Figure 189)
Figure 189.
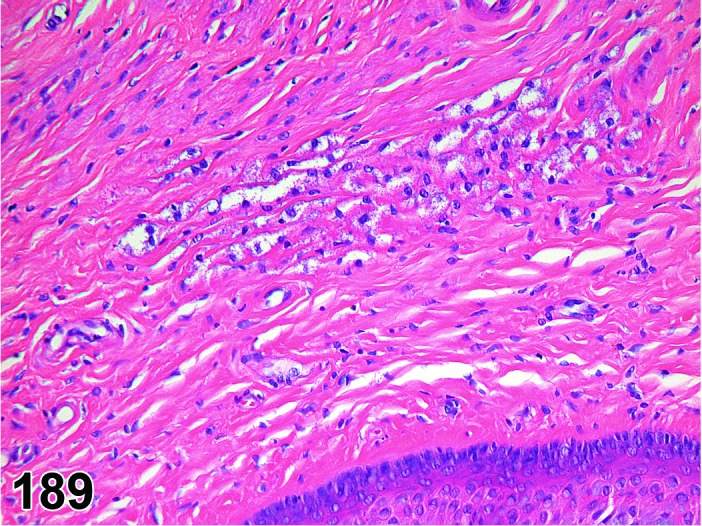
Granular Cell Aggregates, Vagina, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Not established; origin from Schwann cell or mesenchymal cell has been proposed.
Diagnostic Features
・Granular cells are present as occasional scattered single cells or as small clusters.
・No disturbance of the normal tissue architecture.
・Lack of collagen between the granular cells.
・Localization in the wall of the cervix, vagina or adventitia.
・Positive immunohistochemical reaction with S-100.
・Cytoplasmic granules weakly positive with the PAS reaction.
Differential Diagnoses
Hyperplasia, granular cell:
・Disturbance of normal tissue architecture may occur.
・Usually some collagen between the granular cells.
Tumor, granular cell, benign:
・Circumscribed, well-demarcated solid mass composed of large round to oval cells with pale basophilic nuclei and abundant eosinophilic granular cytoplasm and smaller cells with small dark uniform nuclei.
・Prominent interstitial collagen.
Tumor, granular cell, malignant:
・Pleomorphism.
・Tumor mass is composed of typical granular cells located at the periphery as well as of cells with decreased granularity and spindle cell morphology in the center.
・Increased nucleus: cytoplasmic ratio.
・Necrotic areas often present, whereas mitosis may not be common.
Comment
At a diagnostic level at least 3 - 5 cells should be present.
References
Atrophy, epithelial (N) Uterine Cervix; Vagina (Figure 190)
Figure 190.
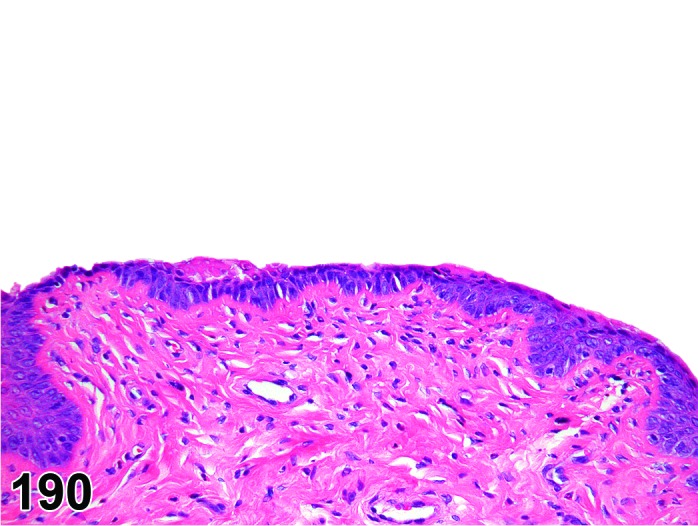
Epithelial Cell Atrophy, Vagina, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Vaginal and cervical epithelium.
Diagnostic Features
・Decreased thickness of the vaginal and cervical epithelium.
・Composed of 2-3 layers of inactive cuboidal cells.
・Squamous cells and keratin are diminished or lost completely.
Differential Diagnoses
Diestrus:
・At its thinnest stage vaginal epithelium is 3 to 5 cell layers thick.
・Epithelium is composed of a stratum basale and a stratum spinosum (stratum germinativum).
Comment
Nonspecific change that can occur in a variety of settings including control rats in persistent anestrus at the end of reproductive senescence, secondary to decreased circulating levels of endogenous ovarian steroid hormones, and administration of xenobiotics as well as a secondary effect due to poor clinical condition.
References
Cyst, NOS (N) Ovary; Uterus; Uterine Cervix, Vagina (See Ovary Section)
Degeneration, epithelial (N) Uterine Cervix; Vagina
Species
Mouse; Rat.
Pathogenesis/cell of origin
Epithelial origin.
Diagnostic Features
・Varying amounts of individual or clusters of apoptotic epithelial cells.
・Loss of normal stratification with or without intercellular edema (spongiosis).
・May include evidence of regeneration and/or hyperkeratosis.
Differential Diagnoses
Autolysis:
・Uniform dissolution of the tissue.
Necrosis, epithelial:
・Displays cellular features of necrosis and often contains acute inflammation. May represent a continuum with degeneration.
Erosion/ulcer:
・May have features of degeneration and/or necrosis, but contains a focal loss of epithelium.
Comment
Nonspecific changes with a spectrum of findings that are often similar to those observed in other squamous epithelial surfaces found elsewhere in the body.
References
Erosion/ulcer (N) Uterine Cervix; Vagina (Figure 191)
Figure 191.
Erosion and Ulceration of the Vaginal and Uterine Epithelium, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Epithelial origin.
Diagnostic Features
・Focal loss of epithelium (erosion).
・Focal loss of epithelium with a breach of the underlying basement membrane (ulcer).
・May have an accompanying acute inflammatory cell infiltrate.
Differential Diagnoses
Degeneration, epithelial:
・Cellular features of degeneration with an intact epithelial surface.
Comment
May be seen in conjunction with degeneration and/or necrosis. Most typically observed secondary to intravaginal administration of xenobiotics, irritating vehicles, and/or intravaginal devices (pessaries).
References
Imperforate vagina (N) Vagina
Species
Mouse.
Synonym(s)
Imperforate hymen; Persistent hymen.
Pathogenesis/cell of origin
Embryologic remnant consisting of a persistent connective tissue membrane within the vaginal vault.
Diagnostic Features
・Varying amounts of connective tissue present within the vaginal vault.
・Complete obstruction may lead to secondary mucometra/hydrometra and distention of the vagina.
・Most readily identified macroscopically and may present with perineal swelling.
Differential Diagnoses
Additional congenital defects such as vaginal agenesis, transverse vaginal septa, or longitudinal vaginal septa may mimic an imperforate vagina; however, all of these defects are exceedingly rare.
Comment
Imperforate vagina has been reported in strains of inbred mice.
References
56Ginty and Hoogstraten-Miller (2008), 187Sundberg and Brown (1994)
Increased keratinization (N) Uterine Cervix; Vagina (Figure 192)
Figure 192.
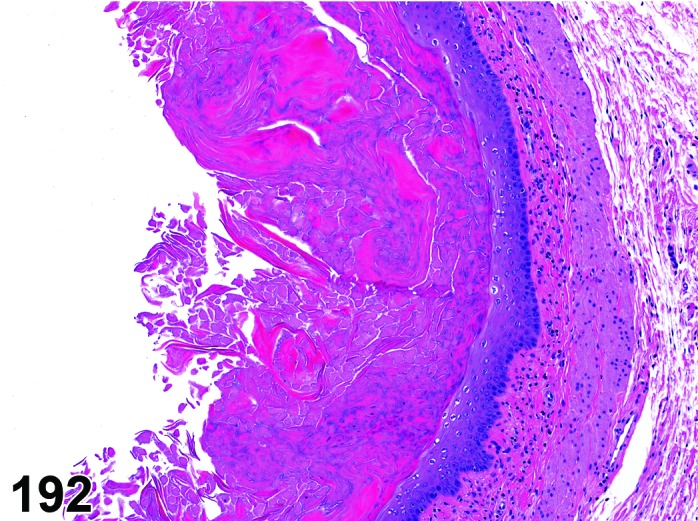
Increased Keratinization, Vagina, rat.
Species
Mouse; Rat.
Synonym(s)
Cornification; Hyperkeratosis; Hyperkeratinization.
Pathogenesis/cell of origin
Superficial vaginal or cervical epithelium.
Diagnostic Features
・Increased thickness of the superficial cornified layer of the vagina (when compared to normal estrus).
・Detachment of portions of the cornified layer may be present.
・May be present in conjunction with diffuse hyperplasia.
Differential Diagnoses
Estrus:
・Vaginal epithelium composed of approximately 8 to 10 cell layers with overlying keratinization, which is in conjunction with the presence of recently ovulated corpora lutea.
・Progressive shedding of keratinized layer with presence of cornified cells within vaginal lumen.
Comment
Hyperkeratinization is observed along with other anomalies of the reproductive tract in female offspring exposed in utero to estrogenic compounds. In adult females exposed to estrogenic compounds vaginal hyperkeratinization and/or hyperplasia may resemble the morphology of estrus in cycling females and needs to be distinguished based on other findings within the reproductive tract (no basophilic corpora lutea suggesting a lack of recent ovulation).
References
7Andrews et al. (2002), 127McLachlan et al. (1980), 182Steinmetz et al. (1998), 214Yuan and Foley (2002)
Increased mucification (N) Uterine Cervix; Vagina (Figure 193)
Figure 193.
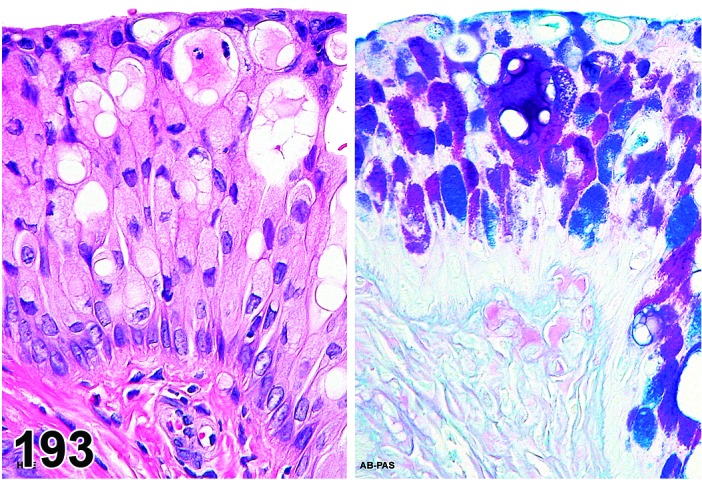
Drug-induced Increased Mucification, Vagina, PAS-Alcian blue, rat.
Species
Mouse; Rat.
Synonym(s)
Hypermucification.
Pathogenesis/cell of origin
Vaginal or cervical epithelium.
Diagnostic Features
・Superficial epithelial cells contain a large cytoplasmic mucus vacuole, which may form a distinct “mucified” layer.
・The thickness of the vaginal epithelium is variable and may be increased, with the vaginal epithelium more involved then the cervical epithelium.
・The ventral vaginal wall is more susceptible to this change and sometimes partial mucification of the vagina can be induced, where the ventral wall is composed of mucified epithelium without a similar change in the dorsal wall.
・Variable amounts of a mixed inflammatory cell infiltrate can be present within the epithelium.
・In more severe cases, intraepithelial microabscesses and epithelial erosions can be present.
・The intracellular mucin stains readily with PAS and/or Alcian blue.
Differential Diagnoses
Proestrus:
・Vaginal epithelium composed of 4 layers: stratum germinativum, stratum granulosum, stratum corneum and a superficial stratum mucification characterized by layers of cuboidal to ovoid cells with mucin-containing vacuoles.
・Stratum mucification overlying stratum corneum (early proestrus) or keratinized stratum corneum visible as a dense “red line”.
・In these cases, it is recommended not to record, if part of the normal cyclic activity.
Vacuolation, epithelial:
・Presence of multiple small intracytoplasmic vacuoles.
Comment
Nonspecific change that can occur in a variety of settings including control rats in repetitive pseudopregnancy during reproductive senescence, secondary to alterations in endogenous prolactin secretion, or administration of hormonally active xenobiotics.
References
10Berger et al. (2005), 84Izumi et al. (2009), 164Rehm et al. (2007), 208Westwood (2008), 214Yuan and Foley (2002)
Infiltrate, inflammatory cell (N) Uterine Cervix; Vagina; Uterus; Oviduct (See Uterus and Oviduct Section)
Inflammation (N) Uterine Cervix; Vagina; Uterus (Figure 194)
Figure 194.
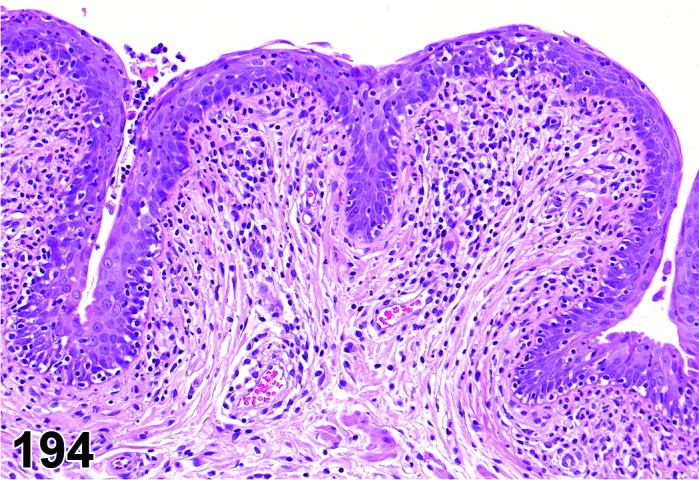
Inflammation, Lymphocytic, Vagina, rat.
