Abstract
This article offers an overview of meditation research: its history, recent developments, and future directions. As the number and scope of studies grow, the field has converged with cognitive and affective neuroscience, and spawned many clinical applications. Recent work has shed light on the mechanisms and effects of diverse practices, and is entering a new phase where consensus and coherent paradigms are within reach. This article suggests an unusual path for future advancement: complementing conventional research with rigorous dialogue with the contemplative traditions that train expert meditators and best know the techniques. It explores the Nalanda tradition developed in India and preserved in Tibet, because its cumulative approach to contemplative methods produced a comprehensive framework that may help interpret data and guide research, and because its naturalistic theories and empirical methods may help bridge the gulf between science and other contemplative traditions. Examining recent findings and models in light of this framework, the article introduces the Indic map of the central nervous system and presents three testable predictions based on it. Finally, it reviews two studies that suggest that the multimodal Nalanda approach to contemplative learning is as well received as more familiar approaches, while showing promise of being more effective.
Keywords: meditation, contemplative science, mindfulness, compassion training, polyvagal theory, Nalanda
Traditional contemplative science: ancient history or timely perspective?
It is a great honor and rare privilege to be invited to share some of my reflections on the past achievements, current advances, and future directions of a field that promises to be increasingly relevant to the future of science, health care, and global well-being. Since a clear survey of current advances in basic research has already been covered by previous reviews, this article will focus on providing an overview of the field from the standpoint of future trends in research and application.
Unlike many other meditation researchers, I come at this field from an interdisciplinary and cross-cultural point of view. As an integrative psychiatrist, contemplative psychotherapist, and Buddhist scholar, my approach to meditation research relies mainly on qualitative and intersubjective methods closer to those used in humanity's ancient traditions of contemplative science than to the modern laboratory methods we think of as defining science. Of course, I also do conventional research with my colleagues at the Weill Cornell Center for Complementary and Integrative Medicine and will offer a snapshot of our latest findings below to illustrate some of the points I hope to convey. But the thrust of my comments will be to share a perspective I believe is neglected in our field: the perspective of the ancient contemplative traditions that train the expert practitioners we study and that best understand the practices we try to teach novice subjects in basic and applied research.
While we are taught to assume that our current methods of science are always and in every way more reliable and definitive than any other, I will ask you for the moment to reflect on this article of faith. As I have done so myself over the years, I have come to appreciate what our philosophers and sociologists of science have been saying for decades: that this belief reflects a relative, culturally specific, and practically limiting point of view.1 For example, as we gain more and more detailed knowledge of the effects of various meditation techniques on different regions, pathways, and neurotransmitters in the brain, we often find ourselves further and further from any broad consensus or coherent model of their diverse mechanisms and effects. As Francisco Varela saw it,2 this challenge is not unique to meditation but is a general limitation of studying a system as complex as the human mind/brain with research methods that privilege analytic thinking and reductive measures over systemic thinking and multidisciplinary assessments.
In struggling with these limits over the course of my career, I have found it helpful to complement the reductive perspective of neuropsychology with two multidisciplinary sciences from very different cultural traditions: modern psychotherapy and Buddhist contemplative science.3 In these comments, I will try to share some of the insights I have gleaned from this complementary approach to meditation research and application.
Past history, current models, and future directions: a topical review
From the first physiological studies of meditation in the 1950s and 1960s4–7 and the first clinical studies by Benson et al. in the 1970s,8–10 meditation research has come a long way. The enormous strides in the field over the last three decades have been mainly driven by two synergistic lines of advancement. The first of these is the convergence of meditation research with the explosive growth of basic neuroscience in recent years. The second is the emergence of mindfulness meditation as the dominant paradigm for clinical research and application in the field.11
Since I hypothesized in my 2000 review12 that meditation shares a common mechanism with psychotherapy and hypnosis—the enrichment of learning through use-dependent neural plasticity—a key study by Lutz et al. helped confirm a link between meditation and the greatest paradigm shift in modern neuroscience.13,14 The 2004 study by Lutz et al.13 showed that Tibetan-trained expert meditators were able to consciously induce electroencephalography (EEG) findings indicative of increased learning and neural plasticity—unprecedented trains of gamma activity and synchrony—at will. As Lutz's colleague Richard Davidson explained, the publication of this finding in the Proceedings of the National Academy of Sciences marked a turning point for meditation research, a landmark on the way to the new field he called “contemplative neuroscience.”15 Putting mechanistic teeth into Jon Kabat-Zinn's prior definition of mindfulness as a “discipline of attention,”16,17 this finding reframed meditation as a missing link in conscious self-regulation, connecting mental training on the one hand to the electrochemical processes of neuronal firing, epigenetic regulation of gene transcription, and new neural connectivity on the other.15,18
As for current advances along this line, a series of findings19–21 show that meditation practice slows or may even stop the progression of global cortical atrophy underlying the normal cognitive decline of aging. But firm conclusions have not been drawn from these studies about basic mechanisms or clinical applications of meditation. More sanguine about the significance of such findings are groups like that of Davidson, which have shown meditation-induced increases in cortical thickness in specific regions, especially areas in the prefrontal cortex (PFC) associated with higher cognitive and social-emotional self-regulation. Specifically, one study by Lazar et al. found increased cortical thickness in the PFC of meditators;22 another related finding is from the earlier work of Davidson on the role of the PFC in increased emotional regulation and resilience in mindfulness meditators.23 Such findings have been linked to the syndrome of hypofrontality, a widely invoked model of psychopathology, to explain how meditation promotes self-healing and positive health. The idea articulated by Davidson et al. is that traumatic stress reactivity in the limbic system is poorly controlled when the left PFC is underactive, but resolves when meditation and/or psychotherapy increases its activity.24 This model is also consistent with the recent finding that meditation reduces prefrontal connectivity with the amygdala.25
