Abstract
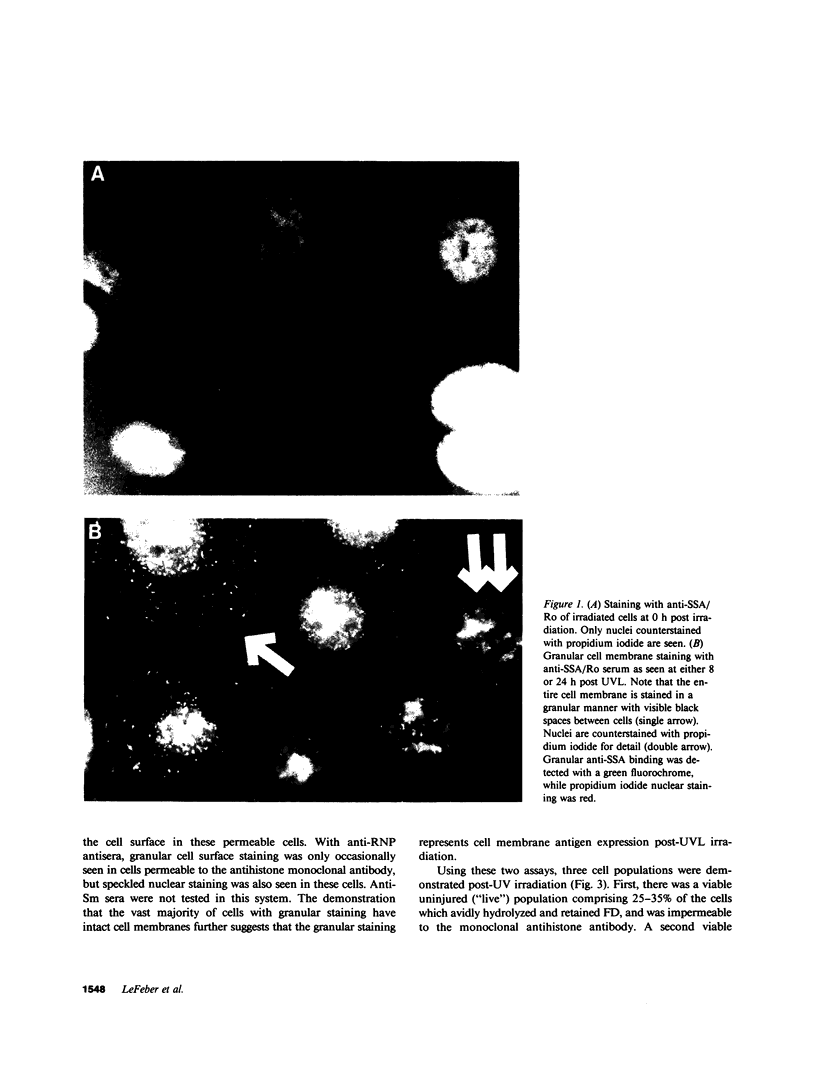
Antibodies which bind to different nuclear antigens in tissue sections or in permeabilized cell cultures are useful markers of subsets of connective tissue disease, especially of lupus erythematosus (LE), but whether these antibodies are able to react with these intracellular sequestered antigens in vivo and cause immunologic tissue damage has been a matter of much debate. We report experiments which show that ultraviolet light-irradiated, cultured human keratinocytes bind IgG antibodies from the sera of LE patients with either monospecific anti-SSA/Ro, anti-RNP, or anti-Sm activity, which implies that these antigens have been made accessible on the cell surface by ultraviolet irradiation. Normal human sera or LE patient's sera with anti-double-stranded DNA, anti-single-stranded DNA, or antihistone activity do not bind to the surface of irradiated human keratinocytes. In control experiments, all antisera produced the expected patterns of nuclear and cytoplasmic staining of fixed permeabilized, unirradiated keratinocytes. Careful study of the viability and permeability of irradiated keratinocytes by several techniques showed that this apparent cell membrane expression of extractable nuclear antigens (SSA/Ro, RNP, and Sm) following irradiation was seen on injured keratinocytes whose cell membranes were intact, but not on dead cells. It is particularly significant that all six monospecific anti-SSA/Ro sera bound to irradiated keratinocytes, since this antibody antigen system is highly associated with photosensitive cutaneous LE.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowden G. T., Hohneck G., Fusenig N. E. DNA excision repair in ultraviolet-irradiated normal and malignantly transformed mouse epidermal cell cultures. Cancer Res. 1977 Jun;37(6):1611–1617. [PubMed] [Google Scholar]
- Boyce S. T., Ham R. G. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J Invest Dermatol. 1983 Jul;81(1 Suppl):33s–40s. doi: 10.1111/1523-1747.ep12540422. [DOI] [PubMed] [Google Scholar]
- Clark G., Reichlin M., Tomasi T. B., Jr Characterization of a soluble cytoplasmic antigen reactive with sera from patients with systemic lupus erythmatosus. J Immunol. 1969 Jan;102(1):117–122. [PubMed] [Google Scholar]
- Cripps D. J., Rankin J. Action spectra of lupus erythematosus and experimental immunofluorescence. Arch Dermatol. 1973 Apr;107(4):563–567. [PubMed] [Google Scholar]
- Galoppin L., Saurat J. H. In vitro study of the binding of antiribonucleoprotein antibodies to the nucleus of isolated living keratinocytes. J Invest Dermatol. 1981 Apr;76(4):264–267. doi: 10.1111/1523-1747.ep12526097. [DOI] [PubMed] [Google Scholar]
