Abstract
Objective:
The objective was to investigate the effect of intracanal antibiotic medicaments followed by ethylenediaminetetraacetic acid (EDTA) on the indentation properties and hardness of radicular dentin using a BioDent reference point indenter and a traditional microhardness technique, respectively.
Materials and Methods:
Specimens with intact root canal dentin surfaces and polished radicular dentin specimens were obtained from immature human premolars. Each type of specimen was randomly assigned (n = 10 per group) and treated with either double antibiotic paste (DAP) for 4-week followed by EDTA for 5 min, triple antibiotic paste (TAP) for 4-week followed by EDTA for 5 min, EDTA for 5 min or Hank's balanced salt solution (control). The BioDent reference point indentor and Vickers microhardness tester were used to measure the indentation properties of root canal surfaces and the hardness of polished dentin specimens, respectively. One-way ANOVA followed by Fisher's protected least significant differences were used for statistical analyses.
Results:
Both types of radicular dentin treated with antibiotic pastes and/or EDTA had a significant increase in the majority of indentation properties and a significant reduction in hardness compared to the untreated dentin. Furthermore, treatment of dentin with antibiotic pastes and EDTA caused significant increases in indentation properties and a significant reduction in hardness compared to EDTA-treated dentin. However, the RPI technique was not able to significantly differentiate between DAP + EDTA and TAP + EDTA-treated dentin.
Conclusion:
Dentin treated with antibiotic medicaments followed by EDTA had a significant increase the indentation properties and significantly reduction in hardness of radicular dentin.
Keywords: Double antibiotic paste, endodontic regeneration, ethylenediaminetetraacetic acid, reference point indentation, triple antibiotic paste
INTRODUCTION
Endodontic regeneration of necrotic immature teeth has gained popularity in the last decade.[1] Regeneration techniques require an effective antibacterial regimen to disinfect the necrotic root canal space of immature teeth.[2] Triple antibiotic paste (TAP), a combination of metronidazole, ciprofloxacin, and minocycline, has been the most commonly used medicament to disinfect the root canal during endodontic regeneration.[3,4] A recent review reported that TAP was used in more than half of all published endodontic regeneration cases.[1] However, tooth discoloration has been associated with the minocyclin present in TAP.[4,5,6] Therefore, studies have suggested eliminating the minocycline in a double antibiotic paste (DAP), keeping only metronidazole and ciprofloxacin,[7,8] or substituting the minocycline with another antibiotic, such as clindamyxin, cefaclor, or amoxicillin.[1] The use of ethylenediaminetetraacetic acid (EDTA) has been suggested as a final irrigation step in endodontic regeneration after the removal of the intracanal medicament.[1] Irrigation with EDTA has been proposed to wash out the remaining intracanal medicament and expose the collagen and other organic proteins that may improve the biological environment for endodontic regeneration.[1,9,10,11] Recently published in vitro studies reported a negative effect of intracanal antibiotic medicaments on hardness, resistance to fracture, and indentation properties of radicular dentin.[12,13] EDTA was also suggested to negatively affect the mechanical properties of radicular dentin.[14,15] However, no previous studies have explored the effect of antibiotic medicaments and EDTA on mechanical properties of radicular dentin.
The predentin is a less mineralized collagen-rich dentin layer adjacent to the pulp of immature teeth with a thickness ranging from 10 to 47 μm.[16,17] This layer may play an important role in endodontic regeneration since minimal to no instrumentation is recommended. Exploring the mechanical properties of the root canal surface that includes predentin and its underlying newly formed dentin in immature teeth is challenging due to the required metallographic preparation of dentin to perform any valid traditional mechanical test such as flexural strength, microhardness, or nanohardness. Recently, a new BioDent indenter was suggested to measure the indentation properties of bone, as well as root canal dentin surfaces without the need for any metallographic preparation using a technique known as reference point indentation (RPI).[13,18,19] The RPI approach relies on a reference probe, which stays on the hard tissue surface, and a test probe, which slides relative to the reference probe while indenting the measured hard tissue. Some parameters obtained from BioDent indenter in bone studies were suggested to correlate with and predict energy to fracture obtained through traditional mechanical testing.[18,20] The purpose of this study was to investigate the effect of intracanal antibiotic medicaments used in endodontic regeneration and EDTA on the microindentation properties and hardness of root canal dentin surfaces and polished radicular dentin using the RPI technique and a traditional microhardness technique, respectively.
