Abstract
Tremendous strides have been made in mapping the complexity of the human gut microbiota in both health and disease states. These analyses have revealed that rather than a constellation of individual species, a healthy microbiota consists of an inter-dependent network of microbes. The microbial and host interactions that shape both this network and the gastrointestinal environment are areas of intense investigation. Here, we review emerging concepts of how microbial metabolic processes control commensal composition, invading pathogens, immune activation, and intestinal barrier function. We posit that all these factors are critical for the maintenance of homeostasis and avoidance of overt inflammatory disease. A greater understanding of the underlying mechanisms will shed light on the pathogenesis of many diseases and guide new therapeutic interventions.
Keywords: Host-microbial interaction, metabolism, epithelial barrier, intestinal pathogen
The microbiota: An inter-dependent network
The microbial communities in the intestinal tract of vertebrates consist of bacteria, viruses, archae, and fungi. In humans the intestine is home to some 1014 bacterial cells and possibly many thousands of individual strains [1, 2]. To convey some perspective on the immensity of this ecosystem, this microbial population contains more than 100 times the number of genes and 10 times the number of cells compared to the host [1]. The microbiota of the gastrointestinal tract is a symbiotic partner of the host as it is crucial for intestinal microvasculature development, metabolism and development of multiple components of the host immune system ([3, 4] and Kabat et. al. in this issue). The specific microbial-derived factors at this interface are the subject of intense investigation as these factors likely play key roles in the regulation of various disease processes. The complexity of the symbiotic relationship between the microbiota and the host has evolved to extract the maximal benefits of a diverse microbiota such as pathogen resistance, metabolism and immune development, while at the same time minimising deleterious effects including imbalanced over-representation of certain members (dysbiosis), microbial translocation, and inflammatory responses. In order for the host to maintain homeostasis, contact between the microbiota and the epithelial cell surface must be limited and carefully controlled. This is achieved by various mechanisms including the host mucus layer, antimicrobial proteins, immunoglobulin A (IgA), and regulation of commensal outgrowth [5-7].
The vast genomic dataset of the Human Microbiome Project has provided unparalleled insight into the composition, structure, and temporal assembly of the microbiota [1]. Moving forward, the next big step will be to utilize this information to gain a better understanding of how the microbiota function in both health and disease. A major theme from recent work is the reciprocal interactions of the microbiota and host, which we are beginning to understand at a mechanistic level. Here we review these recent findings, highlighting current gaps in our understanding of these processes. Specifically, we discuss how commensal microbes determine the composition of their own intestinal ecosystem, operating as an inter-dependent network rather than a vast collection of individual species. We examine how these interactions manifest themselves in the microbe food supply chain, and the manner by which they provide strong resistance to invading pathogens, including engaging in crosstalk with the host immune system. Finally, we highlight the potential of the microbiota in shaping the function of the intestinal epithelial barrier and discuss the significance this may hold in the treatment of disease.
Commensal Establishment and Competition
In humans and mice, the major bacterial phyla that occupy the intestine during homeostasis are Firmicutes, Actinobacteria, Proteobacteria, and Bacteroidetes [1]. Temporal and spatial studies using shotgun metagenomic sequencing or 16S rRNA sequencing have shown that the microbiota appears to operate as an inter-dependent network whereby each member of this community must adapt to its niche within this environment (Reviewed in [8]). The basis of any individual niche can be anatomic (e.g. at the mucosal barrier) and/or metabolic (e.g acquiring nutrients from other microbes, diet or mucus). In humans, gut colonization commences immediately after birth and then undergoes ecological succession with progressive environmental exposures [1, 2]. It appears that most members of the microbiota establish their long term colonization in early childhood [9, 10].
Numerous host factors influence the composition of the microbiota early in life including diet, hygiene, environmental contacts, antibiotic use, and breastfeeding [1, 2, 11]. However, quantification of the relative importance of each of these factors has been challenging. Recent studies have begun to unravel some of the microbial factors and mechanisms involved in establishing the composition of the microbiota (see Figure 1A). Differential oxygen levels along the length of the intestine and during early-life are also major determinants of bacterial composition as the relative amount of oxygen impacts the ability of aerobic, facultative anaerobic, and obligate anaerobic species to thrive. The first bacteria to colonize the gut in early-life are aerobic or facultatively anaerobic bacteria (Enterobacteria, Enterococci, and Staphylococci) because of the higher oxygen levels present [12]. As these bacteria grow they rapidly consume oxygen and release metabolites that make the intestines more suitable for anaerobes like Bifidobacterium, Clostridia and Bacteroides [13]. Thus, as the infant ages there is an increase in the complexity of the microbiota that is inversely correlated with changes in the population of aerobes and facultative anaerobes. Diet, host factors such as mucus, and microbial-derived metabolites are all potentially key factors in shaping the microbiota based on the differential requirements of nutrients by individual commensals. For example, the high concentration of short chain carbohydrates and amino acids in the intestine favours the growth of bacteria that can metabolize these molecules including Proteobacteria and Lactobacillales [1]. In the colon, the majority of nutrients available for bacteria are derived from indigestible or resistant carbohydrates in the diet as well complex carbohydrates in the host mucus layer [14]. Bacteroidetes express clusters of enzymes that are organized in polysaccharide utilization loci (PUL), which can act in concert to breakdown complex carbohydrates and use them as an energy source, likewise Firmicutes are also adept at polysaccharide fermentation [15, 16]. This activity favours colonization of the colon by these phyla. Commensal organisms have evolved to efficiently utilize glycans (polysaccharides) from a number of sources including the diet and mucus layer (Reviewed in [17]). These dietary glycans then undergo microbial fermentation resulting in the production of short chain fatty acid (SCFA) metabolites (discussed below) [18]. Fluctuations in dietary glycans together with the selective microbial glycan preferences would conceivably allow species competition to create a changeable and heterogeneous microbiota. For instance, as little as 24 hours after altering dietary fiber content, detectable shifts in the microbiota can be observed, wherein consumption of more resistant plant starches increases the abundance of Firmicutes such as Eubacterium rectale, Ruminococcus bromii, and Roseburia spp [19-22]. Similar observations have been made in associative studies between European and African children where the high fiber diet of the latter correlates with a major increase in polysaccharide-consuming Bacteroidetes [23]. Overall, the predominant influence on composition of the microbiota appears to be host-related factors, as when germ-free mice are colonized with the microbiota of other species they select for a bacterial composition that is far more similar to other mice than the donors [24].
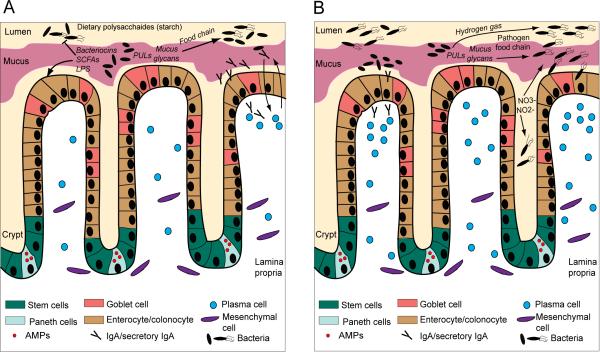
Figure 1. Microbiota exerts control at the epithelial barrier during homeostasis and dysbiosis.