Of course, the idea that meditation enhances prefrontal regulation of stress reactivity and aversive emotions fits well with the growing clinical interest in mindfulness as an adjunct in the cognitive behavioral treatment of anxiety, depression, and personality disorders. One articulate proponent of this convergence, Dan Siegel, is not a meditation researcher but a child psychiatrist who studies the interpersonal neurobiology of early development. His synthesis of the neurobiology of the PFC with current mindfulness applications in psychotherapy and education26 has helped extend a prefrontal model of mind/brain health to the exploding field of clinical research on modalities like dialectical behavior therapy and mindfulness-based cognitive therapy.27,28
So given this growing coalescence, why should our field not adopt the hypothesis of optimizing prefrontal health—or eufrontality—as a general model of meditation? Let me answer by sharing my thoughts about a recent historic conference held in April 2012 in Denver, the First International Symposia on Contemplative Studies. As Yi-Yuan Tang showed by referencing a recent chart of the rise of meditation research, studies of mindfulness alone have climbed dramatically from a handful per year in the 1980s to upward of 450 in the last year.11 Familiar with this historic groundswell, I went to the Denver conference expecting to learn more about mindfulness and its diverse applications. Many familiar faces were there, including mindfulness pioneers Jon Kabat-Zinn, Dan Goleman, and Sharon Salzberg, but to my surprise much if not most of the new research presented focused on the neurobiology of compassion. Most remarkable were the replication of findings first reported in two papers from Davidson's group,13,29 suggesting that the unusual neural activity associated with compassion meditation in expert practitioners can be developed in novices after a short period of traditional compassion training.30,31
As I see it, this latest wave of studies represents a second line of convergence linking meditation research and neuroscience, namely the convergence of research on affective meditations focused on cultivating equanimity, gratitude, love, and compassion, with the exploding field of affective neuroscience and its clinical counterparts, positive psychology and transformational affect therapy. The larger significance of this second line of advancement may be twofold. First, it challenges and expands preconceived notions of meditation as a practice limited to dispassionate, emotionally cool, and metacognitive states of heightened mindfulness, contentless awareness, and nonjudgmental attention. Second, these practices challenge any simplistic model of meditation as a cure for hypofrontality.
Not only does compassion meditation typically rely on positive images of loved ones or loving mentors, but also it uses such mental contents to consciously evoke strong positive feelings of love and care, and gradually extend them toward any and all others. Unlike simple mindfulness models of resilience based on a shift to left PFC activation and downregulation of prefrontal–amygdala circuitry, the neurobiology of compassion involves increased activation of and connectivity with limbic regions including the anterior cingulate cortex (ACC), the insula, and the nucleus acumbens of the ventral striatum.13,29–31 So while mindfulness may work in part by decreasing activation of limbic regions like the amygdala, involved in negative affect and stress reactivity, compassion meditation appears to work by increasing activation of limbic regions involved in social responsiveness, empathy, positive affect, and internal reward.
Another general model of meditation challenged by the new research on compassion meditation is the model of reduced self-reflective presence proposed by Judson Brewer. Brewer bases this model on his finding that the default mode network (DMN), which is active when the mind/brain are off-task and at rest, involves less mental wandering and less self-reflective memory or fantasy in meditators than in nonmeditating controls.32 His recent study found that the two main nodes of the DMN—the medial PFC (mPFC) and the posterior cingulate cortex (PCC)—are less active in mindfulness practitioners while they meditate and are more connected with attention-control regions like the dorsal ACC when they are not meditating.33 Brewer applies his model generally to meditation teaching and clinical interventions using neural feedback of PCC activation as biofeedback markers for novices and practitioners trying to check their mastery of meditation.34
Like the eufrontality model, the DMN model assumes a unitary view of meditation as dispassionate, contentless, and nondiscursive mindfulness, and so is unlikely to apply equally to positive affective practices like compassion meditation. Some of the key regions active in compassion meditation—such as the dorsolateral PFC, the insula, and the temporoparietal junction (TPJ)—involve developing a stronger than usual self-awareness or agency, and using facial recognition and emotional memories to develop empathic models of the suffering of other minds.
One might predict that both these models will have even more difficulty accounting for the poorly studied family of meditation practices that I expect to be the focus of a third and last line of advancement in meditation research. I am referring to an array of advanced meditation practices that use imagery, affirmations, body movements, and gestures, together with intensive breath control practices, to develop exceptional degrees of mind/brain integration and altruistic agency.
As I look to the future advancements of our field, I see research on the diverse array of such integral practices—including Hindu and Buddhist Tantra, Kundalini Yoga, TM Siddhi, Qi-gong, Cabbala, Christian mysticism, and Sufism—converging with research on the parasympathetic nervous system (PNS). I believe this would allow us to study a broad network of related mechanisms linking the practices studied by Zoran Josipovic,35,36 Fred Travis,37,38 Yi-Yuan Tang,39,40 Luciano Bernardi,41,42 and my own work with my colleagues Mary Charlson and Janey Peterson.43,44 Stephen Porges’ groundbreaking synthesis of the neuropsychology of the vagal nerve45 has opened a new horizon for meditation research that goes deeper than the increasingly accepted link between mindfulness and the PFC, and deeper than the emerging link between compassion meditation and the limbic system, promising to help explain how integral meditation may affect the primal centers of mind/body regulation in the brainstem.
Porges’ polyvagal theory explains how the myelinated “smart vagus” that evolved in mammals not only supports voluntary breathing but also helps modulate primitive vagal and sympathetic reflexes—partly via interneurons in the brainstem and partly by the mammalian neuropeptides oxytocin and vasopressin—to support more consistent and expanded use of higher cortical capacities for social engagement. This theory can be invoked to help explain how mindful breathing helps modulate amygdalar fear reactivity, as well as how compassion training may enhance empathy and caretaking responses by stimulating oxytocin release. But the full explanatory power of the theory lies in its ability to synthesize several seemingly unrelated lines of current research in a way that permits a complex convergence of these lines with preliminary studies of the effects of integral meditation techniques.