- Ginsberg B., Keiser H. A Millipore filter assay for antibodies to native DNA in sera of patients with systemic lupus erythematosus. Arthritis Rheum. 1973 Mar-Apr;16(2):199–207. doi: 10.1002/art.1780160210. [DOI] [PubMed] [Google Scholar]
- Huff J. C., Weston W. L., Wanda K. D. Enhancement of specific immunofluorescent findings with use of a para-phenylenediamine mounting buffer. J Invest Dermatol. 1982 May;78(5):449–450. doi: 10.1111/1523-1747.ep12508101. [DOI] [PubMed] [Google Scholar]
- Maddison P. J., Provost T. T., Reichlin M. Serological findings in patients with "ANA-negative" systemic lupus erythematosus. Medicine (Baltimore) 1981 Mar;60(2):87–94. doi: 10.1097/00005792-198103000-00002. [DOI] [PubMed] [Google Scholar]
- Natali P. G., Tan E. M. Experimental skin lesions in mice resembling systemic lupus erythematosus. Arthritis Rheum. 1973 Sep-Oct;16(5):579–589. doi: 10.1002/art.1780160502. [DOI] [PubMed] [Google Scholar]
- Ockleford C. D., Hsi B. L., Wakely J., Badley R. A., Whyte A., Faulk W. P. Propidium iodide as a nuclear marker in immunofluorescence. I. Use with tissue and cytoskeleton studies. J Immunol Methods. 1981;43(3):261–267. doi: 10.1016/0022-1759(81)90173-3. [DOI] [PubMed] [Google Scholar]
- Provost T. T. Lupus band test. Int J Dermatol. 1981 Sep;20(7):475–481. doi: 10.1111/j.1365-4362.1981.tb04906.x. [DOI] [PubMed] [Google Scholar]
- Reichlin M., Wasicek C. A. Clinical and biologic significance of antibodies to Ro/SSA. Hum Pathol. 1983 May;14(5):401–405. doi: 10.1016/s0046-8177(83)80284-6. [DOI] [PubMed] [Google Scholar]
- Sontheimer R. D., Deng J. S., Gilliam J. N. Antinuclear and anticytoplasmic antibodies. Concepts and misconceptions. J Am Acad Dermatol. 1983 Sep;9(3):335–343. doi: 10.1016/s0190-9622(83)70139-8. [DOI] [PubMed] [Google Scholar]
- Sontheimer R. D., Maddison P. J., Reichlin M., Jordon R. E., Stastny P., Gilliam J. N. Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus. Ann Intern Med. 1982 Nov;97(5):664–671. doi: 10.7326/0003-4819-97-5-664. [DOI] [PubMed] [Google Scholar]
- Tuffanelli D. L. Lupus erythematosus. J Am Acad Dermatol. 1981 Feb;4(2):127–142. doi: 10.1016/s0190-9622(81)70016-1. [DOI] [PubMed] [Google Scholar]
- Weston W. L., Harmon C., Peebles C., Manchester D., Franco H. L., Huff J. C., Norris D. A. A serological marker for neonatal lupus erythematosus. Br J Dermatol. 1982 Oct;107(4):377–382. doi: 10.1111/j.1365-2133.1982.tb00380.x. [DOI] [PubMed] [Google Scholar]
- Yeh C. J., Hsi B. L., Faulk W. P. Propidium iodide as a nuclear marker in immunofluorescence. II. Use with cellular identification and viability studies. J Immunol Methods. 1981;43(3):269–275. doi: 10.1016/0022-1759(81)90174-5. [DOI] [PubMed] [Google Scholar]