Abstract
Objective:
To evaluate the surface roughness of acrylic resin submitted to chemical disinfection via 1% sodium hypochlorite (NaClO) or 1% peracetic acid (C2H4O3).
Materials and Methods:
The disc-shaped resin specimens (30 mm diameter ×4 mm height) were polymerized by heated water using two cycles (short cycle: 1 h at 74°C and 30 min at 100°C; conventional long cycle: 9 h at 74°C). The release of substances by these specimens in water solution was also quantified. Specimens were fabricated, divided into four groups (n = 10) depending on the polymerization time and disinfectant. After polishing, the specimens were stored in distilled deionized water. Specimens were immersed in 1% NaClO or 1% C2H4O3 for 30 min, and then were immersed in distilled deionized water for 20 min. The release of C2H4O3 and NaClO was measured via visual colorimetric analysis. Roughness was measured before and after disinfection. Roughness data were subjected to two-way ANOVA and Tukey's test.
Results:
There was no interaction between polymerization time and disinfectant in influencing the average surface roughness (Ra, P = 0.957). Considering these factors independently, there were significant differences between short and conventional long cycles (P = 0.012), but no significant difference between the disinfectants hypochlorite and C2H4O3 (P = 0.366). Visual colorimetric analysis did not detect release of substances.
Conclusion:
It was concluded that there was the difference in surface roughness between short and conventional long cycles, and disinfection at acrylic resins polymerized by heated water using a short cycle modified the properties of roughness.
Keywords: Acrylic resins, dental waste, disinfection, polymerization
INTRODUCTION
For over 60 years, denture bases have been made with acrylic resin, mainly polymethyl methacrylate.[1] A typical fabrication procedure in a denture laboratory involves exothermic chemical polymerization, initiated by light and heat (supplied by heated water), microwave energy, or chemical initiators. The molded acrylic resin is then subjected to a polymerization cycle. A conventional polymerization cycle involves placing a sample in a low-temperature water bath for several hours; for example, the heat-polymerization of acrylic resin, usually, requires 9 h at 74°C. However, it has been reported that a total polymerization time shorter than 2 h is widely preferred over the longer polymerization cycles.[2]
During use, acrylic resins for denture bases have to retain their mechanical and physical properties, be impermeable to oral fluids and resist the bacterial action and growth.[3] The roughness of a resin is very important to prevent biofilm adhesion and staining, which in turn decreases microbial colonization.[4] Polishing of dental bases should provide a smooth and homogenous surface, to improve the denture cleaning and the patient's comfort. However, an unsatisfactory denture cleaning procedure will not efficiently remove the microorganisms entrapped in micro pits and micro porosities of the denture surface.[5]
Transmission of pathogenic microorganisms is an important issue for dental health-care workers.[6] To maintain oral health in the wearers of dentures, the methods used to clean dentures should remove or kill microorganisms and should not cause surface damage to the denture base or oral soft tissue.[5] Guidelines of the American Dental Association and Centers for Disease Control and Prevention recommend that dental prostheses should be disinfected before being sent to the laboratory and before delivery to the patient.[7] To eliminate cross-contamination, all prostheses and dental appliances should be properly disinfected in both the dental office and laboratory and before being inserted intraorally.[8]
Acrylic resins absorb water and disinfecting solutions. These solutions can later be released in the saliva.[9] The disinfectants should be effective in inactivating microorganisms, and have no adverse effects on the denture materials. Common disinfectants used in dental offices are sodium hypochlorite (NaClO)[10] and peracetic acid (C2H4O3). NaClO has been commonly used for disinfecting dentures that are based on acrylic resin.[11,12]
Peracetic acid is a strong chemical disinfectant with a broad antimicrobial spectrum. It is formed from the chemical reaction of acetic acid (CH3 COOH) with hydrogen peroxide (H2O2) in aqueous solution, or by the reaction of tetraacetylethylenediamine with alkaline hydrogen peroxide solution.[13] C2H4O3 is effective for disinfecting acrylic resin, sterilizing dental equipment, demineralizing root canals, and removing smear layers.[14,15]
Thus, the aim of this in vitro study was to evaluate the surface roughness of acrylic resin polymerized by heated water using either the short cycle or the conventional long cycle, and submitted to chemical disinfection with 1% NaClO or 1% C2H4O3. The release of substances by these specimens in water solution was also quantified. The null hypotheses tested were as follows: (1) That the surface roughness of acrylic resin polymerized by heated water is not affected by the polymerization cycle or the disinfectant solution and (2) that the release of substances from the resin is not affected by disinfectant solution.