Given the phylogenetic linkage between the evolution of the smart vagus and its four related cranial nerves—trigeminal, facial, glossopharyngeal, and accessory—the theory overlaps with recent research on the recognition of facial expressions and vocal tone,46,47 clarifying one plausible basis for the calming effects of envisioning benevolent human faces and reciting prayers or mantras that represent a dialogue with a benevolent presence. Finally, the fact that the smart vagus and its related neuropeptides can modulate the defensive fight, flight, or freeze reactions of the primitive sympathetic and vagal systems overlaps with research on embodied cognition and fearless immobilization states like diving, hibernation, and orgasm, clarifying one plausible basis for integral movement and breath-holding practices that induce seemingly paradoxical states of profound physical relaxation and peak heart–brain arousal.48–51
A comparative, interdisciplinary approach to contemplative science
Despite our recent advances and the promise of more ahead, our young field still falls far short of a coherent picture of meditation mechanisms and effects. At this juncture, I believe we can benefit from consulting the explanatory models of traditional contemplative science. In particular, my own research has focused on the models developed at the Buddhist University of Nalanda in ancient India and preserved in Tibet.
Since the Buddhist University of Nalanda was dedicated to developing the most scientific approach to Buddhist teaching and practice, and making it accessible to mainstream students—Buddhist and non-Buddhist, lay and religious—this living tradition represents a useful resource for contemporary meditation research.52 And since the Nalanda tradition is both continuous and cumulative—integrating multiple models and methods in a complete system of contemplative arts and sciences preserved to the present day—it also represents what may be the world's most comprehensive and rigorous framework of ancient meditative teachings and practices.53 While it is specific to a given cultural tradition, its emphasis on explaining meditation mechanistically in terms of mind/body causation, combined with its systematic approach to diverse practices, makes this synthesis a potential Rosetta Stone that can help modern science decipher the symbolic language and ritualized methods of meditation techniques drawn from many different religious traditions.
Of course, this comprehensive approach shares its basic science and technology with the Hindu tradition grounded in Patanjali's Yoga, which lays out four meditation practices over and above the familiar elements of lifestyle, posture, and breathing.54,55 As in the Nalanda tradition, these advanced methods were elaborated on in later systems of Hindu meditation, including the wisdom-compassion methods refined in Vedanta/Bhakti Yoga, and the integral methods of recitation and breath control refined in the Hindu Tantras. Consequently, the Nalanda system has clear analogs from the Hindu side of Indic contemplative science, such as the comprehensive synthesis of Kashmiri Shaivism. These sister traditions integrate classical yoga practice with fully developed methods of compassion meditation and integral breath meditation. The main benefits of the Nalanda version lie in a less theistic explanatory language, and a more extensive and continuous academic tradition of oral and written commentary.
In two recent articles,12,53 I have reviewed the literature and presented a rationale for integrating the Nalanda synthesis into a comparative framework for contemplative research and clinical application. Here I briefly summarize the framework proposed and suggest ways in which it may help interpret ambiguous findings, direct future studies, and improve methodology.
Although the Nalanda framework reflects the historic unfolding of Buddhist teaching and practice from its inception (ca. 500 B.C.E.) to the University's destruction (ca. 1250 C.E.), it was developed as a synchronic mapping of diverse systems of contemplative science and healing arts. The framework maps the field as a continuum of progressively more challenging systems of theory and practice, which yield increasingly more complete degrees of self-regulation based on increasingly more profound levels of mind/brain integration. Before explaining my use of the term brain, let me begin by briefly describing the three (or four) progressive systems of theory and practice defined in the framework. First comes the basic science and contemplative practice of personal healing using mindfulness; second comes the intermediate science and contemplative practice of interpersonal healing using compassion; and third comes the advanced science and contemplative practice of integral or primary process healing, using a combination of visual and auditory imagery with breath-induced transformational affect. The most modern map in effect divides the third system into a moderately advanced integral system and a highly advanced primary process system, yielding a fourfold framework that bears some resemblance to the Vedic model assumed by Fred Travis.56
In light of current meditation research, this framework has both experimental and explanatory value. It makes testable predictions, many of which are seemingly being borne out, while also offering coherent models that account for the distinctions between practices as well as the overall family resemblances that make them part of one spectrum of self-healing and self-regulation. For instance, the threefold framework predicts that the basic science and beneficial effects of simple yoga and mindfulness will differ significantly from those of compassion meditation, and that the science and effects of both these will differ from those of integral practices. In addition, it explains the similarities and differences between the main types of practice by mapping them as a natural spectrum of related methods, each with its own distinct cognitive and affective-behavioral components. One traditional metaphor is that meditative arts and sciences are a polyandrous family born out of the marriage of one mother—cognitive self-transcendence—with three fathers—methods/levels of self-regulation—namely, dispassion (disarming stress emotions and reactions); compassion (cultivating social emotions and actions); and pure passion (tapping blissful flow states and open creative responsiveness). In this, it brings more specificity and variety to David Vago's S-ART model of mindfulness as based on the active ingredients of self-awareness, self-regulation, and self-transcendence.57
This brings us to the most distinctive aspect of the framework: cross-referencing current brain mapping with the contemplative map of the nervous system that the Nalanda tradition shares with many Indic traditions, from classical Yoga to Kashmiri Shaivism. Given the sensitivity and complexity of such cross-cultural comparisons from the perspective of all parties—modern scientists and scholars, traditional scholars and practitioners—I have considered the challenges and feasibility of this aspect at length elsewhere.1,3,53 For now, suffice it to say that many elements of the scientific description of the model called the “subtle body” (skt. suksma-sarira) in Ayruvedic medicine or Tibetan psychiatry make it quite clear that it is meant for use as a map of what we call the central nervous system (CNS).58 Most pertinent are (1) the anatomical congruence between the traditional map and the neuraxis; (2) the use of naturalistic metaphors to describe the structure and function of the system—pathways called “reeds” or “channels” (nadi), complexes called “hubs” or “knots” (chakra, grantha), energies called “breaths” or “winds” (prana, vayu), and a range of different types of fluids called “drops” (bindu); and (3) the use of these descriptive elements to explain the basic functions we attribute to the CNS, including the transmission of sensorimotor and symbolic information based on the movement of winds and drops through, to, and from different channels and complexes; the central control of respiration, excretion, reproduction, digestion, metabolism, circulation, sensation and mentation, and proprioception and motor function, based on the movements of 10 kinds of winds and eight kinds of drops; the support of all activities and state changes of the four levels of consciousness—waking, dreaming, deep sleep, and orgasm—based on the movements of specific winds and drops through channels and complexes at three or four successively deeper, more elementary levels of the subtle material body.
