SUMMARY
We demonstrate that the antibiotic amicoumacin A (AMI) whose cellular target was unknown, is a potent inhibitor of protein synthesis. Resistance mutations in helix 24 of the 16S rRNA mapped the AMI binding site to the small ribosomal subunit. The crystal structure of bacterial ribosome in complex with AMI solved at 2.4 Å resolution revealed that the antibiotic makes contacts with universally conserved nucleotides of 16S rRNA in the E site and the mRNA backbone. Simultaneous interactions of AMI with 16S rRNA and mRNA and the in vivo experimental evidence suggest that it may inhibit the progression of the ribosome along mRNA. Consistent with this proposal, binding of AMI interferes with translocation in vitro. The inhibitory action of AMI can be partly compensated by mutations in the translation elongation factor G.
Keywords: Amicoumacin A, antibiotic, 70S ribosome, X-ray structure, inhibition of translation, translocation inhibitor
INTRODUCTION
The ribosome is one of the main targets of antibiotics in the cell. Multiple copies of rRNA genes in bacterial genomes impedes the development of antibiotic resistance due to target site mutations, accounting in part for the clinical success of the ribosome-targeted drugs. The atomic structures of the ribosome with bound antibiotics reveal how small molecules can inhibit the activity of one of the largest cellular machines and enable rational drug design, paving the way for development of better antibiotics (Franceschi and Duffy, 2006; Wilson, 2014).
Many of the previously discovered antibiotics act upon a limited number of ‘traditional’ functional ribosomal sites, e.g. peptidyl transferase or the decoding center. However, the spread of resistant strains has limited the medical utility of many of these antibiotics. Therefore, there is a high demand for new chemical scaffolds which interact with new ribosomal sites and inhibit translation via novel mechanisms of action.
The antibacterial properties of amicoumacin A (AMI) (Figure 1A) were discovered more than 30 years ago (Itoh et al., 1981; Itoh et al., 1982). AMI is produced by several bacterial species isolated from both soil and marine environments (Itoh et al., 1982; Li et al., 2012). AMI and related compounds are active against a number of pathogenic bacteria, including Helicobacter pylori (Pinchuk et al., 2001) and methicillin-resistant Staphylococcus aureus (Lama et al., 2012). In addition, AMI was reported to exhibit anti-inflammatory (Itoh et al., 1981) and anti-tumor (Canedo et al., 1997) activities. In spite of these attractive medical characteristics, the mechanism of action of AMI is unknown, although studies of a related compound, oosponol, indicated protein synthesis as one of the possible targets (Sonnenbichler and Kovacs, 1997). In a recent functional study, 263 genes were found to be upregulated and 282 genes were downregulated in S. aureus cells treated with AMI (Lama et al., 2012). The majority of those genes were related to the stress response and no definitive conclusion on the exact nature of AMI-induced damage could be drawn. S. aureus cells exposed to subinhibitory concentrations of AMI accumulated mutations in genes related to DNA replication (primase), metabolism (tagatose 1,6-bisphosphate aldolase and glycosyl transferase) and protein biosynthesis (fusA, ksgA), making it difficult to pinpoint the actual mechanism of action (Lama et al., 2012).
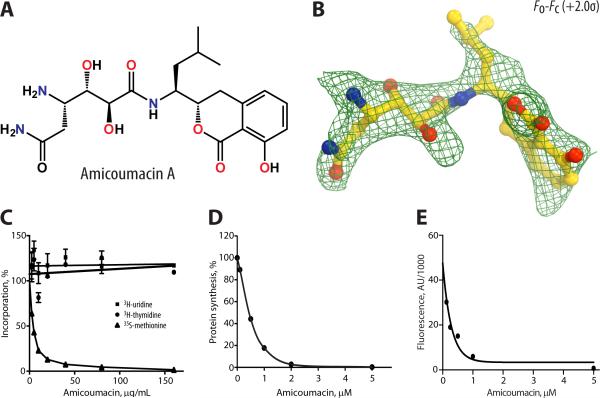
Figure 1. Inhibition of protein synthesis in vitro by AMI and its electron density map.
(A) Chemical structure of amicoumacin A. (B) Difference Fourier map of AMI in complex with the T. thermophilus 70S ribosome. The refined model of AMI (yellow) is displayed in its respective electron density before refinement. The unbiased (Fobs – Fcalc) difference electron density map is contoured at 2σ. Nitrogens are colored blue, oxygens – red. (C) Relative inhibition of RNA, DNA, or protein synthesis by AMI determined by macromolecular synthesis inhibition. The effects of AMI on in vitro protein synthesis carried out in cell lysate. Data are represented as mean +/− SEM. (D) or in the reaction assembled from purified components. (E) Firefly luciferase was translated in the cell extract and green fluorescence protein was translated in the PURE system.
Here, we present biochemical, genetic and structural evidence that AMI targets the ribosome and represents a new class of protein synthesis inhibitors which bind to the small ribosomal subunit. Our data suggest that AMI could interfere with translation by stabilizing the interaction of mRNA with the small ribosomal subunit.
RESULTS
AMI inhibits protein synthesis by acting upon the ribosome
Macromolecular synthesis inhibition experiments (Figure 1C) demonstrated that AMI inhibits protein synthesis (35S-methionine incorporation) in living bacteria at a concentration similar to its minimal inhibitory concentration (MIC) value of 0.5 μg/mL (Table 1). In contrast, inhibition of RNA or DNA synthesis (3H-uridine or 3H-thymidine incorporation, respectively) were not observed at >100-fold higher concentrations (Figure 1C). Results from in vitro protein synthesis assays utilizing an E. coli S30 extract or the PURE system (Shimizu et al., 2005) composed of purified components of the translation reaction (Figure 1D and 1E) confirmed that AMI readily prevented synthesis of the reporter proteins (IC50 = 0.45±0.05 μM in the cell extract and 0.20±0.19 μM in the PURE system) revealing this antibiotic as a potent inhibitor of translation.
Table 1.
Minimal inhibitory concentrations (MIC) of AMI.
| Strain (mutation) | MIC μg/mL, Amicoumacin A |
|---|---|
| SQ110DTCa | 0.5 |
| SQ110DTC (rrsE A794G) | >64 |
| SQ110DTC (rrsE C795U) | 64 |
| SQ171DTCb | 0.5 |
| SQ171DTC (rrsB A794G) | >64 |
| SQ171DTC (rrsB A794C) | >64 |
| SQ171DTC (rrsB A794U) | >64 |
| JM109 (ΔtolC) | 0.5 |
| BW25113 | 32 |
| JW0050c | 64 |
| JW5503d | 0.5 |
| JW5503 (fusA G542V) | 7 |
| JW5503 (fusA Ins544V) | 5 |
| JW5503 (fusA G581A) | 4 |
| JW5503 (ksgA Δ[424-437]) | 1 |
| JW5503 (pCA24fusA) | 0.5 |
| JW5503 (pCA24fusAG542V) | 4 |
| JW5503 (pCA24fusAIns544V) | 2 |
| JW5503 (pCA24fusAG581A) | 2 |
MG1655 ΔtolC, Δ(rrnA, rrnB, rrnC, rrnD, rrnG, rrnH)
MG1655 ΔtolC, Δ(rrnA, rrnB, rrnC, rrnD, rrnE, rrnG, rrnH), pAM552
BW25113 ΔksgA
BW25113 ΔtolC
Protein synthesis can be hindered due to interference with the activity of the ribosome or any of the various other enzymes associated with protein production (translation factors, aminoacyl-tRNA synthetases, etc). Therefore, in order to identify the true target of AMI action, we selected resistant mutants of a recently developed E. coli strain, particularly well-suited for identifying resistance mutations not only in the genes encoding proteins, but also in the genes encoding rRNA (Orelle et al., 2013a). The strain SQ101TDC lacks 6 out of 7 E. coli rrn alleles encoding rRNA. In addition, it is hypersusceptible to many antibiotics due to the lack of the tolC gene, which encodes the outer membrane protein component of the major E. coli efflux pump. Applying 109 SQ101TDC cells to an agar plate containing 2.5 μg/mL AMI (5-fold MIC) led to the appearance of several resistant colonies. In 8 out of 9 randomly selected colonies, the 16S rRNA contained a single A794G mutation, whereas one clone contained a mutation of the neighboring nucleotide C795U. MIC testing confirmed that the C795U mutation increased AMI resistance 128-fold compared to the wild type, whereas the A794G mutation conferred even higher levels of resistance (Table 1). In order to verify that rRNA mutations were responsible for resistance, three possible nucleotide substitutions at position A794 were introduced into the 16S rRNA gene of the rrnB operon of the pAM552 plasmid. The plasmid was then transformed into the tolC− mutant version of the SQ171 strain (Bollenbach et al., 2009; Kannan et al., 2012) where it served as a sole source of rRNA. The AMI MIC value for the engineered strains matched that of the originally selected SQ110TDC mutants, confirming that the presence of the 16S rRNA mutations was sufficient to cause resistance and revealing the ribosome as the likely target of AMI action in the bacterial cell.