(A) The composition of the epithelial barrier is controlled in the stem cell niche, located in the crypt of Lieberkuhn, giving rise to four cell types. These include Paneth cells (small intestine only) that secrete antimicrobial factors (AMPs), goblet cells secreting glycosylated proteins to generate the protective mucus layer, neuroendocrine cells producing metabolic hormones (not depicted here), and the most abundant cell type the absorptive enterocyte (or colonocyte). A mucus layer covers the epithelial interface with the lumen. During homeostasis commensal microbiota exert effects on the intestinal barrier cells through the production of metabolites by fermentation of dietary polysaccharides such as short chain fatty acids (SCFAs), and the microbial molecular ligands (such as LPS) they express. They also control any potential pathogens and pathobionts through secretion of SCFAs (alter pH, and virulence factor expression) and bacteriocins. Certain commensal microbes possess polysaccharide utilization loci (PULs) that enable the enzymatic harvesting of glycans from mucus. These glycans can then also be sequentially utilized as an energy source by other commensals via a ‘food chain’ mechanism. IgA is produced by plasma cells in the lamina propria and trancytosed across the epithelium as secretory IgA. IgA is induced by and binds to commensals, which creates a reciprocal feedback loop between host-symbiont during homeostasis. IgA also prevents colonization by pathogens and overgrowth of pathobionts. (B) During dysbiosis the commensal microbial community becomes unbalanced and exuberant expansion of pathobionts and colonization and invasion by pathogens may also occur. Invasive flagellated pathogens, such as the Salmonella spp., evade commensal resistance and gain a growth advantage through several mechanisms including utilizing the bacterial products of fermentation (hydrogen gas) and mucus degradation (glycans) in order to produce energy. This could be termed a ‘pathogen food chain’ Commensal E. coli species rapidly outgrow by utilizing nitrate and nitrite ions released by the epithelium during inflammation as terminal electron acceptors to facilitate anaerobic respiration. Outgrowth of microbes and epithelial invasion of pathogens induces increased immune cell recruitment (including IgA-secreting plasma cells) to the epithelial barrier. The reciprocal mechanisms of IgA regulation during dysbiosis are largely unknown, however, defects in the IgA pathway or repertoire can initiate dysbiosis.
Competition between commensals for available nutrients begins early in life during breastfeeding, which provides a major source of oligosaccharides, leading to an early-life abundance of Bifdobacterium infantis [25-29]. A recent study in mice suggested that this competition for milk oligosaccharides in early-life actually has long-term impacts on microbial composition and potentially influences DSS-colitis susceptibility in adulthood [30].
However, the identity or function of the protective species have not been discerned Commensal microorganisms can also interact in a mutually beneficial pattern through ‘food chains’, wherein certain species may be dependent on others for partial degradation or production of metabolites [17]. For example, recent studies have shown that co-colonization of germ free mice with both Bacteroides thetaiotaomicron and E. rectale produces a substantially different set of glycan degradation products than in mice undergoing mono-colonization [31]. This suggests that the two bacteria interact metabolically through a food chain mechanism. Indeed, B. thetaiotaomicron is a major producer of the SCFA acetate, which can be consumed as a major carbon source by another member of the Firmicutes Faecalibacterium prausnitzii [32]. Furthermore, B. thetaiotaomicron express PUL enzymes that can harvest certain glycans, such as fucose, from mucus but lack the pathways to utilize them allowing other as yet unidentified commensals to profit from this nutrient source [16]. Interestingly, it has recently been demonstrated that members of the Bacteroidales can package these enzymes into bacterial outer membrane vesicles to be utilized to produce glycan breakdown products to allow the growth of other bacteria at distant sites from the producer [33].
Although the content of any diet can effect bacterial composition, it would be an over-simplification to suggest that diet alone is responsible for the diversity of the microbiota or its variation among individuals. Other host genetic factors (not yet defined) and immune factors (discussed below) play a role in the establishment of the microbiota (i.e. influencing competition for limited sites near the epithelial barrier/mucosal immune system) [34]. Herein, there is an important functional distinction between the microbes that reside in the digesta (luminal) and readily pass through the gut (i.e. Lactobacillaceae and Enterococcaceae) and barrier-associated microbes, which reside within the inner mucus layer in closer contact with the host mucosa (i.e. Lachnospiraceae and Ruminococcaceae) [35, 36]. It can be hypothesized that these two sets of microbial populations play differential roles in regulating intestinal barrier function. A recent demonstration of barrier competition was provided by Lee et al., using one of the most abundant colonic commensal genera, the Bacteroides [15]. The authors aimed to demonstrate that there is a colonization saturation point for many commensals. Mono-colonization with individual Bacteroides species reached a specific saturation level in germ-free mice that was not permissive of additional colonization by the same species. However, other Bacteroides species could efficiently colonize these same mice. This finding suggested that a finite and non-dynamic habitat was available for any given Bacteroides species. Importantly, the phenomenon of habitat saturation was not universal as Escherichia coli did not show this activity. The authors also showed that a novel cluster of Bacteroides genes referred to as commensal colonization factors (ccf), which harvest polysaccharides either from dietary sugars or host mucus glycans, are expressed in close contact with the host epithelium. In turn these genes allowed colonization at the epithelial barrier and resistance to microbial disturbances, such as antibiotics and pathogenic infection, by sequestering a nutrient source for ccf-harbouring bacteria. This report is clearly a provocative idea and runs counterintuitive to the underlying assumption of the field that all bacterial-host interactions are necessarily dynamic.
A recent study of human subjects attempted to put experimental observations on commensal competition into perspective by suggesting that only three human enterotypes exists [37]. An enterotype is defined as a signature composition of microbes that is similar to communities in other individuals based on the presence and relative abundance of certain detectable species [37]. Based on a study of fecal samples from 39 individuals the authors suggested that healthy humans could be categorized into 3 subtypes based on stratified patterns of microbe genera, in particular high abundance of either Bacteroides, Prevotella, or Ruminococcus, and that these were not specific to any given continent. However, the human microbiome project (see Box 1 Human Microbiome Project) using a far larger and more comprehensive dataset (242 healthy adults sampled at 15-18 body sites up to three times, generating 5,177 microbial taxonomic profiles from 16S ribosomal RNA genes and over 3.5 terabases of metagenomic sequence) has largely rebuked this finding of limited enterotypes. Instead it indicates that the gut microbiome may represent more a continuum of inter-individual variation rather than three distinct subsets [1, 2].
The composition of the microbiota is diverse and highly variable between individuals. It is generated early in life through a process of ecological succession dictated by environmental exposures, commensal interaction, and various host factors. The predominant influence on microbial selection is provided by the host through establishment of local microenvironments, nutrition, and as discussed below immune responses to both pathogens and commensals. Additional studies carefully evaluating the gut microbiome composition over time in the same individuals will be useful in furthering our understanding of normal variation (noise) versus disease variations [38].