MATERIALS AND METHODS
A circular metal matrix, with a central opening of 30 mm diameter ×4 mm height, was used to make wax patterns (Wilson; Polidental Manufacturing and Trade Ltd., São Paulo, Brazil). These wax patterns were placed in flasks with type III plaster (Herodent; Vigodent, Petrópolis, Brazil), in a ratio of 100 g powder to 30 ml of water, following the respective manufacturer instructions, vacuum spatulated (Multivac 4; Degussa, Frankfurt am Main, Germany) for 30 s, and poured with mechanical vibration to minimize the occurrence of porosity. The samples were coated with a thin layer of sodium alginate (Cel-Lac; SS White Dental Products, Rio de Janeiro, Brazil). There was used a heat-polymerized acrylic resin Clássico (Classico Ind. e Com. Ltda., São Paulo, Brazil), which is widely used in prosthetics laboratories. Heat-polymerization was performed in a thermostatically controlled water bath (Polimer 180; Zhermack S.p.A, Rovigo, Italy) with two different polymerization cycles, where the short cycle consists of 1 h 74°C, followed by 30 min of terminal boiling at 100°C, and the long cycle consists of 9 h at 74°C.
After resin polymerization, the flasks were cooled at room temperature (26 ± 2°C) for 2 h before opening. The disc-shaped resin specimens were then removed from the flasks. Forty specimens were fabricated, divided into four groups (n = 10) depending on the polymerization time (short cycle versus conventional long cycle) and on the disinfectant used (C2H4O3 or NaClO). Excess acrylic resin was removed with tungsten steel burs #1508 (Edenta, Schweiz, Switzerland) at low speed. The samples were additionally hand smoothed with #320-grit silicon carbide paper (Norton Manufacturing and Trade Ltd., Guarulhos, Brazil) using water as a coolant. Further polishing was performed with #400 and 600-grift silicon carbide papers (Norton Manufacturing and Trade Ltd.). Final polishing was done with a horizontal machine (Struers DPU-10; Panambra, São Paulo, Brazil) using a rag wheel with polishing pastes (pumice/water followed by zinc oxide/water). Specimens were stored in distilled deionized water at room temperature for 7 days before disinfection.
The specimens were numbered and washed under distilled deionized water and dried with absorbent paper. Then, the initial roughness, before disinfection, was measured. To measure the average surface roughness (Ra) of the specimens, a surface roughness tester (SJ-400; Mitutouo, Kawasaki-Shi, Japan) was used at a speed of 0.05 mm/s speed, with a length of 1.25 mm and a cut-off of 0.25 mm. Three measurements in different directions with an angle of 120° among them were recorded, and the Ra was determined for each specimen. Afterwards, the specimens were randomly divided into two groups according to the polymerization time (short cycle and conventional long cycle). Each group was further divided into two subgroups with according to the disinfectant used (1% C2H4O3 or 1% NaClO). The roughness was measured again after disinfection.
The specimens were immersed in 1% NaClO (Solução de Milton; Asfer, São Caetano do Sul, Brazil) or 1% C2H4O3 (Peresal; Ecolab Deutschland GmbH, Düsseldorf, Germany) for 30 min. After the 30 min period of immersion, the specimens were removed from the solutions and washed for 1 min in running water. Each specimen was placed in a receptacle containing 30 ml of distilled deionized water for 20 min.
After soaking the samples in water for 20 min, visual colorimetric analysis on 25 ml of solution was performed to determine the amount of C2H4O3 released, or on 1 ml of solution to determine the amount of NaClO released. The colorimetric analysis for C2H4O3 was performed using the C2H4O3 CHEMets Kit (CHEMets, Midland, USA) with visual reading in the measurement range: 0-1 and 1-5 ppm. The colorimetric analysis of hypochlorite was performed using the Hypochlorite CHEMets Kit (CHEMets) with visual reading in the measurement range: 0-1.55% NaOCl. Both kits use di-1,4-phenylenediamine, at pH 5.5-6.5, to form a pink complex with either C2H4O3 or hypochlorite.
Statistical analysis
Statistical analysis was performed with the Minitab 16 for Windows 8 (Minitab Inc., State College, USA).
The average values of surface roughness (Ra) were subjected to the D’Agostino-Pearson test for normality; and then to Student's t-test for to compare the control groups (without disinfection) with experimental groups (with disinfected) at 5% significance levels; and two-way ANOVA (polymerization time × disinfectant) and the means were compared by Tukey's test at 5% significance levels.
The color of ampoules containing the samples was compared into a color chart, following the manufacturer's guidelines. Readings were made by three raters who were unaware of the purpose of the study.