AMI binding site on the ribosome
To unambiguously identify the mode of binding and action of AMI, we co-crystallized Thermus thermophilus 70S ribosome with mRNA and all three tRNAs and soaked the obtained crystals with 250 μM solution of AMI (because AMI exhibits poor stability in the crystallization solution over the time-course of crystal growth, cocrystallization with AMI was impractical). The stabilizing effect of AMI made it possible to solve the structure of its complex with the bacterial 70S ribosome at 2.4 Å resolution, the highest resolution reported so far for the bacterial 70S ribosome in complex with mRNA and tRNA ligands. The structure was solved by molecular replacement using atomic coordinates of the T. thermophilus 70S ribosome (PDB entries: 4QCM, 4QCN (Polikanov et al., 2014)). Coordinates for AMI were not included in the initial search model and the initial unbiased difference electron density map calculated with the Fobs–Fcalc amplitudes was used to localize the antibiotic.
Strong peaks of positive electron density (Figure 1B) resembling distinct features of the AMI chemical structure (Figure 1A) were observed on the 30S ribosomal subunit between the mRNA backbone in the E site and 16S rRNA (Figure 2). The electron density corresponding to AMI was present in both copies of the ribosome in the asymmetric unit. An atomic model of AMI generated from its known chemical structure, and the restraints based on idealized 3D geometry, were used to fit AMI into the obtained electron density. The final model of AMI, along with difference Fourier maps, are shown in Figure 1B. The statistics for data processing and refinement are shown in Table 2.
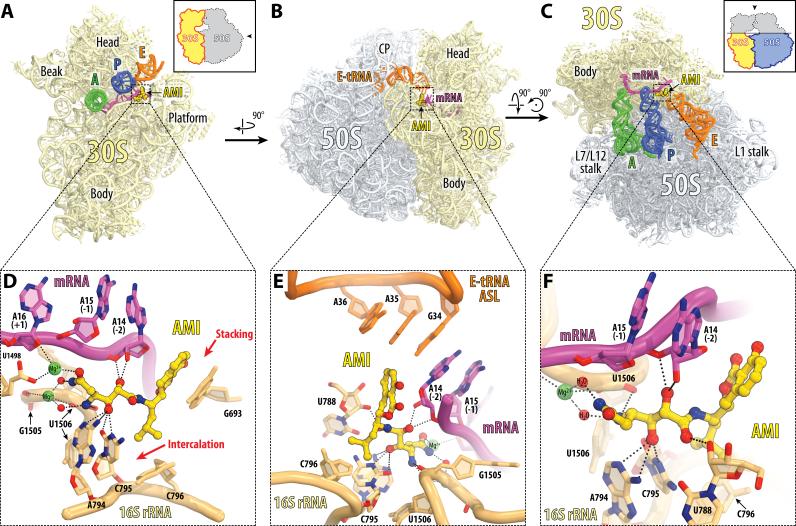
Figure 2. AMI binding site on the 70S ribosome.
(A, B, C) Overview of the AMI binding site (yellow) on the T.thermophilus 70S ribosome viewed from three different perspectives. 30S subunit is shown in light yellow, 50S subunit is in light blue. mRNA is shown in magenta and tRNAs are displayed in green for the A-site, in dark blue for the P-site, and in orange for the E-site. In (A), only the anticodon stems of tRNAs are shown and the 30S subunit is viewed from the intersubunit interface (50S subunit is removed for clarity), as indicated by the inset. The view in (B) is from the cytoplasm onto the E site. The view in (C) is from the top after removing the head of the 30S subunit and protuberances of the 50S subunit, as indicated by the inset. The anticodon stem loop (ASL) of the E-site tRNA is omitted for clarity in (C). (D, E, F) Close-up views of the AMI binding site shown in panels (A), (B), and (C), respectively. Nitrogens are colored blue, oxygens and magnesium-coordinated waters – red, magnesium ions – green. E.coli numbering of the nucleotides in the 16S rRNA is used. See also Figure S1 and Movie S1.
Table 2.
X-ray diffraction data collection and refinement statistics.
| Crystals | 70S-AMI (Soak) | 70S-PCT (Soak) | 70S-PCT (Co-Cryst.) | |
|---|---|---|---|---|
|
Diffraction data
| ||||
| Space Group | P212121 | P212121 | P212121 | |
| Unit Cell Dimensions, Å (a × b × c) | 210.06 × 448.57 × 623.95 | 209.35 × 449.01 × 621.98 | 208.93 × 446.95 × 619.78 | |
| Wavelength, Å | 0.9795 | 0.9795 | 0.9792 | |
| Resolution range (outer shell), Å | 312-2.40 (2.46-2.40) | 311-2.55 (2.62-2.55) | 255-2.70 (2.77-2.70) | |
| I/σI (outer shell with I/σI=1) | 9.89 (1.01) | 7.61 (1.00) | 10.50 (1.07) | |
| Resolution at which I/σI=1 , Å | 2.40 | 2.55 | 2.70 | |
| Resolution at which I/σI=2, Å | 2.60 | 2.78 | 2.88 | |
| CC(1/2) at which I/σI=1, % | 23.5 | 21.0 | 27.6 | |
| Completeness (outer shell), % | 99.7 (99.2) | 99.0 (99.4) | 99.6 (99.5) | |
| Rmerge (outer shell)% | 19.3 (198.1) | 13.9 (124.5) | 18.3 (188.1) | |
| No. of crystals used | 2 | 1 | 2 | |
| No. of Reflections | Observed | 18,807,110 | 6,265,550 | 10,528,583 |
| Used: | Unique | 2,253,324 | 1,857,656 | 1,560,047 |
| Redundancy | 8.35 | 3.37 | 6.75 | |
| Wilson B-factor, Å2 | 45.9 | 48.2 | 53.5 | |
|
Refinement | ||||
| Rwork/Rfree, % | 23.7/27.7 | 22.5/27.3 | 21.5/26.2 | |
| No. of Non-Hydrogen Atoms | ||||
| RNA | 201,135 | 200,019 | 194,597 | |
| Protein | 90,998 | 91,181 | 91,130 | |
| Ions (Mg, K, Zn, Fe) | 2,337 | 2,164 | 2,073 | |
| Waters | 3,351 | 3,070 | 2,763 | |
| Ramachandran Plot | ||||
| Favored regions, % | 95.71 | 93.27 | 92.61 | |
| Allowed regions, % | 3.39 | 5.53 | 6.04 | |
| Outliers, % | 0.90 | 1.20 | 1.35 | |
| Deviations from ideal values (RMSD) | ||||
| Bond, A | 0.005 | 0.005 | 0.007 | |
| Angle, degrees | 0.966 | 1.033 | 1.084 | |
| Chirality | 0.042 | 0.044 | 0.044 | |
| Planarity | 0.005 | 0.005 | 0.005 | |
| Dihedral, degrees | 16.412 | 16.270 | 16.442 | |
| Average B-factor (overall), Å2 | 54.0 | 58.2 | 59.7 | |
Rmerge = Σ |I – <l>| / Σ I, where I is the observed intensity and <I> is the average intensity from multiple measurements.
