Abstract
Controversy exists as to whether the purine nucleotide cycle is important in normal skeletal muscle function. Patients with disruption of the cycle from a deficiency of AMP deaminase exhibit variable degrees of muscle dysfunction. An animal model was used to examine the effect of inhibition of the purine nucleotide cycle on muscle function. When the compound 5-amino-4-imidazolecarboxamide riboside (AICAriboside) is phosphorylated to the riboside monophosphate in the myocyte it is an inhibitor of adenylosuccinate lyase, one of the enzymes of the purine nucleotide cycle. AICAriboside was infused in 28 mice, and 22 mice received saline. Gastrocnemius muscle function was assessed in situ by recording isometric tension developed during stimulation. The purine nucleotide content of the muscle was measured before and after stimulation. Disruption of the purine nucleotide cycle during muscle stimulation was evidenced by a greater accumulation of adenylosuccinate, the substrate for adenylosuccinate lyase, in the animals receiving AICAriboside (0.60 +/- 0.10 vs. 0.05 +/- 0.01 nmol/mumol total creatine, P less than 0.0001). There was also a larger accumulation of inosine monophosphate in the AICAriboside vs. saline-treated animals at end stimulation (73 +/- 6 vs. 56 +/- 5 nmol/mumol total creatine, P less than 0.03). Inhibition of flux through the cycle was accompanied by muscle dysfunction during stimulation. Total developed tension in the AICAriboside group was 40% less than in the saline group (3,023 +/- 1,170 vs. 5,090 +/- 450 g . s, P less than 0.002). An index of energy production can be obtained by comparing the change in total phosphagen content per unit of developed tension in the two groups. This index indicates that less high energy phosphate compounds were generated in the AICAriboside group, suggesting that interruption of the purine nucleotide cycle interfered with energy production in the muscle. We conclude from these studies that defective energy generation is one mechanism whereby disruption of the purine nucleotide cycle produces muscle dysfunction.
Full text
PDF