Species
Mouse; Rat.
Synonym(s)
Vaginitis; Cervicitis; Metritis.
Modifier
Neutrophilic; Lymphocytic; Mononuclear; Mixed. Other modifiers include suppurative, granulomatous.
Pathogenesis/cell of origin
Neutrophils, mononuclear cells, macrophages (histiocytes), mixed cells.
Diagnostic Features
・Extensive cellular infiltrates (mononuclear, neutrophils, histiocytes or mixed cells) in the epithelium or underlying mucosa. May occur in association with inflammation of the uterine body and horns, and involving the endometrium and myometrium (metritis).
・Usually little or no accumulation of exudate in the lumen.
・Edema may be present.
・May be associated in the vagina with retention and accumulation of keratin debris in the fornices.
・May be accompanied by cystic dilation.
Differential Diagnoses
Normal estrous cycle:
・Normal influx of neutrophils during the estrous cycle. In these cases, it is recommended not to record, if part of the normal cyclic activity.
Comment
Must be differentiated from normal presence of inflammatory cells, especially neutrophils, at certain stages of estrous cycle. Inflammation of the vagina and cervix, and transmural involvement of the uterine wall are less common than inflammation of the oviduct, myometrium and endometrium occuring independently. May be seen in conjunction with degeneration and/or necrosis or can also occur as part of an infectious disease (Mycoplasma pulmonalis). In addition, inflammation may be observed secondary to intravaginal administration of xenobiotics, irritating vehicles, and/or intravaginal devices (pessaries).
References
66Greaves (2012), 109Leininger and Jokinen (1990), 214Yuan and Foley (2002)
Necrosis, epithelial (N) Uterine Cervix; Vagina (Figure 195)
Figure 195.
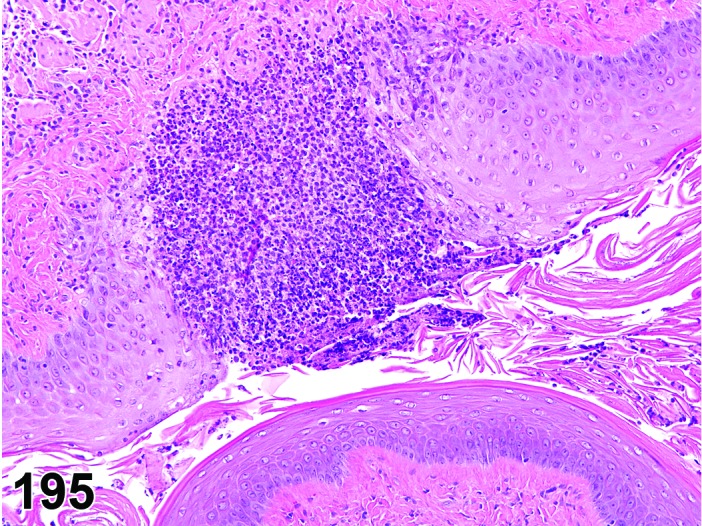
Necrosis with neutrophilic inflammation, Vagina, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Epithelial origin.
Diagnostic Features
・Nuclear pyknosis and/or karyorrhexis.
・Cytoplasmic eosinophilia.
・Cellular swelling or shrinkage.
・Exfoliated cells and/or intraluminal cellular debris.
・Often associated with acute inflammation.
・May result in erosion/ulcer.
Differential Diagnoses
Autolysis:
・Uniform dissolution of tissue.
Degeneration, epithelial:
・Displays cellular features of degeneration and lacks an inflammatory response. May represent a continuum with necrosis.
Comment
Most typically observed secondary to intravaginal administration of xenobiotics, irritating vehicles, and/or intravaginal devices (pessaries).
References
Prolapse (N) Uterus; Uterine Cervix; Vagina (Figure 196)
Figure 196.
Prolapse, Uterus, Vagina, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Uterus.
Diagnostic Features
・Presence of inverted uterine tissue within the cervix and/or vagina.
・Endometrial glands and epithelium present on both internal and external aspects of the uterine tissue.
・Myometrium present between the two layers of endometrium.
Differential Diagnoses
Polyp, endometrial stromal:
・Pedunculated mass that protrudes into uterine lumen and may extend through cervix into vagina, lined externally by epithelium and with prominent loose stroma. Frequently contains glandular elements within the stroma. Myometrium is not present.
Comment
Gross examination and careful histological trimming are important to enable accurate differentiation between prolapse and stromal polyp. In aged mice, prolapse is often associated with other uterine lesions, in particular severe cystic changes and/or tumors.
References
31Davis et al. (1999), 109Leininger and Jokinen (1990), 119Maekawa and Maita (1996)
Prostatic rudiment (N) Vagina (Figures 197 and 198)
Figure 197.
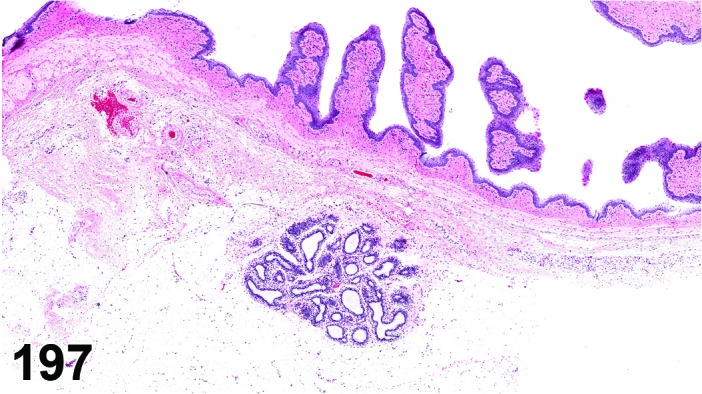
Prostatic Rudiment, Vagina, rat.
Figure 198.
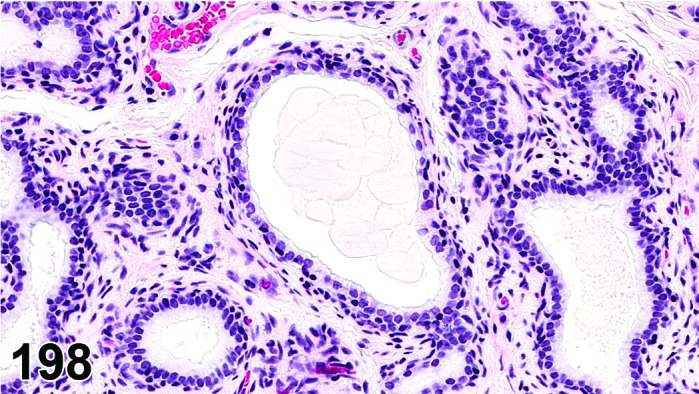
Prostatic Rudiment, Vagina, rat.
Species
Mouse; Rat.
Synonym(s)
Skene’s glands; Skene’s paraurethral glands; Female prostate.
Pathogenesis/cell of origin
Urogenital sinus.
Diagnostic Features
・Small underdeveloped paired glandular structures located along the urethra.
・May contain small amounts of intraluminal secretory material.
・Embedded within a fibromuscular stroma.
・Resembles a ventral prostate from a young male rat.
Differential Diagnoses
Adenoma, endometrial:
・Adenoma is more densely cellular, contains mitotic figures, and lacks a distinct fibromuscular stroma.
Comment
Presence or absence of prostatic rudiment may be encountered in sections of the vagina depending on the location and plane of the section.
References
168Santos et al. (2006)
Vacuolation, epithelial (N) Uterine Cervix; Vagina
Species
Mouse; Rat.
Pathogenesis/cell of origin
Epithelial origin.
Diagnostic Features
・Presence of multiple small intracytoplasmic vacuoles (negative for PAS and/or Alcian blue).
Differential Diagnoses
Mucification, increased:
・Superficial epithelial cells contain a large cytoplasmic mucus vacuole, which may form a distinct “mucified” layer. The mucus can be stained using PAS and/or Alcian blue.
Proestrus:
・Superficial mucoid layer (also called stratum mucification) characterized by layers of cuboidal to ovoid cells with intracytoplasmic mucin vacuoles overlays stratum granulosum (early proestrus) or keratinized stratum corneum (late proestrus). In these cases, it is recommended not to record, if part of the normal cyclic activity.
B. Nonneoplastic Proliferative Lesions
Adenosis (N) Uterine Cervix; Vagina (Figures 199 and 200)
Figure 199.
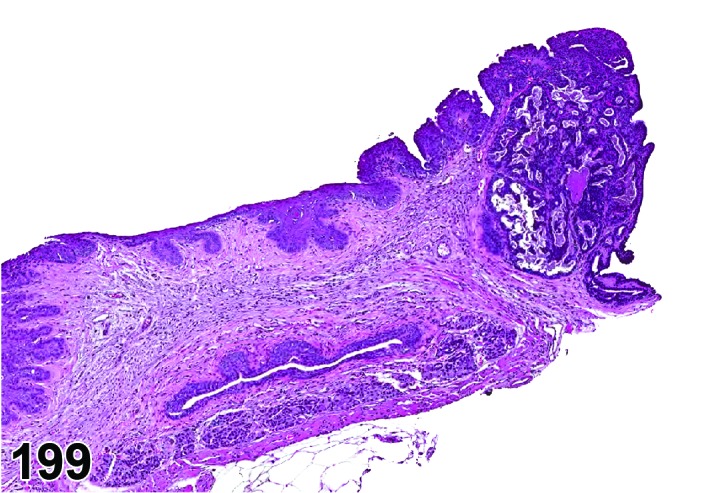
Adenosis, Vagina, mouse.
Figure 200.
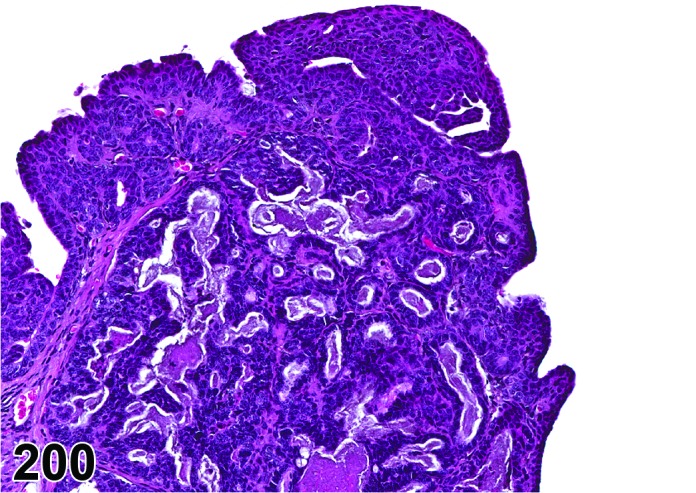
Adenosis, Vagina, mouse.
Species
Mouse.
Synonym(s)
Adenomatous differentiation; Adenomatous hyperplasia.
Pathogenesis/cell of origin
Epithelium of Müllerian ducts.
Diagnostic Features
・Presence of columnar to cuboidal epithelium on the surface and formation of glands in the cervix and vagina.
・Primarily appears in anterior vagina and fornices, but may extend to mid-vagina.
・Heterotopic (columnar) epithelium observed in prepuberal mice with formation of glands at puberty.
Differential Diagnoses
Adenocarcinoma, endometrial:
・Glandular structures in stroma show cellular atypia and/or invasion.
Comment
Extremely rare spontaneous lesion. Developmental abnormality associated with exposure to certain estrogenic compounds perinatally or neonatally. In mice exposed to DES neonatally transformation from the Mullerian epithelium to a two-cell layered vaginal epithelium does not occur. This persistent primitive epithelium can form glands in the underlying stroma following puberty. Also in humans, this phenomenon is known as adenosis. Severity and extent of change varies with strain, compound, dose, and time of exposure.
References
20Chamness et al. (1979), 44Ennis and Davies (1982), 49Forsberg and Kalland (1981), 80Iguchi et al. (1986), 86Johnson (1987), 97Ketani et al. (2002), 145Newbold and McLachlan (1982)
Hyperplasia, epithelial (H) Vagina (Figure 201)
Figure 201.
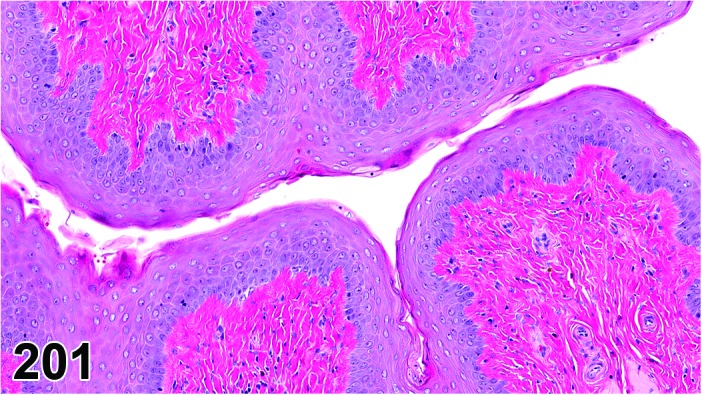
Epithelial Hyperplasia without keratin, Vagina, rat.
Species
Mouse; Rat.
Modifier
Basal cell; Squamous cell.
Pathogenesis/cell of origin
Vaginal epithelium.
Diagnostic Features
・Increased thickness of the vaginal epithelium (when compared to normal estrus).
・Cells are well differentiated with no atypia or invasion of the underlying stroma.
・May form downward projections into the underlying stroma.
・May have overlying keratinization.
・Few mitotic figures present.