Rwork = Σ|Fobs – Fcalc| / Σ Fobs. For calculation of Rfree, 5% of the truncated dataset was excluded from the refinement.
The high resolution of the structure and the presence of the ribosomal ligands made it possible to interpret interactions of the drug with its target in fine detail. In agreement with the location of resistance mutations, AMI binds to the platform of the 30S subunit where it interacts with both 16S rRNA and mRNA (Figures 2A-C; Movie S1). The long hydrophilic 2,3-dihydroxy-5-aminohexandiamide tail of AMI forms multiple hydrogen bonds with the nearly universally conserved 16S rRNA bases U788, A794, and C795 in the loop of the helix 24 (h24) (Figures 2D, 2E, 2F and S1). The terminal amide moiety of AMI forms magnesium-mediated contacts with the phosphate groups of nucleotides G1505 and U1506 (Figures 2D, 2E, 2F and S1). On the opposite side of the binding pocket, the same tail of AMI interacts with the backbone of the nucleotides −1 and −2 (relative to the AUG start codon) of the mRNA, thus “affixing” the E-site codon of mRNA to h24 of the 16S rRNA. The hydrophobic isobutyl extension of AMI intercalates between the bases of C795 and C796 nucleotides (Figures 2D and S1), whereas its large, flat isocoumarin moiety further anchors AMI at the platform of the small subunit by π-stacking with the nucleobase G693 (Figures 2D and S1). Although AMI binds in the E site of the small ribosomal subunit, it does not directly interact with the E-site tRNA (Figure 2E).
The observed location of AMI on the ribosome suggests a possible mechanism of inhibition: by interacting simultaneously with both mRNA and h24, AMI tethers mRNA to 16S rRNA. This interaction is expected to hamper the movement of mRNA relative to the small ribosomal subunit.
The mode of AMI action
We analyzed the mode of AMI action by examining its effects on protein synthesis both in vivo and in vitro. We have previously designed a cell-based reporter system to test the activity of translation elongation inhibitors (Osterman et al., 2012). The reporter utilizes the attenuator of the tryptophan biosynthesis operon: slow progression of the translating ribosomes through the mutant leader ORF activates expression of the downstream gene encoding cerulean fluorescent protein, which can be detected in a spot diffusion assay. When E. coli cells carrying the reporter were exposed to AMI, the expression of the fluorescent protein was strongly activated indicating that the drug decreases the rate of movement of the ribosomes along the leader ORF (Figure 3A). Among other possibilities, this effect is compatible with AMI inhibiting ribosome translocation.
Figure 3. AMI is a likely inhibitor of ribosome translocation.
(A) Induction of a pRFPCER-TrpL2A reporter, sensitive to inhibitors of the ribosome progression. Spots of PCT 1.2 μg, erythromycin 1.5 μg (ERY) and AMI 0.5 μg were placed on the surface of an agar plate containing E. coli cells transformed with the reporter plasmid. Green circles surrounding inhibition zones correspond to induction of cer gene expression. ERY (a positive control) interferes with the ribosome progression along mRNA; PCT (a negative control) inhibits translation initiation (Wilson, 2014). (B) Toe-printing analysis of inhibition of protein synthesis in the PURE system by antibiotics binding in the vicinity of h24 in 16S rRNA, AMI, PCT and EDE. The control lanes contain no antibiotic (“no drug”) or thiostrepton (THS) which arrests ribosome at the start codon (Orelle et al., 2013a; Vazquez-Laslop et al., 2011). Antibiotics THS, PCT and EDE were present in the reaction at 100 μM, AMI – 500 μM. The sequence of mRNA is shown on the left. The band corresponding to the ribosome occupying the initiator codon is indicated by the black triangle. (C) The inhibitory action of AMI in a model translocation assay in which the movement of the ribosome from the initiator codon to the second codon of the phage T4 gene 32 is monitored by toe-printing (Shoji et al., 2006). The translocation reaction, initiated by addition of EF-G, was terminated after 30 seconds while translocation in the absence of the inhibitor is still incomplete. The extent of the inhibition of translocation by AMI was calculated relative to the ‘no AMI’ control, which was taken as 100%. Data are represented as mean +/− SEM. Triangles indicate the toe-printing bands corresponding to the position of the ribosome at the initiator codon (white triangle – empty A-site; grey triangle – Phe-tRNAPhe-occupied A-site) or the ribosome translocated to the second codon (black triangle). The intensities of the bands indicated by grey and black triangles were used for calculating the extent of translocation. (D) Inhibition of in vitro synthesis of firefly luciferase (white bars) by AMI (black bars) without or with addition of extra 1 μM of EF-Tu, wild type EF-G or of the G542V mutant EF-G. Data are represented as mean +/− SEM. See also Figures S2 and S3.
The addition of a high concentration of AMI to the cell-free translation reaction prior to initiation of protein synthesis freezes the ribosome at the initiator codon (Figure 3B). In contrast to edeine B (EDE), which inhibits formation of the initiation complex, AMI allows proper binding of the ribosome to the initiator AUG codon. However, AMI prevents ribosome departure from the start codon. At lower concentrations, AMI was able to slow progression of the ribosome through the internal codons of the gene, leading to the appearance of several weak toe-printing bands lacking in the sample without the antibiotic (Figure S2); this effect could be counterbalanced by increasing the concentration of EF-G in the translation reaction (data not shown). These results are consistent with the possibility that AMI inhibits translocation.
To directly test whether AMI is able to inhibit translocation, we used a toe-printing-based translocation assay where the P-site of the ribosome is prefilled with tRNAiMet, N-acetyl-Phe-tRNAPhe is placed in the A-site and translocation is catalyzed by the elongation factor EF-G in the absence or presence of antibiotics (Shoji et al., 2006). As can be seen in Figure 3C, AMI inhibits relocation of the ribosome from the Met codon to the second Phe codon in a concentration-dependent manner, confirming that inhibition of translocation is an important component of the antibiotic action.