Commensal Suppression of Pathogens
One of the primary functions of the microbiota is to prevent invasion of pathogens (such as Salmonella spp, Shigella flexneri) and the inflammation that can be stimulated by an overrepresentation of specific commensals (such as Escherichia coli) (see Figure 1B) [39-41]. Certain members of the indigenous bacterial community, termed pathobionts, have inflammatory potential in either a genetically susceptible host or a host where the microbiota has been perturbed (i.e. through antibiotics). Rather than enteric epithelial invasion or systemic dissemination, overgrowth of these pathobiont microbes can precipitate pathologic immune responses. For example, in the presence of a diminished enteric microbiota following antibiotic treatment of mice, indigenous E. coli can expand and induce pathologic inflammation through the activation of the inflammasome [40]. The precise molecular differences between such indigenous commensals and exogenous pathogens are still being unravelled. Due to the diverse microbiota and pathogen carrier states between individuals, it is likely that a spectrum exists between pure commensals and pure pathogens that is filled by the concept of the pathobiont.
Nevertheless, the commensal microbiota has similar mechanisms for dealing with both inflammatory pathobionts and pathogens. These mechanisms include competition for limited nutrients and sites of epithelial adherence, along with the production of antimicrobial factors such as specific SCFAs, and bacteriocins. Certain commensal bacteria, including the members of Clostridiales, produce SCFAs that can alter the local enteric pH and prevent growth of intestinal pathogens in mice [42-44]. Fukuda et al., recently demonstrated in mice how the Bifidobacterium metabolite acetate can potently inhibit the growth of enterohaemorrhagic E. coli O157:H7 and lethality induced by translocation of its Shiga toxin into the blood [43]. Butyrate, another prominent SCFA produced by carbohydrate fermenters, down regulates the expression of virulence factors from Salmonella enterica in vitro, including genes localized to the Salmonella pathogenicity island 1 (SPI1) [45]. Bacteriocins are a set of toxins that specifically inhibit different strains of the same or closely related bacterial species (Reviewed in [46]). For example, commensal E. coli produces bacteriocin to inhibit the growth of the pathogenic enterohaemorrhagic E. coli O157:H7 strains in anaerobic liquid cultures [47].
Competition for nutrients is also vital in maintaining the stability of the endogenous microbial community. For example, commensal E. coli has been shown in vitro at least, to compete with human pathogenic adherent-invasive E. coli strains for consumption of critical carbohydrate metabolites [48]. Moreover, Citrobacter rodentium, which is a pathogenic mouse E. coli modelling human attaching and effacing bacteria, cannot be eradicated when mono-colonized in germ-free mice but is eventually outcompeted and expelled from the intestines of conventionally-reared mice [49]. Kamada et al. questioned the mechanisms that might control this commensal resistance. They discovered that C. rodentium initially upregulates virulence factors (LEE gene cluster) that were required for growth in conventional mice but not in germ-free mice. These virulence factors enabled it to attach to and grow on carbohydrate molecules similar to those used by commensals. However, eventually this pathogen is outcompeted for utilization of these carbohydrates by the indigenous microbes and expelled from the intestine.
The strongest evidence that a healthy balanced microbiota controls pathogen invasion is that disruption of this equilibrium by antibiotic treatment in mice or humans leads to proliferation and colonization by three well-studied enteric pathogens: Salmonella enterica, Clostridium difficle, and Vancomycin-resistant enterococcus (VRE) [50-53]. Typically these pathogens would poorly colonize the intestinal tract in the presence of an intact microbial repertoire. Interestingly, a similar concept appears to be true for certain indigenous commensals in a susceptible host. Ayers et al., recently demonstrated that some strains of mice contain multi-antibiotic resistant endogenous E. coli. Following broad spectrum antibiotic disruption of the microbiota, this E. coli rapidly expands [40]. The authors showed that this expansion in the absence of commensals poses a risk of systemic dissemination and septic shock if the epithelial barrier is breached. Others studies investigating commensal bacterial-driven models of ulcerative colitis in mice have observed pathobionts that expand and induce a chronic inflammatory intestinal disease, including B. thetaiotaomicron, Klebsiella pneumonia, and Proteus mirabilis [54, 55]. Interestingly, these studies did not observe an indigenous E. coli that is multi-antibiotic resistant or one that induces inflammation arguing perhaps that the observations of Ayres et al., may be mouse strain or colony specific.
The microbiota of the intestinal tract utilizes multiple mechanisms (including secretion of soluble factors and competition for nutrients) to resist invasion by various opportunistic pathogens and expansion of pathobionts. However, as is often the case with microbes some pathogens have evolved to subvert these processes for their own benefit.
Pathogen Subversion of Commensal Resistance to Colonization
Some pathogens can overcome commensal resistance and flourish in the host intestinal tract (see Figure 1B). Recent work from multiple groups elucidated the molecular mechanisms utilized by specific bacteria to subvert commensal defences. Ng et al. recently found that certain diarrheal pathogens expand in numbers after antibiotic-mediated disruption of the indigenous microbiota [16]. The authors demonstrated that B. thetaiotaomicron in monocolonized germ free mice could harvest, but not utilize, mucus-derived sialic acid and fucose. This led to the up-regulation of the nan operon pathway (a cluster of genes linked to a single promoter involved in regulation of related catabolic function) in S. enterica and C. difficile to enable metabolism of these sialic acid and fucose nutrients. This allowed for greater expansion of these pathogens than that which occurred in the absence of the commensal B. thetaiotaomicron. Similar results were obtained by treating the complex microbiota of conventionally-reared mice with streptomycin, leading to an increase in sialic acid, as the commensal microbes (as yet not identified) that normally consume sialic acid were likely eliminated. This also led to subsequent pathogen expansion upon inoculation. These findings suggest that commensal resistance to pathogens also arises from competition for by-products catabolized by other commensals like the model symbiont B. thetaiotaomicron. This might well be termed a ‘pathogen food chain’. A critical question arising from this study is the identity of the streptomycin-sensitive sialic acid consuming commensals. These bacteria may well be of therapeutic value in prevention of diarrheal diseases.
Another newly appreciated strategy that pathogens employ to effectively colonize is to promote host inflammation leading to excess free radicals [56, 57]. This concept arose primarily through studies of Salmonella pathogenesis. Free radicals can act as electron acceptors for which the majority of commensal species do not express the metabolic pathways, in particular the tetrathionate reductase (ttrA) cluster, to utilize in respiration [57]. Pathogens that express this enzyme, can therefore gain a growth advantage. Infection by Salmonella typhimurium induces inflammation and reactive oxygen species (ROS) generation via NAPDH oxidase activity during the respiratory burst of phagocytes [57].
Under homeostatic conditions, commensals produce H2S during fermentation, which is then normally converted to an innocuous thiosulfate by the epithelium in order to prevent toxicity from H2S [58]. ROS oxidizes the thiosulfate produced by the epithelium, resulting instead in the production of tetrathionate. This in turn facilitates respiration by Salmonella (expressing the ttrA cluster), thus imparting a growth advantage on this pathogen [57]. Maier et al. recently examined the initial phase of gut ecosystem invasion by S. typhimurium, in the presence of a low complexity or Schaedler microbiota, which consists of less than 20 species [56]. Having a reduced microbiota but still maintaining many symbionts, including Lachnospiraceae and Bacteroides, facilitated Salmonella infection. Importantly, the authors specifically examined the period prior to any inflammation and reactive oxygen species production [56]. The findings revealed that in order to initially expand, Salmonella expressed a hydrogenase enzyme, which enabled it to utilize the hydrogen gas output from commensal fermentation as an energy source.