Whatever the feasibility of such comparisons in principle, in practice I believe they permit testable predictions and heuristically valid models of the neural mechanisms and benefits of meditation that may help us design and interpret current research. To illustrate this, I very briefly allude to three examples drawn from the application of the traditional map to explain and guide contemplative practice. First, the traditional model predicts that mindful control of attention and body awareness will mainly involve the higher structures of the nervous system behind the forehead and under the crown; compassion meditation will involve intermediate structures that support language, mental imagery, and emotion; and integral meditation will involve deeper structures that support basic bodily functions like heart rate, respiration, sense input, states of consciousness, digestion, and reproduction. As I mentioned earlier, this prediction seems to have been borne out by mounting research on mindfulness22,25 and compassion,29–31,59 and there is preliminary evidence that the third aspect of this prediction may be borne out as well (see Figure 1).49–52,60
Fig 1.

Color-coded structural–functional congruence in modern and Indic central nervous system (CNS) maps. Illustration by Daniel Hakansson.
Second, the traditional model predicts that conscious self-regulation depends not only on vertical integration of different levels of structure and process, but also on the lateral integration of three systems of autonomic regulation present at each level: a right-sided system based in a “masculine” activating channel, with activating winds and drops; a left-sided system based in a “feminine” calming channel, with calming winds and drops; and a central system based in a “neuter” bliss-love channel, with blissful winds and drops, accessed by balanced, voluntary nostril breathing and conscious, prolonged breath holding. In my view, this prediction has found unexpected support from current research on the autonomic nervous system (ANS), not only from the unlikely finding that the sympathetic and parasympathetic systems show lateral dominance at all levels of the brain and body,61 but also from the finding that that dominance, though variable and alternating, generally lines up with the traditional map, showing right-sided sympathetic dominance and left-sided vagal dominance.62 Finally, this second prediction also finds support from current studies of alternate nostril breathing,63 breath-slowing and breath-holding practices,64 as well as from the emerging consensus that conscious breathing exerts smart-vagal dominance over the primitive sympathetic nervous system (SNS) and PNS,65 balancing and integrating them to support social engagement with the help of the mammalian neuropeptides vasopressin and oxytocin.66 Of note, this tradition would weigh in on current discussions of whether the slowing of cortical atrophy by meditation is due to neuroplasticity or to stress protection, predicting that these two mechanisms are linked and synergistic aspects of one general process of lateral integration are employed at all mind/brain levels in different ways by different meditative practices (see Figure 2).
Fig 2.

Autonomic laterality in modern and Indic autonomic nervous system (ANS) maps. Illustration by Daniel Hakansson.
Thirdly, the traditional model predicts that there is a central locus of self-regulation at the level of central cardiorespiratory control, that breath is controlled by two binary circuits above and below that level, and that voluntary breathing, breath slowing, and breath holding can help link the circuits, yielding full access and control of primal winds and drops that support motivation and reward as well as the vital rhythms of life. This prediction is supported by current research and thinking on the ANS, since the new brainstem nucleus that gives rise to the smart vagus—the ventral nucleus ambiguous—is close not just to the older dorsomedial nucleus of the vagus but also to the cardiorespiratory oscillator and the dopaminergic nuclei that innervate the ventral striatum, close enough for the growth of interneurons that permit the extension of smart-vagal control to these neighboring nuclei.46
While the second and third predictions based on the traditional model of neural mechanisms of meditation have not been widely entertained or studied, they are broadly consistent with current research and testable by current methods. In response to the conventional wisdom that consulting such ancient models and methods is anachronistic and introduces unscientific beliefs and biases that obstruct solid research, I draw your attention to the fact that the most prolific and cutting-edge teams in the field work closely with traditional contemplative scientists and traditionally trained expert practitioners.13–15,37–40 In response to the objection that integrating traditional models and methods of meditation limits the accessibility and efficacy of clinical applications, we need only consider the broad appeal and impact of mindfulness methods drawn from traditional Buddhist teaching and practice. In fact, an additional benefit of these models is that their qualitative, intersubjective approach to mapping the CNS, and their holistic elegance, makes them far more user friendly as heuristic learning guides for meditators trying to regulate their nervous system first-hand than are modern brain maps.1,56 In that vein, I would now like to share with you the approach and findings of our own research, practically applying the multimodal approach to contemplative self-healing that Mary Charlson, Janey Peterson, and I have studied in women recovering from breast cancer treatment.43–45
Contemplative self-healing: integrating the Nalanda synthesis in practice
The Nalanda 20-week program in contemplative self-healing combines simple yoga and mindfulness with compassion training and integral methods of role-modeling imagery, affirmation, and breath holding, based on the Nalanda tradition of gradual mind training.43 In two recent pilot studies of breast cancer survivors conducted by the Weill Cornell Center for Complementary and Integrative Medicine and funded by the Avon Foundation, the Nalanda program had stronger effects on quality of life and traumatic avoidance than any other psychosocial interventions studied, while also showing promising effects on biomarkers of stress, and matching or exceeding the efficacy of interventions like mindfulness-based stress reduction (MBSR), aerobic exercise three times per week, and antidepressants.44,45 The program in both these studies combined an 8-week contemplative skills learning component with a 12-week contemplative outlook and lifestyle-change program.
