Abstract
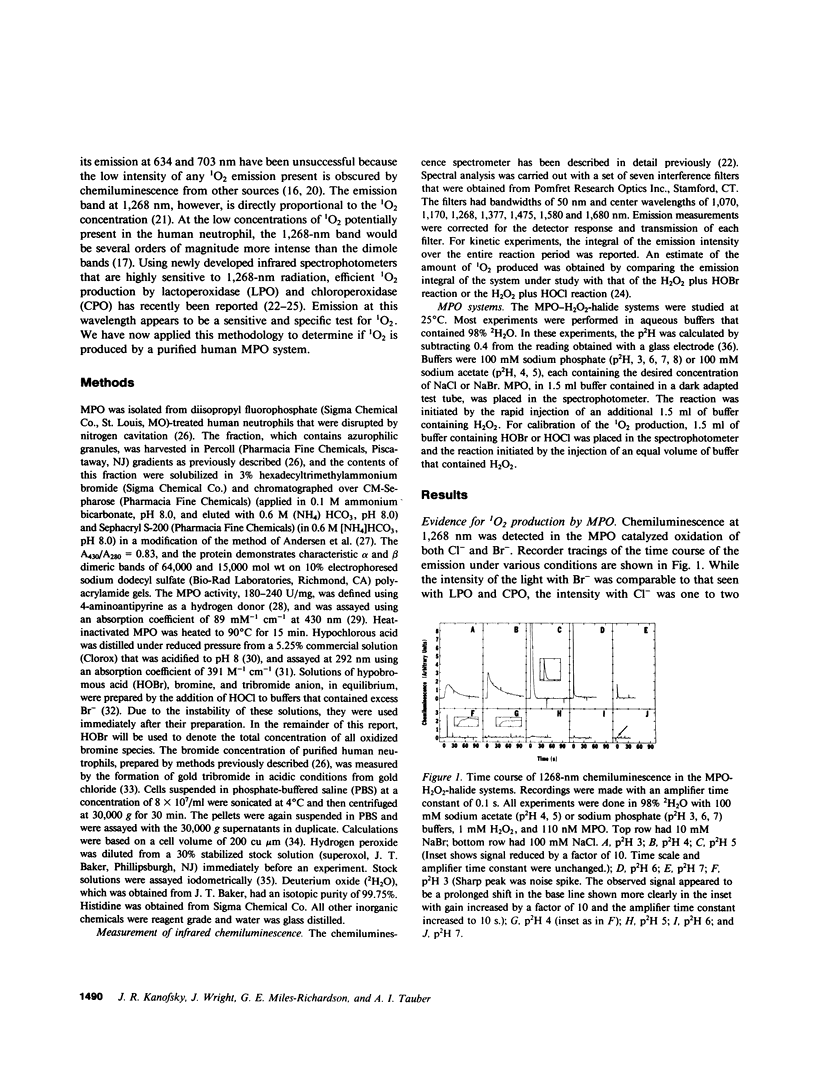
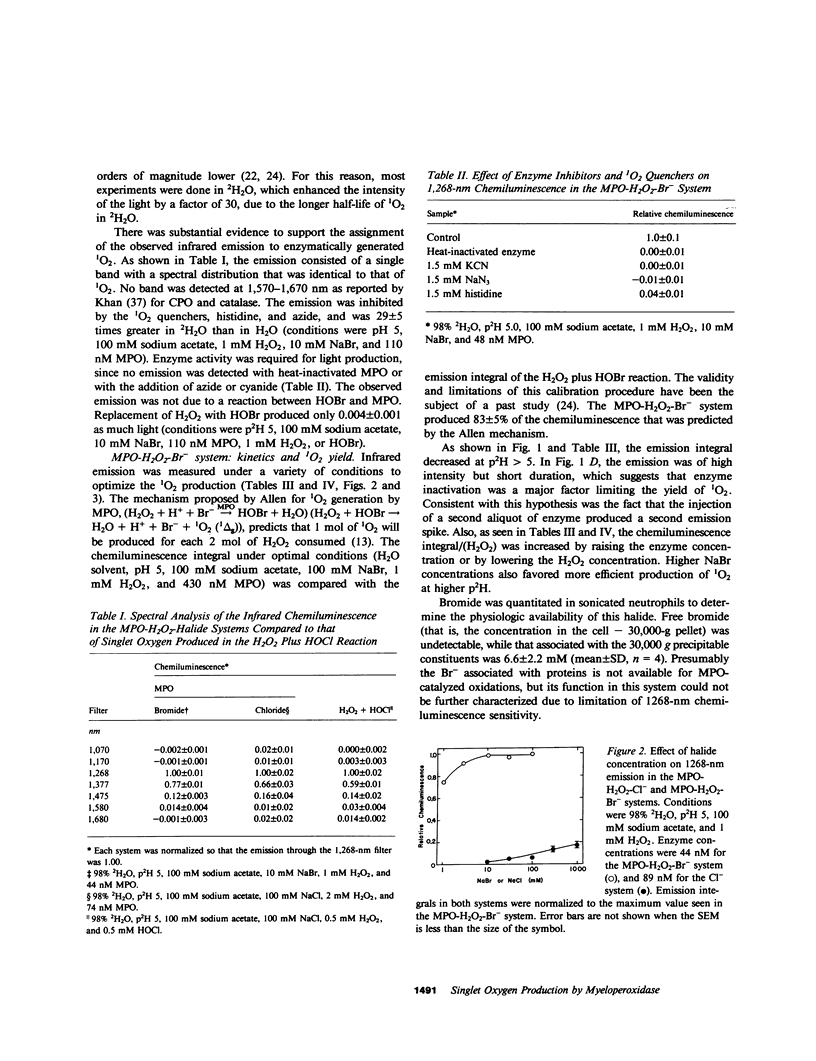
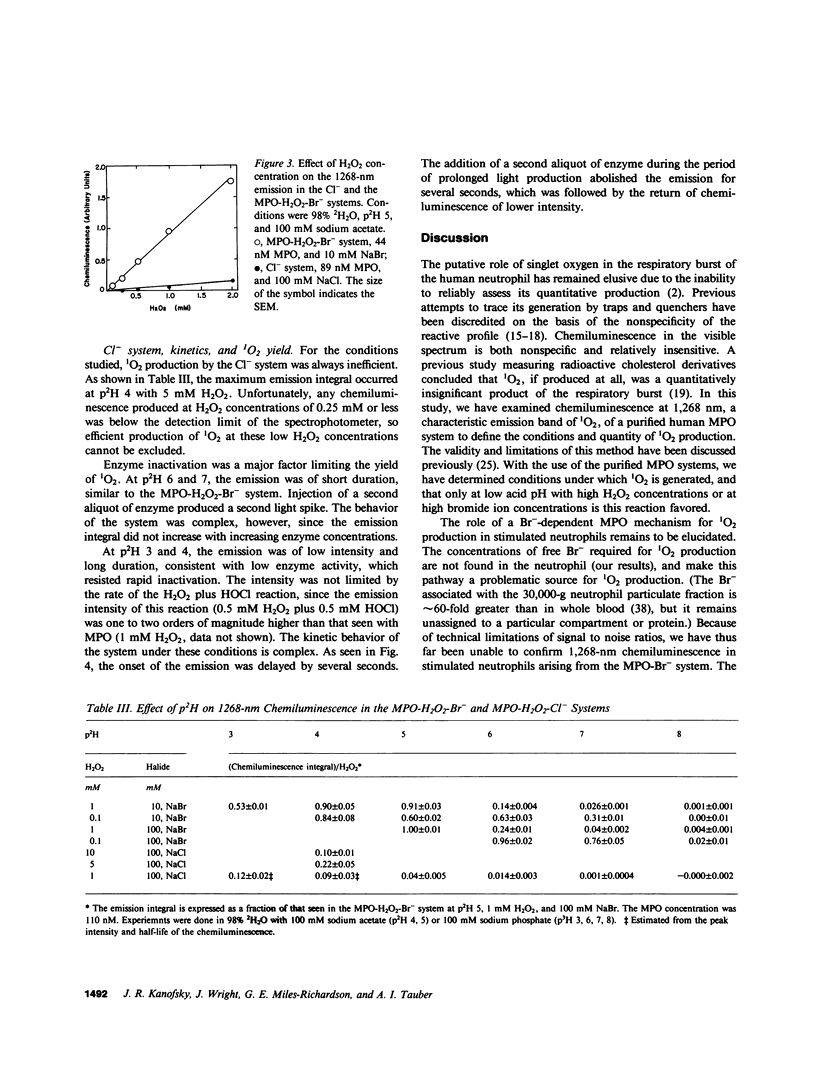
The myeloperoxidase (MPO)-hydrogen peroxide (H2O2)-halide systems were found to produce chemiluminescence at 1,268 nm, a characteristic emission band for singlet oxygen (1O2). The emission was enhanced by a factor of 29 +/- 5 in deuterium oxide and was inhibited by the 1O2 quenchers, histidine and azide ion. Inactivation of MPO with heat or with cyanide ion prevented light production. The combined weight of all data strongly supported the production of 1O2 by these enzyme systems. The amount of 1O2 produced was sensitive to the conditions employed. Under optimal conditions at pH 5, the MPO-H2O2-bromide (Br-) system produced 0.42 +/- 0.03 mol 1O2/mol H2O2 consumed, close to the theoretical value of 0.5 that was predicted by the reaction stoichiometry. In contrast, the MPO-H2O2-chloride (Cl-) system was much less efficient. The maximum yield of 1O2 was 0.09 +/- 0.02 mol/mol H2O2 consumed and required pH 4 and 5 mM H2O2. At higher pH, the 1O2 production rapidly decreased. The yield at pH 7 was 0.0004 +/- 0.0002 mol/mol H2O2 consumed. Enzyme inactivation was a major factor limiting the yield of 1O2 with both Cl- and Br-. While the MPO-H2O2-halide systems can efficiently produce 1O2, the conditions required are not physiologic, which suggests that the chemiluminescence of the stimulated neutrophil does not derive from 1O2 generated by a MPO mechanism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C. Halide dependence of the myeloperoxidase-mediated antimicrobial system of the polymorphonuclear leukocyte in the phenomenon of electronic excitation. Biochem Biophys Res Commun. 1975 Apr 7;63(3):675–683. doi: 10.1016/s0006-291x(75)80437-2. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Andersen M. R., Atkin C. L., Eyre H. J. Intact form of myeloperoxidase from normal human neutrophils. Arch Biochem Biophys. 1982 Mar;214(1):273–283. doi: 10.1016/0003-9861(82)90031-5. [DOI] [PubMed] [Google Scholar]
