Abstract
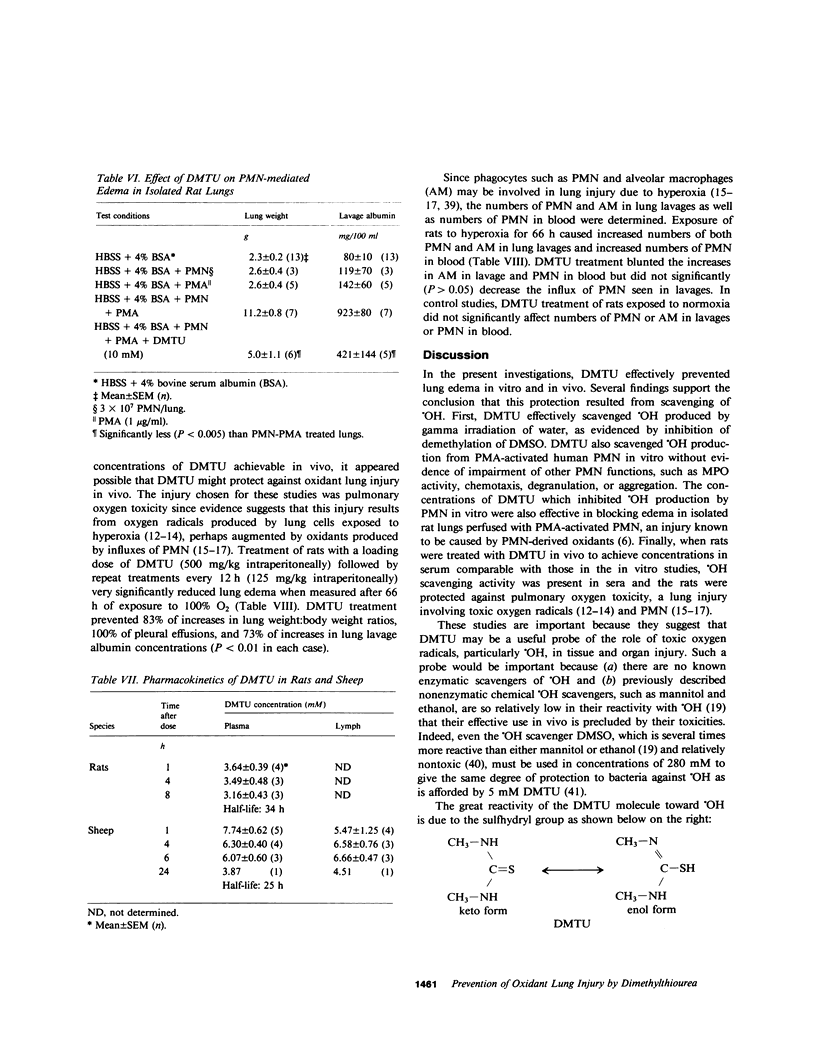
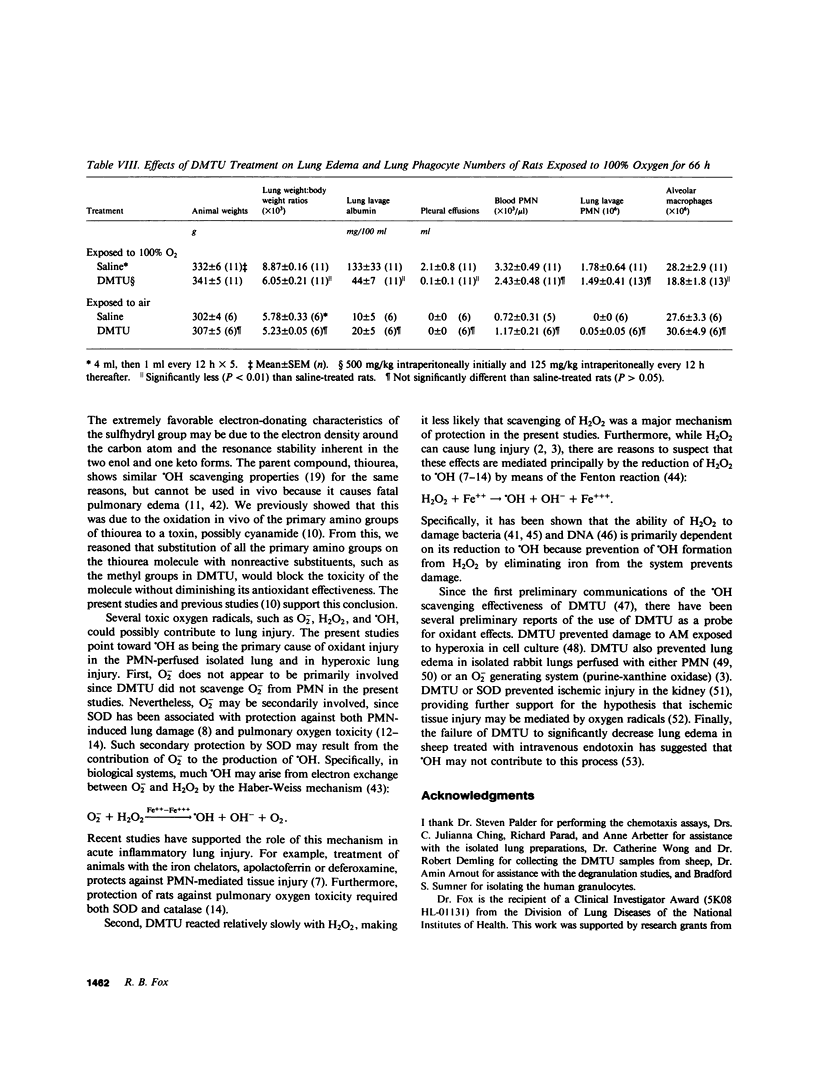
Toxic, partially reduced metabolites of oxygen (toxic oxygen radicals) are increasingly implicated in acute leukocyte-mediated tissue injury. To further probe the roles of oxygen radicals in acute lung edema, I studied the effects of a recently described and very potent oxygen radical scavenger, dimethylthiourea (DMTU) (Fox, R. B., R. N. Harada, R. M. Tate, and J. E. Repine, 1983, J. Appl. Physiol., 55:1456-1459) on polymorphonuclear leukocyte (PMN) oxidant function and on two types of lung injury mediated by oxygen radicals and PMN. DMTU (10 mM) blocked 79% of hydroxyl radical (OH) production by PMN in vitro without interfering with other PMN functions, such as O-2 production, myeloperoxidase activity, chemotaxis, degranulation, or aggregation. When isolated rat lung preparations were perfused with PMN activated to produce OH, lung weights were increased from 2.3 +/- 0.2 to 11.2 +/- 0.8 g. DMTU (10 mM) prevented 70% of these increases (lung weights, 5.0 +/- 1.1 g, P less than 0.005). Finally, when intact rats were exposed to 100% O2 for 66 h, lung weight:body weight ratios were increased from 5.78 +/- 0.33 to 8.87 +/- 0.16 g. DMTU (500 mg/kg) prevented 83% of this hyperoxia-induced lung edema in vivo (lung:body weight ratios, 6.05 +/- 0.21, P less than 0.001). Pharmacokinetic studies showed that DMTU diffused effectively into lung interstitial fluids and had a relatively long half-life (25-35 h) in the circulation. Because a variety of oxygen radicals, such as superoxide (O-2), hydrogen peroxide (H2O2), or OH are produced by PMN, there is usually some uncertainty about which one is responsible for injury. However, in these studies, DMTU did not scavenge O-2 and scavenged H2O2 only very slowly while scavenging OH very effectively. Therefore, DMTU may be useful in the investigation of the roles of oxygen radicals, especially OH, in acute granulocyte-mediated tissue injury.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- CHANCE B. The properties of the enzyme-substrate compounds of horseradish peroxidase and peroxides; the effect of pH upon the rate of reaction of complex II with several acceptors and its relation to their oxidation-reduction potential. Arch Biochem. 1949 Dec;24(2):410–421. [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Dalmasso A. P., Brighan K. L., Jacob H. S. Hemodialysis leukopenia. Pulmonary vascular leukostasis resulting from complement activation by dialyzer cellophane membranes. J Clin Invest. 