The first study in 68 female cancer survivors41 found unprecedented within-patient improvements in quality of life as measured by the Functional Assessment of Cancer Therapy (FACT) scale and the Short Form 36 (SF-36). Figure3 shows an overall FACT General (FACT-G) improvement of 6.4, along with significant improvements in emotional (2.3) and role functioning (2.0). Figure4 shows significant improvements (>10 points) in quality of life as measured by the SF-36, specifically in social, role-emotional, and mental functioning. Biologic measures in a small cohort of participants also showed statistically significant within-patient improvements in biomarkers of stress, specifically normalized waking cortisol and decreased resting heart rate.
Fig 3.
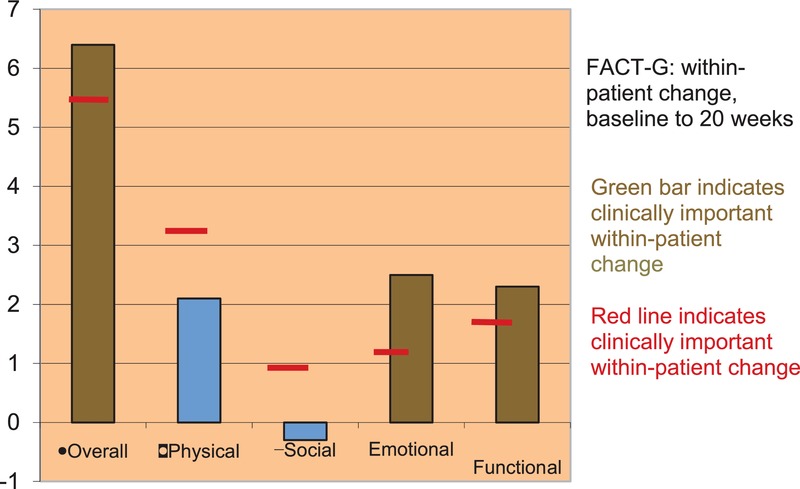
Within-patient improvements in quality of life measured by the Functional Assessment of Cancer Therapy (FACT) scale.
Fig 4.
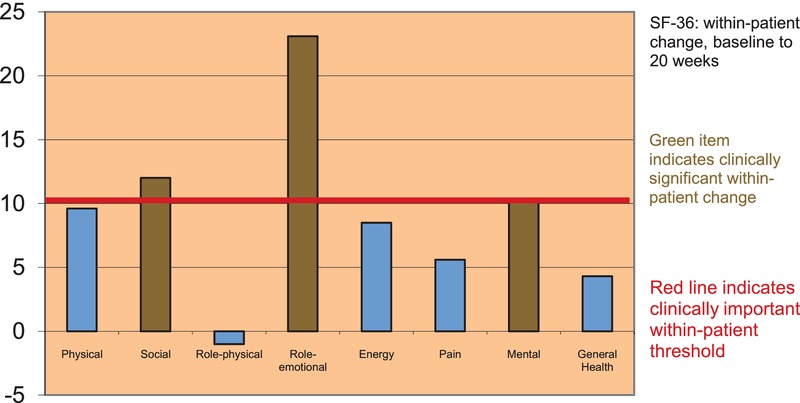
Within-patient improvements in quality of life measured by the Short Form 36 (SF-36).
In the second study of the Nalanda 20-week program45 in an underserved population of 42, mostly minority female breast cancer survivors, the intervention had a positive effect of the same order of magnitude (3.7 on the FACT-G, and 4.6 corrected for women with recurrences during the study). This finding is remarkable considering both the women's unusually high levels of stress and their reported low adherence to the reading and homework program consisting of practice sessions. It is also notable in that the intervention was delivered by four clinicians newly trained for the study. The most remarkable finding of this second study was the strong intervention effect on the trauma symptoms reported by breast cancer survivors, as measured by the Impact of Events Scale (IES). Specifically, our study showed more than twice the reduction in overall symptoms (–6.3) and in traumatic avoidance (–3.9) than was found in a Danish observational study67 and in a study of a 10-week cognitive behavioral intervention.68
Although still preliminary, these findings suggest that applying the comprehensive Nalanda approach to tough clinical challenges, such as fear of recurrence and traumatic avoidance in cancer survivors, may improve the efficacy of familiar interventions involving mindfulness or cognitive behavioral techniques alone. Qualitative feedback from very diverse populations strongly supports our anecdotal experience that this traditional step-by-step tool-chest approach to contemplative interventions is both broadly accessible and effective.
Concluding thoughts and future directions: a modest proposal
Although still in its adolescence, the field of meditation research has come a long way. As an increasing number and range of studies has shed light on the neural mechanisms and beneficial effects of a wide array of contemplative practices, I believe we are entering a new phase where a broad consensus and coherent research and practice paradigms are within reach. I hope this article has helped to clarify the potential of bringing interdisciplinary, cross-cultural perspective and dialogue to our work at this critical phase. In particular, my aim in this article was to explore one such dialogue—with the Nalanda tradition developed in India and preserved in Tibet—for two key reasons. First, given its cumulative approach to contemplative methods, I believe the Nalanda tradition can help us interpret a diverse field of data in light of a comprehensive framework that helps reconcile conflicting findings and integrate partial models. Second, given its naturalistic causal theories and intersubjective empirical methods, this rare tradition may serve as a missing link or bridge between modern reductive science and traditional contemplative science in general.