Differential Diagnoses
Estrus:
・Vaginal epithelium composed of approximately 8 to 10 cell layers, with overlying keratinization, which is in conjunction with the presence of basophilic corpora lutea indicating recent ovulation. In these cases, it is recommended not to record, if part of the normal cyclic activity.
Papilloma, squamous cell:
・Papillary proliferation of the mucosa with squamous differentiation.
Carcinoma, squamous cell:
・Squamous cell carcinomas may be well differentiated, but cells have varying degrees of atypia and often infiltrate into submucosa and muscularis, and serosa. Differentiation from basal to squamous cells - if detectable - is often less organized.
Polyp, vaginal:
・Lesion is comprised predominantly of a fibromuscular core covered by normal or slightly hyperkeratinized epithelium.
Comment
Nonspecific change that can occur in a variety of settings including control rats in persistent estrus during reproductive senescence, secondary to increases in endogenous estradiol production, or administration of hormonally active xenobiotics.
References
32Davis et al. (2001), 39Dixon et al. (1999), 64Gopinath et al. (1987), 187Sundberg and Brown (1994), 214Yuan and Foley (2002)
Hyperplasia, granular cell (H) Uterus; Uterine Cervix; Vagina (Figures 202 and 203)
Figure 202.
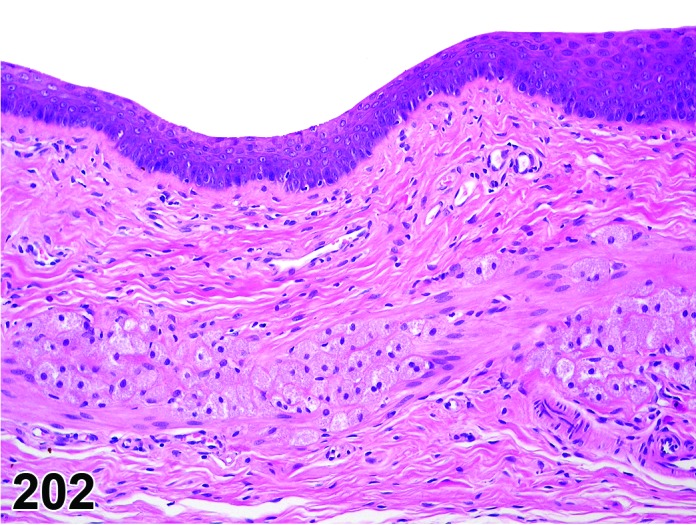
Granular Cell Hyperplasia, Vagina, rat.
Figure 203.
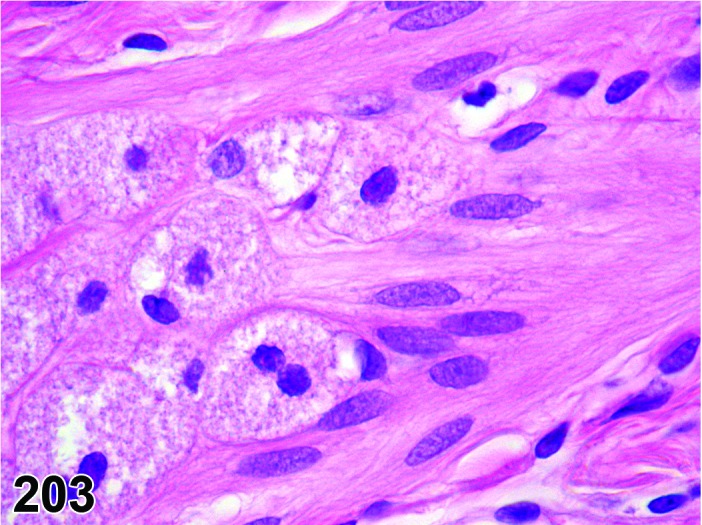
Granular Cell Hyperplasia, Vagina, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Not established; origin from Schwann cell or mesenchymal cell has been proposed.
Diagnostic Features
・Granular cells similar to those of granular cell tumors are present as numerous scattered single cells or as small clusters.
・No compression of the adjacent tissue.
・Few collagen bundles between the granular cells in the clusters.
・Localization in the wall of the cervix, vagina or adventitia.
・Minimal disturbance of normal tissue architecture may occur.
・Positive immunohistochemical reaction with S-100.
・Cytoplasmic granules weakly positive with the PAS reaction.
Differential Diagnoses
Aggregate, granular cell:
・Single cells (at least 3-5) without interstitial collagen.
・No disturbance of normal tissue architecture.
Tumor, granular cell, benign:
・Circumscribed, well demarcated mass, which causes compression of adjacent tissue.
・Prominent interstitial collagen is present.
Comment
Granular cell hyperplasia is considered a rare lesion. As this cell is not present in normal tissue, this can be considered an early form of transformation.
References
78Hollander et al. (1976), 103Krinke et al. (2000), 124Markovits and Sahota (2000b), 158Picut et al. (2009), 169Sasahara et al. (1998)
Hyperplasia, stroma (H) Uterine Cervix (Figure 204)
Figure 204.
Stromal Hyperplasia, Uterine Cervix, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Stromal cell.
Diagnostic Features
・Focal to diffuse increased proliferation of primarily stromal cells within a fibrovascular stroma.
・Increased cellularity primarily due to stromal cell proliferation.
Differential Diagnoses
Leiomyoma:
・Typically, well-circumscribed masses that may be solitary or multiple and compress adjacent structures.
・Tumor cells form interlacing bundles and whorls reminiscent of normal smooth muscle cells.
・Varying amounts of collagenous tissue and vascularity.
・Tumor cells are spindle-shaped, have abundant eosinophilic cytoplasm, distinct borders and “cigar-shaped” or blunt-ended nuclei.
Fibroma:
・Tumors cause compression of surrounding tissues and usually are moderately to poorly cellular depending on extent of mature collagen present.
・Cells are spindled or fusiform and contain elongated nuclei with observable nuclei.
・Varying amounts of mature collagen forming interwoven bands.
Hypertrophy, stroma:
・Diffuse increased proliferation of the fibromuscular stroma of the portio vaginalis uteri without distortion of the tissue architecture.
Hypertrophy, stroma (H) Uterine Cervix (Figure 205)
Figure 205.
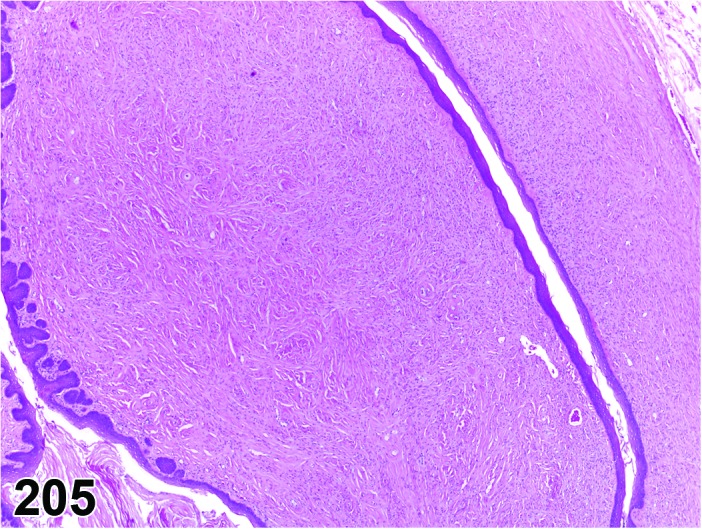
Stromal Hypertrophy, Cervix, rat.
Species
Rat.
Synonym(s)
Hypertrophy of the Portio Vaginalis.
Pathogenesis/cell of origin
Fibromuscular stroma.
Diagnostic Features
・Diffuse increased amounts of fibromuscular stroma of the portio vaginalis uteri without distortion of the tissue architecture.
Differential Diagnoses
Leiomyoma:
・Typically, well-circumscribed masses that may be solitary or multiple and compress adjacent structures.
・Tumor cells form interlacing bundles and whorls reminiscent of normal smooth muscle cells.
・Varying amounts of collagenous tissue and vascularity.
・Tumor cells are spindle-shaped, have abundant eosinophilic cytoplasm, distinct borders and “cigar-shaped” or blunt-ended nuclei.
Fibroma:
・Tumors cause compression of surrounding tissues and usually are moderately to poorly cellular depending on extent of mature collagen present.
・Cells are spindled or fusiform and contain elongated nuclei with observable nuclei.
・Varying amounts of mature collagen forming interwoven bands.
Hyperplasia, stroma:
・Focal to diffuse increased proliferation of primarily stromal cells within a fibrovascular stroma. More stromal cell nuclei are typically present.
Comment
This lesion is often observed in aged Fisher 344 rats. It has also been observed in mice following transplacental exposure to DES.
References
39Dixon et al. (1999), 109Leininger and Jokinen (1990), 145Newbold and McLachlan (1982), 148Nomura and Kanzaki (1977)
C. Neoplastic Proliferative Lesions
Keratoacanthoma (B) Uterine Cervix; Vagina
Species
Mouse; Rat.
Synonym(s)
Epithelioma.
Modifier
Keratinizing; Non-keratinizing.
Pathogenesis/cell of origin
Surface epithelium.
Diagnostic Features
・Resemble keratoacanthomas of the skin.
・Well encapsulated and composed of cavities that may be single or multiple that are lined by stratified cornifying squamous cell epithelium.
・There may be papillary projections into the lumens or cavities may be formed and contain concentrically arranged laminated keratin or homogeneous material that may contain cholesterol crystals or proteinaceous fluid.
・Cells are well differentiated with no atypia or invasion of the adjacent tissues, and a low nuclear to cytoplasmic ratio of proliferating epithelium is present.
Differential Diagnoses
Hyperplasia, epithelial, squamous cell:
・Restricted to the normal mucosal surface and does not form a mass with a cavity filled with laminated keratin or homogeneous material.
Carcinoma, squamous cell:
・Squamous cell carcinomas may be well differentiated but cells have varying degrees of atypia and often infiltrate into submucosa and muscularis, and serosa.
Reference
32Davis et al. (2001)
Papilloma, squamous cell (B) Vagina; Uterine cervix; Uterus (Figures 206 and 207)
Figure 206.
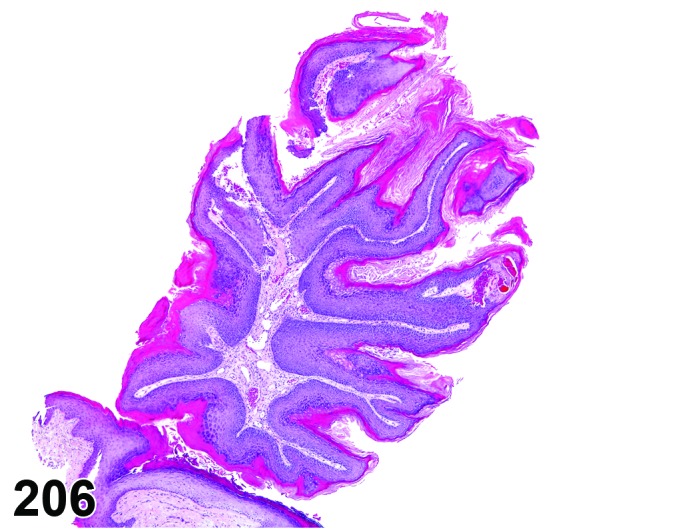
Squamous Cell Papilloma, Vagina, mouse.
Figure 207.
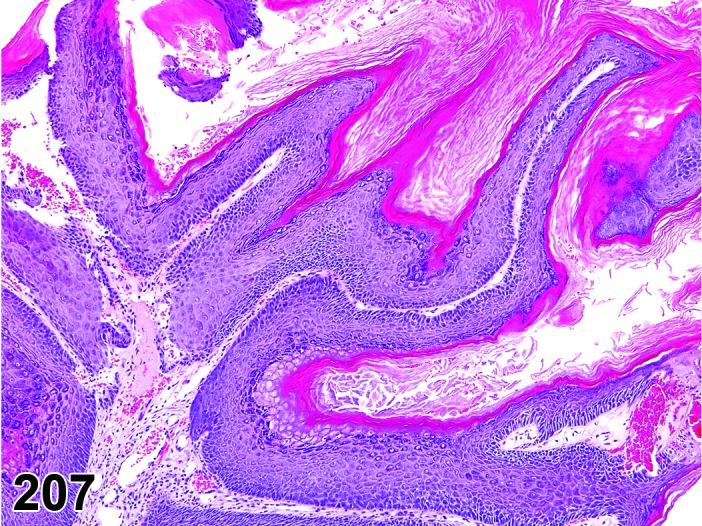
Squamous Cell Papilloma, Vagina, mouse.
Species
Mouse; Rat.
Modifier
Keratinizing; Non-keratinizing.
Pathogenesis/cell of origin
Surface epithelium of vagina, uterine cervix or uterus.
Diagnostic Features
・Marked papillary/exophytic proliferation of the mucosa with squamous differentiation.
・Moderately dense fibrovascular core comprises less of the lesion than the epithelial component.
・Cells are well differentiated with no atypia or invasion of the underlying stroma.
・Frequently associated with chronic suppurative inflammation.
Differential Diagnoses
Hyperplasia, epithelial, squamous cell:
・Restricted to the normal mucosal surface and does not form a mass that protrudes into the lumen.
Carcinoma, squamous cell:
・Squamous cell carcinomas may be well differentiated but cells have varying degrees of atypia and often infiltrate into submucosa and muscularis, and serosa.
Polyp, vaginal:
・Lesion is comprised predominantly of a fibromuscular core covered by normal or slightly hyperkeratinized epithelium.
Comment
Squamous cell papillomas of the vagina, uterine cervix or uterus are similar to those of the skin and oral cavity.