This mechanism identifies how inflammation enables the expansion of the exogenous pathogen S. typhimurium, however the question remains how inflammation effects the expansion of indigenous microbes that are associated with human chronic inflammatory diseases. In a state of dysbiosis, often associated with various inflammatory pathologies such as IBD, commensals have substantially altered relative abundance due to overgrowth (or reduction) of certain species [59, 60]. For example, it is well-established from multiple independent human metagenomic and 16S sequencing studies that the increased abundance of Enterobacteriaceae (E. coli is a major family member) is positively correlated with IBD diagnosis [59, 60]. More specifically E. coli has been linked to IBD through both culture and antibody detection methods in mucosal biopsies [61, 62]. Despite these correlations, it has not been possible to establish a causal relationship between commensal E. coli and colitis. In a recent elegant study, Winter et al. attempted to dissect the question of whether E. coli expansion is a cause versus secondary effect of inflammation [63]. The authors demonstrated that intestinal inflammation (generated by use of the epithelial damaging agent DSS) leads to the production of nitrate and nitrite ions in vivo. These ions were selective for facultative anaerobes such as E. coli. Unlike most commensals, E. coli expresses nitrate reductases that sequester nitrate ions as terminal electron acceptors to facilitate anaerobic respiration [63]. This process confers a growth advantage to E. coli (but not mutants lacking these reductase enzymes) as this bacteria is able to utilize the nitrate products of inflammation to produce an energy advantage over most intestinal anaerobes, which strictly rely on fermentation to provide their energy requirements. These data suggest that E. coli expansion occurs as a secondary result of nitrate production during inflammation, and thus it is more representative of a marker of intestinal inflammation rather than a causal factor.
Although the microbiota of the intestinal tract maintains a strong cooperative resistance to pathogen invasion, there are specific pathogens and indigenous commensals that have evolved to subvert these processes by taking advantage of commensal- and host-derived metabolites. The secondary line of defence against pathogen colonization involves the immune defences of the intestinal barrier, which interestingly also play a pivotal role in shaping the composition of the microbiota.
Crosstalk between Microbes and Intestinal Barrier Defences
The intestinal barrier defence includes a physical component (a layer of epithelial cells), and a chemical component that includes the mucus layer, secretory IgA, and the antimicrobial peptides (AMPs). We now understand that this physical-chemical barrier forms part of the intestinal immune system and plays a critical role in determining the composition of the microbiota. Likewise, the microbiota itself modulates the function of the intestinal immune system through the production of metabolites and immune-stimulatory ligands (reviewed in greater depth by Kabat et. al. in this issue). There is now increasing interest in how soluble factors released by commensal organisms may influence intestinal epithelial cells, which form the physical barricade between activation of immune cell populations and the microbiota.
The Role of Barrier Immune Defences: IgA
IgA is secreted across the epithelium into the intestinal lumen and coats bacteria providing a key defence against pathogens (Reviewed in [64]). In germ-free mice one of the most pronounced immunological defects is a loss of secretory IgA and lack of plasma cells [65]. This defect is reversible by colonizing germ-free mice with commensals, demonstrating that the microbiota directs the production of intestinal plasma cells and secretory IgA [66]. This process is believed to operate by dendritic cells (DCs) acquiring gut commensal bacteria and migrating to mesenteric lymph nodes to interact with naive B cells to induce production of IgA [67]. A recent study has taken this analysis of IgA induction one step further by examining the early stages of B cell development and V(D)J recombination in various mouse tissues [68]. Surprisingly, the authors observed that B cell receptor editing actually occurs not only in fetal liver and bone marrow, but also in the intestinal lamina propria. Intestine-specific editing of B cell receptors appeared to be regulated by signals produced by the developing microbiota, which did not exist in germ-free mice [68]. This study offers an explanation of how IgA-specific responses to commensals arise after intestinal colonization takes place. The commensal microbiota therefore regulates the initial development of intestine-specific B cell IgA repertoires. It has been well established that the Toll-like receptor (TLR) ligands of many commensal bacteria direct the function of both intestinal epithelial cells and mucosal DCs to induce IgA class switching (Reviewed in [69]). Moreover, mice deficient in the polymeric IgA receptor (pIgR) cannot trancytose IgA into the intestinal lumen and consequently these mice have an increased presence of bacteria in gut-draining mesenteric lymph nodes [70]. Overall, this indicates an important role for the microbiota in shaping intestinal immune responses required to prevent the translocation of commensal bacteria across the gut epithelial barrier. Although the microbiota is clearly important in these processes the specific bacteria responsible for IgA induction have not been identified.
Interestingly, there is mounting evidence to suggest that a reciprocal relationship also exists, such that intestinal IgA specificity controls the composition of the microbiota. In order to understand the effect of somatic hypermutation on intestinal B cell immunity, Wei et al. generated mice with a point mutation in the activation-induced cytidine deaminase gene thus impairing B cell capacity to undergo somatic hypermutation and limiting the diversity of their secretory IgA repertoire [71]. The authors demonstrated that these mice spontaneously develop dysbiosis with overgrowth of specific bacteria, such as Yersinia enterocolitica, suggesting that normal diverse IgA repertoires are necessary to control these commensals [71]. Moreover, recent seminal work from Kawamoto et al. elegantly demonstrated that a regulatory feedback loop exists in the gut between the commensal Firmicutes and IgA selection [72]. Using transfer of Foxp3+ T cells into T cell-deficient mice the authors were able to show that these cells select for a highly diverse IgA repertoire in germinal centres of Peyer's Patches. This in turn contributed to maintenance of a more diversified microbiota, in particular Firmicutes, which facilitated the further expansion of Foxp3+ T cells in the Peyer's patches. Thus, IgA and the microbiota can regulate each other creating a symbiotic mutualism between the host and microbes during homesostasis. We now need to understand how these processes break down during pathogen invasion or chronic inflammation in IBD.