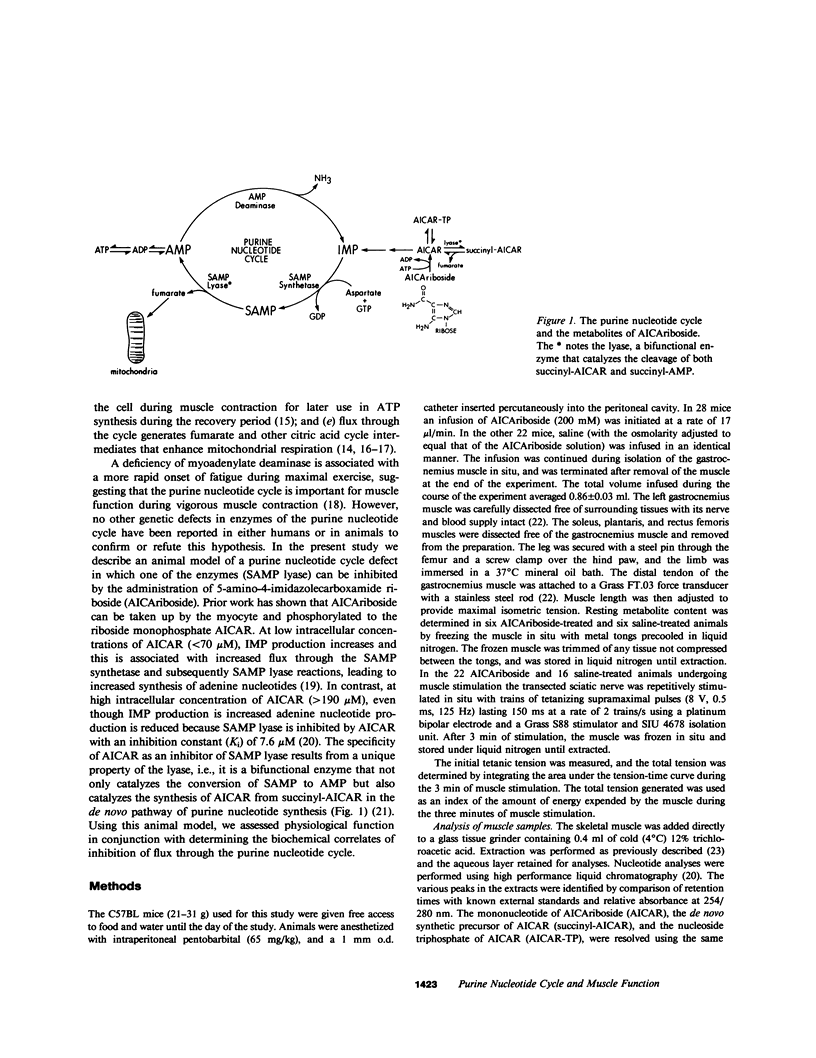
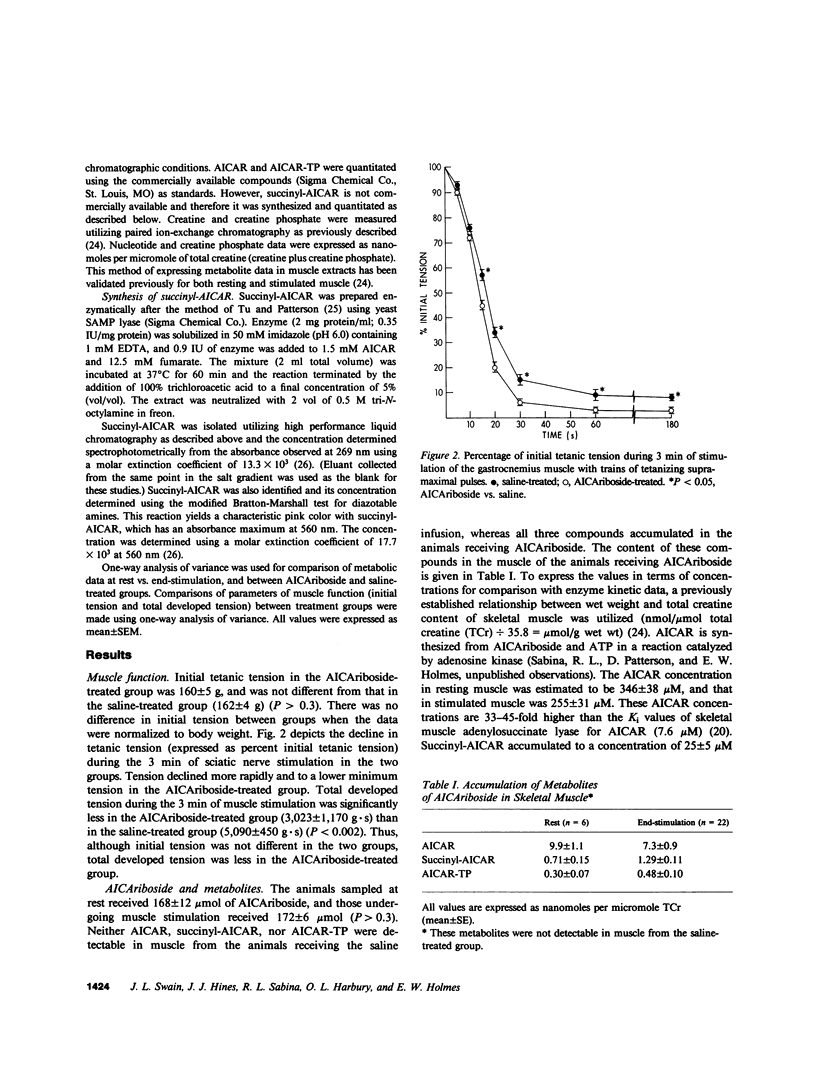



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aragón J. J., Lowenstein J. M. The purine-nucleotide cycle. Comparison of the levels of citric acid cycle intermediates with the operation of the purine nucleotide cycle in rat skeletal muscle during exercise and recovery from exercise. Eur J Biochem. 1980 Sep;110(2):371–377. doi: 10.1111/j.1432-1033.1980.tb04877.x. [DOI] [PubMed] [Google Scholar]
- Aragón J. J., Tornheim K., Goodman M. N., Lowenstein J. M. Replenishment of citric acid cycle intermediates by the purine nucleotide cycle in rat skeletal muscle. Curr Top Cell Regul. 1981;18:131–149. doi: 10.1016/b978-0-12-152818-8.50014-1. [DOI] [PubMed] [Google Scholar]
- Aragón J. J., Tornheim K., Lowenstein J. M. On a possible role of IMP in the regulation of phosphorylase activity in skeletal muscle. FEBS Lett. 1980 Aug 25;117 (Suppl):K56–K64. doi: 10.1016/0014-5793(80)80570-9. [DOI] [PubMed] [Google Scholar]
- Goodman M. N., Lowenstein J. M. The purine nucleotide cycle. Studies of ammonia production by skeletal muscle in situ and in perfused preparations. J Biol Chem. 1977 Jul 25;252(14):5054–5060. [PubMed] [Google Scholar]
- Hettleman B. D., Sabina R. L., Drezner M. K., Holmes E. W., Swain J. L. Defective adenosine triphosphate synthesis. An explanation for skeletal muscle dysfunction in phosphate-deficient mice. J Clin Invest. 1983 Aug;72(2):582–589. doi: 10.1172/JCI111006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka P. W., Mommsen T. P. Protons and anaerobiosis. Science. 1983 Mar 25;219(4591):1391–1397. doi: 10.1126/science.6298937. [DOI] [PubMed] [Google Scholar]
- Infante A. A., Davies R. E. The effect of 2,4-dinitrofluorobenzene on the activity of striated muscle. J Biol Chem. 1965 Oct;240(10):3996–4001. [PubMed] [Google Scholar]
- LUKENS L. N., BUCHANAN J. M. Biosynthesis of the purines. XXIII. The enzymatic synthesis of N-(5-amino-1-ribosyl-4-imidazolylcarbonyl)-L-aspartic acid 5'-phosphate. J Biol Chem. 1959 Jul;234(7):1791–1798. [PubMed] [Google Scholar]