References
8Anisimov and Nikonov (1990), 9Ashley (1990), 32Davis et al. (2001), 39Dixon et al. (1999), 64Gopinath et al. (1987), 68Greaves and Faccini (1984), 109Leininger and Jokinen (1990)
Carcinoma, squamous cell (M) Vagina; Uterine Cervix; Uterus (See Uterus and Oviduct Section)
Polyp, vaginal (B) Vagina (Figure 208)
Figure 208.
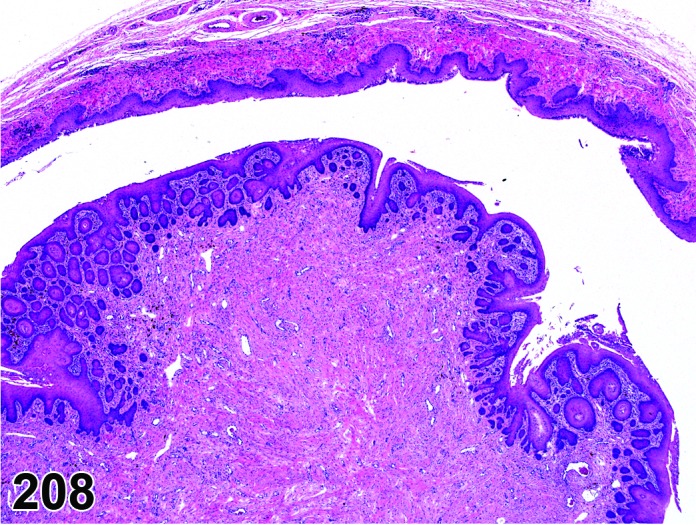
Vaginal Polyp with Epithelial Hyperplasia, rat.
Species
Mouse; Rat.
Synonym(s)
Polyp, squamous.
Pathogenesis/cell of origin
Vaginal epithelium and submucosal stroma.
Diagnostic Features
・Polyp covered by normal to hyperplastic squamous epithelium.
・Epithelium may have areas of hyperkeratosis.
・Epithelium may have mitotic figures, but is not infiltrative into the stroma.
・Bulk of the lesion is generally a dense fibrous or fibromuscular core, less commonly loose more highly vascularized connective tissue.
・The lesion may be solitary or multiple.
・Lesion may be edematous or infarcted.
Differential Diagnoses
Polyp, endometrial stromal:
・Presence of endometrial epithelium on or covering the polyp.
・Evidence of origin in uterus, projection into vagina.
Polyp, glandular:
・Presence of prominent and often hyperplastic glandular structures throughout a major part of the polyp.
Comment
Endometrial stromal polyps commonly project through the cervix and appear in the vagina. Key determinants of a vaginal polyp are evidence of the stalk arising from the vaginal stroma and characteristic vaginal epithelium on the surface.
References
39Dixon et al. (1999), 68Greaves and Faccini (1984), 109Leininger and Jokinen (1990)
Sarcoma, stromal (M) Uterine Cervix (Figures 209 and 210)
Figure 209.
Stromal Sarcoma, Cervix, rat.
Figure 210.
Stromal Sarcoma, Cervix, rat.
Species
Mouse; Rat.
Pathogenesis/cell of origin
Stromal cell.
Diagnostic Features
・Anaplastic cells resembling endocervical stromal cells.
・Moderate mitotic activity.
・Increased numbers of cells with small, basophilic nuclei.
・Necrotic areas may be present.
・Focal areas of hemorrhage, numerous dilated and congested blood vessels.
・May infiltrate the vagina and uterus.
・May metastasize to distant sites.
Differential Diagnoses
Hyperplasia, stroma:
・Focal to diffuse increased proliferation of primarily typical stromal cells within the dense fibromuscular stroma.
・No atypia or cellular pleomorphism is present.
Leiomyosarcoma:
・Poorly delineated mass with disorganized and invasive growth patterns, although metastasis is not common.
・Appear hypercellular due to decrease in amount of cytoplasm of anaplastic cells.
・Tumor cells are spindled to pleomorphic and have blunt ended to oval nuclei.
・Nuclei may be pleomorphic and have high mitotic activity, prominent nucleoli and hyperchromasia.
・Typically, well-circumscribed masses that may be solitary or multiple and infiltrate into adjacent structures.
・Varying amounts of collagenous tissue, vascularity, hemorrhage and necrosis may be present.
Fibrosarcoma:
・Tumor consists of pleomorphic spindle-shaped cells that often form interlacing bundles or a “herring bone” cellular pattern.
・Varying amounts of collagen can be seen between tumor cells depending on the degree of differentiation.
・Numerous mitotic figures are typically present.
・Areas of necrosis and hemorrhage can be seen.
・Local invasion and extension into adjacent structures.
Comment
There is a report of a CD-1 mouse exposed to DES that had a stromal sarcoma that infiltrated the uterus and vagina and metastasized to the liver, spleen, ovary, and oviduct.
References
Leiomyoma (B) Uterine Cervix; Vagina; Uterus (See Uterus and Oviduct Section)
Leiomyosarcoma (M) Uterine Cervix; Vagina; Uterus (See Uterus and Oviduct Section)
Schwannoma, benign (B) Uterine Cervix; Vagina; Uterus (See Uterus and Oviduct Section)
Schwannoma, malignant (M) Uterine Cervix; Vagina; Uterus (See Uterus and Oviduct Section)
Tumor, granular cell, benign (B) Uterine Cervix; Vagina; Uterus (Figures 211 and 212)
Figure 211.
Granular Cell Tumor, Benign, Vagina, rat.
Figure 212.
Granular Cell Tumor, Benign, Vagina, rat.
Species
Mouse; Rat.
Synonym(s)
Abrikossoff’s tumor, benign; Myoblastoma.
Pathogenesis/cell of origin
Not established; an origin from Schwann cells or mesenchymal cells has been proposed.
Diagnostic Features
・Circumscribed, well-demarcated solid mass composed of large round to oval cells with pale basophilic nuclei and abundant eosinophilic granular cytoplasm and smaller cells with small dark uniform nuclei.
・Prominent interstitial collagen.
・Cytoplasmic granules are weakly positive with PAS, diastase-resistant, and react immunohistochemically positive with S-100 and vimentin. The granules are considered to be various stages of lysosomes.
・Expansive growth causes compression and atrophy of adjacent tissue. No capsule formation and often with some local infiltration in the adjacent tissues, but no metastasis.
・Often located within the muscularis, but also expanding into the adventitia.
Differential Diagnoses
Hyperplasia, granular cell:
・No compression of the adjacent tissue.
・Few collagen bundles between the granular cells.
Tumor, granular cell, malignant:
・Prominent pleomorphism.
・Increased nucleus: cytoplasmic ratio.
・Necrosis common.
Comment
Uncommon tumor, but can be produced experimentally with certain chemicals. The older name of “myoblastoma” originated from the early idea that these tumors derived from skeletal muscle cells and while the histogenesis is still uncertain, this is no longer widely accepted. Granular cell tumors also occur at other anatomical sites (e.g., the meninges). Granular cell tumors of the brain show a slightly different morphology and immunohistochemical staining pattern suggesting that they may have a different histogenesis.
References
29Courtney et al. (1992), 78Hollander et al. (1976), 102Krinke et al. (1985), 103Krinke et al. (2000), 124Markovits and Sahota (2000b), 150Nyska et al. (1991), 158Picut et al. (2009), 200Veit et al. (2008)
Tumor, granular cell, malignant (M) Uterine Cervix; Vagina; Uterus (Figures 213 and 214)
Figure 213.
Granular Cell Tumor, Malignant, Vagina, rat.
Figure 214.
Granular Cell Tumor, Malignant, rat.
Species
Rat.
Pathogenesis/cell of origin
Not established; an origin from Schwann cells or mesenchymal cells has been proposed.
Diagnostic Features
・Pleomorphic granular cells.
・Tumor mass is composed of typical granular cells located in the periphery as well as of cells with decreased granularity and spindle cell morphology in the center.
・Increased nucleus: cytoplasmic ratio.
・Necrotic areas often present, whereas mitosis is not common.
・Cytoplasmic granules, if present in sufficient numbers, are weakly positive with PAS, diastase-resistant, and react immunohistochemically with S-100 and vimentin. The granules are considered to be various stages of lysosomes.
Differential Diagnoses
Tumor, granular cell, benign:
・No pleomorphism.
・Solid mass of typical granular cells.
Comment
The malignant granular cell tumor is a rare lesion described in the literature. Infiltrative growth does not appear to be a diagnostic feature used to distinguish benign from malignant granular cell tumors in the female reproductive tract, although infiltration has been cited as a characteristic of malignant granular cell tumors described in the central nervous system.
References
29Courtney et al. (1992), 78Hollander et al. (1976), 102Krinke et al. (1985), 103Krinke et al. (2000), 123Markovits and Sahota (2000a), 124Markovits and Sahota (2000b), 200Veit et al. (2008), 92Kaufmann et al. (2012)
References
- 1.Alison RH, Lewis DJ, and Montgomery CA. Ovarian choriocarcinoma in the mouse. Vet Pathol. 24(3): 226-230 1987. a. [DOI] [PubMed] [Google Scholar]
- 2.Alison RH, and Morgan KT. Ovarian neoplasms in F344 rats and B6C3F1 mice. Environ Health Perspect. 73: 91-106 1987. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alison RH, and Morgan KT. Granulosa cell tumor, ovary, mouse. In: Monographs on pathology of laboratory animals. Genital System. Jones TC, Mohr U, and Hunt RD (eds). Springer-Verlag, Berlin, Heidelberg, New York. 22-30 1987. b. [Google Scholar]
- 4.Alison RH, Morgan KT, Haseman JK, and Boorman GA. Morphology and classification of ovarian neoplasms in F344 rats and (C57BL/6 X C3H)F1 mice. J Natl Cancer Inst. 78(6): 1229-1243 1987. b. [PubMed] [Google Scholar]
- 5.Alison RH, Morgan KT, and Montgomery CA. Ovary. In: Pathology of the Fischer rat. Reference and atlas. Boorman GA, Eustis SL, Elwell MR, Montgomery CA, Jr, and MacKenzie WF (eds). Academic Press, San Diego, New York, London. 429-442 1990. [Google Scholar]
- 6.Amadi K, Nwana EJ, and Otubu JA. Morphology and function of the rat oviduct: effects of thyroidectomy and thyroxine administration. Afr J Med Med Sci. 36(4): 353-358 2007. [PubMed] [Google Scholar]
- 7.Andrews P, Freyberger A, Hartmann E, Eiben R, Loof I, Schmidt U, Temerowski M, Folkerts A, Stahl B, and Kayser M. Sensitive detection of the endocrine effects of the estrogen analogue ethinylestradiol using a modified enhanced subacute rat study protocol (OECD Test Guideline no. 407). Arch Toxicol. 76(4): 194-202 2002. [DOI] [PubMed] [Google Scholar]
- 8.Anisimov VN, and Nikonov AA. Tumours of the vagina, uterus and oviduct. In: Pathology of tumours in laboratory animals. Vol I. Tumours of the rat, 2nd edition. Turusov VS, and Mohr U (eds). IARC Scientific Publications No. 99, Lyon. 445-471 1990. [PubMed] [Google Scholar]
- 9.Ashley DJB. Evan’s histological appearances of tumors. Churchill Livingstone, Edinburgh. 1990. [Google Scholar]
- 10.Berger L, El-Alfy M, Martel C, and Labrie F. Effects of dehydroepiandrosterone, Premarin and Acolbifene on histomorphology and sex steroid receptors in the rat vagina. J Steroid Biochem Mol Biol. 96(2): 201-215 2005. [DOI] [PubMed] [Google Scholar]
- 11.Biegel LB, Flaws JA, Hirshfield AN, O’Connor JC, Elliott GS, Ladics GS, Silbergeld EK, Van Pelt CS, Hurtt ME, Cook JC, and Frame SR. 90-day feeding and one-generation reproduction study in Crl:CD BR rats with 17 beta-estradiol. Toxicol Sci. 44(2): 116-142 1998. [DOI] [PubMed] [Google Scholar]
- 12.Bolon B, Bucci TJ, Warbritton AR, Chen JJ, Mattison DR, and Heindel JJ. Differential follicle counts as a screen for chemically induced ovarian toxicity in mice: results from continuous breeding bioassays. Fundam Appl Toxicol. 39(1): 1-10 1997. [DOI] [PubMed] [Google Scholar]
- 13.Branham WS, Zehr DR, Chen JJ, and Sheehan DM. Alterations in developing rat uterine cell populations after neonatal exposure to estrogens and antiestrogens. Teratology. 38(3): 271-279 1988. [DOI] [PubMed] [Google Scholar]
- 14.Brossia LJ, Roberts CS, Lopez JT, Bigsby RM, and Dynlacht JR. Interstrain differences in the development of pyometra after estrogen treatment of rats. J Am Assoc Lab Anim Sci. 48(5): 517-520 2009. [PMC free article] [PubMed] [Google Scholar]
- 15.Brown H, and Leininger JR. Alterations of the uterus. In: Pathobiology of the aging rat. Mohr U, Dungworth DL, and Capen CC (eds). ILSI Press, Washington DC. 377-388 1992. [Google Scholar]
- 16.Busch K, and Naglić T. Natural uterine Mycoplasma pulmonis infection in female rats. Vet Med (Praha). 40(8): 253-255 1995. [PubMed] [Google Scholar]
- 17.Campbell JS. Adenoacanthoma, uterus, rat. In: Monographs on pathology of laboratory animals. Genital system. Jones TC, Mohr U, and Hunt RD (eds). Springer, Berlin, Heidelberg, New York, Tokyo. 110-115 1987. [Google Scholar]