Mutations in translation elongation factor can partially alleviate the AMI inhibitory action
In addition to biochemical data, the results of mutational analysis also point to translocation as the primary mode of action of AMI. In a recent study, multiple mutations were identified in the genome of S. aureus exposed to subinhibitory concentrations of AMI, including a few identified in the fusA gene encoding elongation factor EF-G (Lama et al., 2012). However, the multitude of other mutations identified in those studies made it difficult to establish a causative link between the EF-G mutations and AMI resistance. Because our structural and biochemical data strongly point to translocation as the reaction inhibited by AMI, we were curious as to whether the EF-G mutations could indeed overcome, at least partially, the effect of the antibiotic on translation. To this end, we repeated our selection of AMI-resistant mutants, this time utilizing the E. coli strain carrying intact copies of the seven chromosomal rrn alleles. The redundancy of rRNA genes prevents isolation of recessive rRNA mutations, confining the possible resistance mutations primarily to single copy genes. The E. coli strain JW5503 that lacks the tolC gene (Baba et al., 2006) was plated onto the agar plate containing 4 μg/mL AMI. Several resistant clones grew on the plate after 40-hr incubation at 37°C. The fusA gene was PCR-amplified from 7 resistant clones and sequenced. Four of the seven mutants carried nucleotide alterations leading to amino acid changes in the segment of the fusA gene encoding domain IV of EF-G: G542V (two clones), G581A (one clone), and an insertion of an extra valine residue before V544 (one clone) (Figure S3). Of these, only the G542V was observed previously in S. aureus (Lama et al., 2012). In order to verify that the EF-G domain IV mutations were sufficient to increase MIC, the mutant fusA gene variants were cloned and expressed in the parental JW5503 strain. Ectopic expression of mutant fusA was sufficient to confer 4-14 fold higher MIC values (Table 1). We purified EF-G carrying the G542V mutation and tested it for an ability to suppress inhibition of in vitro translation of firefly luciferase by AMI (Figure 3D). Increasing concentration of the wild type EF-G (but not EF-Tu) partially relieved AMI-dependent inhibition of luciferase synthesis. Substitution of the wild-type EF-G with G542V-mutant resulted in a nearly 3-fold increase in the yield of the active luciferase synthesized in the presence of AMI. These data demonstrate that the G542V-mutant can partially counterbalance the inhibition of translocation by AMI both in vivo and in vitro.
Of the three analyzed AMI-resistant clones that lacked fusA mutations, two clones exhibited increased resistance to several antibiotics indicating that the mutations might affect intracellular drug accumulation rather than its action (data not shown). The last resistant clone carried a 14 nucleotide deletion (Δ424-437) in the ksgA gene. This mutation likely inactivates KsgA methyltransferase and, therefore, should prevent dimethylation of 16S rRNA residues A1518/A1519 located in a close vicinity of the AMI binding site (see Discussion). To determine if inactivation of ksgA is sufficient to render cells resistant to AMI, we evaluated the MIC of the ΔksgA strain JW0050 from the E. coli knockout Keio collection and compared it to the MIC of the BW25113 parental strain (Baba et al., 2006). Inactivation of KsgA methyltransferase increased the MIC value approximately two-fold (Table 1) suggesting that the lack of A1518/A1519 dimethylation affects either binding or action of AMI.
AMI and pactamycin (PCT) bind to the same site but exhibit different modes of action
The AMI binding site on the ribosome overlaps with that of another antibiotic – pactamycin (PCT) (Figure S4A), which was previously suggested to influence translocation (Dinos et al., 2004). While binding of PCT or its derivative, de-6-MSA-pactamycin, to the 30S ribosomal subunit from T. thermophilus have been analyzed crystallographically in previous studies (Brodersen et al., 2000; Tourigny et al., 2013), its structure in complex with 70S ribosome carrying mRNA and tRNAs was unknown. To bridge this gap, we determined the structure of the T. thermophilus 70S ribosome bearing mRNA/tRNAs complexed with PCT. Interestingly, co-crystallization and soaking experiments yielded slightly different orientations of PCT in its binding site on the small ribosomal subunit (Figures S4B and S4C; Movie S2). When stabilized crystals were soaked in the antibiotic solution, PCT appeared in a conformation almost identical to that reported previously (Brodersen et al., 2000) (Figure S4D). Besides the density for PCT, this structure solved at 2.55 Å resolution contained all three tRNAs and mRNA, whose (−1) and (−2) nucleotides are slightly displaced from their normal locations (Figure S4E). Surprisingly, when PCT was co-crystallized with 70S ribosomes, it appeared in a more extended conformation (Figure S4D), which leads to a more severe displacement of mRNA (Figure S4F). As a result of the mRNA shift, the stacking contact between mRNA and the E-site tRNA becomes broken and the E-site tRNA disappeared from the density (compare panels (E) and (F) in Figure S4). Even though all tRNAs and PCT were included in the crystallization mixture, the structure solved at 2.7 Å resolution contained no A-site tRNA.
Taken together, our structural data suggest that two distinct antibiotics – AMI and PCT – bind to overlapping sites, but most likely exhibit principally different mechanisms of inhibition of translation with PCT displacing mRNA from its normal path and AMI fastening mRNA to the ribosome. Consistent with these results, fusA mutations that conferred AMI resistance had no effect upon the MIC of PCT, indicating distinct modes of action of these two antibiotics.
DISCUSSION
Our results establish that AMI is a new translation inhibitor whose chemical scaffold is distinct from the other known antibiotics targeting protein synthesis. AMI binds to the ribosome in a unique way and inhibits translation by a novel mechanism.
The location of AMI adjacent to the undisturbed mRNA and 16S rRNA in the E-site of the small ribosomal subunit suggests that, unlike many other ribosome inhibitors that act by altering the structure of the ribosomal functional centers or clashing with its ligands, AMI functions differently. The structure suggests that AMI interferes with translocation by locking the mRNA in the mRNA-binding channel of the 30S subunit. Simultaneous interaction of AMI with both 16S rRNA and mRNA should impede their relative motion and thus will increase the activation energy of translocation. In addition to increasing the “friction” between mRNA and the ribosome, the drug may decrease the general conformational flexibility of the 30S subunit required for efficient translocation. The possibility that AMI rigidifies the ribosome and thus limits motions of its domains, especially the head of the 30S subunit, is consistent with the observed high resolution of diffraction to 2.4 Å that indicates high degree of order in the corresponding crystals compared to the crystals without AMI (data not shown). Furthermore, binding of AMI may stabilize the ridge between nucleotides 790 and 1338 of the 16S rRNA, which separates P- and E-site bound tRNAs (Schuwirth et al., 2005) and whose opening is likely a prerequisite for translocation (Yamamoto et al., 2014).
The base-specific interactions of AMI with 16S rRNA in its binding site are confined to conserved rRNA residues, indicating that AMI should interfere with bacterial, eukaryotic and archaeal translation. Indeed, AMI readily inhibits translation in the rabbit reticulocyte cell-free translation system as well as growth of halophylic archaea (data not shown). The ribosome-targeting activity of AMI in eukaryotic cells could be the reason for its reported anticancer and anti-inflammatory properties (Canedo et al., 1997; Itoh et al., 1981).
Several different mechanisms account for bacterial resistance to AMI. While mutations of 16S rRNA residues 794 and 795 in the site of antibiotic action directly interfere with the binding of AMI to the ribosome, the mutation in the ksgA gene that eliminates dimethylation of the adenine residues 1518 and 1519 in the 16S rRNA helix 45 likely achieves the same effect by allosterically altering the conformation of the AMI binding site. A similar explanation was proposed for the effect of KsgA inactivation on binding of the antibiotic kasugamycin (KSG) (Schluenzen et al., 2006), whose site of action (Demirci et al., 2010) partially overlaps with that of AMI (Figure 4D). Because of a small conformational change observed in this region in the T. thermophilus small ribosomal subunit lacking A1518/A1519 methylation (Demirci et al., 2010), it is possible that the lack of the ksgA mutation can additionally neutralize AMI action by facilitating the opening of the 790-1338 ridge required for translocation. In contrast to these ribosomal mutations, the resistance that is associated with mutations in EF-G must be based upon a different mechanism. The tip of EF-G domain IV, where the AMI-resistance mutations are located, does not come into close proximity with the AMI binding site in the ribosome (Figure S3). One possibility is that the mutant EF-G protein acts similarly to TetO/TetM ribosome protection proteins, whose transient binding promotes dissociation of the antibiotic from its ribosomal binding site, thereby shifting the chemical equilibrium towards a drug-free ribosome (Nguyen et al., 2014). Alternatively, such mutations may change functional properties of EF-G, helping it counteract the antibiotic action.