MATERIALS AND METHODS
BioDent experiment
Specimen preparation
Ten immature human mandibular premolars previously stored in 0.1% thymol at 4°C were used within 6 months of extraction after obtaining Indiana university Institutional Review Board approval to use human teeth (IRB number; 1305011353). The inclusion criteria were the absence of caries, root cracks, or restorations. Furthermore, each immature tooth had to have at least a 1 mm-diameter opening at the apical foramen with two-thirds of the root formed. Each tooth was decoronated, and two 4 mm root dentin cylinders were obtained from the coronal and middle thirds of each root utilizing a water-cooled low-speed diamond saw (Buehler, Lake Bluff, IL, USA). The pulp tissue was extirpated with a barbed broach without touching the root canal surface. Four specimens were obtained from each root by sectioning each cylinder longitudinally across the maximum diameter of the root canal into two specimens without touching the root canal dentin surface.
Treatment procedure
A TAP was prepared by mixing USP-grade antibiotic powders compounded of equal portions of metronidazole, ciprofloxacin, and minocycline (Champs Pharmacy, San Antonio, TX, USA) with sterile water (1 g/mL). A DAP was prepared by mixing USP-grade antibiotic powders compounded of equal portions of metronidazole and ciprofloxacin (Champs) with sterile water (1 g/mL). The four specimens obtained from each root were randomly assigned to three treatment groups (TAP + EDTA, DAP + EDTA, and only EDTA) and one Hank's balanced salt solution (HBSS) control group. Then, specimens were placed in a 2 mL conical sample cup (Fisher Scientific, Florence, KY, USA) containing 0.15 mL of one of the treatment pastes or HBSS for the EDTA and control groups. Thus, the root canal dentin surface of each specimen in the antibiotic groups was covered with 0.3 mm layer of the treatment paste. The containers were stored at 37°C with an approximately 100% relative humidity for 4-week. After that, the specimens were taken out and rinsed thoroughly with sterile water for 30s. Each specimen in the three treatment groups was immersed for 5 min in a magnetic stirrer bath that contained 5 mL of 17% EDTA (Henry Schein, Melville, NY, USA) followed by immersion in sterile water for 5 min before being subjected to RPI testing.
Reference point indentation
RPI was performed using a BioDent Hfc [Figure 1] (Active Life Scientific, Santa Barbara, CA, USA) with Bone Probe 3 (Active Life Scientific) as described in a previous study.[13] In summary, each root dentin specimen was immobilized on an acrylic block, the Bone Probe assembly was passively placed on the root canal surface and three indentations 1 mm apart were measured and averaged from each dentin specimen. Ten indentation cycles of a 5 N indentation force were applied at a frequency of 2 Hz. The most common outcome variables reported in the literature[13,18,19] were included in this study. Those are the first cycle indentation distance (ID) which is the distance indented on the first of the 10 cycles, the ID increase (IDI) which is the increase in an ID from the first to the tenth cycle, and the total ID (total ID) which is the maximum indentation after the end of the tenth cycle. Furthermore, the hardness values were estimated using first cycle ID according to the following equation of cone geometry:
Figure 1.
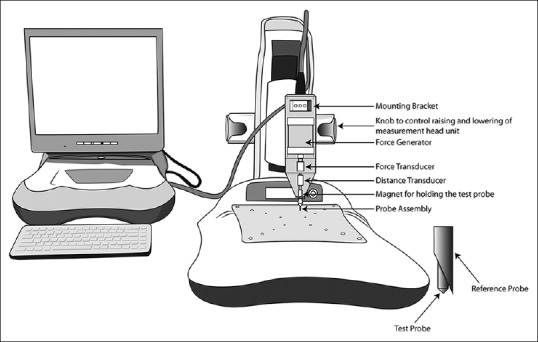
Illustration of the major components of the BioDent reference point indenter. A force generator is used to move a test probe relative to a reference probe and the force in monitored by a force transducer. A distance transducer is used to monitor the movement of the test probe relative to the reference probe. The test probe is attached to the instrument body through a magnet. The reference probe, a modified hypodermic needle, is connected to the instrument body with a Luer lock fitting. The probe assembly (test probe and reference probe) is disposable and replaced after every 150 indentations
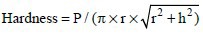
Where P is the constant load applied = 5 Newton; r and h are the first cycle ID values obtained from BioDent.