- Lowenstein J. M., Goodman M. N. The purine nucleotide cycle in skeletal muscle. Fed Proc. 1978 Jul;37(9):2308–2312. [PubMed] [Google Scholar]
- Meyer R. A., Dudley G. A., Terjung R. L. Ammonia and IMP in different skeletal muscle fibers after exercise in rats. J Appl Physiol Respir Environ Exerc Physiol. 1980 Dec;49(6):1037–1041. doi: 10.1152/jappl.1980.49.6.1037. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Terjung R. L. AMP deamination and IMP reamination in working skeletal muscle. Am J Physiol. 1980 Jul;239(1):C32–C38. doi: 10.1152/ajpcell.1980.239.1.C32. [DOI] [PubMed] [Google Scholar]
- PURZYCKA J. AMP and adenosine aminohydrolases in rat tissues. Acta Biochim Pol. 1962;9:83–93. [PubMed] [Google Scholar]
- Sabina R. L., Holmes E. W., Becker M. A. The enzymatic synthesis of 5-amino-4-imidazolecarboxamide riboside triphosphate (ZTP). Science. 1984 Mar 16;223(4641):1193–1195. doi: 10.1126/science.6199843. [DOI] [PubMed] [Google Scholar]
- Sabina R. L., Kernstine K. H., Boyd R. L., Holmes E. W., Swain J. L. Metabolism of 5-amino-4-imidazolecarboxamide riboside in cardiac and skeletal muscle. Effects on purine nucleotide synthesis. J Biol Chem. 1982 Sep 10;257(17):10178–10183. [PubMed] [Google Scholar]
- Sabina R. L., Swain J. L., Hines J. J., Holmes E. W. A comparison of methods for quantitation of metabolites in skeletal muscle. J Appl Physiol Respir Environ Exerc Physiol. 1983 Aug;55(2):624–627. doi: 10.1152/jappl.1983.55.2.624. [DOI] [PubMed] [Google Scholar]
- Sabina R. L., Swain J. L., Olanow C. W., Bradley W. G., Fishbein W. N., DiMauro S., Holmes E. W. Myoadenylate deaminase deficiency. Functional and metabolic abnormalities associated with disruption of the purine nucleotide cycle. J Clin Invest. 1984 Mar;73(3):720–730. doi: 10.1172/JCI111265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabina R. L., Swain J. L., Patten B. M., Ashizawa T., O'Brien W. E., Holmes E. W. Disruption of the purine nucleotide cycle. A potential explanation for muscle dysfunction in myoadenylate deaminase deficiency. J Clin Invest. 1980 Dec;66(6):1419–1423. doi: 10.1172/JCI109995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz V., Lowenstein J. M. Purine nucleotide cycle. Evidence for the occurrence of the cycle in brain. J Biol Chem. 1976 Jan 25;251(2):485–492. [PubMed] [Google Scholar]