To flesh out this suggestion, I briefly surveyed a few current research trends and explanatory models, and then tried to put these into the broad perspective of a comparative framework for contemplative research and application that draws on the Nalanda synthesis. On the basis of decades of integrative study and practice, I made the seemingly anachronistic suggestion that even our research and understanding of the neural mechanisms and effects of different meditation techniques could be strengthened and enriched by consulting the contemplative map of the CNS developed in ancient India and preserved in Tibet. I mentioned three testable predictions based on that map and reviewed some of the evidence that could be seen to support them. And I suggested that research teams that have engaged in ongoing dialogue with traditional contemplative scientists and practitioners have reaped tangible results in terms of the novelty and cogency of their work. Finally, I shared two studies conducted by my own team at Weill Cornell, which suggest that the multimodal approach to contemplative learning developed at Nalanda appears to be as well received and as reproducible as single-method approaches, while showing some promise of being more effective.
Although perspectives like the one shared here may not be common in today's research circles and literature, I believe there is nothing new or controversial in what I have shared. Rather, I submit that the simple anthropology of respecting traditional know-how and practical expertise has been a secret ingredient to successful meditation research for decades.15,56 In a sense, I am simply suggesting that our young field has proven its rigor and relevance enough that we are ready to enter a new phase of open, rigorous, and systematic interdisciplinary dialogue with traditional contemplative science. My vision for the future of the field is that such an open, mutually respectful, and rigorous partnership promises to speed the advancement and align the direction of our field toward optimal science and maximal human benefit, as much or more than any conventional line of advancement through technical breakthroughs and new methodologies.
Conflicts of interest
The author declares no conflicts of interest.
References
- 1.Loizzo J. Blackhall L. Traditional alternatives as complementary sciences: the case of Indo-Tibetan medicine. J. Altern. Complement. Med. 1998;4::311–319. doi: 10.1089/acm.1998.4.3-311. [DOI] [PubMed] [Google Scholar]
- 2.Varela F, Thompson E. Rosch E. The Embodied Mind: Cognitive Science and Human Experience. Cambridge, MA: MIT Press; 1996. [Google Scholar]
- 3.Loizzo J. Sustainable Happiness: The Mind Science of Well-Being, Altruism, and Inspiration. New York: Routledge Press; 2012. [Google Scholar]
- 4.Das N. Gastaut H. Variations de l'activite electrique de cerveau du Coeur et des muscles squelletiques au cours de la meditation et de l'extase yogique. Electroencephalogr. Clin. Neurophysiol. Suppl. 1955;6::211–219. [Google Scholar]
- 5.Bagchi B. Wegner M. Electrophysiological correlates of some yogi exercises. Electroencephalogr. Neurophysiol. Suppl. 1957;7::132–149. [Google Scholar]
- 6.Anand B, Chinna G. Singh B. Some aspects of electroencephalographic studies in yogis. Electroencephalogr. Neurophysiol. 1961;13::452–456. [Google Scholar]
- 7.Kasamatsu A. Harai T. An electroencephalographic study on Zen meditation (zazen) Folia Psychiatr. Neurol. Jpn. 1966;20::315–336. doi: 10.1111/j.1440-1819.1966.tb02646.x. [DOI] [PubMed] [Google Scholar]
- 8.Wallace R, Benson H. Wilson A. A wakeful hypometabolic physiologic state. Am. J. Physiol. 1971;221::795–799. doi: 10.1152/ajplegacy.1971.221.3.795. [DOI] [PubMed] [Google Scholar]
- 9.Beary J. Benson H. A simple psychophysiologic technique which elicits the hypometabolic changes of the relaxation response. Psychosom. Med. 1974;36::115–120. doi: 10.1097/00006842-197403000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Benson H. Systematic hypertension and the relaxation response. N. Engl. J. Med. 1977;296::1152–1156. doi: 10.1056/NEJM197705192962008. [DOI] [PubMed] [Google Scholar]
- 11. Yi-yuan Tang's keynote cited the compelling chart compiled by David Black of USC, and posted in his online Mindfulness Research Guide: www.mindfulexperience.org/mindfo.php.
- 12.Loizzo J. Meditation and psychotherapy: stress, allostasis and enriched learning. In: Muskin P, editor. Complementary and Alternative Medicine and Psychiatry. Washington: American Psychiatric Association Press; 2000. pp. 147–197. [Google Scholar]
- 13.Lutz A, Grieschar L, Rawlings N, et al. Long-term meditators self-induce high amplitude gamma synchrony during mental practice. Proc. Natl. Acad. Sci. USA. 2004;101::16369–16373. doi: 10.1073/pnas.0407401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidson RJ. Affective style, psychopathology and resilience: brain mechanisms and plasticity. Am. Psychol. 2000;55::1196–1214. doi: 10.1037//0003-066x.55.11.1196. [DOI] [PubMed] [Google Scholar]
- 15.Davidson R. The Emotional Life of Your Brain. New York: Plume Books; 2013. [Google Scholar]
- 16.Kabat-Zinn J. An outpatient program based in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen. Hosp. Psychiatr. 1982;4::33–47. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- 17.Kabat-Zinn J, Lipworth L. Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. J. Behav. Med. 1985;8::163–190. doi: 10.1007/BF00845519. [DOI] [PubMed] [Google Scholar]
