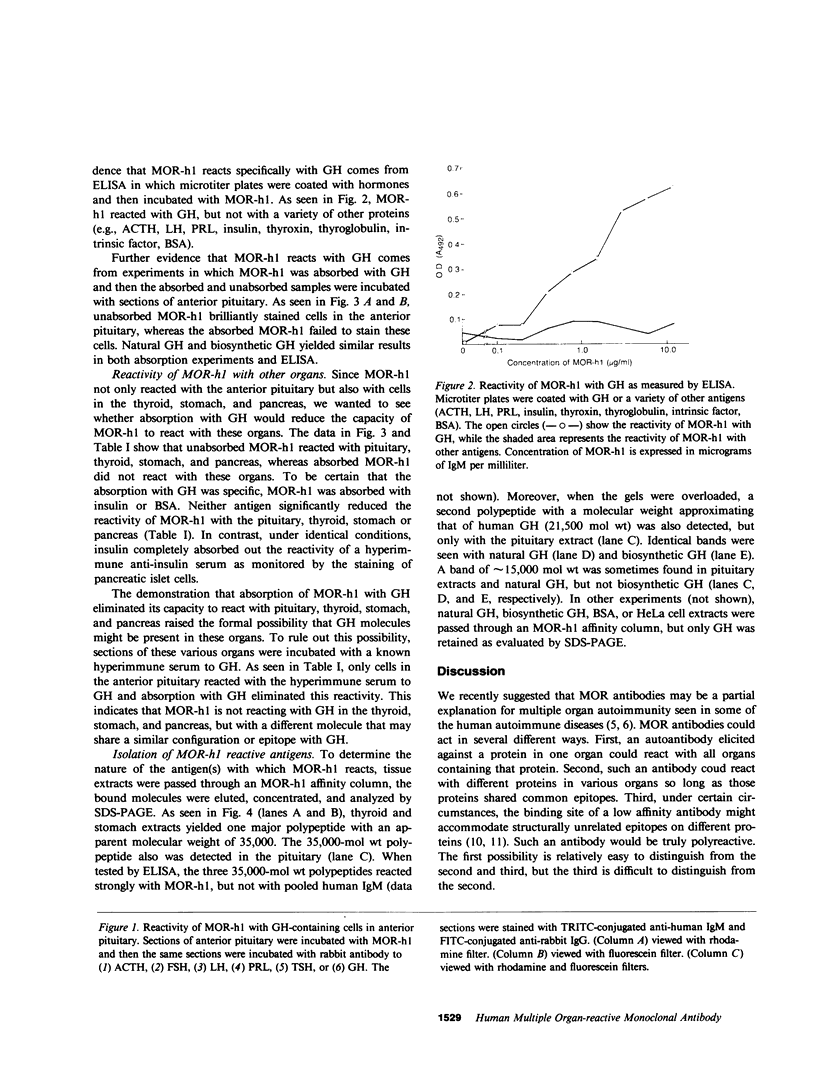
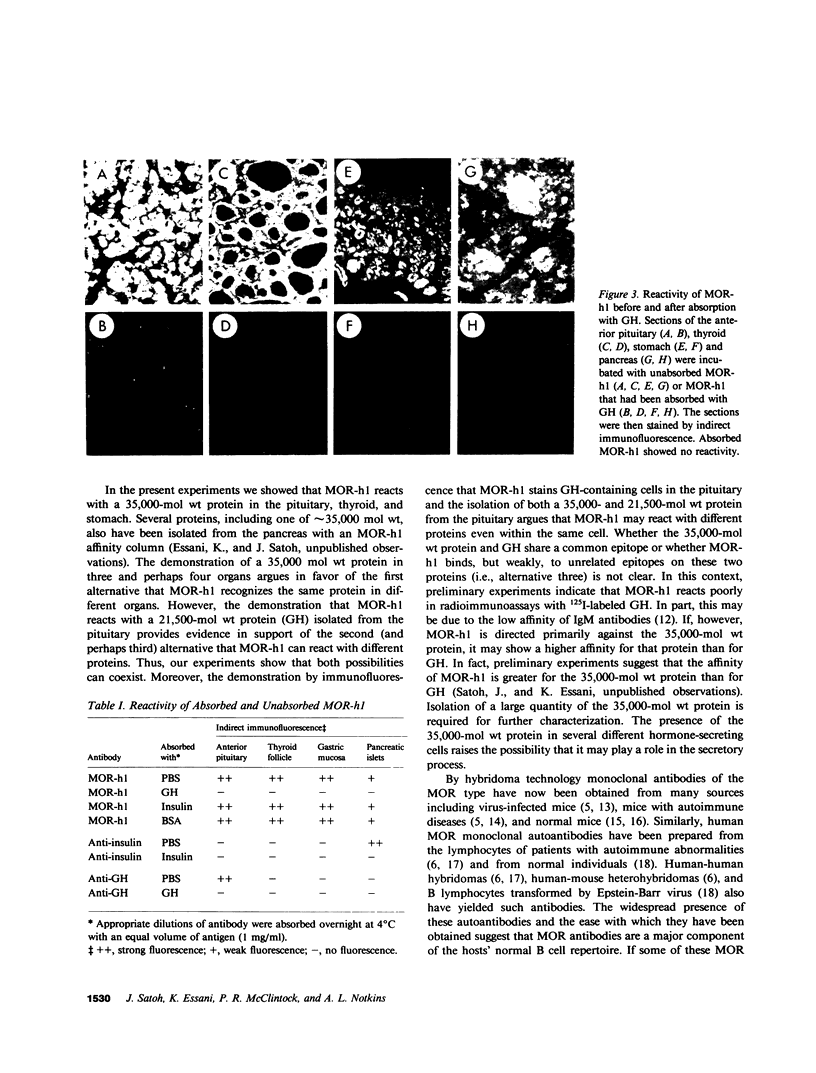
Abstract
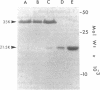
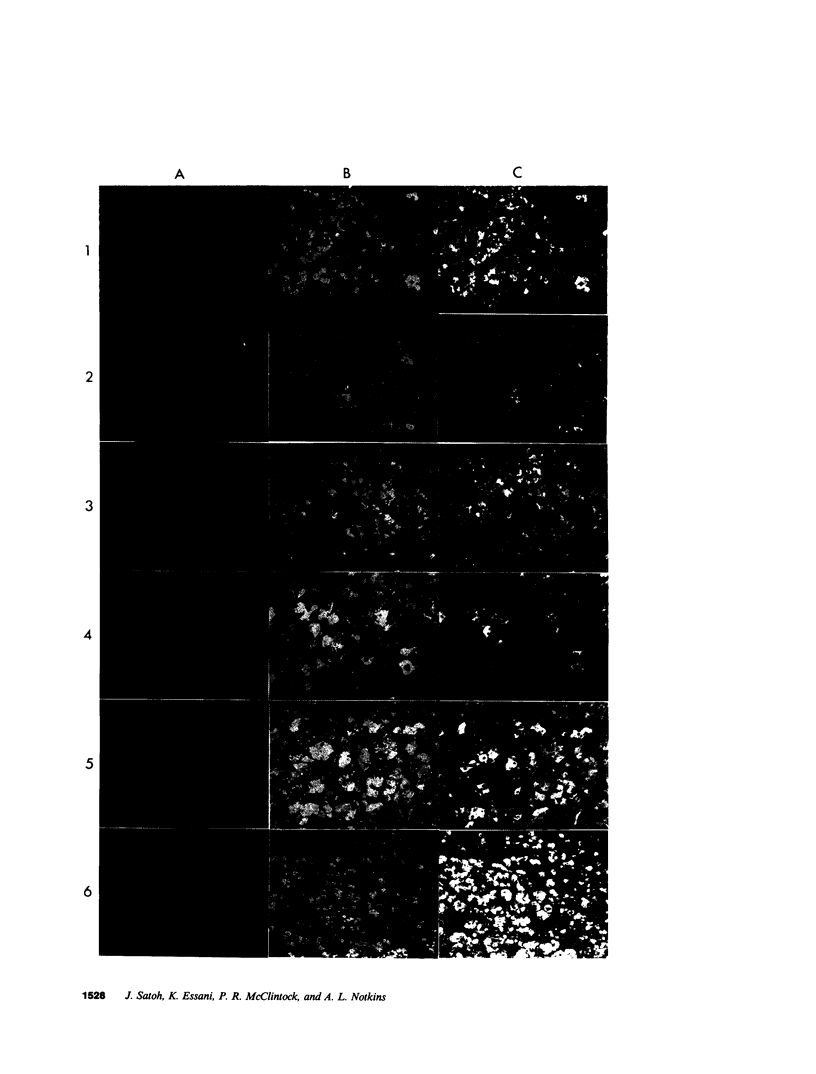
By fusing peripheral leukocytes from a patient with insulin-dependent diabetes with mouse myeloma cells, a heterohybridoma was isolated that, for over one year, has secreted a human monoclonal autoantibody, designated MOR-h1 (multiple organ-reactive human 1). This antibody reacts with antigens in several endocrine organs including the pituitary, thyroid, stomach, and pancreas. By double immunofluorescence, MOR-h1 was found to react specifically with growth hormone (GH)-containing cells in the anterior pituitary and, by enzyme-linked immunosorbent assay, MOR-h1 was shown to react with both natural and biosynthetic GH. Absorption experiments revealed that GH could remove the capacity of MOR-h1 to react not only with cells in the anterior pituitary, but also with cells in the thyroid, stomach, and pancreas. The demonstration with hyperimmune serum that these organs do not contain GH indicated that MOR-h1 was reacting with a different molecule(s) in these organs. By passing extracts of pituitary, thyroid, and stomach through an MOR-h1 affinity column and analyzing the eluted antigens by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, a 35,000-mol wt polypeptide was isolated from each of these organs. In addition, a 21,500-mol wt polypeptide with an electrophoretic mobility identical to purified human GH was isolated from the pituitary, but not the other organs. It is concluded that MOR-h1 reacts with a 35,000-mol wt polypeptide present in the pituitary, thyroid, and stomach and that this antibody also recognizes a determinant on GH.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dales S., Fujinami R. S., Oldstone M. B. Infection with vaccinia favors the selection of hybridomas synthesizing autoantibodies against intermediate filaments, one of them cross-reacting with the virus hemagglutinin. J Immunol. 1983 Sep;131(3):1546–1553. [PubMed] [Google Scholar]