- 18.Cardesa A, Ribalta T, Vogeley KT, Reifenberger G, Wechsler W, and Turusov VS. Tumours of the peripheral nervous system. In: Pathology of tumours in laboratory animals. Vol I. Tumours of the rat, 2nd edition. Turusov VS, Mohr U (eds). IARC Scientific Publications No. 99, Lyon. 699-724 1990. [PubMed] [Google Scholar]
- 19.Carthew P, Edwards RE, Nolan BM, Martin EA, and Smith LL. Tamoxifen associated uterine pathology in rodents: relevance to women. Carcinogenesis. 17(8): 1577-1582 1996. [DOI] [PubMed] [Google Scholar]
- 20.Chamness GC, Bannayan GA, Landry LA, Jr, Sheridan PJ, and McGuire WL. Abnormal reproductive development in rats after neonatally administered antiestrogen (tamoxifen). Biol Reprod. 21(5): 1087-1090 1979. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, and Pepling ME. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology. 148(8): 3580-3590 2007. [DOI] [PubMed] [Google Scholar]
- 22.Ciaccio LA, Hill JL, and Kincl FA. Observation on toxic effects of quinacrine hydrochloride (in rodents). Contraception. 17(3): 231-236 1978. [DOI] [PubMed] [Google Scholar]
- 23.Clement PB. Non-neoplastic lesions of the ovary. In: Blaustein’s Pathology of the Female Genital Tract. Kurman RJ (ed). Springer-Verlag, New York. 676-677 2002. [Google Scholar]
- 24.Clow OL, Hurst PR, and Fleming JS. Changes in the mouse ovarian surface epithelium with age and ovulation number. Mol Cell Endocrinol. 191(1): 105-111 2002. [DOI] [PubMed] [Google Scholar]
- 25.Comereski CR, Peden WM, Davidson TJ, Warner GL, Hirth RS, and Frantz JD. BR96-doxorubicin conjugate (BMS-182248) versus doxorubicin: a comparative toxicity assessment in rats. Toxicol Pathol. 22(5): 473-488 1994. [DOI] [PubMed] [Google Scholar]
- 26.Corbeil LB, Chatterjee A, Foresman L, and Westfall JA. Ultrastructure of cyclic changes in the murine uterus, cervix, and vagina. Tissue Cell. 17(1): 53-68 1985. [DOI] [PubMed] [Google Scholar]
- 27.Cornillie FJ, and Lauweryns JM. Phagocytotic and iron-storing capacities of stromal cells in the rat endometrium. A histochemical and ultrastructural study. Cell Tissue Res. 239(3): 467-476 1985. [DOI] [PubMed] [Google Scholar]
- 28.Coskun A, Coban YK, and Ciralik H. Critical ischemic time for the rat ovary: experimental study evaluating early histopathologic changes. J Obstet Gynaecol Res. 35(2): 330-334 2009. [DOI] [PubMed] [Google Scholar]
- 29.Courtney CL, Hawkins KL, Meierhenry EF, and Graziano MJ. Immunohistochemical and ultrastructural characterization of granular cell tumors of the female reproductive tract in two aged Wistar rats. Vet Pathol. 29(1): 86-89 1992. [DOI] [PubMed] [Google Scholar]
- 30.Davis BJ, Almekinder JL, Flagler N, Travlos G, Wilson R, and Maronpot RR. Ovarian luteal cell toxicity of ethylene glycol monomethyl ether and methoxy acetic acid in vivo and in vitro. Toxicol Appl Pharmacol. 142(2): 328-337 1997. [DOI] [PubMed] [Google Scholar]
- 31.Davis BJ, Dixon D, and Herbert RA. Ovary, oviduct, uterus, cervix, and vagina. In: Pathology of the mouse. Reference and atlas. Maronpot RR, Boorman GA, and Gaul BW (eds). Cache River Press, Vienna. 409-443 1999. [Google Scholar]
- 32.Davis BJ, Harleman JH, Heinrichs M, Maekawa A, McConnell RF, Reznik G, and Tucker M. Female genital system. In: International Classification of Rodent Tumors: The Mouse. Mohr U (ed). Springer-Verlag, Heidelberg, Berlin, New York. 211-268 2001. [Google Scholar]
- 33.Davis JK, Gaertner DJ, Cox NR, Lindsey JR, Cassell GH, Davidson MK, Kervin KC, and Rao GN. The role of Klebsiella oxytoca in utero-ovarian infection of B6C3F1 mice. Lab Anim Sci. 37(2): 159-166 1987. [PubMed] [Google Scholar]
- 34.de la Maza LM, Pal S, Khamesipour A, and Peterson EM. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun. 62(5): 2094-2097 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deerberg F, Pohlmeyer G, Lörcher K, and Petrow V. Total suppression of spontaneous endometrial carcinoma in BDII/Han rats by melengestrol acetate. Oncology. 52(4): 319-325 1995. [DOI] [PubMed] [Google Scholar]
- 36.Deerberg F, Rehm S, and Pittermann W. Uncommon frequency of adenocarcinomas of the uterus in virgin Han:Wistar rats. Vet Pathol. 18(6): 707-713 1981. [DOI] [PubMed] [Google Scholar]
- 37.Diters RW, Funk KA, Fang H, Durham SK, and Mense MG. Hermaphroditism in a Sprague-Dawley rat. Vet Pathol. 44(3): 418-420 2007. [DOI] [PubMed] [Google Scholar]
- 38.Dixon D, Couse JF, and Korach KS. Disruption of the estrogen receptor gene in mice. Toxicol Pathol. 25(5): 518-520 1997. [DOI] [PubMed] [Google Scholar]
- 39.Dixon D, Leininger JR, Valerio MG, Johnson AN, Stabinski LG, and Frith CH. Proliferative lesions of the ovary, uterus, vagina, cervix and oviduct in rats. In Guides for Toxicologic Pathology. STP/ARP/AFIP, Washington, DC. 1999. [Google Scholar]
- 40.Dodo T, Taketa Y, Sugiyama M, Inomata A, Sonoda J, Okuda Y, Mineshima H, Hosokawa S, and Aoki T. Collaborative work on evaluation of ovarian toxicity. 11) Two- or four-week repeated-dose studies and fertility study of ethylene glycol monomethyl ether in female rats. J Toxicol Sci. 34(Suppl 1): SP121-SP128 2009. [DOI] [PubMed] [Google Scholar]
- 41.Durlinger AL, Kramer P, Karels B, Grootegoed JA, Uilenbroek JT, and Themmen AP. Apoptotic and proliferative changes during induced atresia of pre-ovulatory follicles in the rat. Hum Reprod. 15(12): 2504-2511 2000. [DOI] [PubMed] [Google Scholar]
- 42.Elwell M, Mahler JF, and Ruecker FA. Proliferative and non-proliferative lesions in the heart and vasculature in mice. In Guides for Toxicologic Pathology. STP/ARP/AFIP, Washington, DC. 2004. [Google Scholar]
- 43.Engle ET. Tubular adenomas and testis-like tubules of the ovaries of aged rats. Cancer Res. 6: 578-582 1946. [PubMed] [Google Scholar]
- 44.Ennis BW, and Davies J. Reproductive tract abnormalities in rats treated neonatally with DES. Am J Anat. 164(2): 145-154 1982. [DOI] [PubMed] [Google Scholar]
- 45.Ernst H, Carlton WW, Courtney C, Rinke M, Greaves P, Isaacs KR, Krinke G, Konishi Y, Mesfin GM, and Sandusky G. Soft tissue and skeletal muscle. In: International Classification of Rodent Tumors. The Mouse. Mohr U (ed). Springer, Heidelberg, Berlin, New York. WHO IARK 361-387. 2001a. [Google Scholar]
- 46.Ernst H, Carlton WW, Courtney C, Rinke M, Greaves P, Isaacs KR, Krinke G, Konishi Y, Mesfin GM, and Sandusky G. Soft tissue and skeletal muscle. In: International Classification of Rodent Tumors. The Mouse. Mohr U (ed). Springer, Heidelberg, Berlin, New York. WHO IARK. 2001b. [Google Scholar]
- 47.Faccini JM, Abbott DP, and Paulus GJJ. Mouse histopathology. A glossary for use in toxicity and carcinogenicity studies. Elsevier, Amsterdam, New York, Oxford. 1990. [Google Scholar]
- 48.Fleming JS, McQuillan HJ, Millier MJ, Beaugié CR, and Livingstone V. E-cadherin expression and bromodeoxyuridine incorporation during development of ovarian inclusion cysts in age-matched breeder and incessantly ovulated CD-1 mice. Reprod Biol Endocrinol. 5: 14 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forsberg JG, and Kalland T. Neonatal estrogen treatment and epithelial abnormalities in the cervicovaginal epithelium of adult mice. Cancer Res. 41(2): 721-734 1981. [PubMed] [Google Scholar]
- 50.Frith CH, and Chandra M. Incidence, distribution, and morphology of amyloidosis in Charles Rivers CD-1 mice. Toxicol Pathol. 19(2): 123-127 1991. [DOI] [PubMed] [Google Scholar]
- 51.Frith CH, and Ward JM. Color atlas of neoplastic and non-neoplastic lesions in aging mice. Elsevier, Amsterdam, New York, Tokyo. 1988. [Google Scholar]
- 52.Frith CH, Ward JM, Harleman JH, Stromberg PC, Halm S, Inoue T, and Wright JA. Hematopoietic System. In: International classification of rodent tumors in the mouse. Mohr U (ed). Springer, Berlin. 417-451. 2001. [Google Scholar]
- 53.Gaytán M, Bellido C, Morales C, Sánchez-Criado JE, and Gaytán F. Effects of selective inhibition of cyclooxygenase and lipooxygenase pathways in follicle rupture and ovulation in the rat. Reproduction. 132(4): 571-577 2006. [DOI] [PubMed] [Google Scholar]
- 54.Geist SH, and Lullmann-Rauch R. Experimentally induced lipidosis in uterine and vaginal epithelium of rats. Ann Anat. 176(1): 3-9 1994. [DOI] [PubMed] [Google Scholar]
- 55.Genadry R, Parmley T, and Woodruff JD. The origin and clinical behavior of the parovarian tumor. Am J Obstet Gynecol. 129(8): 873-880 1977. [DOI] [PubMed] [Google Scholar]
- 56.Ginty I, and Hoogstraten-Miller S. Perineal swelling in a mouse. Diagnosis: imperforate vagina with secondary mucometra. Lab Anim (NY). 37(5): 196-199 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldman JM, Murr AS, and Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 80(2): 84-97 2007. [DOI] [PubMed] [Google Scholar]
- 58.Goodman DG, and Hildebrandt PK. Adenocarcinoma, endometrium, rat. In: Monographs on pathology of laboratory animals. Genital system. Jones TC, Mohr U, and Hunt RD (eds). Springer, Berlin, Heidelberg, New York, Tokyo. 80-82. 1987a. [Google Scholar]
- 59.Goodman DG, and Hildebrandt PK. Stromal polyp, endometrium, rat. In: Monographs on pathology of laboratory animals. Genital system. Jones TC, Mohr U, and Hunt RD (eds). Springer, Berlin, Heidelberg, New York, Tokyo. 146-148. 1987b. [Google Scholar]
- 60.Goodman DG, and Hildebrandt PK. Papillary adenoma, endometrium, rat. In: Monographs on pathology of laboratory animals. Genital system. Jones TC, Mohr U, and Hunt RD (eds). Springer, Berlin, Heidelberg, New York, Tokyo. 78-80. 1987c. [Google Scholar]
- 61.Goodman DG, and Hildebrandt PK. Squamous cell carcinoma, endometrium/ cervix, rat. In: Monographs on pathology of laboratory animals. Genital system. Jones TC, Mohr U, and Hunt RD (eds). Springer, Berlin, Heidelberg, New York, Tokyo. 82-83. 1987d. [Google Scholar]
- 62.Goodman DG, and Hildebrandt PK. Stromal sarcoma, endometrium, rat. In: Monographs on pathology of laboratory animals. Genital system. Jones TC, Mohr U, and Hunt RD (eds). Springer, Berlin, Heidelberg, New York, Tokyo. 70-72. 1987e. [Google Scholar]
- 63.Gopinath C, and Gibson WA. Mesovarian leiomyomas in the rat. Environ Health Perspect. 73: 107-113 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gopinath C, Prentice DE, and Lewis DJ. The respiratory system. In: Atlas of experimental pathology. MTP Press, Lancaster, Boston, The Hague, Dordrecht. 1987. [Google Scholar]
- 65.Graham CE. Cyclic changes in the squamo-columnar junction of the mouse cervix uteri. Anat Rec. 155(2): 251-260 1966. [DOI] [PubMed] [Google Scholar]
- 66.Greaves P. Female genital tract. In: Histopathology of Preclinical Toxicity Studies. 4th ed. Elsevier, Amsterdam. 667-723. 2012. [Google Scholar]
- 67.Greaves P, Carlton WW, Courtney CL, Ernst H, Halm S, Isaacs KR, Konishi Y, Krinke GJ, Mesfin GM, Rinke M, and Sandusky G. Non-proliferative and proliferative lesions of soft tissues and skeletal muscle in mice. In: Guides for Toxicologic Pathology. STP/ARP/AFIP, Washington DC. 1-18. 2004. [Google Scholar]
- 68.Greaves P, and Faccini JM. Rat histopathology. A glossary for use in toxicity and carcinogenicity studies. Elsevier, Amsterdam, New York, Oxford. 1984. [Google Scholar]