Microhardness experiment
Specimen preparation
Twenty immature premolars were obtained for the microhardness test. The inclusion criteria were the same as described for the BioDent experiment. Forty 4 × 4 radicular dentin specimens were obtained from the coronal and middle thirds of immature roots. Each specimen was embedded in acrylic resin (Varidur, Buehler, Lake Bluff, IL, USA) with the pulpal surface facing outside. The resulting blocks (10 × 10 × 8 mm) were ground flat and polished according to a standardized protocol described in a previous study.[12] As a final cleaning step, the polished specimens were sonicated in neutral detergent solution (2% Micro 90 liquid soap, International Product Corporation, Burlington, NJ, USA) and rinsed with de-ionized water for 3 min.
Treatment procedure
The specimens were randomly assigned to the three treatment groups and a control group as described in the BioDent experiment. For antibiotic-treated groups, approximately 0.15 mL of TAP or DAP was applied on each embedded specimen to cover each specimen with a 0.3 mm layer of antibiotic paste. A close fit custom made ethylene vinyl acetate dome shaped caps (Soft-Try, Ultradent Products, South Jordan, UT, USA) were used to cover each treated embedded specimen. The EDTA and control groups were immersed in HBSS. All specimens were stored at 37°C with an approximately 100% relative humidity for 4-week. After that, each specimen was rinsed thoroughly with sterile water for 30s. Then, each specimen from the three treatment groups was immersed in 17% EDTA followed by sterile water as described in BioDent experiment before being subjected to Vickers hardness testing.
Vickers microhardness testing
Microhardness measurements were performed using a Vickers Microhardness Tester (Leco, LM247, St. Joseph, MI, USA) on the polished pulpal side of the specimen. Three indentations, spaced 200 μm apart, were made on each specimen using a 50-g load and a 10-s dwell time. The indentations were measured using an optical microscope with a digital camera. The representative hardness value for each specimen was obtained as the mean of the results for the three indentations.
Scanning electron microscopy
One root specimen from each group of the BioDent experiment was randomly selected for scanning electron microscopy (SEM) analysis to confirm the presence of indentations on the intact root canal surfaces after various treatments. Each selected specimen was irrigated with 5 mL of distilled water, sonicated in de-ionized water for 10 min, and desiccated for 48 h. Then, specimens were sputter coated for 2 min with gold/palladium and images were taken with a JEOL 6390 LV scanning electron microscope (Peabody, MA, USA).
Statistical analyses
The effect of treatment type on BioDent parameters and Vickers microhardness values was examined using one-way ANOVA. Pair-wise comparisons were performed using Fisher's protected least significant differences to control the overall significance level at 5%. To satisfy the ANOVA assumptions, the analyses were performed using the natural-log transformed hardness data.
RESULTS
The effect of treatment type on root canal dentin surfaces as well as on polished radicular dentin was significant for indentation properties and microhardness measurements. Figure 2a shows that first-cycle ID of TAP + EDTA and DAP + EDTA-treated dentin were significantly greater than EDTA-treated and control dentin (P < 0.0001). Furthermore, first-cycle ID of the EDTA group was significantly greater than the control dentin (P = 0.0126). However, first-cycle IDs of TAP + EDTA and DAP + EDTA-treated dentin were not significantly different from each other (P = 0.26).
Figure 2.
(a) Mean and standard error (SE) of the first-cycle indentation distance (ID) of root canal dentin exposed to various treatments and a no-treatment control group. Different upper-case letters represent a significant difference. (b) Mean and SE of estimated hardness of intact root canal dentin exposed to various treatments and a no-treatment control group. Different upper-case letters represent a significant difference. (c) Mean and SE of ID increase of intact root canal dentin exposed to various treatments and a no-treatment control group. Different upper-case letters represent a significant difference. (d) Mean and SE of Total ID of intact root canal dentin exposed to various treatments and a no-treatment control group. Different upper-case letters represent a significant difference. (e) Mean and SE of Vickers microhardness of polished radicular dentin exposed to various treatments and a no-treatment control group. Different upper-case letters represent a significant difference
The estimated hardness was significantly higher for the control group compared to EDTA (P = 0.0021), DAP + EDTA (P < 0.0001), and TAP + EDTA (P < 0.0001) treated dentin [Figure 2b]. In addition, the estimated hardness was significantly higher for EDTA treated dentin than for DAP + EDTA (P < 0.0001) and TAP + EDTA (P < 0.0001) treated dentin. However, no significant difference in estimated hardness was observed between DAP + EDTA and TAP + EDTA treated dentin (P = 0.27).