RESULTS
On comparing, the control groups with the disinfected groups, statistically higher roughness was only observed in samples that had been prepared by a short cycle [Table 1].
Table 1.
Mean and SD of surface roughness (Ra, μm) for groups with or without disinfection
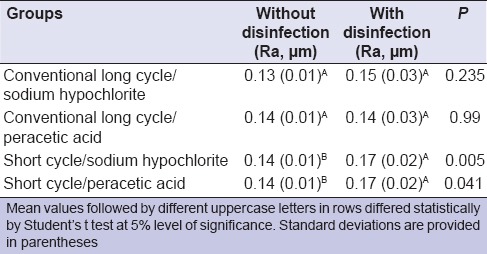
Interaction between polymerization time and disinfectant was not evident in the Ra (polymerization time × disinfectant, P = 0.957). Considering each factor independently, polymerization time significantly influenced the surface roughness (polymerization time factor, P = 0.012) [Table 2], but the type of disinfectant did not (disinfectant factor, P = 0.366) [Table 3].
Table 2.
Mean values of surface roughness (Ra, μm) for different polymerization times

Table 3.
Mean values of surface roughness (Ra, μm) for different disinfectants

The visual colorimetric analysis of water specimens from each disinfectant revealed no detectable release of NaClO or C2H4O3 for different polymerization times.
DISCUSSION
Polishing of denture surfaces removes irregularities that are unavoidably introduced during the construction of the appliance and reduces the adherence of microorganisms, food, and other debris to the denture surface.[3,4,13] Complete immersion of dentures in denture cleansers may roughen the surface of denture base resins. A previous report indicates that higher numbers of Candida albicans were observed on rough acrylic resins compared with smooth resins.[8] A smooth surface is thus essential to promote effective disinfection of dentures. The critical threshold surface roughness for bacterial adhesion is 0.2 μm.[16]
In this study [Table 1], after the polishing agents were used to remove excess acrylic resin, the critical surface roughness of the control was 0.17 μm. However, when the samples were polymerized via the short cycle, Ra values were statistically higher for disinfected specimens when compared to the nondisinfected specimens. In this study, Ra represents the arithmetic mean of all the roughness values measured within a given area on the surface.[13,17] Therefore, being the most indicated value.[13,17]
Porosity of the denture base resin depends on the material and polymerization method used, and it is a complex phenomenon with a multifactorial origin. Controlling the temperature during the polymerization process is important to prevent the absorption of disinfectant substances. Heat-polymerization is the most widely used method for acrylic resin denture base fabrication and usually involves immersion in a heated water bath for several hours, typically 9 h at 74°C. This method allows the sample to continuously absorb water, which can act as a plasticizer, reducing the glass transition temperature and influencing other mechanical properties.[18] It has been reported that a total polymerization time shorter than 2 h is widely preferred over the long polymerization cycle in clinical use.[2] However, the present study shows that the polymerization time affected the Ra after disinfection [Table 2].
Microorganisms can remain on the surface of a dental prosthesis after cleaning, especially if the dentures have surface irregularities, which act as a reservoir of infection.[8] A rough surface may promote biofilm accumulation. Therefore, acrylic resin used for denture bases must be thoroughly finished and polished to maintain ideal surface characteristics even under the action of disinfectants such as NaClO and C2H4O3. NaClO and C2H4O3 diluted in water are commonly used as denture cleansers and included in several regimens for complete denture hygiene.[13] This method is effective in reducing Candida albicans in patients with denture-induced stomatitis, depending on the concentration and the immersion time. Both NaClO and C2H4O3 are recommended by the American Dental Association, where NaClO can be used to disinfect acrylic resin dentures and C2H4O3 for cleaning and disinfecting, as alternatives to the chemical disinfectant glutaraldehyde, which can be highly toxic.[19] C2H4O3 is a more powerful oxidant than chloride or chloride dioxide, causing the rupture of the cell membrane by means of protein denaturing.[13] Moreover, C2H4O3 can disinfect specimens contaminated with Bacillus subtilis and Bacillus stearothermophilus.[14] Testing with these microorganisms proves the antimicrobial efficacy of C2H4O3-based disinfectant because these microorganisms are routinely used as controls to test the sterilizing capacity of ovens (B. subtilis) and autoclaves (B. stearothermophilus). If a physical or chemical agent is able to kill B. subtilis and B. stearothermophilus, the assumption is that the agent can destroy any other microorganism under the same temperature and time conditions.[20] Thus, though the disinfectants in this study did not produce a statistical difference in roughness [Table 3], the disinfectant of choice appears to be C2H4O3 based on these literature reports.