- 18.Saatcioglu F. Regulation of gene expression by yoga, meditation and related practices: a review of recent studies. Asian J. Psychiatr. 2013;8::e61910. doi: 10.1016/j.ajp.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Paganoni G. Cekic M. Age effects on gray matter volume and attentional performance in Zen meditation. Neurobiol. Aging. 2007;28::1623–1627. doi: 10.1016/j.neurobiolaging.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Holtzel B, Carmody J, Vangel M, et al. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatr. Res. 2011;191::36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luders E, Phillips O, Clark K, et al. Bridging the hemispheres in meditation: thicker collosal regions and enhanced fractional anisotropy (FA) in long-term practitioners. Neuroimage. 2012;61::181–187. doi: 10.1016/j.neuroimage.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lazar SW, et al. Meditation experience is associated with increased cortical thickness. NeuroReport. 2005;16::1893–1897. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davidson R, Kabat-Zinn J, Schumacher J, et al. Alterations in brain and immune functions produced by mindfulness meditation. Psychosom. Med. 2003;65::564–570. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- 24.Davidson R, Pizagalli D, Nitschke J. Putnam K. Depression: perspectives from affective neuroscience. Ann. Rev. Psychol. 2002;53::545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- 25.Van den Hurk P, Janssen B, Giommi F, et al. Mindfulness meditation associated with alterations in bottom-up processing: psychophysiological evidence for reduced reactivity. Int. J. Psychophysiol. 2010;78::151–157. doi: 10.1016/j.ijpsycho.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Siegel D. The Mindful Brain: Reflection and Attunement in the Cultivation of Wellbeing. New York: W.W. Norton & Company; 2007. [Google Scholar]
- 27.Feliu-Soler A, Pascual J, Borras X, et al. Effects of dialectical behavior therapy mindfulness training on emotional reactivity in borderline personality disorder: preliminary results. Clin. Psychol. Psychother. Mar. 2013;14 doi: 10.1002/cpp.1837. doi: 10.1002/cpp.1837 epub. [DOI] [PubMed] [Google Scholar]
- 28.Teasdale J, Segal Z. Williams J. How does cognitive therapy prevent depressive relapse and why should attentional control (mindfulness) training help? Behav. Res. Ther. 1995;33::25–39. doi: 10.1016/0005-7967(94)e0011-7. [DOI] [PubMed] [Google Scholar]
- 29.Lutz A, Brefcyznyski-Lewis J, Johnstone T. Davidson R. Voluntary regulation of the neural circuitry of emotion by compassion meditation: effects of expertise. PLoS One. 2008;3::e1897. doi: 10.1371/journal.pone.0001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desbordes G, Negi L, Pace T, et al. Effects of mindful attention and compassion meditation training on amygdala response to emotional stimuli in an ordinary, non-meditative state. Front. Hum. Neurosci. 2012;6::292. doi: 10.3389/fnhum.2012.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klimecki O, Leiberg S, Lamm C. Singer T. Functional neuroplasticity and associated changes in positive affect after compassion training. Cereb. Cortex. 2013;23::1552–1561. doi: 10.1093/cercor/bhs142. [DOI] [PubMed] [Google Scholar]
- 32.Berkovich-Ohana A, Glicksohn J. Goldstein A. Mindfulness-induced changes in gamma band activity—implications for the default mode network, self-reference and attention. Clin. Neurophysiol. 2012;123:700–710. doi: 10.1016/j.clinph.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 33.Brewer J, Worhunsky P, Gray J, et al. Meditation experience is associated with differences in default mode network activity and connectivity. Proc. Natl. Acad. Sci. USA. 2011;108::20254–20259. doi: 10.1073/pnas.1112029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brewer J, Sinha R. Chen J, et al. Mindfulness training and stress reactivity in substance abuse: results from a randomized, controlled stage I pilot study. Subst. Abus. 2009;30::306–317. doi: 10.1080/08897070903250241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kozhevnikov M, Louchakova O, Josipovic Z. Motes M. The enhancement of visuospatial processing efficiency through Buddhist Deity meditation. Psychol. Sci. 2009;20::645–653. doi: 10.1111/j.1467-9280.2009.02345.x. [DOI] [PubMed] [Google Scholar]
- 36.Josipovic Z. Influence of meditation on anti-correlated networks in the brain. Front. Hum. Neurosci. 2011;5::183. doi: 10.3389/fnhum.2011.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mason L, Alexander C, Travis F, et al. Electrophysiological correlates of higher states of consciousness during sleep in long-term practitioners of the transcendental meditation program. Sleep. 1997;20::102–110. doi: 10.1093/sleep/20.2.102. [DOI] [PubMed] [Google Scholar]
- 38.Travis F. Comparison of coherence, amplitude, and eLORETA patterns during transcendental meditation and TM-Sidhi practice. Int. J. Psychophysiol. 2011;81::198–202. doi: 10.1016/j.ijpsycho.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 39.Tang Y, Lu Q, Geng X, et al. Short-term meditation induces white matter changes in the anterior cingulate. Proc. Natl. Acad. Sci. USA. 2010;107::15649–15652. doi: 10.1073/pnas.1011043107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang Y, Ma Y, Fan Y, et al. Central and autonomic nervous system interaction is altered by short-term meditation. Proc. Natl. Acad. Sci. USA. 2009;106::8865–8870. doi: 10.1073/pnas.0904031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haider T, Casucci G, Linser T, et al. Interval hypoxic training improves autonomic cardiovascular and respiratory control in patients with mild chronic obstructive pulmonary disease. J. Hypertens. 2009;27::1648–1654. doi: 10.1097/HJH.0b013e32832c0018. [DOI] [PubMed] [Google Scholar]
- 42.Bernardi L, Spadacini G, Bellwon J, et al. Effect of breathing rate on oxygen saturation and exercise performance in chronic heart failure. Lancet. 1998;351::1308–1311. doi: 10.1016/S0140-6736(97)10341-5. [DOI] [PubMed] [Google Scholar]