Figure 2c shows that the IDI of TAP + EDTA and DAP + EDTA-treated groups were significantly greater than EDTA-treated and control dentin (P < 0.0001). However, the IDI of TAP + EDTA and DAP + EDTA-treated dentin was not significantly different from each other (P = 0.17), nor were EDTA-treated and control groups (P = 0.63).
Figure 2d shows that the total ID of TAP + EDTA and DAP + EDTA-treated dentin was significantly greater than EDTA-treated and control dentin (P < 0.0001). However, the total ID of TAP + EDTA and DAP + EDTA-treated dentin was not significantly different from each other (P = 0.26). EDTA-treated dentin had significantly greater total ID than the control group (P = 0.020).
Vickers microhardness was significantly higher for the control dentin than for EDTA (P = 0.0039), DAP + EDTA (P < 0.0001), and TAP + EDTA (P < 0.0001) treated dentin [Figure 2e]. In addition, microhardness was higher for EDTA-treated dentin than for DAP + EDTA (P < 0.0001) and TAP + EDTA (P < 0.0001) treated dentin. Furthermore, microhardness was higher for DAP + EDTA than for TAP + EDTA (P < 0.0001) treated dentin. The order of means of the four study groups was the same for both estimated hardness using RPI and Vickers microhardness.
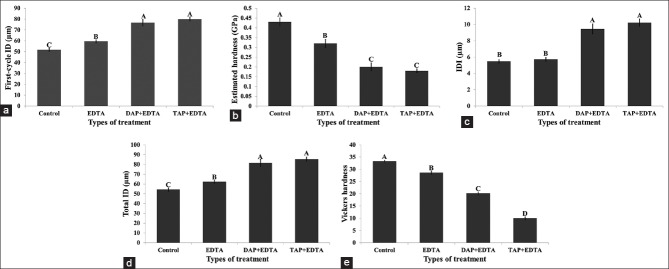
Scanning electron microscopy images taken at ×750 magnification showed the presence of microindentations as well as microcracks created during the repetitive loading. Qualitatively, the micro-indentations were larger and deeper in TAP + EDTA [Figure 3a] and DAP + EDTA-treated dentin [Figure 3b] compared to EDTA-treated dentin [Figure 3c] and untreated control [Figure 3d].
Figure 3.
(a) BioDent indentation in triple antibiotic paste + ethylenediaminetetraacetic acid (TAP + EDTA) treated root canal dentin surface. (b) BioDent indentation in DAP + EDTA treated root canal dentin surface. (c) BioDent indentation in EDTA treated root canal dentin surface. (d) BioDent indentation in untreated root canal dentin surface
DISCUSSION
The use of various indentation techniques to determine the hardness of radicular dentin after exposure to various root canal irrigants and medicaments is a common approach in endodontic research.[14,21] Previous studies have used segments of polished radicular dentin due to the difficulties in performing a standardized indentation test on the actual root canal surface. However, polishing root canal dentin would remove the superficial predentin layer as well as the underlying newly formed inner radicular dentin with higher tubular density. Therefore, trying to study the indentation properties of the intact root canal surface without metallographic manipulation would be more representative of the actual clinical situation. This study proposes a novel direct mechanical test to characterize the intact root canal dentin surface after exposure to antibiotic medicaments and EDTA solution. A traditional microhardness mechanical test was also performed in this study on polished radicular dentin to compare the magnitude of hardness reduction in both approaches. However, no statistical correlation was performed between the two types of hardness due to the different nature of the examined dentin (unpolished intact dentin versus polished dentin). Dentine hardness property has been related to other mechanical properties such as modulus of elasticity, compressive strength and tensile strength.[22] Indeed, a recent investigation observed that a significant reduction in root fracture resistance after 3 months intracanal treatment with DAP and TAP was proceeded by a significant reduction in dentin microhardness after 1-month treatment with the same medicaments.[12]
In the current study, the percentage reduction in estimated dentin hardness from the RPI experiment were significantly higher in DAP + EDTA (53%) and TAP + EDTA (58%) treated dentin compared to EDTA-treated dentin (26%). Furthermore, the percentage reduction in hardness of DAP + EDTA and TAP + EDTA-treated dentin reported in this study was higher than 4-week DAP, and TAP treated dentin reported in a recent study using the same RPI technique, which was 40% and 50%, respectively.[13] This indicates that the use of antibiotic intracanal medicaments followed by EDTA irrigation have an additive effect on the reduction of dentin hardness. This could be explained by the ability of EDTA to chelate calcium and cause superficial dentin demineralization as well as the acidic nature of antibiotic medicaments and their ability to demineralize dentin.[23]