The Role of Barrier Immune Defences: anti-microbial proteins
Similarly to IgA, the AMPs also seem to share a reciprocal relationship with the microbiota. These evolutionarily ancient innate immune peptides are produced and secreted by most mucosal epithelial cells (and some immune cells), however a specialized cell type, known as Paneth cells, located exclusively in the small intestinal crypts of Lieberkuhn, are the major source of these peptides in the intestinal tract (Reviewed in [73]). The primary AMPs produced in the intestines include the α-defensins (produced by Paneth cells), β-defensins (produced by colonocytes), C-type lectins (Regenerating islet-derived protein (REG) family; produced by Paneth cells and enterocytes) and cathelicidins (produced by colonocytes), Lysozyme, Phospholipase A2, and RNases (all produced by Paneth cells) [73]. The mechanism of action of these peptides varies widely. Broad spectrum bacterial targeting defensins and cathelicidins, possess cationic residues that are attracted to negatively charged bacterial lipid rich membranes creating pores leading to cell lysis, whereas other AMPs, including C-type lectins, selectively target Gram-positive bacteria by exclusively binding peptidoglycan [74, 75]. Despite the potency of AMPs, just as with antibiotics some pathogens have also developed mechanisms to evade AMP killing. For example, Salmonella and Shigella spp evade defensins by reducing the negative charge on their anionic cell walls to repulse cationic AMPs, or by inhibiting enteric synthesis of these molecules [76, 77]. More recently we have begun to understand the regulation of AMP secretion, which appears to be tightly controlled in order to minimize the toxic effect of some AMPs on mammalian cell membranes. Studies in germ-free mice have demonstrated that some intestinal AMPs require microbiota-derived signals, whereas others are dependent solely on the host. For instance, β-defensin 2, REG3γ, and angiogenin 4 (RNase) are either expressed at very low levels or absent in germ-free mice but upregulated upon colonization with the microbiota of conventionally-reared mice [74, 78-80]. Furthermore, other studies have begun to unlock the molecular basis for this microbiota-driven AMP expression. For example, stimulation of TLRs directly on epithelial cells is required for REG3γ expression, as mice lacking myeloid differentiation primary response protein 88 (MyD88) specifically on intestinal epithelial cells produce significantly reduced REG3γ and have diminished Paneth cell activity [81-83]. In addition, the intracellular pathogen recognition receptor (PRR) nucleotide oligomerization domain (NOD2), is activated by muramyl dipeptide ligands of Gram-positive and –negative bacteria to induce release of multiple AMPs from Paneth cells [84]. As a consequence of this role Nod2-deficient mice have substantially altered compositions of their small intestinal microbiota compared to littermate controls. Microbial signals may also induce AMPs via a non-PRR-dependent mechanism. For example, the production of the SCFA metabolite butyrate potently induces the expression of cathelicidins in human intestinal epithelial cell lines, thus enhancing resistance to infection [85]. Another important aspect of AMP regulation is that when these molecules are isolated from different tissue compartments it can be observed that they undergo selective post-translational activation by the reducing environment within the intestinal lumen, in contrast to the oxidizing conditions inside the cell, which are associated with weak AMP activity [86].
AMPs are not only regulated by commensals but also play a fundamental role in shaping the composition of the microbiota. Functional studies employing antibody-mediated neutralization of REG3γ in mice have provided direct evidence that this AMP is intimately involved in resistance to the opportunistic intestinal pathogen VRE [50, 87]. Importantly, Salzman et. al. demonstrated that α-defensins in particular, exert a profound effect on the composition of major phyla in the intestinal microbiota [88]. The authors compared the microbiota of Mmp7-deficient mice (unable to cleave and activate α-defensin) and α-defensin-overexpressing transgenic mice and discovered that reciprocal differences emerged in bacterial composition of the Firmicutes, and Bacteroidetes [88]. Finally, it appears that an integral part of the role of AMPs in shaping the microbiota involves achieving separation between the host epithelial surface and microbes. Recent evidence suggests that this effect is established in large part by the intestinal mucus layers concentrating the AMP activity [83, 89]. At baseline in Reg3γ-deficient mice, there is a pronounced increase in colonization of the intestinal surfaces by the Reg3γ targeted Gram-positive bacteria, however there are no changes in the bacterial contents of the luminal digesta, suggesting that activity of the AMP is limited to the mucus layer and epithelial surface [83].
Overall, there is increasing evidence that the host barrier immune defences not only prevent microbial invasion but also play an essential role in shaping the composition of the normal commensal population. A reciprocal relationship exists such that the commensals provide feedback signals that act to tune the control of the immune system in order to maintain separation between the microbiota and the physical epithelial barrier.
Microbial Modulation of the Intestinal Epithelial Barrier
The intestinal epithelial barrier is initiated by the intestinal stem cell (ISC) niche that gives rise to the differentiated cell types, including Paneth cells (small intestine), enteroendocrine cells, goblet cells, and absorptive colonocytes (colon) or enterocytes (small intestine) (see Figure1A) [90]. Bacterial metabolites and molecular patterns are the conduits through which intestinal microbes communicate with the intestinal epithelium. As described above, SCFAs are the major bacterial metabolites of gut fermentation. Regardless of which glycan or fibrous substrate is degraded, these molecules play critical roles at the epithelial surface. Butyrate in particular is a preferred energy source for absorptive colonocytes improving differentiated colonocyte survival using tissue explants in vitro (Reviewed in [91]). Propionate and acetate are more efficiently absorbed into the blood stream than butyrate, and have dramatic systemic effects on host function and immunity [92, 93]. Locally these SCFAs promote various host metabolic hormone and digestive processes by binding to the G-protein coupled receptors (GPCR) 41, 43, and 109a, which are differentially expressed on various intestinal and immune cell subsets (reviewed in [94]). Seminal work by Maslowski et. al. investigated how bacterial metabolites may impact on the immune system in particular the SCFAs as these molecules are diminished in multiple inflammatory diseases [92]. By studying the loss of function of GPR43 in mice, the authors uncovered a pivotal role for these molecules in suppressing inflammation in models of asthma, colitis, and arthritis.
Recently, Smith et. al. expanded upon these observations to demonstrate that germ-free mice have reduced numbers of regulatory T cells (Treg) in the colon and that this defect was reversible through colonization in a microbiota-, SCFA-, and GPR43-dependent manner [93]. The authors went on to show that SCFAs could directly induce Treg proliferation, through binding GPR43, and thus suppress colitogenic inflammation in mice during the T cell-transfer colitis model. SCFAs may be of significant clinical relevance in IBD as the levels of these molecules are lower in IBD patients than healthy controls, and as we have discussed SCFAs act to control both microbial composition and reduce inflammation [45, 85, 92, 95]. . These studies of SCFAs are likely only the first of many discoveries to evolve in this field. There are potentially many other metabolites that will influence the immune system or the epithelium through effects on epithelial turnover and barrier integrity.
Recent studies have demonstrated evidence to suggest bacterial localization adjacent to ISCs in crypts [15, 96], therefore, there is much interest in understanding whether and how the microbiota acts on ISCs to stimulate their proliferation/differentiation during homesostasis and repair. The bulk of studies so far suggest an indirect interaction. In Drosophila, Buchon et al. employed re-colonization of axenic (germ-free) flies with the commensal bacterium Erwinia carotovora [97]. They found that the re-colonization stimulated an increase in intestinal epithelial proliferation [97]. The authors suggested that ISCs were responsive to ROS signals produced by the host immune system following colonization, rather than the bacteria themselves. A similar process was identified in mice, whereby ISC proliferation was induced by the commensal Lactobacillus, due to its Nox1-mediated induction of ROS in the intestinal epithelium [98]. In a study examining the effects of aging on intestinal homeostasis and epithelial proliferation in Drosophila, it was demonstrated that as flies age they experience chronic activation of the transcription factor Foxo in the intestine [99]. Foxo induced microbial dysbiosis and increased innate immune signalling via dysregulation of Rel/NFkB activity. As the flies aged this dysregulation led to increased ROS-driven ISC hyperproliferation [99]. This process did not occur in axenic flies suggesting there is a role for microbiota-induced ROS in epithelial barrier dysplasia.