- Dighiero G., Lymberi P., Mazié J. C., Rouyre S., Butler-Browne G. S., Whalen R. G., Avrameas S. Murine hybridomas secreting natural monoclonal antibodies reacting with self antigens. J Immunol. 1983 Nov;131(5):2267–2272. [PubMed] [Google Scholar]
- Gearhart P. J., Johnson N. D., Douglas R., Hood L. IgG antibodies to phosphorylcholine exhibit more diversity than their IgM counterparts. Nature. 1981 May 7;291(5810):29–34. doi: 10.1038/291029a0. [DOI] [PubMed] [Google Scholar]
- Haspel M. V., Onodera T., Prabhakar B. S., Horita M., Suzuki H., Notkins A. L. Virus-induced autoimmunity: monoclonal antibodies that react with endocrine tissues. Science. 1983 Apr 15;220(4594):304–306. doi: 10.1126/science.6301002. [DOI] [PubMed] [Google Scholar]
- Haspel M. V., Onodera T., Prabhakar B. S., McClintock P. R., Essani K., Ray U. R., Yagihashi S., Notkins A. L. Multiple organ-reactive monoclonal autoantibodies. Nature. 1983 Jul 7;304(5921):73–76. doi: 10.1038/304073a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lafer E. M., Rauch J., Andrzejewski C., Jr, Mudd D., Furie B., Furie B., Schwartz R. S., Stollar B. D. Polyspecific monoclonal lupus autoantibodies reactive with both polynucleotides and phospholipids. J Exp Med. 1981 Apr 1;153(4):897–909. doi: 10.1084/jem.153.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D., Koprowski H. Molecular recognition and the future of monoclonal antibodies. Nature. 1982 Mar 18;296(5854):200–202. doi: 10.1038/296200a0. [DOI] [PubMed] [Google Scholar]