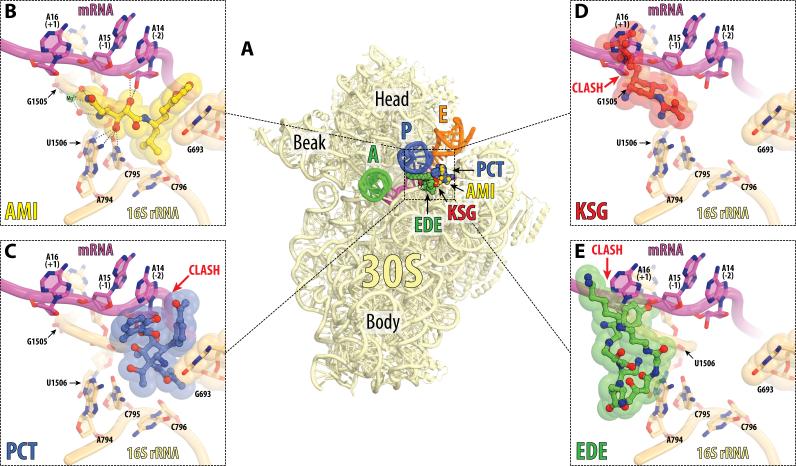
Figure 4. Antibiotics in the E site on the small ribosomal subunit.
(A) Overview of the superimposed binding sites of AMI (yellow), PCT (blue), KSG (red), and EDE (green) on the 30S subunit. The view and the coloring of 16S rRNA, mRNA and tRNAs are the same as in Figure 2A. AMI and PCT structures are from the current work, KSG is from PDB entry 1VS5 (Schuwirth et al., 2006), and EDE is from PDB entry 1I95 (Pioletti et al., 2001). All four structures were aligned based on h24 of the 16S rRNA (nucleotides 769-810). (B, C, D, E) Close-up views of the binding sites shown in (A) for AMI, PCT, KSG, and EDE, respectively. Steric clashes between antibiotics and parts of the ribosome are indicated by red arrows. Note, that AMI tethers mRNA to the 16S rRNA and does not clash with any parts of the ribosome, while PCT, KSG and EDE clash with mRNA. See also Figure S4 and Movie S2.
The AMI binding site in h24 of 16S rRNA overlaps with that of three other previously known antibiotics: PCT, KSG and EDE (Figures 4). PCT is generally assumed to be an inhibitor of translation initiation. However, inhibition of translocation was also suggested (Dinos et al., 2004). Toe-printing analysis demonstrated that, similar to AMI, PCT freezes the ribosome at the initiator codon during cell-free translation (Figure 3B). However, these two antibiotics bind to the ribosome in significantly different ways. Comparison of our structure of the AMI-ribosome complex to the newly determined structure of PCT bound to programmed T. thermophilus 70S ribosomes shows that in contrast to AMI, PCT does not contact either A794 or G1505 (compare panels (B) and (C) in Figure 4). While both antibiotics stack upon the nucleobase of G693, PCT occludes significantly more space than AMI, due to its second 6-methylsalicylyl aromatic ring. As a result, and unlike AMI, PCT displaces mRNA from its normal position in the E site of the small subunit, leading to disruption of mRNA contacts with the E-site tRNA (Figure S4F). Consistent with our structural data, PCT was previously suggested to interfere with accommodation of the tRNA into the E site (Dinos et al., 2004). In contrast, AMI stabilizes the conformation of mRNA typically seen in the structures of programmed 70S ribosomes with bound A-, P-, and E-site tRNAs. This clear difference between the modes of action of AMI and PCT is further supported by the lack of resistance to PCT of the AMI-resistant fusA mutants. Therefore, in spite of their overlapping binding sites, AMI and PCT exhibit nearly opposite modes of action: one stabilizes mRNA in the E site while the other one displaces it.
Importantly, in our structural studies we used mRNA and tRNA species which cannot form base-pairing interactions in the E site of the ribosome. This resembles the ribosome's in vivo state right before the first act of translocation after productive initiation, when there should be no codon-anticodon interactions in the E site. A previous crystal structure of the ribosome with a cognate E-site tRNA (Jenner et al., 2010) revealed a slightly different mRNA path as compared with mRNA paths in current AMI or PCT structures. Both AMI and PCT clash with this path. This observation is consistent with the proposed mode of action for PCT, known as initiation inhibitor. Likely, PCT is unable to bind to the elongating ribosome, whose E site, most of the time, is occupied with the cognate tRNA. In contrast, our results (Figure S2) show that AMI can exert its inhibitory action even on the ribosome translating the internal codons of the gene. It is conceivable that kinetic or thermodynamic parameters of AMI binding account for its ability to inhibit translation elongation. A faster association rate would be favorable for invading the E-site transiently devoid of tRNA; alternatively, tighter binding of AMI to the ribosome could stimulate mRNA displacement and inhibition of translocation even when cognate tRNA is present in the E-site. Currently available data do not allow distinguishing between these possibilities.
Two other antibiotics that bind in the vicinity of h24 are KSG (Schluenzen et al., 2006) and EDE (Pioletti et al., 2001). Their modes of action are also principally different from that of AMI. Both KSG and EDE sterically clash with the mRNA codon located in the P site of the ribosome and interfere with mRNA binding and with the formation of the translation initiation complex (Figures 4D and 4E), evident in the case of EDE from the lack of an initiation complex toe-print (Figure 3B).
Overlap of the binding sites of four chemically unrelated antibiotics (AMI, PCT, KSG and EDE) could be used for designing new antibiotic hybrids with potentially superior antibacterial properties. The several hydroxyl, amino and amido groups that are present in the relatively simple AMI chemical structure offer obvious venues for its derivatization that can be used to improve its activity and possibly provide selectivity to its action. The lack of known resistance enzymes that act upon h24 and the fact that only rRNA mutations, which are rarely seen in clinical pathogens, confer high level of resistance, make this compound attractive for exploration by medicinal chemists. Such exploration can be assisted by structural information that we provided here and can be stimulated by the unusual mode of action of this novel protein synthesis inhibitor.
EXPERIMENTAL PROCEDURES
AMI purification
The AMI-producing bacterial strain was isolated from a sample of leached chernozem soil (Krasnodar region, Russia). It was identified using a dual fluorescent protein reporter (Osterman et al., 2012) and was classified as Bacillus pumilus on the basis of 16S rRNA sequencing. The strain was deposited at the Culture Collection of the Gause Institute of New Antibiotics, Russian Academy of Medical Sciences, under the numbered INA 01087.