- Andrews P. C., Krinsky N. I. A kinetic analysis of the interaction of human myeloperoxidase with hydrogen peroxide, chloride ions, and protons. J Biol Chem. 1982 Nov 25;257(22):13240–13245. [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Bakkenist A. R., Wever R., Vulsma T., Plat H., van Gelder B. F. Isolation procedure and some properties of myeloperoxidase from human leucocytes. Biochim Biophys Acta. 1978 May 11;524(1):45–54. doi: 10.1016/0005-2744(78)90101-8. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Tauber A. I. Subcellular localization of the human neutrophil NADPH oxidase. b-Cytochrome and associated flavoprotein. J Biol Chem. 1984 Jan 10;259(1):47–52. [PubMed] [Google Scholar]
- Cech P., Lehrer R. I. Phagolysosomal pH of human neutrophils. Blood. 1984 Jan;63(1):88–95. [PubMed] [Google Scholar]
- Cheson B. D., Christensen R. L., Sperling R., Kohler B. E., Babior B. M. The origin of the chemiluminescence of phagocytosing granulocytes. J Clin Invest. 1976 Oct;58(4):789–796. doi: 10.1172/JCI108530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K., Archibald A. C., Robinson M. F. The origin of chemiluminescence produced by neutrophils stimulated by opsonized zymosan. J Immunol. 1983 May;130(5):2324–2329. [PubMed] [Google Scholar]
- Foote C. S., Goyne T. E., Lehrer R. I. Assessment of chlorination by human neutrophils. Nature. 1983 Feb 24;301(5902):715–716. doi: 10.1038/301715a0. [DOI] [PubMed] [Google Scholar]
- Harrison J. E., Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976 Mar 10;251(5):1371–1374. [PubMed] [Google Scholar]