- 69.Greaves P, Faccini JM, and Courtney CL. Proliferative lesions of soft tissues and skeletal muscle in rats. In: Guides for Toxicologic Pathology. STP/ARP/AFIP, Washington DC. 1-14. 1992. [Google Scholar]
- 70.Greaves P, and Seely JC. Non-proliferative lesions of soft tissues and skeletal muscle in rats. In: Guides for Toxicologic Pathology. STP/ARP/AFIP. MST-1. Washington DC. 292-299. 1996. [Google Scholar]
- 71.Gregson RL, Lewis DJ, and Abbott DP. Spontaneous ovarian neoplasms of the laboratory rat. Vet Pathol. 21(3): 292-299. [DOI] [PubMed] [Google Scholar]
- 72.Hao X, Fredrickson TN, Chattopadhyay SK, Han W, Qi CF, Wang Z, Ward JM, Hartley JW, and Morse HC., 3rd The histopathologic and molecular basis for the diagnosis of histiocytic sarcoma and histiocyte-associated lymphoma of mice. Vet Pathol. 47(3): 434-445 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harada M, Kishimoto K, Hagiwara R, Nakashima Y, Kurisu K, and Kawaguchi Y. Infertility observed in female rats treated with N-acetyl-L-cysteine: Histopathological examination of ovarian follicles and recovery of fertility. Congenit Anom (Kyoto). 43(3): 168-176 2003. [DOI] [PubMed] [Google Scholar]
- 74.Hart-Elcock L, Stuart BP, Mueller RE, and Hoss HE. Deciduoma, uterus, rat. In: Monographs on pathology of laboratory animals. Genital system. Jones TC, Mohr U, and Hunt RD (eds). Springer, Berlin, Heidelberg, New York, Tokyo. 140-146. 1987. [Google Scholar]
- 75.Hendry WJ, 3rd, Zheng X, Leavitt WW, Branham WS, and Sheehan DM. Endometrial hyperplasia and apoptosis following neonatal diethylstilbestrol exposure and subsequent estrogen stimulation in both host and transplanted hamster uteri. Cancer Res. 57(10): 1903-1908 1997. [PubMed] [Google Scholar]
- 76.Heywood R, and Wadsworth PF. The experimental toxicology of estrogens. In: Pharmacology of Estrogens (International Encyclopedia of Pharmacology and Therapeutics). Chaudhury RR (ed). Pergamon Press, New York. Vol. Section 106, 68. 1981. [Google Scholar]
- 77.Hirshfield AN, and Midgley AR., Jr Morphometric analysis of follicular development in the rat. Biol Reprod. 19(3): 597-605 1978. [DOI] [PubMed] [Google Scholar]
- 78.Hollander CF, Burek JD, Boorman GA, Snell KC, and Laqueur GL. Granular cell tumors of the central nervous system of rats. Arch Pathol Lab Med. 100(8): 445-447 1976. [PubMed] [Google Scholar]
- 79.Hoyer PB. Can the clock be turned back on ovarian aging? Sci SAGE KE. 2004(10): pe11 2004. [DOI] [PubMed] [Google Scholar]
- 80.Iguchi T, Hirokawa M, and Takasugi N. Occurrence of genital tract abnormalities and bladder hernia in female mice exposed neonatally to tamoxifen. Toxicology. 42(1): 1-11 1986. [DOI] [PubMed] [Google Scholar]
- 81.Ioannidis N. Lipidosis induced in rat uteri by high doses of tamoxifen. Ann Anat. 180(4): 315-319 1998. [DOI] [PubMed] [Google Scholar]
- 82.Ishii S, Ube M, Okada M, Adachi T, Sugimoto J, Inoue Y, Uno Y, and Mutai M. Collaborative work on evaluation of ovarian toxicity. 17) Two- or four-week repeated-dose studies and fertility study of sulpiride in female rats. J Toxicol Sci. 34(Suppl 1): SP175-SP188 2009. [DOI] [PubMed] [Google Scholar]
- 83.Ito A, Mafune N, and Kimura T. Collaborative work on evaluation of ovarian toxicity. 4) Two- or four-week repeated dose study of 4-vinylcyclohexene diepoxide in female rats. J Toxicol Sci. 34(Suppl 1): SP53-SP58 2009. [DOI] [PubMed] [Google Scholar]
- 84.Izumi Y, Watanabe T, Awasaki N, Hikawa K, Minagi T, and Chatani F. Collaborative work on evaluation of ovarian toxicity. 16) Effects of 2 or 4 weeks repeated dose studies and fertility study of Chlorpromazine hydrochloride in rats. J Toxicol Sci. 34(Suppl 1): SP167-SP174 2009. [DOI] [PubMed] [Google Scholar]
- 85.Jarrett RJ. Some endocrine effects of two phenothiazine derivatives, chlorpromazine and perphenazine, in the female mouse. Br Pharmacol Chemother. 20: 497-506 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johnson LD. Lesions of the female genital system caused by diethylstilbestrol in humans, subhuman primates, mice. In: Monographs on pathology of laboratory animals. Genital system. Jones TC, Mohr U, and Hunt RD (eds). Springer, Berlin, Heidelberg, New York, Tokyo. 84-109. 1987. [Google Scholar]
- 87.Jones TC, Hunt RD, and King NW. Veterinary pathology. Williams and Wilkins, Baltimore. 1997. [Google Scholar]
- 88.Kai K, Satoh N, Watanabe A, Shiraiwa K, Sasano H, and Furuhama K. Case report of rat true hermaphroditism: colocalization of oocytes and granulosa and sertoli cells in the germinal cord. Toxicol Pathol. 31(3): 290-294 2003. [DOI] [PubMed] [Google Scholar]
- 89.Kaipia A, and Hsueh AJ. Regulation of ovarian follicle atresia. Annu Rev Physiol. 59: 349-363 1997. [DOI] [PubMed] [Google Scholar]
- 90.Kao SW, Sipes IG, and Hoyer PB. Early effects of ovotoxicity induced by 4-vinylcyclohexene diepoxide in rats and mice. Reprod Toxicol. 13(1): 67-75 1999. [DOI] [PubMed] [Google Scholar]
- 91.Kaspareit-Rittinghausen J, and Deerberg F. Spontaneous malignant mixed mullerian tumors and rhabdomyosarcoma of the uterus in rats. Toxicol Pathol. 18(3): 417-422 1990. [DOI] [PubMed] [Google Scholar]
- 92.Kaufmann W, Bolon B, Bradley A, Butt M, Czasch S, Garman RH, George C, Gröters S, Krinke G, Little P, McKay J, Narama I, Rao D, Shibutani M, and Sills R. Proliferative and nonproliferative lesions of the rat and mouse central and peripheral nervous systems. Toxicol Pathol. 40(4 Suppl): 87S-157S 2012. [DOI] [PubMed] [Google Scholar]
- 93.Kaushic C, Frauendorf E, Rossoll RM, Richardson JM, and Wira CR. Influence of the estrous cycle on the presence and distribution of immune cells in the rat reproductive tract. Am J Reprod Immunol. 39(3): 209-216 1998. [DOI] [PubMed] [Google Scholar]
- 94.Kelly WA, Marler RJ, and Weikel JH. Drug-induced mesovarial leiomyomas in the rat - a review and additional data. J Am Coll Toxicol. 12: 13-22 1993. [Google Scholar]
- 95.Kent HA., Jr Polyovular follicles and multinucleate ova in the ovaries of young mice. Anat Rec. 137: 521-524 1960. [DOI] [PubMed] [Google Scholar]
- 96.Kent HA., Jr Polyovular follicles and multinucleate ova in the ovaries of young rats. Anat Rec. 142: 25-29 1962. [DOI] [PubMed] [Google Scholar]
- 97.Ketani MA, Ketani S, Kaloğlu C, and Güney B. Effects of tamoxifen administration in rat vaginas: an ultrastructural and light microscopy study. Eur J Gynaecol Oncol. 23(6): 557-560 2002. [PubMed] [Google Scholar]
- 98.Kim H, Nakajima T, Hayashi S, Chambon P, Watanabe H, Iguchi T, and Sato T. Effects of diethylstilbestrol on programmed oocyte death and induction of polyovular follicles in neonatal mouse ovaries. Biol Reprod. 81(5): 1002-1009 2009. [DOI] [PubMed] [Google Scholar]
- 99.King A, Gardner L, and Loke YW. Evaluation of oestrogen and progesterone receptor expression in uterine mucosal lymphocytes. Hum Reprod. 11(5): 1079-1082 1996. [DOI] [PubMed] [Google Scholar]
- 100.Kodama T, Yoshida J, Miwa T, Hasegawa D, and Masuyama T. Collaborative work on evaluation of ovarian toxicity. 4) Effects of fertility study of 4-vinylcyclohexene diepoxide in female rats. J Toxicol Sci. 34(Suppl 1): SP59-SP63 2009. [DOI] [PubMed] [Google Scholar]
- 101.Kon Y, Konno A, Hashimoto Y, and Endoh D. Ovarian cysts in MRL/MpJ mice originate from rete ovarii. Anat Histol Embryol. 36(3): 172-178 2007. [DOI] [PubMed] [Google Scholar]
- 102.Krinke G, Naylor DC, Schmid S, Fröhlich E, and Schnider K. The incidence of naturally-occurring primary brain tumours in the laboratory rat. J Comp Path. 95: 175-192 1985. [DOI] [PubMed] [Google Scholar]
- 103.Krinke GJ, Kaufmann W, Mahrous AT, and Schaetti P. Morphologic characterization of spontaneous nervous system tumors in mice and rats. Toxicol Pathol. 28(1): 178-192 2000. [DOI] [PubMed] [Google Scholar]
- 104.Kumazawa T, Nakajima A, Ishiguro T, Jiuxin Z, Tanaharu T, Nishitani H, Inoue Y, Harada S, Hayasaka I, and Tagawa Y. Collaborative work on evaluation of ovarian toxicity. 15) Two- or four-week repeated-dose studies and fertility study of bromocriptine in female rats. J Toxicol Sci. 34(Suppl 1): SP157-SP165 2009. [DOI] [PubMed] [Google Scholar]
- 105.Löseke A, and Spanel-Borowski K. Simple or repeated induction of superovulation: a study on ovulation rates and microvessel corrosion casts in ovaries of golden hamsters. Ann Anat. 178(1): 5-14 1996. [DOI] [PubMed] [Google Scholar]
- 106.Lúllmann-Rauch R, and Reil GH. Fenfluramine-induced ultrastructural alterations in tissues of rats and guinea pigs. Naunyn Schmiedebergs Arch Pharmacol. 285(2): 175-184 1974. [DOI] [PubMed] [Google Scholar]
- 107.Landes C, Heider K, Krinke AL, Krinke GJ, Mahrous AT, and Hess R. Contribution of immunohistochemistry toward the diagnosis of tumors of laboratory rats. Exp Pathol. 40(4): 239-250 1990. [DOI] [PubMed] [Google Scholar]
- 108.Lee S-H, Ichii O, Otsuka S, Elewa YH, Namiki Y, Hashimoto Y, and Kon Y. Ovarian cysts in MRL/MpJ mice are derived from the extraovarian rete: a developmental study. J Anat. 219(6): 743-755 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Leininger JR, and Jokinen MP. Oviduct, uterus and vagina. In: Pathology of the Fischer rat. Reference and atlas. Boorman GA, Eustis SL, Elwell MR, Montgomery CA, Jr, and MacKenzie WF (eds). Academic Press, San Diego, New York, London. 443-459. 1990. [Google Scholar]
- 110.Lemon PG, and Gubareva AV. Tumours of the ovary. In: Pathology of tumours in laboratory animals. Vol. II. Tumours of the mouse. Turusov VS (ed). IARC Scientific Publications No. 23, Lyon. 385-409. 1979. [PubMed] [Google Scholar]
- 111.Lerner LJ, Hilf R, Turkheimer AR, Michel I, and Engel SL. Effects of hormone antagonists on morphological and biochemical changes induced by hormonal steroids in the immature rat uterus. Endocrinology. 78(1): 111-124 1966. [DOI] [PubMed] [Google Scholar]
- 112.Lewis DJ. Ovarian neoplasia in the Sprague-Dawley rat. Environ Health Perspect. 73: 77-90 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li S, and Davis B. Evaluating rodent vaginal and uterine histology in toxicity studies. Birth Defects Res B Dev Reprod Toxicol. 80(3): 246-252 2007. [DOI] [PubMed] [Google Scholar]
- 114.Long GG. Apparent mesonephric duct (rete anlage) origin for cysts and proliferative epithelial lesions in the mouse ovary. Toxicol Pathol. 30(5): 592-598 2002. [DOI] [PubMed] [Google Scholar]
- 115.Long GG, Cohen IR, Gries CL, Young JK, Francis PC, and Capen CC. Proliferative lesions of ovarian granulosa cells and reversible hormonal changes induced in rats by a selective estrogen receptor modulator. Toxicol Pathol. 29(6): 719-726 2001. [DOI] [PubMed] [Google Scholar]
- 116.Maekawa A. Tumours of the ovary. In: Pathology of tumours in laboratory animals. Vol I. Tumours of the rat, 2nd edition. Turusov VS, and Mohr U (eds). IARC Scientific Publications No. 99, Lyon. 473-497. 1990. [PubMed] [Google Scholar]
- 117.Maekawa A. Genital tumors in female rats-influence of chemicals and/or hormones and host factors on their occurrence. J Toxicol Sci. 19(3): 119-132 1994. [DOI] [PubMed] [Google Scholar]
- 118.Maekawa A, and Hayashi Y. Granulosa/theca cell tumor, ovary, rat. In: Monographs on pathology of laboratory animals. Genital system. Jones TC, Mohr U, and Hunt RD (eds). Springer, Berlin, Heidelberg, New York, Tokyo. 15-22. 1987. [Google Scholar]