1977 May;59(5):879–888. doi: 10.1172/JCI108710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapo J. D., DeLong D. M., Sjostrom K., Hasler G. R., Drew R. T. The failure of aerosolized superoxide dismutase to modify pulmonary oxygen toxicity. Am Rev Respir Dis. 1977 Jun;115(6):1027–1033. doi: 10.1164/arrd.1977.115.6.1027. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Tierney D. F. Superoxide dismutase and pulmonary oxygen toxicity. Am J Physiol. 1974 Jun;226(6):1401–1407. doi: 10.1152/ajplegacy.1974.226.6.1401. [DOI] [PubMed] [Google Scholar]
- Doumas B. T., Watson W. A., Biggs H. G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta. 1971 Jan;31(1):87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Fox R. B., Harada R. N., Tate R. M., Repine J. E. Prevention of thiourea-induced pulmonary edema by hydroxyl-radical scavengers. J Appl Physiol Respir Environ Exerc Physiol. 1983 Nov;55(5):1456–1459. doi: 10.1152/jappl.1983.55.5.1456. [DOI] [PubMed] [Google Scholar]
- Frank L., Summerville J., Massaro D. Potection from oxygen toxicity with endotoxin. Role of the endogenous antioxidant enzymes of the lung. J Clin Invest. 1980 May;65(5):1104–1110. doi: 10.1172/JCI109763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTTLIEBLAU K. S., WASSERMAN L. R., HERBERT V. RAPID CHARCOAL ASSAY FOR INTRINSIC FACTOR (IF), GASTRIC JUICE UNSATURATED B12 BINDING CAPACITY, ANTIBODY TO IF, AND SERUM UNSATURATED B12 BINDING CAPACITY. Blood. 1965 Jun;25:875–884. [PubMed] [Google Scholar]
- Granger D. N., Rutili G., McCord J. M. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981 Jul;81(1):22–29. [PubMed] [Google Scholar]
- Hammerschmidt D. E., White J. G., Craddock P. R., Jacob H. S. Corticosteroids inhibit complement-induced granulocyte aggregation. A possible mechanism for their efficacy in shock states. J Clin Invest. 1979 Apr;63(4):798–803. doi: 10.1172/JCI109365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada R. N., Vatter A. E., Repine J. E. Oxygen radical scavengers protect alveolar macrophages from hyperoxic injury in vitro. Am Rev Respir Dis. 1983 Oct;128(4):761–762. doi: 10.1164/arrd.1983.128.4.761. [DOI] [PubMed] [Google Scholar]
- Hoidal J. R., Fox R. B., LeMarbe P. A., Perri R., Repine J. E. Altered oxidative metabolic responses in vitro of alveolar macrophages from asymptomatic cigarette smokers. Am Rev Respir Dis. 1981 Jan;123(1):85–89. doi: 10.1164/arrd.1981.123.1.85. [DOI] [PubMed] [Google Scholar]