- Harrison J. E., Watson B. D., Schultz J. Myeloperoxidase and singlet oxygen: a reappraisal. FEBS Lett. 1978 Aug 15;92(2):327–332. doi: 10.1016/0014-5793(78)80780-7. [DOI] [PubMed] [Google Scholar]
- Held A. M., Hurst J. K. Ambiguity associated with use of singlet oxygen trapping agents in myeloperoxidase-catalyzed oxidations. Biochem Biophys Res Commun. 1978 Apr 14;81(3):878–885. doi: 10.1016/0006-291x(78)91433-x. [DOI] [PubMed] [Google Scholar]
- Holzbecher J., Ryan D. E. The rapid determination of total bromine and iodine in biological fluids by neutron activation. Clin Biochem. 1980 Dec;13(6):277–278. doi: 10.1016/s0009-9120(80)80009-9. [DOI] [PubMed] [Google Scholar]
- Jacques Y. V., Bainton D. F. Changes in pH within the phagocytic vacuoles of human neutrophils and monocytes. Lab Invest. 1978 Sep;39(3):179–185. [PubMed] [Google Scholar]
- Kanofsky J. R. Singlet oxygen production by chloroperoxidase-hydrogen peroxide-halide systems. J Biol Chem. 1984 May 10;259(9):5596–5600. [PubMed] [Google Scholar]
- Kanofsky J. R. Singlet oxygen production by lactoperoxidase. J Biol Chem. 1983 May 25;258(10):5991–5993. [PubMed] [Google Scholar]
- Khan A. U., Gebauer P., Hager L. P. Chloroperoxidase generation of singlet Delta molecular oxygen observed directly by spectroscopy in the 1- to 1.6-mum region. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5195–5197. doi: 10.1073/pnas.80.17.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinsky N. I. Singlet excited oxygen as a mediator of the antibacterial action of leukocytes. Science. 1974 Oct 25;186(4161):363–365. doi: 10.1126/science.186.4161.363. [DOI] [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Travis J. Isolation and properties of human neutrophil myeloperoxidase. Biochemistry. 1981 Jan 20;20(2):325–330. doi: 10.1021/bi00505a015. [DOI] [PubMed] [Google Scholar]