In this study, the percentage reduction in hardness reported using RPI of both EDTA-treated dentin (26%) and DAP + EDTA (53%) treated dentin were higher than the percentage reduction in Vickers hardness, which was 14% for EDTA-treated dentin and 39% for DAP + EDTA treated dentin. This could be explained by the ability of the RPI technique to test the root canal surface dentin which is expected to be weaker due to the high density of dentin tubules in this area and the presence of predentin. On the other hand, the percentage reduction in hardness reported using RPI of TAP + EDTA (58%) treated dentin were lower than the percentage reduction in Vickers microhardness using a traditional experiment, which was 70% for TAP + EDTA-treated dentin. The inability of the RPI technique to detect more reduction in microhardness for TAP + EDTA treated dentin compared to the Vickers microhardness technique could be explained by the severe dentin erosive effect caused by the highly acidic nature of TAP. A recent study has shown that 4-week dentin treatment with TAP followed by EDTA caused an average surface loss of 67 μm.[24] The estimated hardness values obtained from first-cycle ID depends mainly on the depth of penetration of the test probe into dentin in relative to the reference probe. The presence of surface loss on the measured surface after treatment may greatly decrease the first cycle ID and thus affect the magnitude of the estimated hardness. This might be considered as one of the limitations of the RPI technique when used to measure the effect of solutions/medicaments with high erosive potential on a hard tissue. The above reason could also explain the inability of the RPI technique to significantly differentiate between DAP + EDTA and TAP + EDTA treated dentin. A significant difference was detected between DAP + EDTA and TAP + EDTA treated dentin when Vickers microhardness was used. The significantly lower Vickers microhardness of TAP compared to DAP in the current study could be explained by the relatively low pH of TAP compared to DAP[23] as well as the presence of minocyclin in TAP, which was suggested to chelate calcium and demineralize dental hard tissues.[25]
Additional indentation properties are obtained from the RPI technique by applying repetitive loading.[26] Therefore, 10 indentation cycles were used in the current study as an attempt to obtain more quantitative variables regarding the mechanical behavior of the treated dentin. The total ID variable reported in this study showed the same significant trends to first cycle ID and the estimated hardness, which might be considered as another reliable variable for differentiating between various dentin treatments. The IDI of TAP + EDTA and DAP + EDTA-treated dentin was significantly greater than EDTA-treated and control dentin. Recent studies on bone have shown a significant correlation between IDI and modulus of toughness estimated by three-point bending mechanical tests[18] as well as crack growth toughness.[20] However, IDI of TAP + EDTA and DAP + EDTA treated dentin were not significantly different from each other, nor were EDTA-treated and control dentin. This indicates that the IDI variable was less sensitive in detecting significant differences after various dentin treatments compared to first cycle ID and total ID.
In the current study, 1 g/mL of TAP and DAP were used since these are the concentration required to create a pasty consistency that can be applied into the infected immature root canal.[27,28] Some concerns have been raised regarding the use of these relatively high concentrations during endodontic regeneration. These concerns include the negative effects of TAP and DAP on the mechanical properties of radicular dentin[12,13] and direct or indirect cytotoxic effects of TAP and DAP on human stem cells of the apical papilla[27,29] and human dental pulp cells.[30] The American Association of Endodontists has recommended the use of low concentrations of the antibiotic medicaments.[31] However, nondiluted TAP is still the most commonly used medicaments in the majority of the recently published clinical studies[6,32] and there is no clinical evidence supporting the efficiency of low concentrations of antibiotic medicaments in endodontic regeneration. The use of lower concentrations of these antibiotic medicaments might be a good approach to minimize their negative effect on the mechanical properties of radicular dentin. However, further studies are required to support this suggestion.