- 119.Maekawa A, and Maita K. Changes in the uterus and vagina. In: Pathobiology of the Aging Mouse. Mohr U, Dungworth DL, Capen CC, Carlton WW, JP Sundberg JP, and Ward JM (eds). ILSI Press, Washington, DC. 469-480. 1996. [Google Scholar]
- 120.Maekawa A, Maita K, and Harleman JH. Changes in the ovary. In: Pathobiology of the Aging Mouse. Vol 1. Mohr U, Dungworth DL, Capen CC, Carlton WW, JP Sundberg JP, and Ward JM (eds). ILSI Press, Washington, DC. 466. 1996. [Google Scholar]
- 121.Maekawa A, and Mitsumori K. Spontaneous occurrence and chemical induction of neurogenic tumors in rats-influence of host factors and specificity of chemical structure. Crit Rev Toxicol. 20(4): 287-310 1990. [DOI] [PubMed] [Google Scholar]
- 122.Majeed SK, Alison RH, Boorman GA, and Gopinath C. Ovarian yolk sac carcinoma in mice. Vet Pathol. 23(6): 776-778 1986. [DOI] [PubMed] [Google Scholar]
- 123.Markovits JE, and Sahota PS. Aromatase inhibitors prevent spontaneous granular cell tumors in the distal female reproductive tract of Sprague-Dawley rats. Toxicol Pathol. 28(6): 799-801 2000a. [DOI] [PubMed] [Google Scholar]
- 124.Markovits JE, and Sahota PS. Granular cell lesions in the distal female reproductive tract of aged Sprague-Dawley rats. Vet Pathol. 37(5): 439-448 2000b. [DOI] [PubMed] [Google Scholar]
- 125.Mattheij JA, and Swarts HJ. Induction of luteinized unruptured follicles in the rat after injection of luteinizing hormone early in pro-oestrus. Eur J Endocrinol. 132(1): 91-96 1995. [DOI] [PubMed] [Google Scholar]
- 126.McIntyre A, and La Perle KM. Hermaphroditism in 3 chimeric mice. Vet Pathol. 44(2): 249-252 2007. [DOI] [PubMed] [Google Scholar]
- 127.McLachlan JA, Newbold RR, and Bullock BC. Long-term effects on the female mouse genital tract associated with prenatal exposure to diethylstilbestrol. Cancer Res. 40(11): 3988-3999 1980. [PubMed] [Google Scholar]
- 128.Medlock KL, Branham WS, and Sheehan DM. Effects of toremifene on neonatal rat uterine growth and differentiation. Biol Reprod. 56(5): 1239-1244 1997. [DOI] [PubMed] [Google Scholar]
- 129.Mirsky ML, Sivaraman L, Houle C, Potter DM, Chapin RE, and Cappon GD. Histologic and cytologic detection of endocrine and reproductive tract effects of exemestane in female rats treated for up to twenty-eight days. Toxicol Pathol. 39(4): 589-605 2011. [DOI] [PubMed] [Google Scholar]
- 130.Mitsuhashi N, Takahashi T, Nozaki M, Matsumoto H, Sakurai H, Takahashi M, and Niibe H. Establishment and characterization of a rat yolk sac tumor cell line, NMT-1, producing alpha-fetoprotein, with potential for lymphatic metastasis. Jpn J Cancer Res. 84(12): 1287-1291 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Miwa M, Kojima M, Ohtani T, and Tsuji K. [A recessive mutant causing testicular/ovarian teratoma in rats (Tera strain)]. Jikken Dobutsu. 36(2): 205-208 1987. (In Japanese) [DOI] [PubMed] [Google Scholar]
- 132.Mobini Far HR, Agren G, Lindqvist AS, Marmendal M, Fahlke C, and Thiblin I. Administration of the anabolic androgenic steroid nandrolone decanoate to female rats causes alterations in the morphology of their uterus and a reduction in reproductive capacity. Eur J Obstet Gynecol Reprod Biol. 131(2): 189-197 2007. [DOI] [PubMed] [Google Scholar]
- 133.Montgomery CA, and Alison RH. Nonneoplastic lesions of the ovary in Fischer 344 rats and B6C3F1 mice. Environ Health Perspect. 73: 53-75 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Moore AB, He H, Yoshida A, Rico PJ, Haseman JK, and Dixon D. Transforming growth factor-alpha, epidermal growth factor receptor, and PCNA immunoexpression in uterine leiomyosarcomas and leiomyomas in B6C3F1 mice. Exp Toxicol Pathol. 52(3): 195-200 2000. [DOI] [PubMed] [Google Scholar]
- 135.Morgan KT, and Alison RH. Tubular adenoma, ovary, mouse. In: Monographs on pathology of laboratory animals. Genital system. Jones TC, Mohr U, and Hunt RD (eds). Springer, Berlin, Heidelberg New York, Tokyo. 36-41. 1987. [Google Scholar]
- 136.Myers RK, and McGavin MD. Cellular and tissue responses to injury. In: Pathologic Basis of Veterinary Disease. Fourth Ed. McGavin MD, and Zachary JF (eds). Mosby Elsevier, St. Louis. 2007. [Google Scholar]
- 137.Nagaoka T, Takeuchi M, Onodera H, Matsushima Y, Ando-Lu J, and Maekawa A. Sequential observation of spontaneous endometrial adenocarcinoma development in Donryu rats. Toxicol Pathol. 22(3): 261-269 1994. [DOI] [PubMed] [Google Scholar]
- 138.National Toxicology ProgramCarcinogenesis Bioassay of Zearalenone (CAS No. 17924-92-4) in F344/N Rats and B6C3F1 Mice (Feed Study). Natl Toxicol Program Tech Rep Ser. 235: 1-155 1982. [PubMed] [Google Scholar]
- 139.National Toxicology Program NTP Toxicology and Carcinogenesis Studies of Oxazepam (CAS No. 604-75-1) in Swiss-Webster and B6C3F1 Mice (Feed Studies). Natl Toxicol Program Tech Rep Ser. 443: 1-321. 1993. [PubMed]
- 140.Nelson LW, Kelly WA, and Weikel JH, Jr . Mesovarial leiomyomas in rats in a chronic toxicity study of mesuprine hydrochloride. Toxicol Appl Pharmacol. 23(4): 731-737 1972. [DOI] [PubMed] [Google Scholar]
- 141.Newbold RR, Banks EP, Bullock B, and Jefferson WN. Uterine adenocarcinoma in mice treated neonatally with genistein. Cancer Res. 61(11): 4325-4328 2001. [PubMed] [Google Scholar]
- 142.Newbold RR, Bullock BC, and McLachlan JA. Diverticulosis and salpingitis isthmica nodosa (SIN) of the fallopian tube. Estrogen-induced diverticulosis and SIN of the mouse oviduct. Am J Pathol. 117(2): 333-335 1984. [PMC free article] [PubMed] [Google Scholar]
- 143.Newbold RR, Jefferson WN, and Padilla-Banks E. Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod Toxicol. 24(2): 253-258 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Newbold RR, Jefferson WN, and Padilla-Banks E. Prenatal exposure to bisphenol a at environmentally relevant doses adversely affects the murine female reproductive tract later in life. Environ Health Perspect. 117(6): 879-885 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Newbold RR, and McLachlan JA. Vaginal adenosis and adenocarcinoma in mice exposed prenatally or neonatally to diethylstilbestrol. Cancer Res. 42(5): 2003-2011 1982. [PubMed] [Google Scholar]
- 146.Newbold RR, Moore AB, and Dixon D. Characterization of uterine leiomyomas in CD-1 mice following developmental exposure to diethylstilbestrol (DES). Toxicol Pathol. 30(5): 611-616 2002. [DOI] [PubMed] [Google Scholar]
- 147.Nielsen SW, Misdorp W, and McEntee K. Tumours of the ovary. Bull World Health Organ. 53(2-3): 203-215 1976. [PMC free article] [PubMed] [Google Scholar]
- 148.Nomura T, and Kanzaki T. Induction of urogenital anomalies and some tumors in the progeny of mice receiving diethylstilbestrol during pregnancy. Cancer Res. 37(4): 1099-1104 1977. [PubMed] [Google Scholar]
- 149.Nozaki Y, Furubo E, Matsuno T, Fukui R, Kizawa K, Kozaki T, and Sanzen T. Collaborative work on evaluation of ovarian toxicity. 6) Two- or four-week repeated-dose studies and fertility study of cisplatin in female rats. J Toxicol Sci. 34(Suppl 1): SP73-SP81 2009. [DOI] [PubMed] [Google Scholar]
- 150.Nyska A, Pirak M, Shahar A, Scolnik M, and Waner T. Spontaneous uterine granular cell tumour in a Fischer 344 rat. Lab Anim. 25(4): 299-302 1991. [DOI] [PubMed] [Google Scholar]
- 151.Ohtake S, Fukui M, and Hisada S. Collaborative work on evaluation of ovarian toxicity. 1) Effects of 2- or 4-week repeated-dose administration and fertility studies with medroxyprogesterone acetate in female rats. J Toxicol Sci. 34(Suppl 1): SP23-SP29 2009. [DOI] [PubMed] [Google Scholar]
- 152.Pears AGE. Histochemistry, Theoretical and Applied, Vol Two: Analytical Technology. Churchill Livingstone, Edinburgh, London, Melbourne, New York. 1985. [Google Scholar]
- 153.Pedersen T, and Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil. 17(3): 555-557 1968. [DOI] [PubMed] [Google Scholar]
- 154.Peluso JJ. Morphologic and physiologic features of the ovary. In: Mohr U, Dungworth DL, and Capen CC (eds). Pathobiology of the Aging Rat. ILSI Press, Washington DC. 337-349. 1992. [Google Scholar]
- 155.Peluso JJ, and Gordon LR. Nonneoplastic and neoplastic changes in the ovary. In: Pathobiology of the aging rat. Vol 1.Mohr U, Dungworth DL, and Capen CC (eds). ILSI Press, Washington DC. 351-364. 1992. [Google Scholar]
- 156.Picut CA, Aoyama H, Holder JW, Gold LS, Maronpot RR, and Dixon D. Bromoethane, chloroethane and ethylene oxide induced uterine neoplasms in B6C3F1 mice from 2-year NTP inhalation bioassays: pathology and incidence data revisited. Exp Toxicol Pathol. 55(1): 1-9 2003. [DOI] [PubMed] [Google Scholar]
- 157.Picut CA, Remick AK, Asakawa MG, Simons ML, and Parker GA. Histologic features of prepubertal and pubertal reproductive development in female Sprague-Dawley rats. Toxicol Pathol. 42(2): 403-413 2014. [DOI] [PubMed] [Google Scholar]
- 158.Picut CA, Swanson CL, Parker RF, Scully KL, and Parker GA. The metrial gland in the rat and its similarities to granular cell tumors. Toxicol Pathol. 37(4): 474-480 2009. [DOI] [PubMed] [Google Scholar]
- 159.Plowchalk DR, Meadows MJ, and Mattison DR. Reproductive toxicity of cyclophosphamide in the C57BL/6N mouse: 2. Effects on uterine structure and function. Reprod Toxicol. 6(5): 423-429 1992. [DOI] [PubMed] [Google Scholar]
- 160.Putti R, and Varano L. Histological and histochemical modifications of the uterine and vaginal mucosa of the mouse during the oestrus cycle. Basic Appl Histochem. 23(1): 25-37 1979. [PubMed] [Google Scholar]
- 161.Rao GN, Hickman RL, Seilkop SK, and Boorman GA. Utero-ovarian infection in aged B6C3F1 mice. Lab Anim Sci. 37(2): 153-158 1987. [PubMed] [Google Scholar]
- 162.Regan KS, Cline JM, Creasy D, Davis B, Foley GL, Lanning L, Latendresse JR, Makris S, Morton D, Rehm S, Stebbins K. STP Ovary Evaluation Working GroupSTP position paper: ovarian follicular counting in the assessment of rodent reproductive toxicity. Toxicol Pathol. 33(3): 409-412 2005. [DOI] [PubMed] [Google Scholar]
- 163.Rehm S, Dierksen D, and Deerberg F. Spontaneous ovarian tumors in Han:NMRI mice: histologic classification, incidence, and influence of food restriction. J Natl Cancer Inst. 72(6): 1383-1395 1984. [PubMed] [Google Scholar]
- 164.Rehm S, Stanislaus DJ, and Wier PJ. Identification of drug-induced hyper- or hypoprolactinemia in the female rat based on general and reproductive toxicity study parameters. Birth Defects Res B Dev Reprod Toxicol. 80(3): 253-257 2007. [DOI] [PubMed] [Google Scholar]
- 165.Ruben Z, Arceo RJ, Bishop SP, Elwell MR, Kerns WD, Mesfin GM, Sandusky GE, and Van Vleet JF. Proliferative lesions of the heart and vasculature in rats. In: Guides for Toxicologic Pathology. STP/ARP/AFIP CV-1, Washington, DC. 1997. [Google Scholar]
- 166.Sakurada Y, Kudo S, Iwasaki S, Miyata Y, Nishi M, and Masumoto Y. Collaborative work on evaluation of ovarian toxicity. 5) Two- or four-week repeated-dose studies and fertility study of busulfan in female rats. J Toxicol Sci. 34(Suppl 1): SP65-SP72 2009. [DOI] [PubMed] [Google Scholar]
- 167.Sanbuissho A, Yoshida M, Hisada S, Sagami F, Kudo S, Kumazawa T, Ube M, Komatsu S, and Ohno Y. Collaborative work on evaluation of ovarian toxicity by repeated-dose and fertility studies in female rats. J Toxicol Sci. 34(Suppl 1): SP1-SP22 2009. [DOI] [PubMed] [Google Scholar]