- Johnson K. J., Fantone J. C., 3rd, Kaplan J., Ward P. A. In vivo damage of rat lungs by oxygen metabolites. J Clin Invest. 1981 Apr;67(4):983–993. doi: 10.1172/JCI110149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane S. P., Peters T. J. Analytical subcellular fractionation of human granulocytes with reference to the localization of vitamin B12-binding proteins. Clin Sci Mol Med. 1975 Aug;49(2):171–182. doi: 10.1042/cs0490171. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Kobayashi H., Matano O., Goto S. Simultaneous quantitation of thioureas in rat plasma by high-performance liquid chromatography. J Chromatogr. 1981 Mar 20;207(2):281–285. doi: 10.1016/s0021-9673(00)89945-8. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., Simmons R. L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975 Dec;115(6):1650–1656. [PubMed] [Google Scholar]
- Paller M. S., Hoidal J. R., Ferris T. F. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984 Oct;74(4):1156–1164. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Eaton J. W., Anders M. W., Hoidal J. R., Fox R. B. Generation of hydroxyl radical by enzymes, chemicals, and human phagocytes in vitro. Detection with the anti-inflammatory agent, dimethyl sulfoxide. J Clin Invest. 1979 Dec;64(6):1642–1651. doi: 10.1172/JCI109626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Fox R. B., Berger E. M., Harada R. N. Effect of staphylococcal iron content on the killing of Staphylococcus aureus by polymorphonuclear leukocytes. Infect Immun. 1981 Apr;32(1):407–410. doi: 10.1128/iai.32.1.407-410.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Fox R. B., Berger E. M. Hydrogen peroxide kills Staphylococcus aureus by reacting with staphylococcal iron to form hydroxyl radical. J Biol Chem. 1981 Jul 25;256(14):7094–7096. [PubMed] [Google Scholar]
- Repine J. E., Pfenninger O. W., Talmage D. W., Berger E. M., Pettijohn D. E. Dimethyl sulfoxide prevents DNA nicking mediated by ionizing radiation or iron/hydrogen peroxide-generated hydroxyl radical. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1001–1003. doi: 10.1073/pnas.78.2.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin L. F. Toxicologic update of dimethyl sulfoxide. Ann N Y Acad Sci. 1983;411:6–10. doi: 10.1111/j.1749-6632.1983.tb47278.x. [DOI] [PubMed] [Google Scholar]
- Sawchuk R. J., Zaske D. E. Pharmacokinetics of dosing regimens which utilize multiple intravenous infusions: gentamicin in burn patients. J Pharmacokinet Biopharm. 1976 Apr;4(2):183–195. doi: 10.1007/BF01086153. [DOI] [PubMed] [Google Scholar]