CONCLUSION
Both polished and intact root canal dentin treated with antibiotic pastes followed by EDTA had significant increases in indentation properties and significant reduction in microhardness compared to untreated control dentin and EDTA-treated dentin. However, the RPI technique was not able to significantly differentiate between DAP + EDTA- and TAP + EDTA-treated dentin. The currently used clinical endodontic regeneration protocols that include intracanal application of TAP followed by EDTA irrigation may cause a significant reduction in hardness of radicular dentin ranging from 58% to 70%.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared
REFERENCES
- 1.Diogenes A, Henry MA, Teixeira FB, Hargreaves KM. An update on clinical regenerative endodontics. Endod Topics. 2013;28:2–23. [Google Scholar]
- 2.Fouad AF, Nosrat A. Pulp regeneration in previously infected root canal space. Endod Topics. 2013;28:24–37. [Google Scholar]
- 3.Lovelace TW, Henry MA, Hargreaves KM, Diogenes A. Evaluation of the delivery of mesenchymal stem cells into the root canal space of necrotic immature teeth after clinical regenerative endodontic procedure. J Endod. 2011;37:133–8. doi: 10.1016/j.joen.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Miller EK, Lee JY, Tawil PZ, Teixeira FB, Vann WF., Jr Emerging therapies for the management of traumatized immature permanent incisors. Pediatr Dent. 2012;34:66–9. [PubMed] [Google Scholar]
- 5.Petrino JA, Boda KK, Shambarger S, Bowles WR, McClanahan SB. Challenges in regenerative endodontics: A case series. J Endod. 2010;36:536–41. doi: 10.1016/j.joen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Nagata JY, Gomes BP, Rocha Lima, TF, Murakami LS, de Faria DE, Campos GR, et al. Traumatized immature teeth treated with 2 protocols of pulp revascularization. J Endod. 2014;40:606–12. doi: 10.1016/j.joen.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 7.Iwaya SI, Ikawa M, Kubota M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent Traumatol. 2001;17:185–7. doi: 10.1034/j.1600-9657.2001.017004185.x. [DOI] [PubMed] [Google Scholar]
- 8.Trope M. Treatment of the immature tooth with a non-vital pulp and apical periodontitis. Dent Clin North Am. 2010;54:313–24. doi: 10.1016/j.cden.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Huang X, Zhang J, Huang C, Wang Y, Pei D. Effect of intracanal dentine wettability on human dental pulp cell attachment. Int Endod J. 2012;45:346–53. doi: 10.1111/j.1365-2591.2011.01982.x. [DOI] [PubMed] [Google Scholar]
- 10.Galler KM, D’Souza RN, Federlin M, Cavender AC, Hartgerink JD, Hecker S, et al. Dentin conditioning codetermines cell fate in regenerative endodontics. J Endod. 2011;37:1536–41. doi: 10.1016/j.joen.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Trevino EG, Patwardhan AN, Henry MA, Perry G, Dybdal-Hargreaves N, Hargreaves KM, et al. Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet-rich plasma scaffold in human root tips. J Endod. 2011;37:1109–15. doi: 10.1016/j.joen.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Yassen GH, Vail MM, Chu TG, Platt JA. The effect of medicaments used in endodontic regeneration on root fracture and microhardness of radicular dentine. Int Endod J. 2013;46:688–95. doi: 10.1111/iej.12046. [DOI] [PubMed] [Google Scholar]
- 13.Yassen GH, Chu TM, Gallant MA, Allen MR, Vail MM, Murray PE, et al. A novel approach to evaluate the effect of medicaments used in endodontic regeneration on root canal surface indentation. Clin Oral Investig. 2014;18:1569–75. doi: 10.1007/s00784-013-1125-x. [DOI] [PubMed] [Google Scholar]
- 14.De-Deus G, Paciornik S, Mauricio MH. Evaluation of the effect of EDTA, EDTAC and citric acid on the microhardness of root dentine. Int Endod J. 2006;39:401–7. doi: 10.1111/j.1365-2591.2006.01094.x. [DOI] [PubMed] [Google Scholar]
- 15.Uzunoglu E, Aktemur S, Uyanik MO, Durmaz V, Nagas E. Effect of ethylenediaminetetraacetic acid on root fracture with respect to concentration at different time exposures. J Endod. 2012;38:1110–3. doi: 10.1016/j.joen.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 16.Couve E. Changes in the predentin thickness and mineralization front configuration in developing human premolars. Acta Anat (Basel) 1987;130:324–8. doi: 10.1159/000146464. [DOI] [PubMed] [Google Scholar]