The direct host-commensal interaction is dictated by the presence of the intestinal mucus layer, produced by epithelial goblet cells and overlying the intestinal epithelium. Its importance is demonstrated by loss of function mutants, such as Muc2, that lead to spontaneous colonic inflammation and mucosal thickening in untreated mice (at 5 weeks of age), and more severe colitis than wild-type littermates following treatment with DSS [100]. We described in detail above (see Commensal Establishment and Competition) how the mucus layer and its glycan content shape the microbiota. Interestingly, the commensal communities have also been proposed to regulate mucus production, with the most convincing evidence coming from studies in germ free rodents. Goblet cells are both fewer in number and smaller in size in germ free mice as compared to conventionally raised mice [101]. Furthermore, the overall thickness of the mucus layer is decreased in germ-free rodents [102]. This phenomenon is reversible by addition of microbiota from conventionally-reared rodents [102]. Despite this broad evidence of a clear role, our current knowledge of the mechanism of goblet cell sensitivity to commensal microbial products is extremely limited and is now the subject of intense investigation in IBD pathogenesis.
Both the epithelium and mucus layer appear to respond to signals derived from the microbiota, including microbial molecular patterns and metabolites. We are only now beginning to investigate the cellular and molecular mechanisms underlying these effects.
Many unanswered question remain and future studies will aim to determine the effects of microbial ligands and metabolites on ISCs. It will be interesting to determine if shifts in metabolites secondary to the changes in bacterial composition can directly influence the function of the epithelial barrier in diseases such as IBD.
Emerging Concepts in our Understanding of the Microbiota and Disease
An emerging concept in studies of the microbiota is that the particular bacterial genera that are present in an individuals’ microbiome are perhaps less important and less indicative of a disease state than are the networks of bacterial genes present [1, 60, 103]. Based on analysis within a population of healthy individuals, the inter-individual variation in bacterial genes is comparatively much less than genus level composition [60]. Similarly, this pattern of bacterial genes is also more predictive of the disease state in IBD than is the bacterial genera signature [60]. Therefore, it may be more informative to examine the gene pathways present in an individual's microbiome rather than taxonomically categorizing the specific bacteria present. This type of gene pathway analysis has identified that bacterial metabolic pathways, such as the oxidative stress pathway, are more dramatically shifted in IBD than the taxonomical bacterial composition. Furthermore, the evolutionary link of phylogeny and microbial gene function may be sufficient such that simply sequencing the 16S marker genes can predict (using the PICRUSt algorithm) the metabolic roles of the uncultivated microbes living in the human intestinal tract [103]. Extending on this notion, recent publications conveyed that the general loss of microbiota richness (and thus bacterial genes) is predictive of diseases such as obesity [11, 104]. A similar observation has been made in IBD where the number of bacterial genes is at least 20% lower in diseased patients compared to healthy controls [105]. Whether these findings have potential implications for causation and therapeutic intervention remains to be seen.
Concluding Remarks
Over the next few years, there is much progress anticipated in understanding the impact of microbial populations on host physiology (see Box 2 Important areas of future research). One of the major caveats in the past has been the inability to examine the direct effects of specific microbes or metabolites on primary intestinal epithelial cells in vitro. The advent of new techniques for perpetuating cultures of these cells and their lineage differentiation may provide important new insights into the role of epithelial-microbe interactions [106, 107]. Furthermore, with a greater understanding of the manner in which commensals interact in inter-dependent networks and interface with the host we will hopefully begin to determine whether microbial community changes result from disease or are implicit in instigating disease. This will be a key future development in guiding therapeutic strategies, including those that would entail engineering microbial genomes. An important step forward will be the translating of the Human Microbiome Project findings into relevant animal models, in order to experimentally assess the functional roles of various microbes and networks of commensals in host health and disease, and also examine the influence of environmental perturbations on these microbial populations. For example, metagenomic screens have shown that the bacterial redox pathway is dysregulated in IBD. Given the possible importance of this pathway in epithelial proliferation, as discussed above, a critical question to be addressed is whether this acts as a dysbiotic trigger factor in IBD.
An improved understanding of the mechanisms underlying host-microbe interactions in homeostasis and disease would potentially enable targeted therapies involving manipulation of specific microbial populations. Although fecal transplant has produced exciting results in the treatment of antibiotic resistant C. difficile, this is a highly variable treatment with unknown long-term consequences and uncertain applicability to other intestinal diseases [108]. More specific manipulations of the microbiota may prove more beneficial. Similarly, a deeper understanding of these interactions may also lead us to adopt a more conservative stance on the use of antibiotics, especially in early-life, due to long-term effects on symbiotic commensal-host relationships. Despite the recent advances in our understanding of commensal colonization, competition and impact on immune cell development and differentiation, many aspects of the host-microbe interaction remain unknown. Developing approaches to examine how a complex microbiota interacts as a spatially and temporally dynamic network rather than distinct static species should be a priority.
Highlights.
Microbiota is an inter-dependent network of microbes influencing pathogen invasion
Diet, mucus, and microbiota-derived metabolites regulate commensal colonization
Microbiota maintains a reciprocal relationship with IgA and AMPs during homeostasis
Box1: Human Microbiome Project.
An initiative launched in 2008 by the National Institute of Health with the aim of culture-independent identification and molecular characterization of the microorganisms found in association with both healthy and diseased states in humans. This initiative was termed ‘The Human Microbiome Project’ (http://www.hmpdacc.org/), or HMP. The ultimate goal was to understand the role of the human microbiome in the maintenance of health and causation of disease, and to use this knowledge to improve the ability to prevent and treat disease. The HMP has generated a comprehensive data resource for various body sites (including mouth, nose, skin, intestine, and vagina) using directed 16S and also metagenomic sequencing of DNA to identify microbes and predict functional gene sets. Some of the principal findings have included evidence that there is strong body site (habitat) specificity among subjects but that the diversity and abundance of each habitat's microbes varies substantially between healthy subjects. Despite this diversity there was strong conservation of functionality of the bacterial genes between subjects. Thus, there are many different compositions of bacteria that can lead to the same overall functional potential. Another interesting finding was that human body sites varied widely in their alpha diversity (diversity of taxonomical units at a given body surface within an individual). For example, saliva was shown to have very high alpha diversity (many different taxa). Other interesting findings included correlations of groups of organisms with healthy host phenotypes. For example, ethnicity appeared to strongly correlate with microbiota composition across all body sites, and age of the individual appeared to have a significant influence on the microbiota of the skin and oral cavity. Furthermore, there was a correlation of microbial composition and body mass index.
The future goal of this project is that by providing access to this microbiome data resource (much like the human genome project) this will enable investigators to characterize the functional relationship between the human microbiome at various body sites and its role in human disease [1, 2]. One shortcoming of the HMP is that it has thus far largely overlooked the human virome, in particular the RNA genomes that exist for both mammalian viruses and bacteriophages. The other major question emanating from this project is that given the substantial inter-individual variation in diversity and abundance of the healthy microbiome what is the influence of diet and host genetics on this composition.
Box 2: Important areas of future research.
Determining the functional role (cause vs effect) of microbial dysbiosis in disease settings, such as in animal models of IBD.
Determining the impact of microbial-derived metabolites on intestinal epithelial and stem cells.
Determining the impact of microbes and their metabolites on immune education of different immune cell subsets at the gastrointestinal interface.
Determining the impact of microbes and their metabolites on systemic immune education including in the liver (the main site of metabolism), and lymphoid organs.
Temporal (longitudinal) analysis of the impact of the intestinal microbiota in immune cell development, differentiation and function, so as to assess whether there are critical ‘windows of opportunity’ early in life that corresponds to the development of the stable adult microbiota.