Inflammatory reactions in the oral mucosa are commonly observed in patients that use their dentures continuously. The acrylic polymers can release several compounds, including residual monomer, methyl methacrylate and other additives, such as hydroquinone, benzoyl peroxide, N, N-dimethyl-p-toluidine, and formaldehyde (formed from residual monomer). Upon release, these compounds diffuse into the saliva and come into contact with the oral mucosa, causing flushing and a burning sensation in the adjacent areas.[21] Chemical disinfectants that have become absorbed into the resin can also be released and cause similar irritation to the oral mucosa. In order to choose the correct disinfectant, factors to be considered include cost, risk of toxicity to the patient or dental professional, potential instrument damage, stability, antimicrobial activity, and ability to inactivate the microorganisms rapidly.[22] In the present study, visual colorimetric analysis showed that the specimens that received chemical polishing did not release C2H4O3 or NaClO after disinfection. This may be attributed to the surface finishing, which formed a film that covered the resin surface, conferring protection from the disinfectants used in the study and preventing penetration of liquids[23] during the rinsing of the specimens in running water for 1 min after disinfection. The rinse procedure was meant to reproduce a typical patient's routine for cleaning dentures, and further removed the disinfectant from the acrylic resin.
The results of the present study indicate that the first null hypothesis was not valid, because the polymerization cycle affected the surface roughness of acrylic resins polymerized by heated water, and the second null hypothesis was valid because there was no difference in the release of substances from resin prepared via different methods and subjected to disinfection. The disinfectant solutions evaluated in the study did not influence the surface roughness of acrylic resins polymerized by heated water. However, C2H4O3 remains the disinfectant of choice due its greater effectiveness in disinfection when compared to hypochlorite.
CONCLUSION
Higher surface roughness was detected in acrylic resins for denture bases, subjected to a short polymerization cycle in heated water and disinfected by either NaClO or C2H4O3. The conventional long polymerization cycle resulted in lower surface roughness than the short cycle. Thus, disinfection of acrylic resin polymerized by heated water using a short cycle modified the properties of roughness. Both disinfectants have no effect on surface roughness. Visual colorimetric analysis of water specimens, in which each disinfected sample had soaked, revealed no detectable release of NaClO or C2H4O3 from the specimens.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared
REFERENCES
- 1.Lai CP, Tsai MH, Chen M, Chang HS, Tay HH. Morphology and properties of denture acrylic resins cured by microwave energy and conventional water bath. Dent Mater. 2004;20:133–41. doi: 10.1016/s0109-5641(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 2.Austin AT, Basker RM. Residual monomer levels in denture bases. The effects of varying short curing cycles. Br Dent J. 1982;153:424–6. doi: 10.1038/sj.bdj.4804962. [DOI] [PubMed] [Google Scholar]
- 3.Rahal JS, Mesquita MF, Henriques GE, Nóbilo MA. Water sorption and solubility of acrylic resins. Influence of chemical and mechanical polishing on water sorption and solubility of denture base acrylic resins. Braz Dent J. 2004;15:225–30. doi: 10.1590/s0103-64402004000300012. [DOI] [PubMed] [Google Scholar]
- 4.Berger JC, Driscoll CF, Romberg E, Luo Q, Thompson G. Surface roughness of denture base acrylic resins after processing and after polishing. J Prosthodont. 2006;15:180–6. doi: 10.1111/j.1532-849X.2006.00098.x. [DOI] [PubMed] [Google Scholar]
- 5.Kulak-Ozkan Y, Kazazoglu E, Arikan A. Oral hygiene habits, denture cleanliness, presence of yeasts and stomatitis in elderly people. J Oral Rehabil. 2002;29:300–4. doi: 10.1046/j.1365-2842.2002.00816.x. [DOI] [PubMed] [Google Scholar]