For AMI isolation, B. pumilus INA 01087 culture was grown in a 750-mL flask with 100 mL medium containing 1% glucose, 0.5% peptone, 0.3 % tryptone, 0.5% NaCl, pH 7.2-7.4. The medium was inoculated with 105/mL of spore suspension prepared by growing cells on agar plates containing the same medium and grown at 37°C. Cultures were then grown for 48 hrs at 28°C with constant shaking at 220 rpm. Antibiotic was isolated from culture supernatant by adsorption on Amberlyte XAD- 2, followed by desorption with n-butanol-acetone-water mixture (1:1:1) at neutral pH. The obtained eluates were evaporated at 37°C and the dry residue was dissolved in 60% ethanol. Chromatographic pre-fractionation was carried out on Kieselgel 60 column (Merck), using the gradient elution from chloroform to chloroform-ethanol (7:3). Final chromatographic purification was on Phenomenex HPLC column Luna 5u C18(2) 100A 250x4.60mm. AMI and amicoumacin B were separated in acetonitrile gradient (0-80%) in ammonium acetate buffer (pH 7.5). Amicoumacin-containing fractions were lyophilized, frozen and stored in the dark at −70°C. The purity of AMI was verified by mass spectrometry in the MALDI-TOF-TOF mode.
Crystallographic structure determination
Tth 70S ribosomes, unmodified tRNAiMet and tRNAPhe from E. coli were purified as previously described (Junemann et al., 1996; Polikanov et al., 2012; Schmitt et al., 1999). Synthetic mRNA with the sequence 5’-GGC AAG GAG GUA AAA AUG UUC UAA-3’ was obtained from IDT (Coralville, IA).
Ribosome-mRNA-tRNA complexes were formed by programming of 5 μM 70S Tth ribosomes with 10 μM mRNA and incubation at 55°C for 10 minutes, followed by addition of 20 μM P- and A-site tRNA substrates (with minor changes from (Voorhees et al., 2009)). Each of the last two steps was allowed to reach equilibrium for 10 minutes at 37°C. In the co-crystallization experiments with PCT (Sigma), the antibiotic was added to a final concentration of 100 μM and the complex was left at room temperature for an additional 15 minutes prior to crystallization. For soaking experiments with either AMI or PCT, the antibiotics were not added at this point, and instead were included into the crystal-stabilization solutions at a later step. The programmed 70S ribosomes were crystallized in the buffer containing 5 mM HEPES-KOH (pH 7.6), 50 mM KCl, 10 mM NH4Cl and 10 mM Mg(CH3COO)2.Crystals were grown by the vapor diffusion method in sitting drops at 19°C and stabilized as described (Polikanov et al., 2012) with antibiotics included in the stabilization buffers (250 μM AMI or 100 μM PCT). Diffraction data were collected using beamline 24ID-C at the Advanced Photon Source and beamline X25 at the Brookhaven National Laboratory. All crystals belonged to the primitive orthorhombic space group P212121 with approximate unit cell dimensions of 210Å × 450Å × 620Å and contained two copies of the 70S ribosome per asymmetric unit. The initial molecular replacement solutions were refined by rigid body refinement with the ribosome split into multiple domains, followed by positional and individual B-factor refinement. The statistics for data processing and refinement are shown in Table 2.
See also Supplemental Experimental Procedures for detailed structure determination protocol.
Testing in vivo activity of AMI
MIC values were determined by serial dilutions in 96-well plates using exponential E. coli cultures in LB medium with the starting density of A650= 0.002. After addition of antibiotic, the plates were incubated overnight at 37°C and cell growth was assessed by visual inspection.
Macromolecular synthesis inhibition experiments were carried out as previously described (Cunningham et al., 2013) using the ΔtolC variant of the E. coli strain JM109. The in vivo effect of AMI on translation elongation activity was tested by spot diffusion assay using the pRFPCER-TrpL2A reporter as described (Osterman et al., 2012).
Selection of AMI resistance mutants
The single rrn allele, ΔtolC E. coli strain SQ110DTC (Orelle et al., 2013a) was grown overnight in LB medium supplemented with 50 μg/mL of kanamycin. Cells were diluted 100 fold into fresh LB, grown for 3 hours, and 1.2 A650 (ca. 109) cells were plated on an LB agar plate containing 50 μg/mL kanamycin and 2.5 μg/mL AMI. Several colonies appeared after a 24-hr incubation. rDNA was PCR-amplified from 9 colonies and sequenced.
To screen for the non-rRNA AMI resistant mutations, 109 cells of the overnight culture of the E. coli strain JW5503 (Baba et al., 2006) were plated on LB-agar plates supplemented with 4 μg/mL AMI. Plates were incubated 40 hr at 37°C. The fusA and ksgA genes from resistant clones were PCR-amplified using primers (CCTTCAGGAGAGAGCACGG) and (CGGTGTGGTTAACTCTGG) for fusA, or (CAATGTAGACGCTTTGAACCTG) and (GGCGCTTAATCTCGCCATC) for ksgA, and the resulting PCR products were sequenced.
Testing AMI resistance mutations
All three possible mutations at position A794 were introduced by site directed mutagenesis in the 16S rRNA gene in plasmid pAM552 (a derivative of pLK35 (Douthwaite et al., 1989)) containing the entire E. coli rrnB operon under the control of the PL promoter. The mutagenized plasmids were initially transformed in the POP2136 cells which were grown at 30°C to prevent expression of the mutant rrnB operon (Rottmann et al., 1988). Once mutant plasmids were identified, they were transformed into the SQ171ΔtolC (SQ171DTC) strain and the cells were then cured of wild type plasmid pCSacB by plating onto an LB/Amp plate supplemented with 5% sucrose (Zaporojets et al., 2003). The complete replacement of wild type plasmid with the mutant one and expression of a homogeneous population of mutant ribosomes was verified by primer extension. MIC values of the engineered mutants were tested by microbroth dilution.
The plasmids pCA24fusAG542V, pCA24fusAIns544V and pCA24fusAG581A expressing mutant forms of EF-G were generated by PCR-amplifying mutant fusA genes from the respective AMI-resistant clones using primers (CCTTCAGGAGAGAGCACGG) and (CGGTGTGGTTAACTCTGG) and cloning the PCR product between DraIII and AhdI restriction sites of the plasmid pCA24fusA (Kitagawa et al., 2005). The resulting plasmids, along with the parent pCA24fusA plasmids, were transformed into the E. coli strain JW5503 (Baba et al., 2006). Cells were grown in LB media supplemented with 34 μg/mL chloramphenicol and expression of fusA was induced by 1 mM IPTG. MIC values were determined as described.
In vitro translation and toe-printing analysis
Translation of superfolder GFP was carried out using the PURExpress system (New England Biolabs). The reactions (10 μL), assembled according to the manufacturer's protocol, were supplemented with 100 ng of PCR-amplified sfGPF template and antibiotic, when needed. The reactions were placed in the 384-well black-wall plate and the progression of the reactions was monitored over 5 hours by a TECAN microplate scanner. The 3-hr time point, which corresponded to the kinetic slope of the translation reaction, was used for calculating the IC50 values.
The in vitro transcribed firefly luciferase mRNA was translated in the E. coli S30 cell-free system prepared according to (Svetlov et al., 2006). Reactions programmed with 200 μg mRNA were carried out in 10 μL at 37°C for 30 min and the activity of in vitro synthesized luciferase was assessed using the Steady-Glo® Luciferase Assay System (Promega). When required, the concentration of EF-G was increased to 1 μM by addition of purified wild type or mutant variants of the elongation factor G.
Toe-printing analysis was carried out as previously described (Orelle et al., 2013a; Vazquez-Laslop et al., 2008), using the synthetic RST1 gene as a template for protein translation (Orelle et al., 2013b).