Systems-based approaches to define the underlying network architectures that characterize the interactions among intestinal microbial populations in homeostasis, and how these change in disease settings.
Further analysis of the fungal component of the microbiota and its correlation with human disease.
Metagenomic screening of the human virome including both DNA and RNA mammalian viruses and bacteriophages, and their correlation with disease.
Acknowledgements
This work was funded by DK097079 and the CCFA microbiome initiative. The Washington University Digestive Disease Research Core Center is supported by a grant from the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) (P30DK052574). National Health and Medical Research Council (NHMRC) of Australia Fellowship (GEK).
Glossary Box
- 16S sequencing
DNA sequencing of the variable region of bacterial ribosomal RNA genes, which are typically conserved within a bacterial species. Frequently used to taxonomically classify bacteria and determine the composition of bacterial communities.
- Bacteriocins
proteinaceous toxins produced by a particular species of bacteria that specifically inhibit different strains of the same bacterial species
- Commensal microbe
a bacterial, viral, fungal, or achaea organism that under normal circumstances resides in or on human tissue and does not cause disease, and forms a symbiotic relationship with the host in which one derives some benefit while the other is unaffected.
- Dysbiosis
is a state in which certain members of the indigenous microbial community become unbalanced due to overgrowth or reduced growth.
- Facultative anaerobe
is an organism that produces energy and ATP by aerobic respiration in environments where oxygen is present, and is also capable of switching to anaerobic respiration in environments where oxygen is absent.
- Germ-free animals
animals re-derived and reared in isolators without exposure to exogenous microorganisms
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Consortium HMP. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–14. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Consortium H. A framework for human microbiome research. Nature. 2012;486(7402):215–21. doi: 10.1038/nature11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10(3):159–69. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 4.Lee WJ, Hase K. Gut microbiota-generated metabolites in animal health and disease. Nat Chem Biol. 2014;10(6):416–24. doi: 10.1038/nchembio.1535. [DOI] [PubMed] [Google Scholar]
- 5.Hansson GC. Role of mucus layers in gut infection and inflammation. Curr Opin Microbiol. 2012;15(1):57–62. doi: 10.1016/j.mib.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaetzel CS. Cooperativity among secretory IgA, the polymeric immunoglobulin receptor, and the gut microbiota promotes host-microbial mutualism. Immunol Lett. 2014 doi: 10.1016/j.imlet.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salzman NH, Bevins CL. Dysbiosis--a consequence of Paneth cell dysfunction. Semin Immunol. 2013;25(5):334–41. doi: 10.1016/j.smim.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Faust K, Raes J. Microbial interactions: from networks to models. Nat Rev Microbiol. 2012;10(8):538–50. doi: 10.1038/nrmicro2832. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa M, et al. Transitions in oral and intestinal microflora composition and innate immune receptor-dependent stimulation during mouse development. Infect Immun. 2010;78(2):639–50. doi: 10.1128/IAI.01043-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matamoros S, et al. Development of intestinal microbiota in infants and its impact on health. Trends Microbiol. 2013;21(4):167–73. doi: 10.1016/j.tim.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Cotillard A, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500(7464):585–8. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- 12.Adlerberth I, et al. Reduced enterobacterial and increased staphylococcal colonization of the infantile bowel: an effect of hygienic lifestyle? Pediatr Res. 2006;59(1):96–101. doi: 10.1203/01.pdr.0000191137.12774.b2. [DOI] [PubMed] [Google Scholar]
- 13.Benno Y, Sawada K, Mitsuoka T. The intestinal microflora of infants: composition of fecal flora in breast-fed and bottle-fed infants. Microbiol Immunol. 1984;28(9):975–86. doi: 10.1111/j.1348-0421.1984.tb00754.x. [DOI] [PubMed] [Google Scholar]
- 14.Cummings JH, et al. Digestion and physiological properties of resistant starch in the human large bowel. Br J Nutr. 1996;75(5):733–47. doi: 10.1079/bjn19960177. [DOI] [PubMed] [Google Scholar]
- 15.Lee SM, et al. Bacterial colonization factors control specificity and stability of the gut microbiota. Nature. 2013;501(7467):426–9. doi: 10.1038/nature12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng KM, et al. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature. 2013;502(7469):96–9. doi: 10.1038/nature12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koropatkin NM, Cameron EA, Martens EC. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol. 2012;10(5):323–35. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mortensen PB, Nordgaard-Andersen I. The dependence of the in vitro fermentation of dietary fibre to short-chain fatty acids on the contents of soluble non-starch polysaccharides. Scand J Gastroenterol. 1993;28(5):418–22. doi: 10.3109/00365529309098242. [DOI] [PubMed] [Google Scholar]
- 19.Martinez I, et al. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS One. 2010;5(11):e15046. doi: 10.1371/journal.pone.0015046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker AW, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5(2):220–30. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu GD, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–8. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.David LA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Filippo C, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107(33):14691–6. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rawls JF, et al. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell. 2006;127(2):423–33. doi: 10.1016/j.cell.2006.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Favier CF, et al. Molecular monitoring of succession of bacterial communities in human neonates. Appl Environ Microbiol. 2002;68(1):219–26. doi: 10.1128/AEM.68.1.219-226.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harmsen HJ, et al. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr. 2000;30(1):61–7. doi: 10.1097/00005176-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 27.Mariat D, et al. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123. doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sela DA. Bifidobacterial utilization of human milk oligosaccharides. Int J Food Microbiol. 2011;149(1):58–64. doi: 10.1016/j.ijfoodmicro.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 29.Sela DA, et al. An infant-associated bacterial commensal utilizes breast milk sialyloligosaccharides. J Biol Chem. 2011;286(14):11909–18. doi: 10.1074/jbc.M110.193359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuhrer A, et al. Milk sialyllactose influences colitis in mice through selective intestinal bacterial colonization. J Exp Med. 2010;207(13):2843–54. doi: 10.1084/jem.20101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahowald MA, et al. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc Natl Acad Sci U S A. 2009;106(14):5859–64. doi: 10.1073/pnas.0901529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wrzosek L, et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013;11:61. doi: 10.1186/1741-7007-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rakoff-Nahoum S, Coyne MJ, Comstock LE. An ecological network of polysaccharide utilization among human intestinal symbionts. Curr Biol. 2014;24(1):40–9. doi: 10.1016/j.cub.2013.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benson AK, et al. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc Natl Acad Sci U S A. 2010;107(44):18933–8. doi: 10.1073/pnas.1007028107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eckburg PB, et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–8. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nava GM, Friedrichsen HJ, Stappenbeck TS. Spatial organization of intestinal microbiota in the mouse ascending colon. ISME J. 2011;5(4):627–38. doi: 10.1038/ismej.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arumugam M, et al. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–80. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caporaso JG, et al. Moving pictures of the human microbiome. Genome Biol. 2011;12(5):R50. doi: 10.1186/gb-2011-12-5-r50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Croswell A, et al. Prolonged impact of antibiotics on intestinal microbial ecology and susceptibility to enteric Salmonella infection. Infect Immun. 2009;77(7):2741–53. doi: 10.1128/IAI.00006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ayres JS, Trinidad NJ, Vance RE. Lethal inflammasome activation by a multidrug-resistant pathobiont upon antibiotic disruption of the microbiota. Nat Med. 2012;18(5):799–806. doi: 10.1038/nm.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marteyn B, et al. Modulation of Shigella virulence in response to available oxygen in vivo. Nature. 2010;465(7296):355–8. doi: 10.1038/nature08970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cherrington CA, et al. Short-chain organic acids at ph 5.0 kill Escherichia coli and Salmonella spp. without causing membrane perturbation. J Appl Bacteriol. 1991;70(2):161–5. doi: 10.1111/j.1365-2672.1991.tb04442.x. [DOI] [PubMed] [Google Scholar]