- Onodera T., Jenson A. B., Yoon J. W., Notkins A. L. Virus-induced diabetes mellitus: reovirus infection of pancreatic beta cells in mice. Science. 1978 Aug 11;201(4355):529–531. doi: 10.1126/science.208156. [DOI] [PubMed] [Google Scholar]
- Onodera T., Toniolo A., Ray U. R., Jenson A. B., Knazek R. A., Notkins A. L. Virus-induced diabetes mellitus. XX. Polyendocrinopathy and autoimmunity. J Exp Med. 1981 Jun 1;153(6):1457–1473. doi: 10.1084/jem.153.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodwell J. D., Gearhart P. J., Karush F. Restriction in IgM expression. IV. Affinity analysis of monoclonal anti-phosphorylcholine antibodies. J Immunol. 1983 Jan;130(1):313–316. [PubMed] [Google Scholar]
- Satoh J., Prabhakar B. S., Haspel M. V., Ginsberg-Fellner F., Notkins A. L. Human monoclonal autoantibodies that react with multiple endocrine organs. N Engl J Med. 1983 Jul 28;309(4):217–220. doi: 10.1056/NEJM198307283090405. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y., Rauch J., Massicotte H., Datta S. K., André-Schwartz J., Stollar B. D., Schwartz R. S. Polyspecificity of monoclonal lupus autoantibodies produced by human-human hybridomas. N Engl J Med. 1983 Feb 24;308(8):414–420. doi: 10.1056/NEJM198302243080802. [DOI] [PubMed] [Google Scholar]
- TALMAGE D. W. Immunological specificity, unique combinations of selected natural globulins provide an alternative to the classical concept. Science. 1959 Jun 19;129(3364):1643–1648. doi: 10.1126/science.129.3364.1643. [DOI] [PubMed] [Google Scholar]
- Teillaud J. L., Desaymard C., Giusti A. M., Haseltine B., Pollock R. R., Yelton D. E., Zack D. J., Scharff M. D. Monoclonal antibodies reveal the structural basis of antibody diversity. Science. 1983 Nov 18;222(4625):721–726. doi: 10.1126/science.6356353. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]