- 168.Santos FC, Leite RP, Custódio AM, Carvalho KP, Monteiro-Leal LH, Santos AB, Góes RM, Carvalho HF, and Taboga SR. Testosterone stimulates growth and secretory activity of the female prostate in the adult gerbil (Meriones unguiculatus). Biol Reprod. 75(3): 370-379 2006. [DOI] [PubMed] [Google Scholar]
- 169.Sasahara K, Ando-Lu J, Nishiyama K, Takahashi M, Yoshida M, and Maekawa A. Granular cell foci of the uterus in Donryu rats. J Comp Pathol. 119(2): 195-199 1998. [DOI] [PubMed] [Google Scholar]
- 170.Sass B, and Rehm S. Tumours of the ovary. In: Pathology of tumours in laboratory animals. Vol 2. Tumours of the mouse, 2nd edition. Turusov VS, and Mohr U (eds). IARC Scientific Publications No. 111, Lyon. 493-526. 1994. [PubMed] [Google Scholar]
- 171.Sato M, Shiozawa K, Uesugi T, Hiromatsu R, Fukuda M, Kitaura K, Minami T, and Matsumoto S. Collaborative work on evaluation of ovarian toxicity. 7) Effects of 2- or 4- week repeated dose studies and fertility study of cyclophosphamide in female rats. J Toxicol Sci. 34(Suppl 1): SP83-SP89 2009a. [DOI] [PubMed] [Google Scholar]
- 172.Sato N, Uchida K, Nakajima M, Watanabe A, and Kohira T. Collaborative work on evaluation of ovarian toxicity. 13) Two- or four-week repeated dose studies and fertility study of PPAR alpha/gamma dual agonist in female rats. J Toxicol Sci. 34(Suppl 1): SP137-SP146 2009b. [DOI] [PubMed] [Google Scholar]
- 173.Seaman WJ. Postmortem change in the rat: A histologic characterization. Iowa State University Press, Ames. 1987. [Google Scholar]
- 174.Serov SF, Scully RE, and Sobin LH. International histological classification of tumours No 9. Histological typing of ovarian tumours. WHO, Geneva. 1-56. 1973. [Google Scholar]
- 175.Shibayama H, Kotera T, Shinoda Y, Hanada T, Kajihara T, Ueda M, Tamura H, Ishibashi S, Yamashita Y, and Ochi S. Collaborative work on evaluation of ovarian toxicity. 14) Two- or four-week repeated-dose studies and fertility study of atrazine in female rats. J Toxicol Sci. 34(Suppl 1): SP147-SP155 2009. [DOI] [PubMed] [Google Scholar]
- 176.Shirai M, Sakurai K, Saitoh W, Matsuyama T, Teranishi M, Furukawa T, Sanbuissho A, and Manabe S. Collaborative work on evaluation of ovarian toxicity. 8) Two- or four-week repeated-dose studies and fertility study of Anastrozole in female rats. J Toxicol Sci. 34(Suppl 1): SP91-SP99 2009. [DOI] [PubMed] [Google Scholar]
- 177.Sobis H. Yolk sac carcinoma, rat. In: Monographs on pathology of laboratory animals. Genital system. Jones TC, Mohr U, and Hunt RD (eds). Springer, Berlin, Heidelberg, New York, Tokyo. 127-134. 1987. [Google Scholar]
- 178.Sobis H. Choriocarcinoma, uterus, rat. In: Monographs on pathology of laboratory animals. Genital system. Jones TC, Mohr U, and Hunt RD (eds). Springer, Berlin, Heidelberg, New York, Tokyo. 138-140. 1987a. [Google Scholar]
- 179.Sobis H. Embryonal carcinoma, uterus, rat. In: Monographs on pathology of laboratory animals. Genital system. Jones TC, Mohr U, and Hunt RD (eds). Springer, Berlin, Heidelberg, New York, Tokyo. 134-138. 1987b. [Google Scholar]
- 180.Sobis H. Teratoma, uterus, rat. In: Monographs on pathology of laboratory animals. Genital system. Jones TC, Mohr U, and Hunt RD (eds). Springer, Berlin, Heidelberg, New York, Tokyo. 120-126. 1987c. [Google Scholar]
- 181.Squire RA, Goodman DG, Valerio MG, Fredrickson T, Strandberg JD, Levitt MH, Lingeman CH, Harshbarger JC, and Dawe CJ. Tumors. Female reproductive system. In: Pathology of laboratory animals Vol II. Benirschke K, Garner FM, and Jones TC (eds). Springer, Berlin, Heidelberg, New York, Tokyo. 1172-1194. 1978. [Google Scholar]
- 182.Steinmetz R, Mitchner NA, Grant A, Allen DL, Bigsby RM, and Ben-Jonathan N. The xenoestrogen bisphenol A induces growth, differentiation, and c-fos gene expression in the female reproductive tract. Endocrinology. 139(6): 2741-2747 1998. [DOI] [PubMed] [Google Scholar]
- 183.Stewart HL, Deringer MK, Dunn TB, and Snell KC. Malignant schwannomas of nerve roots, uterus, and epididymis in mice. J Natl Cancer Inst. 53(6): 1749-1758 1974. [PubMed] [Google Scholar]
- 184.Stoica G, Capen CC, and Koestner A. Sertoli’s cell tumor, ovary, rat. In: Monographs on pathology of laboratory animals. Genital system. Jones TC, Mohr U, and Hunt RD (eds). Springer, Berlin, Heidelberg, New York, Tokyo. 30-36. 1987. [Google Scholar]
- 185.Suckow M, Weisbroth SH, and Franklin CL. The Laboratory Rat. Elsevier Academic Press, Burlington. 2006. [Google Scholar]
- 186.Sundberg JP. Imperforate vagina and mucometra in mice. In: JAX NOTES Issue 441, Spring 1990. 1990. [Google Scholar]
- 187.Sundberg JP, and Brown KS. Imperforate vagina and mucometra in inbred laboratory mice. Lab Anim Sci. 44(4): 380-382 1994. [PubMed] [Google Scholar]
- 188.Taketa Y, Yoshida M, Inoue K, Takahashi M, Sakamoto Y, Watanabe G, Taya K, Yamate J, and Nishikawa A. Differential stimulation pathways of progesterone secretion from newly formed corpora lutea in rats treated with ethylene glycol monomethyl ether, sulpiride, or atrazine. Toxicol Sci. 121(2): 267-278 2011. [DOI] [PubMed] [Google Scholar]
- 189.Tamura T, Yokoi R, Okuhara Y, Harada C, Terashima Y, Hayashi M, Nagasawa T, Onozato T, Kobayashi K, Kuroda J, and Kusama H. Collaborative work on evaluation of ovarian toxicity. 2) Two- or four-week repeated dose studies and fertility study of mifepristone in female rats. J Toxicol Sci. 34(Suppl 1): SP31-SP42 2009. [DOI] [PubMed] [Google Scholar]
- 190.Tan OL, Hurst PR, and Fleming JS. Location of inclusion cysts in mouse ovaries in relation to age, pregnancy, and total ovulation number: implications for ovarian cancer? J Pathol. 205(4): 483-490 2005. [DOI] [PubMed] [Google Scholar]
- 191.Telfer E, and Gosden RG. A quantitative cytological study of polyovular follicles in mammalian ovaries with particular reference to the domestic bitch (Canis familiaris). J Reprod Fertil. 81(1): 137-147 1987. [DOI] [PubMed] [Google Scholar]
- 192.Toaff ME, Abramovici A, Sporn J, and Liban E. Selective oocyte degeneration and impaired fertility in rats treated with the aliphatic monoterpene, Citral. J Reprod Fertil. 55(2): 347-352 1979. [DOI] [PubMed] [Google Scholar]
- 193.Tryphonas L, and Buttar HS. Genital tract toxicity of nonoxynol-9 in female rats: temporal development, reversibility and sequelae of the induced lesions. Fundam Appl Toxicol. 2(5): 211-219 1982. [DOI] [PubMed] [Google Scholar]
- 194.Tsubota K, Kushima K, Yamauchi K, Matsuo S, Saegusa T, Ito S, Fujiwara M, Matsumoto M, Nakatsuji S, Seki J, and Oishi Y. Collaborative work on evaluation of ovarian toxicity. 12) Effects of 2- or 4-week repeated dose studies and fertility study of indomethacin in female rats. J Toxicol Sci. 34(Suppl 1): SP129-SP136 2009. [DOI] [PubMed] [Google Scholar]
- 195.Tsujioka S, Ban Y, Wise LD, Tsuchiya T, Sato T, Matsue K, Ikeda T, Sasaki M, and Nishikibe M. Collaborative work on evaluation of ovarian toxicity. 3) Effects of 2- or 4- week repeated-dose toxicity and fertility studies with tamoxifen in female rats. J Toxicol Sci. 34(Suppl 1): SP43-SP51 2009. [DOI] [PubMed] [Google Scholar]
- 196.Tucker MJ. Diseases of the Wistar Rat. Taylor & Francis, London, Bristol. 1997. [Google Scholar]
- 197.Turusov MS, Munoz N, and Dunn TB. Tumours of the vagina and uterus. In: Pathology of Tumours in Laboratory Animals, Vol 2. Tumours of the Mouse. Turusov V, and Mohr U (eds). IARC Scientific Publications. 1994. [PubMed] [Google Scholar]
- 198.Usta U, Inan M, Erbas H, Aydogdu N, Oz Puyan F, and Altaner S. Tissue damage in rat ovaries subjected to torsion and detorsion: effects of L-carnitine and N-acetyl cysteine. Pediatr Surg Int. 24(5): 567-573 2008. [DOI] [PubMed] [Google Scholar]
- 199.van den Brink-Knol H, and van Esch E. Spontaneous malignant mixed Müllerian tumor in a Wistar rat: a case report including immunohistochemistry. Vet Pathol. 47(6): 1105-1110 2010. [DOI] [PubMed] [Google Scholar]
- 200.Veit AC, Painter JT, Miller RA, Hardisty JF, and Dixon D. Characterization of uterine granular cell tumors in B6C3F1 mice: a histomorphologic, immunohistochemical, and ultrastructural study. Vet Pathol. 45(5): 654-662 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Vidal JD, Mirsky ML, Colman K, Whitney KM, and Creasy DM. Reproductive System and Mammary Gland. In: Toxicologic Pathology: Nonclinical Safety Assessment. Sahota P, Popp JA, Hardisty JF, and Gopinath C (eds). CRC Press, Boca Raton. 717-830. 2013. [Google Scholar]
- 202.Vollmer G. Endometrial cancer: experimental models useful for studies on molecular aspects of endometrial cancer and carcinogenesis. Endocr Relat Cancer. 10(1): 23-42 2003. [DOI] [PubMed] [Google Scholar]
- 203.Vrcić H, Horvat B, and Damjanov I. Lectin histochemistry of mouse vagina during the estrous cycle. J Histochem Cytochem. 39(12): 1685-1692 1991. [DOI] [PubMed] [Google Scholar]
- 204.Walker VE, Morgan KT, Zimmerman HM, and Innes JRM. Tumours of the central and peripheral nervous system. In: Pathology of tumours in laboratory animals. Vol 2. Tumours of the mouse, 2nd edition. Turusov VS, and Mohr U (eds). IARC Scientific Publications No. 111, Lyon. 731-776. 1994. [PubMed] [Google Scholar]
- 205.Wang F, Yu B, Yang W, Liu J, Lu J, and Xia X. Polycystic ovary syndrome resembling histopathological alterations in ovaries from prenatal androgenized female rats. J Ovarian Res. 5(1): 15 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ward JM, Goodman DG, Squire RA, Chu KC, and Linhart MS. Neoplastic and nonneoplastic lesions in aging (C57BL/6N x C3H/HeN)F1 (B6C3F1) mice. J Natl Cancer Inst. 63(3): 849-854 1979. [DOI] [PubMed] [Google Scholar]
- 207.Wenzel JG, and Odend’hal S. The mammalian rete ovarii: a literature review. Cornell Vet. 75(3): 411-425 1985. [PubMed] [Google Scholar]
- 208.Westwood FR. The female rat reproductive cycle: a practical histological guide to staging. Toxicol Pathol. 36(3): 375-384 2008. [DOI] [PubMed] [Google Scholar]
- 209.Yoshida M, Katsuda S, Tanimoto T, Asai S, Nakae D, Kurokawa Y, Taya K, and Maekawa A. Induction of different types of uterine adenocarcinomas in Donryu rats due to neonatal exposure to high-dose p-t-octylphenol for different periods. Carcinogenesis. 23(10): 1745-1750 2002. [DOI] [PubMed] [Google Scholar]
- 210.Yoshida M, Sanbuissyo A, Hisada S, Takahashi M, Ohno Y, and Nishikawa A. Morphological characterization of the ovary under normal cycling in rats and its viewpoints of ovarian toxicity detection. J Toxicol Sci. 34(Suppl 1): SP189-SP197 2009. [DOI] [PubMed] [Google Scholar]
- 211.Yoshida M, Shiraki K, Kudoh K, Ando-Lu J, Takahashi M, and Maekawa A. A uterine choriocarcinoma in a virgin Donryu rat. Toxicol Pathol. 25(6): 644-646 1997. [DOI] [PubMed] [Google Scholar]
- 212.Yoshida M, Watanabe G, Shirota M, Maekawa A, and Taya K. Reduction of primordial follicles caused by maternal treatment with busulfan promotes endometrial adenocarcinoma development in donryu rats. J Reprod Dev. 51(6): 707-714 2005. [DOI] [PubMed] [Google Scholar]
- 213.Yuan Y. Structure, cyclic change, and function, vagina and vulva, rat. In: Monographs on Pathology of Laboratory Animals. Genital System. Springer-Verlag, Berlin, Heidelberg, New York. 161-168. 1987. [Google Scholar]
- 214.Yuan Y, and Foley G. Female reproductive system. In: Handbook of Toxicologic Pathology, Vol. 2. Hascheck WM, Rousseaux CG, and Wallig MA (eds). Academic Press, San Diego. 847-894. 2002. [Google Scholar]