- 6.Guler U, Budak Y, Ruh E, Ocal Y, Canay S, Akyon Y. Effect of mixing techniques on bacterial attachment and disinfection time of polyether impression material. Eur J Dent. 2013;7:S54–9. doi: 10.4103/1305-7456.119074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Council on Dental Materials, Instrument, and Equipment, Council on Dental Practice, Council on Dental Therapeutics. Infection control recommendations for the dental office and the dental laboratory. J Am Dent Assoc. 1996;127:672–80. doi: 10.14219/jada.archive.1996.0280. [DOI] [PubMed] [Google Scholar]
- 8.Verran J, Maryan CJ. Retention of Candida albicans on acrylic resin and silicone of different surface topography. J Prosthet Dent. 1997;77:535–9. doi: 10.1016/s0022-3913(97)70148-3. [DOI] [PubMed] [Google Scholar]
- 9.Mähönen K, Virtanen K, Larmas M. The effect of prosthesis disinfection on salivary microbial levels. J Prosthet Dent. 1997;77:535–9. doi: 10.1111/j.1365-2842.1998.00231.x. [DOI] [PubMed] [Google Scholar]
- 10.Elkassas DW, Fawzi EM, El Zohairy A. The effect of cavity disinfectants on the micro-shear bond strength of dentin adhesives. Eur J Dent. 2014;8:184–90. doi: 10.4103/1305-7456.130596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shang C, Blatchley ER., 3rd Chlorination of pure bacterial cultures in aqueous solution. Water Res. 2001;35:244–54. doi: 10.1016/s0043-1354(00)00248-7. [DOI] [PubMed] [Google Scholar]
- 12.Guiraldo RD, Borsato TT, Berger SB, Lopes MB, Gonini-Jr A, Sinhoreti MA. Surface detail reproduction and dimensional accuracy of stone models: Influence of disinfectant solutions and alginate impression materials. Braz Dent J. 2012;23:417–21. doi: 10.1590/s0103-64402012000400018. [DOI] [PubMed] [Google Scholar]
- 13.Fernandes FH, Orsi IA, Villabona CA. Effects of the peracetic acid and sodium hypochlorite on the colour stability and surface roughness of the denture base acrylic resins polymerised by microwave and water bath methods. Gerodontology. 2013;30:18–25. doi: 10.1111/j.1741-2358.2012.00640.x. [DOI] [PubMed] [Google Scholar]
- 14.Chassot AL, Poisl MI, Samuel SM. In vivo and in vitro evaluation of the efficacy of a peracetic acid-based disinfectant for decontamination of acrylic resins. Braz Dent J. 2006;17:117–21. doi: 10.1590/s0103-64402006000200006. [DOI] [PubMed] [Google Scholar]
- 15.Lottanti S, Gautschi H, Sener B, Zehnder M. Effects of ethylenediaminetetraacetic, etidronic and peracetic acid irrigation on human root dentine and the smear layer. Int Endod J. 2009;42:335–43. doi: 10.1111/j.1365-2591.2008.01514.x. [DOI] [PubMed] [Google Scholar]
- 16.Yap AU, Wu SS, Chelvan S, Tan ES. Effect of hygiene maintenance procedures on surface roughness of composite restoratives. Oper Dent. 2005;30:99–104. [PubMed] [Google Scholar]
- 17.Joniot S, Salomon JP, Dejou J, Grégoire G. Use of two surface analyzers to evaluate the surface roughness of four esthetic restorative materials after polishing. Oper Dent. 2006;31:39–46. doi: 10.2341/04-166. [DOI] [PubMed] [Google Scholar]
- 18.Barbosa DB, de Souza RF, Pero AC, Marra J, Compagnoni MA. Flexural strength of acrylic resins polymerized by different cycles. J Appl Oral Sci. 2007;15:424–8. doi: 10.1590/S1678-77572007000500010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orsi IA, Andrade VG, Bonato PS, Raimundo LB, Herzog DS, Borie E. Glutaraldehyde release from heat-polymerized acrylic resins after disinfection and chemical and mechanical polishing. Braz Dent J. 2011;22:490–6. doi: 10.1590/s0103-64402011000600009. [DOI] [PubMed] [Google Scholar]
- 20.Andrés MT, Tejerina JM, Fierro JF. Reliability of biologic indicators in a mail-return sterilization-monitoring service: A review of 3 years. Quintessence Int. 1995;26:865–70. [PubMed] [Google Scholar]
- 21.Koda T, Tsuchiya H, Yamauchi M, Ohtani S, Takagi N, Kawano J. Leachability of denture-base acrylic resins in artificial saliva. Dent Mater. 1990;6:13–6. doi: 10.1016/0109-5641(90)90037-f. [DOI] [PubMed] [Google Scholar]
- 22.Angelillo IF, Bianco A, Nobile CG, Pavia M. Evaluation of the efficacy of glutaraldehyde and peroxygen for disinfection of dental instruments. Lett Appl Microbiol. 1998;27:292–6. [PubMed] [Google Scholar]
- 23.Orsi IA, Andrade VG. Effect of chemical disinfectants on the transverse strength of heat-polymerized acrylic resins submitted to mechanical and chemical polishing. J Prosthet Dent. 2004;92:382–8. doi: 10.1016/j.prosdent.2004.07.015. [DOI] [PubMed] [Google Scholar]