- 43.Loizzo J, Charlson M. Peterson J. A program in contemplative self-healing: stress, allostasis and learning in the Indo-Tibetan tradition. Ann. N.Y. Acad. Sci. 2009;1172::123–147. doi: 10.1111/j.1749-6632.2009.04398.x. [DOI] [PubMed] [Google Scholar]
- 44.Loizzo J, Peterson J, Wolf E, et al. Effects of a contemplative self-healing program on quality of life in women with breast and other gyn. Altern. Therap. Health Med. 2010;16::30–37. [PubMed] [Google Scholar]
- 45.Charlson M, Loizzo J, Moadel A, et al. Contemplative self-healing improves quality of life and reduces stress symptoms in women breast cancer survivors: a pilot study in minority women. 2013. In Press. [DOI] [PMC free article] [PubMed]
- 46.Porges S. The Polyvagal Theory: Neurophysiological Foundations of Emotions, Attachment, Communication and Self-Regulation. New York: W.W. Norton & Co; 2011. [Google Scholar]
- 47.Ekman P, Davidson R. Friesen W. The Duchene smile: emotional expression and brain physiology II. J. Pers. Soc. Psychol. 1990;58::342–353. [PubMed] [Google Scholar]
- 48.Liu G, Cui R, Li G, et al. Changes in brainstem and cortical auditory potentials during qi-gong meditation. Am. J. Chin. Med. 1990;18::95–103. doi: 10.1142/S0192415X90000137. [DOI] [PubMed] [Google Scholar]
- 49.Heller C, Elsner R. Rao N. Voluntary hypometabolism in an Indian Yogi. J. Ther. Biol. 1987;2::171–173. [Google Scholar]
- 50.Benson H, Lehman J, Malhotra M, et al. Body temperature changes during the practice of gTummo heat yoga. Nature. 1982;295::234–236. doi: 10.1038/295234a0. [DOI] [PubMed] [Google Scholar]
- 51.Benson H, Malhotra M, Goldman R, et al. Three case reports of the metabolic and electroencephalographic changes during advanced Buddhist meditative techniques. Behav. Med. 1990;16::90–95. doi: 10.1080/08964289.1990.9934596. [DOI] [PubMed] [Google Scholar]
- 52.Loizzo J. Kalachakra and the Nalanda Tradition. In: Arnold E, editor. As Long as Space Endures: Essays in Honor of His Holiness the Dalai Lama. Ithaca: Snow Lion Press; 2009a. pp. 333–336. [Google Scholar]
- 53.Loizzo J. Optimizing learning and quality of life throughout the lifespan: a global framework for research and application. Ann. N.Y. Acad. Sci. 2009;1172::186–198. doi: 10.1196/annals.1393.006. [DOI] [PubMed] [Google Scholar]
- 54.Telles S, Raghavendra B, Naveen K, et al. Changes in autonomic variables following two meditative states described in yoga texts. Altern. Complement. Med. 2013;19::35–42. doi: 10.1089/acm.2011.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Telles S, Raghavendra B, Naveen K, et al. Mid-latency auditory evoked potentials in 2 meditative states. Clin. EEG Neurosci. 2012;43::154–160. doi: 10.1177/1550059412439963. [DOI] [PubMed] [Google Scholar]
- 56.Travis F. Focused attention, open monitoring and automatic self-transcending: categories to organize meditations from Vedic, Buddhist and Chinese traditions. Conscious Cogn. 2010;19::1256–1264. doi: 10.1016/j.concog.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 57.Vago D. Silbersweig D. Self-awareness, self-regulation, and self-transcendence (S-ART): a framework for understanding the neurobiological mechanisms of mindfulness. Front. Hum. Neurosci. 2012;6::296. doi: 10.3389/fnhum.2012.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loizzo J, Blackhall LJ. Rapgay L. Tibetan medicine: a complementary science of optimal health. Ann. N.Y. Acad. Sci. 2009c;1172::218–230. doi: 10.1196/annals.1393.008. [DOI] [PubMed] [Google Scholar]
- 59.Yu X, Fumoto M, Nakatani Y, et al. Activation of the anterior prefrontal cortex and serotonergic system is associated with improvements in mood and EEG changes induced by Zen meditation practice in novices. Int. J. Psychophysiol. 2011;80::103–111. doi: 10.1016/j.ijpsycho.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 60.Vestergaard-Poulsen P, van Beek M. Skewes J, et al. Long-term meditation is associated with increased gray matter density in the brain stem. Neuroreport. 2009;20::170–174. doi: 10.1097/WNR.0b013e328320012a. [DOI] [PubMed] [Google Scholar]
- 61.Shannahoff-Khalsa D. Selective unilateral autonomic activation: implications for psychiatry. CNS Spectr. 2007;12::625–634. doi: 10.1017/s1092852900021428. [DOI] [PubMed] [Google Scholar]
- 62.Hilz M, Dutsch M, Perrine K, et al. Hemispheric influence on autonomic modulation and baroreflex sensitivity. Ann. Neurol. 2001;49::578–584. [PubMed] [Google Scholar]
- 63.Jella S. Shannahoff-Khalsa D. The effects of forced nostril breathing on cognitive performance. Int. J. Neurosci. 1993;73::61–68. doi: 10.3109/00207459308987211. [DOI] [PubMed] [Google Scholar]
- 64.Brown R. Gerbarg P. Sudarshan Kriya yogic breathing in the treatment of stress, anxiety and depression. Part I: neurophysiologic model. J. Altern. Complement. Med. 2005a;11::189–201. doi: 10.1089/acm.2005.11.189. [DOI] [PubMed] [Google Scholar]
- 65.Morse D, Cohen L, Furst L, et al. A physiological evaluation of the yoga concept of respiratory control of autonomic system activity. Int. J. Psychosom. 1984;31::3–19. [PubMed] [Google Scholar]
- 66.O'Halloran J, Jevning R, Wilson A, et al. Hormonal control in a state of decreased activation: potentiation of argenine vasopressin secretion. Physiol. Behav. 1985;35::591–595. doi: 10.1016/0031-9384(85)90146-5. [DOI] [PubMed] [Google Scholar]
- 67.O'Connor M, Christensen S, Jensen A, et al. How traumatic is breast cancer? Post-traumatic stress symptoms (PTSS) and risk factors for severe PTSS at 3 and 15 months after surgery in a nationwide cohort of Danish women treated for primary breast cancer. Br. J. Cancer. 2011;104::419–426. doi: 10.1038/sj.bjc.6606073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koopman C, Butler L, Classen C, et al. Traumatic stress symptoms among women with recently diagnosed primary breast cancer. J. Trauma Stress. 2002;15::277–287. doi: 10.1023/A:1016295610660. [DOI] [PubMed] [Google Scholar]