- 17.Butler WT, Brunn JC, Qin C. Dentin extracellular matrix (ECM) proteins: Comparison to bone ECM and contribution to dynamics of dentinogenesis. Connect Tissue Res. 2003;44(Suppl 1):171–8. [PubMed] [Google Scholar]
- 18.Gallant MA, Brown DM, Organ JM, Allen MR, Burr DB. Reference-point indentation correlates with bone toughness assessed using whole-bone traditional mechanical testing. Bone. 2013;53:301–5. doi: 10.1016/j.bone.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammond MA, Gallant MA, Burr DB, Wallace JM. Nanoscale changes in collagen are reflected in physical and mechanical properties of bone at the microscale in diabetic rats. Bone. 2014;60:26–32. doi: 10.1016/j.bone.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diez-Perez A, Güerri R, Nogues X, Cáceres E, Peña MJ, Mellibovsky L, et al. Microindentation for in vivo measurement of bone tissue mechanical properties in humans. J Bone Miner Res. 2010;25:1877–85. doi: 10.1002/jbmr.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Twati WA, Wood DJ, Liskiewicz TW, Willmott NS, Duggal MS. An evaluation of the effect of non-setting calcium hydroxide on human dentine: A pilot study. Eur Arch Paediatr Dent. 2009;10:104–9. doi: 10.1007/BF03321610. [DOI] [PubMed] [Google Scholar]
- 22.Kinney JH, Marshall SJ, Marshall GW. The mechanical properties of human dentin: A critical review and re-evaluation of the dental literature. Crit Rev Oral Biol Med. 2003;14:13–29. doi: 10.1177/154411130301400103. [DOI] [PubMed] [Google Scholar]
- 23.Yassen GH, Chu TM, Eckert G, Platt JA. Effect of medicaments used in endodontic regeneration technique on the chemical structure of human immature radicular dentin: An in vitro study. J Endod. 2013;39:269–73. doi: 10.1016/j.joen.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Andrew NN. Thesis. Indiana University; 2014. Effect of Two Concentrations of Triple Antibiotic Paste and Ethylenediaminetetraacetic Acid on Surface Loss and Surface Roughness of Radicular Dentin. [Google Scholar]
- 25.Maruyama H, Aoki A, Sasaki KM, Takasaki AA, Iwasaki K, Ichinose S, et al. The effect of chemical and/or mechanical conditioning on the Er: YAG laser-treated root cementum: Analysis of surface morphology and periodontal ligament fibroblast attachment. Lasers Surg Med. 2008;40:211–22. doi: 10.1002/lsm.20609. [DOI] [PubMed] [Google Scholar]
- 26.Randall C, Mathews P, Yurtsev E, Sahar N, Kohn D, Hansma P. The bone diagnostic instrument III: Testing mouse femora. Rev Sci Instrum. 2009;80:065108. doi: 10.1063/1.3147383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruparel NB, Teixeira FB, Ferraz CC, Diogenes A. Direct effect of intracanal medicaments on survival of stem cells of the apical papilla. J Endod. 2012;38:1372–5. doi: 10.1016/j.joen.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 28.Berkhoff JA, Chen PB, Teixeira FB, Diogenes A. Evaluation of triple antibiotic paste removal by different irrigation procedures. J Endod. 2014;40:1172–7. doi: 10.1016/j.joen.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 29.Althumairy RI, Teixeira FB, Diogenes A. Effect of dentin conditioning with intracanal medicaments on survival of stem cells of apical papilla. J Endod. 2014;40:521–5. doi: 10.1016/j.joen.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Labban N, Yassen GH, Windsor LJ, Platt JA. The direct cytotoxic effects of medicaments used in endodontic regeneration on human dental pulp cells. Dent Traumatol. 2014 doi: 10.1111/edt.12108. Doi: 10.1111/edt.12108. [DOI] [PubMed] [Google Scholar]
- 31.American Association of Endodontists. Considerations for Regenerative Procedures. 2013. [Last accessed on 2014 Aug 12]. Available from: http://www.aae.org/clinical-resources/regenerative-endodontics/considerations-for-regenerative-procedures.aspx .
- 32.Nagy MM, Tawfik HE, Hashem AA, Abu-Seida AM. Regenerative potential of immature permanent teeth with necrotic pulps after different regenerative protocols. J Endod. 2014;40:192–8. doi: 10.1016/j.joen.2013.10.027. [DOI] [PubMed] [Google Scholar]