Translocation assay was carried out using the phage T4 gene 32 template (m291) as described by (Shoji et al., 2006). Briefly, tight-coupled ribosomes (0.7 μM) were incubated with 0.5 μM mRNA and 1 μM tRNAiMet for 20 min at 37°C in the Pure System Buffer (5 mM potassium phosphate (pH 7.3), 9 mM Mg(CH3COO)2, 95 mM potassium glutamate, 5 mM NH4Cl, 0.5 mM CaCl2, 1 mM spermidine, 8 mM putrescine, 1 mM dithiothreitol) and then for 10 min at 37°C with 2 μM of N-acetyl-Phe-tRNAPhe. When needed, AMI was included at the time of N-acetyl-Phe-tRNAPhe addition. The reaction volume was 5 μL. The translocation reaction was initiated by addition of 1 μL EF-G/GTP mixture. After 30 seconds of incubation at 37°C, 2 μL of reverse transcriptase/dNTP mixture was added and the reactions were incubated for 3 more minutes at 37°C. The reactions were stopped by the addition of 200 μL of the stop buffer (0.3 M sodium acetate (pH 5.5), 5 mM EDTA, 0.5% SDS) and phenol extraction. After ethanol precipitation, the reaction products were analyzed in sequencing gels.
Supplementary Material
ACKNOWLEDGMENTS
We thank the staff at the Advanced Photon Source (beamline 24ID) and at the National Synchrotron Light Source (beamline X25) for help during data collection, and the staff at the Richards Center at Yale University for computational support. We thank N. Vazquez-Laslop, C. Orelle, A. Ochabowicz, J. Lin, and M. Gagnon for their help, advice and critical reading of the manuscript, R.L. Grodzicki for preparation of the unmodified tRNAs and critical reading of the manuscript, K. Fredrick for providing the template for translocation experiments and advice and members of the A.S.M., T.A.S., and P.V.S. laboratories for discussions. This work was supported by NIH grants GM104370 (A.S.M.), GM022778 (T.A.S.), RFBR grants 13-04-00836 (P.V.S.), 13-04-40212 (A.L.K.), 13-04-40211 (A.A.B.), 14-04-01061 (O.A.D.), RSF grant 14-14-00072 (P.V.S.) and Moscow State University development program PNR 5.13 (O.A.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
Y.S.P. and T.A.S. designed the crystallography experiments; I.A.O., P.V.S. and A.S.M. designed the biochemistry and genetics experiments; Y.S.P., I.A.O., T.S., V.N.T., P.K., T.A.E., O.V.E., I.A.M., D.B., A.L.K. and A.S.M. performed the experiments; Y.S.P., I.A.O., P.V.S., T.A.S., A.S.M., A.A.B., M.V.R., O.A.D, K.S.J., interpreted the results; A.S.M, P.V.S., Y.S.P. and T.A.S. wrote the manuscript.
ACCESSION NUMBERS
Coordinates and structure factors were deposited in the RCSB Protein Data Bank with accession codes 4RB5, 4RB6, 4RB7 and 4RB8 for the T. thermophilus 70S ribosome in complex with AMI (soaked); 4RB9, 4RBA, 4RBB and 4RBC for the T. thermophilus 70S ribosome in complex with PCT (soaked); 4RBD, 4RBE, 4RBF and 4RBG for the T. thermophilus 70S ribosome in complex with PCT (co-crystallized).
REFERENCES
- Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2006;2:2006–2008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollenbach T, Quan S, Chait R, Kishony R. Nonoptimal microbial response to antibiotics underlies suppressive drug interactions. Cell. 2009;139:707–718. doi: 10.1016/j.cell.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen DE, Clemons WM, Jr., Carter AP, Morgan-Warren RJ, Wimberly BT, Ramakrishnan V. The structural basis for the action of the antibiotics tetracycline, pactamycin, and hygromycin B on the 30S ribosomal subunit. Cell. 2000;103:1143–1154. doi: 10.1016/s0092-8674(00)00216-6. [DOI] [PubMed] [Google Scholar]
- Canedo LM, Fernandez Puentes JL, Perez Baz J, Acebal C, de la Calle F, Garcia Gravalos D, Garcia de Quesada T. PM-94128, a new isocoumarin antitumor agent produced by a marine bacterium. J. Antibiot. 1997;50:175–176. doi: 10.7164/antibiotics.50.175. [DOI] [PubMed] [Google Scholar]
- Cunningham ML, Kwan BP, Nelson KJ, Bensen DC, Shaw KJ. Distinguishing on-target versus off-target activity in early antibacterial drug discovery using a macromolecular synthesis assay. J. Biomol. Screen. 2013;18:1018–1026. doi: 10.1177/1087057113487208. [DOI] [PubMed] [Google Scholar]
- Demirci H, Murphy F.t., Belardinelli R, Kelley AC, Ramakrishnan V, Gregory ST, Dahlberg AE, Jogl G. Modification of 16S ribosomal RNA by the KsgA methyltransferase restructures the 30S subunit to optimize ribosome function. RNA. 2010;16:2319–2324. doi: 10.1261/rna.2357210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinos G, Wilson DN, Teraoka Y, Szaflarski W, Fucini P, Kalpaxis D, Nierhaus KH. Dissecting the ribosomal inhibition mechanisms of edeine and pactamycin: the universally conserved residues G693 and C795 regulate P-site RNA binding. Mol. Cell. 2004;13:113–124. doi: 10.1016/s1097-2765(04)00002-4. [DOI] [PubMed] [Google Scholar]
- Douthwaite S, Powers T, Lee JY, Noller HF. Defining the structural requirements for a helix in 23 S ribosomal RNA that confers erythromycin resistance. J. Mol. Biol. 1989;209:655–665. doi: 10.1016/0022-2836(89)93000-3. [DOI] [PubMed] [Google Scholar]
- Franceschi F, Duffy EM. Structure-based drug design meets the ribosome. Biochem. Pharmacol. 2006;71:1016–1025. doi: 10.1016/j.bcp.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Itoh J, Omoto S, Shomura T, Nishizawa N, Miyado S, Yuda Y, Shibata U, Inouye S. Amicoumacin-A, a new antibiotic with strong antiinflammatory and antiulcer activity. J. Antibiot. 1981;34:611–613. doi: 10.7164/antibiotics.34.611. [DOI] [PubMed] [Google Scholar]
- Itoh J, Shomura T, Omoto S, Miyado S, Yuda Y, Shibata U, Inouye S. Isolation, Physicochemical Properties and Biological Activities of Amicoumacins Produced by Bacillus pumilus. Agric. Biol. Chem. 1982;46:1255–1259. [Google Scholar]
- Jenner LB, Demeshkina N, Yusupova G, Yusupov M. Structural aspects of messenger RNA reading frame maintenance by the ribosome. Nat. Struct. Mol. Biol. 2010;17:555–560. doi: 10.1038/nsmb.1790. [DOI] [PubMed] [Google Scholar]
- Junemann R, Wadzack J, Triana-Alonso FJ, Bittner JU, Caillet J, Meinnel T, Vanatalu K, Nierhaus KH. In vivo deuteration of transfer RNAs: overexpression and large-scale purification of deuterated specific tRNAs. Nucleic. Acids Res. 1996;24:907–913. doi: 10.1093/nar/24.5.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan K, Vazquez-Laslop N, Mankin AS. Selective protein synthesis by ribosomes with a drug-obstructed exit tunnel. Cell. 2012;151:508–520. doi: 10.1016/j.cell.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Ara T, Arifuzzaman M, Ioka-Nakamichi T, Inamoto E, Toyonaga H, Mori H. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res. 2005;12:291–299. doi: 10.1093/dnares/dsi012. [DOI] [PubMed] [Google Scholar]