- Naskalski J. W. Myeloperoxidase inactivation in the course of catalysis of chlorination of taurine. Biochim Biophys Acta. 1977 Dec 8;485(2):291–300. doi: 10.1016/0005-2744(77)90165-6. [DOI] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Formation of singlet oxygen by the myeloperoxidase-mediated antimicrobial system. J Biol Chem. 1977 Jul 25;252(14):4803–4810. [PubMed] [Google Scholar]
- Schmid-Schönbein G. W., Shih Y. Y., Chien S. Morphometry of human leukocytes. Blood. 1980 Nov;56(5):866–875. [PubMed] [Google Scholar]
- Takayama K., Noguchi T., Nakano M. Reactivities of diphenylfuran (a singlet oxygen trap) with singlet oxygen and hydroxyl radical in aqueous systems. Biochem Biophys Res Commun. 1977 Apr 25;75(4):1052–1058. doi: 10.1016/0006-291x(77)91488-7. [DOI] [PubMed] [Google Scholar]
- Tauber A. I., Borregaard N., Simons E., Wright J. Chronic granulomatous disease: a syndrome of phagocyte oxidase deficiencies. Medicine (Baltimore) 1983 Sep;62(5):286–309. [PubMed] [Google Scholar]
- Thomas E. L., Grisham M. B., Jefferson M. M. Myeloperoxidase-dependent effect of amines on functions of isolated neutrophils. J Clin Invest. 1983 Aug;72(2):441–454. doi: 10.1172/JCI110992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L. Myeloperoxidase, hydrogen peroxide, chloride antimicrobial system: nitrogen-chlorine derivatives of bacterial components in bactericidal action against Escherichia coli. Infect Immun. 1979 Feb;23(2):522–531. doi: 10.1128/iai.23.2.522-531.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Klein R., Slivka A., Wei M. Chlorination of taurine by human neutrophils. Evidence for hypochlorous acid generation. J Clin Invest. 1982 Sep;70(3):598–607. doi: 10.1172/JCI110652. [DOI] [PMC free article] [PubMed] [Google Scholar]