- Shasby D. M., Fox R. B., Harada R. N., Repine J. E. Reduction of the edema of acute hyperoxic lung injury by granulocyte depletion. J Appl Physiol Respir Environ Exerc Physiol. 1982 May;52(5):1237–1244. doi: 10.1152/jappl.1982.52.5.1237. [DOI] [PubMed] [Google Scholar]
- Shasby D. M., Shasby S. S., Bowman C. M., Fox R. B., Harada R. M., Tate R. M., Repine J. E. Angiotensin converting enzyme concentrations in the lung lavage of normal rabbits and rabbits treated with nitrogen mustard exposed to hyperoxia. Am Rev Respir Dis. 1981 Aug;124(2):202–203. doi: 10.1164/arrd.1981.124.2.202. [DOI] [PubMed] [Google Scholar]
- Shasby D. M., Vanbenthuysen K. M., Tate R. M., Shasby S. S., McMurtry I., Repine J. E. Granulocytes mediate acute edematous lung injury in rabbits and in isolated rabbit lungs perfused with phorbol myristate acetate: role of oxygen radicals. Am Rev Respir Dis. 1982 Apr;125(4):443–447. doi: 10.1164/arrd.1982.125.4.443. [DOI] [PubMed] [Google Scholar]
- Staub N. C., Bland R. D., Brigham K. L., Demling R., Erdmann A. J., 3rd, Woolverton W. C. Preparation of chronic lung lymph fistulas in sheep. J Surg Res. 1975 Nov;19(5):315–320. doi: 10.1016/0022-4804(75)90056-6. [DOI] [PubMed] [Google Scholar]
- Tate R. M., Repine J. E. Neutrophils and the adult respiratory distress syndrome. Am Rev Respir Dis. 1983 Sep;128(3):552–559. doi: 10.1164/arrd.1983.128.3.552. [DOI] [PubMed] [Google Scholar]
- Tate R. M., Vanbenthuysen K. M., Shasby D. M., McMurtry I. F., Repine J. E. Oxygen-radical-mediated permeability edema and vasoconstriction in isolated perfused rabbit lungs. Am Rev Respir Dis. 1982 Nov;126(5):802–806. doi: 10.1164/arrd.1982.126.5.802. [DOI] [PubMed] [Google Scholar]
- Till G. O., Johnson K. J., Kunkel R., Ward P. A. Intravascular activation of complement and acute lung injury. Dependency on neutrophils and toxic oxygen metabolites. J Clin Invest. 1982 May;69(5):1126–1135. doi: 10.1172/JCI110548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens J. F., Crapo J. D., Freeman B. A. Protection against oxygen toxicity by intravenous injection of liposome-entrapped catalase and superoxide dismutase. J Clin Invest. 1984 Jan;73(1):87–95. doi: 10.1172/JCI111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Till G. O., Kunkel R., Beauchamp C. Evidence for role of hydroxyl radical in complement and neutrophil-dependent tissue injury. J Clin Invest. 1983 Sep;72(3):789–801. doi: 10.1172/JCI111050. [DOI] [PMC free article] [PubMed] [Google Scholar]