- Scisłowski P. W., Aleksandrowicz Z., Swierczyński J. Purine nucleotide cycle as a possible anaplerotic process in rat skeletal muscle. Experientia. 1982 Sep 15;38(9):1035–1037. doi: 10.1007/BF01955351. [DOI] [PubMed] [Google Scholar]
- Swain J. L., Hines J. J., Sabina R. L., Holmes E. W. Accelerated repletion of ATP and GTP pools in postischemic canine myocardium using a precursor of purine de novo synthesis. Circ Res. 1982 Jul;51(1):102–105. doi: 10.1161/01.res.51.1.102. [DOI] [PubMed] [Google Scholar]
- Swain J. L., Sabina R. L., McHale P. A., Greenfield J. C., Jr, Holmes E. W. Prolonged myocardial nucleotide depletion after brief ischemia in the open-chest dog. Am J Physiol. 1982 May;242(5):H818–H826. doi: 10.1152/ajpheart.1982.242.5.H818. [DOI] [PubMed] [Google Scholar]
- Tornheim K., Lowenstein J. M. The purine nucleotide cycle. 3. Oscillations in metabolite concentrations during the operation of the cycle in muscle extracts. J Biol Chem. 1973 Apr 25;248(8):2670–2677. [PubMed] [Google Scholar]
- Tornheim K., Lowenstein J. M. The purine nucleotide cycle. Control of phosphofructokinase and glycolytic oscillations in muscle extracts. J Biol Chem. 1975 Aug 25;250(16):6304–6314. [PubMed] [Google Scholar]
- Tornheim K., Lowenstein J. M. The purine nucleotide cycle. IV. Interactions with oscillations of the glycolytic pathway in muscle extracts. J Biol Chem. 1974 May 25;249(10):3241–3247. [PubMed] [Google Scholar]
- Tornheim K., Lowenstein J. M. The purine nucleotide cycle. The production of ammonia from aspartate by extracts of rat skeletal muscle. J Biol Chem. 1972 Jan 10;247(1):162–169. [PubMed] [Google Scholar]
- Tu A. S., Patterson D. Biochemical genetics of Chinese hamster cell mutants with deviant purine metabolism. VI. Enzymatic studies of two mutants unable to convert inosinic acid to adenylic acid. Biochem Genet. 1977 Feb;15(1-2):195–210. doi: 10.1007/BF00484561. [DOI] [PubMed] [Google Scholar]