- Lama A, Pane-Farre J, Chon T, Wiersma AM, Sit CS, Vederas JC, Hecker M, Nakano MM. Response of methicillin-resistant Staphylococcus aureus to amicoumacin A. PLoS One. 2012;7:e34037. doi: 10.1371/journal.pone.0034037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu Y, Liu L, Han Z, Lai PY, Guo X, Zhang X, Lin W, Qian PY. Five new amicoumacins isolated from a marine-derived bacterium Bacillus subtilis. Mar. Drugs. 2012;10:319–328. doi: 10.3390/md10020319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen F, Starosta AL, Arenz S, Sohmen D, Donhofer A, Wilson DN. Tetracycline antibiotics and resistance mechanisms. Biol. Chem. 2014;395:559–575. doi: 10.1515/hsz-2013-0292. [DOI] [PubMed] [Google Scholar]
- Orelle C, Carlson S, Kaushal B, Almutairi MM, Liu H, Ochabowicz A, Quan S, Pham VC, Squires CL, Murphy BT, et al. Tools for characterizing bacterial protein synthesis inhibitors. Antimicrob. Agents. Chemother. 2013a;57:5994–6004. doi: 10.1128/AAC.01673-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orelle C, Szal T, Klepacki D, Shaw KJ, Vazquez-Laslop N, Mankin AS. Identifying the targets of aminoacyl-tRNA synthetase inhibitors by primer extension inhibition. Nucleic Acids Res. 2013b;41:e144. doi: 10.1093/nar/gkt526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterman IA, Prokhorova IV, Sysoev VO, Boykova YV, Efremenkova OV, Svetlov MS, Kolb VA, Bogdanov AA, Sergiev PV, Dontsova OA. Attenuation-based dual-fluorescent-protein reporter for screening translation inhibitors. Antimicrob. Agents. Chemother. 2012;56:1774–1783. doi: 10.1128/AAC.05395-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinchuk IV, Bressollier P, Verneuil B, Fenet B, Sorokulova IB, Megraud F, Urdaci MC. In vitro anti-Helicobacter pylori activity of the probiotic strain Bacillus subtilis 3 is due to secretion of antibiotics. Antimicrob. Agents. Chemother. 2001;45:3156–3161. doi: 10.1128/AAC.45.11.3156-3161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pioletti M, Schlunzen F, Harms J, Zarivach R, Gluhmann M, Avila H, Bashan A, Bartels H, Auerbach T, Jacobi C, et al. Crystal structures of complexes of the small ribosomal subunit with tetracycline, edeine and IF3. EMBO J. 2001;20:1829–1839. doi: 10.1093/emboj/20.8.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polikanov YS, Blaha GM, Steitz TA. How hibernation factors RMF, HPF, and YfiA turn off protein synthesis. Science. 2012;336:915–918. doi: 10.1126/science.1218538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polikanov YS, Steitz TA, Innis CA. A proton wire to couple aminoacyl tRNA accommodation and peptide bond formation on the ribosome. Nat. Struct. Mol. Biol. 2014;21:787–793. doi: 10.1038/nsmb.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottmann N, Kleuvers B, Atmadja J, Wagner R. Mutants with base changes at the 3'-end of the 16S RNA from Escherichia coli. Construction, expression and functional analysis. Eur. J. Biochem. 1988;177:81–90. doi: 10.1111/j.1432-1033.1988.tb14347.x. [DOI] [PubMed] [Google Scholar]
- Schluenzen F, Takemoto C, Wilson DN, Kaminishi T, Harms JM, Hanawa-Suetsugu K, Szaflarski W, Kawazoe M, Shirouzu M, Nierhaus KH, et al. The antibiotic kasugamycin mimics mRNA nucleotides to destabilize tRNA binding and inhibit canonical translation initiation. Nat. Struct. Mol. Biol. 2006;13:871–878. doi: 10.1038/nsmb1145. [DOI] [PubMed] [Google Scholar]
- Schmitt E, Blanquet S, Mechulam Y. Crystallization and preliminary X-ray analysis of Escherichia coli methionyl-tRNAMet(f) formyltransferase complexed with formyl-methionyl-tRNAMet(f). Acta Crystallogr. D Biol. Crystallogr. 1999;55:332–334. doi: 10.1107/S0907444998011780. [DOI] [PubMed] [Google Scholar]
- Schuwirth BS, Borovinskaya MA, Hau CW, Zhang W, Vila-Sanjurjo A, Holton JM, Cate JH. Structures of the bacterial ribosome at 3.5 A resolution. Science. 2005;310:827–834. doi: 10.1126/science.1117230. [DOI] [PubMed] [Google Scholar]
- Schuwirth BS, Day JM, Hau CW, Janssen GR, Dahlberg AE, Cate JH, Vila-Sanjurjo A. Structural analysis of kasugamycin inhibition of translation. Nat. Struct. Mol. Biol. 2006;13:879–886. doi: 10.1038/nsmb1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y, Kanamori T, Ueda T. Protein synthesis by pure translation systems. Methods. 2005;36:299–304. doi: 10.1016/j.ymeth.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Shoji S, Walker SE, Fredrick K. Reverse translocation of tRNA in the ribosome. Mol. Cell. 2006;24:931–942. doi: 10.1016/j.molcel.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenbichler J, Kovacs T. Influence of the Gloeophyllum metabolite oosponol and some synthetic analogues on protein and RNA synthesis in target cells. Eur. J. Biochem. 1997;246:45–49. doi: 10.1111/j.1432-1033.1997.t01-2-00045.x. [DOI] [PubMed] [Google Scholar]
- Svetlov MS, Kommer A, Kolb VA, Spirin AS. Effective cotranslational folding of firefly luciferase without chaperones of the Hsp70 family. Protein Sci. 2006;15:242–247. doi: 10.1110/ps.051752506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourigny DS, Fernandez IS, Kelley AC, Vakiti RR, Chattopadhyay AK, Dorich S, Hanessian S, Ramakrishnan V. Crystal structure of a bioactive pactamycin analog bound to the 30S ribosomal subunit. J. Mol. Biol. 2013;425:3907–3910. doi: 10.1016/j.jmb.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Laslop N, Klepacki D, Mulhearn DC, Ramu H, Krasnykh O, Franzblau S, Mankin AS. Role of antibiotic ligand in nascent peptide-dependent ribosome stalling. Proc. Natl. Acad. Sci. USA. 2011;108:10496–10501. doi: 10.1073/pnas.1103474108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Laslop N, Thum C, Mankin AS. Molecular mechanism of drug-dependent ribosome stalling. Mol. Cell. 2008;30:190–202. doi: 10.1016/j.molcel.2008.02.026. [DOI] [PubMed] [Google Scholar]
- Voorhees RM, Weixlbaumer A, Loakes D, Kelley AC, Ramakrishnan V. Insights into substrate stabilization from snapshots of the peptidyl transferase center of the intact 70S ribosome. Nat. Struct. Mol. Biol. 2009;16:528–533. doi: 10.1038/nsmb.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DN. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat. Rev. Microbiol. 2014;12:35–48. doi: 10.1038/nrmicro3155. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Qin Y, Achenbach J, Li C, Kijek J, Spahn CM, Nierhaus KH. EF-G and EF4: translocation and back-translocation on the bacterial ribosome. Nat. Rev. Microbiol. 2014;12:89–100. doi: 10.1038/nrmicro3176. [DOI] [PubMed] [Google Scholar]
- Zaporojets D, French S, Squires CL. Products transcribed from rearranged rrn genes of Escherichia coli can assemble to form functional ribosomes. J. Bacteriol. 2003;185:6921–6927. doi: 10.1128/JB.185.23.6921-6927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.