- 43.Fukuda S, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469(7331):543–7. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 44.Shin R, Suzuki M, Morishita Y. Influence of intestinal anaerobes and organic acids on the growth of enterohaemorrhagic Escherichia coli O157:H7. J Med Microbiol. 2002;51(3):201–6. doi: 10.1099/0022-1317-51-3-201. [DOI] [PubMed] [Google Scholar]
- 45.Gantois I, et al. Butyrate specifically down-regulates salmonella pathogenicity island 1 gene expression. Appl Environ Microbiol. 2006;72(1):946–9. doi: 10.1128/AEM.72.1.946-949.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cotter PD, Ross RP, Hill C. Bacteriocins - a viable alternative to antibiotics? Nat Rev Microbiol. 2013;11(2):95–105. doi: 10.1038/nrmicro2937. [DOI] [PubMed] [Google Scholar]
- 47.Schamberger GP, Diez-Gonzalez F. Selection of recently isolated colicinogenic Escherichia coli strains inhibitory to Escherichia coli O157:H7. J Food Prot. 2002;65(9):1381–7. doi: 10.4315/0362-028x-65.9.1381. [DOI] [PubMed] [Google Scholar]
- 48.Fabich AJ, et al. Comparison of carbon nutrition for pathogenic and commensal Escherichia coli strains in the mouse intestine. Infect Immun. 2008;76(3):1143–52. doi: 10.1128/IAI.01386-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamada N, et al. Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science. 2012;336(6086):1325–9. doi: 10.1126/science.1222195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brandl K, et al. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008;455(7214):804–7. doi: 10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Endt K, et al. The microbiota mediates pathogen clearance from the gut lumen after nontyphoidal Salmonella diarrhea. PLoS Pathog. 2010;6(9):e1001097. doi: 10.1371/journal.ppat.1001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brandt LJ, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107(7):1079–87. doi: 10.1038/ajg.2012.60. [DOI] [PubMed] [Google Scholar]
- 53.Theriot CM, et al. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat Commun. 2014;5:3114. doi: 10.1038/ncomms4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bloom SM, et al. Commensal Bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell Host Microbe. 2011;9(5):390–403. doi: 10.1016/j.chom.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garrett WS, et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8(3):292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maier L, et al. Microbiota-derived hydrogen fuels salmonella typhimurium invasion of the gut ecosystem. Cell Host Microbe. 2013;14(6):641–51. doi: 10.1016/j.chom.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Winter SE, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467(7314):426–9. doi: 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levitt MD, et al. Detoxification of hydrogen sulfide and methanethiol in the cecal mucosa. J Clin Invest. 1999;104(8):1107–14. doi: 10.1172/JCI7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gevers D, et al. The treatment-naive microbiome in new-onset Crohn's disease. Cell Host Microbe. 2014;15(3):382–92. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morgan XC, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13(9):R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Magin WS, Van Kruiningen HJ, Colombel JF. Immunohistochemical search for viral and bacterial antigens in Crohn's disease. J Crohns Colitis. 2013;7(2):161–6. doi: 10.1016/j.crohns.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 62.Negroni A, et al. Characterization of adherent-invasive Escherichia coli isolated from pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2012;18(5):913–24. doi: 10.1002/ibd.21899. [DOI] [PubMed] [Google Scholar]
- 63.Winter SE, et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science. 2013;339(6120):708–11. doi: 10.1126/science.1232467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cerutti A. The regulation of IgA class switching. Nat Rev Immunol. 2008;8(6):421–34. doi: 10.1038/nri2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moreau MC, et al. Increase in the population of duodenal immunoglobulin A plasmocytes in axenic mice associated with different living or dead bacterial strains of intestinal origin. Infect Immun. 1978;21(2):532–9. doi: 10.1128/iai.21.2.532-539.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hapfelmeier S, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328(5986):1705–9. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303(5664):1662–5. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 68.Wesemann DR, et al. Microbial colonization influences early B-lineage development in the gut lamina propria. Nature. 2013;501(7465):112–5. doi: 10.1038/nature12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bessa J, Bachmann MF. T cell-dependent and -independent IgA responses: role of TLR signalling. Immunol Invest. 2010;39(4-5):407–28. doi: 10.3109/08820131003663357. [DOI] [PubMed] [Google Scholar]
- 70.Johansen FE, et al. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J Exp Med. 1999;190(7):915–22. doi: 10.1084/jem.190.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei M, et al. Mice carrying a knock-in mutation of Aicda resulting in a defect in somatic hypermutation have impaired gut homeostasis and compromised mucosal defense. Nat Immunol. 2011;12(3):264–70. doi: 10.1038/ni.1991. [DOI] [PubMed] [Google Scholar]
- 72.Kawamoto S, et al. Foxp3(+) T cells regulate immunoglobulin a selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity. 2014;41(1):152–65. doi: 10.1016/j.immuni.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 73.Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. 2012;12(7):503–16. doi: 10.1038/nri3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cash HL, et al. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313(5790):1126–30. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kagan BL, et al. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc Natl Acad Sci U S A. 1990;87(1):210–4. doi: 10.1073/pnas.87.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gunn JS, et al. Genetic and functional analysis of a PmrA-PmrB-regulated locus necessary for lipopolysaccharide modification, antimicrobial peptide resistance, and oral virulence of Salmonella enterica serovar typhimurium. Infect Immun. 2000;68(11):6139–46. doi: 10.1128/iai.68.11.6139-6146.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Islam D, et al. Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat Med. 2001;7(2):180–5. doi: 10.1038/84627. [DOI] [PubMed] [Google Scholar]
- 78.O'Neil DA, et al. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol. 1999;163(12):6718–24. [PubMed] [Google Scholar]
- 79.Hooper LV, et al. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol. 2003;4(3):269–73. doi: 10.1038/ni888. [DOI] [PubMed] [Google Scholar]
- 80.Becker S, et al. Bacteria regulate intestinal epithelial cell differentiation factors both in vitro and in vivo. PLoS One. 2013;8(2):e55620. doi: 10.1371/journal.pone.0055620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brandl K, et al. MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection. J Exp Med. 2007;204(8):1891–900. doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vaishnava S, et al. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci U S A. 2008;105(52):20858–63. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vaishnava S, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334(6053):255–8. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Petnicki-Ocwieja T, et al. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc Natl Acad Sci U S A. 2009;106(37):15813–8. doi: 10.1073/pnas.0907722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schauber J, et al. Expression of the cathelicidin LL-37 is modulated by short chain fatty acids in colonocytes: relevance of signalling pathways. Gut. 2003;52(5):735–41. doi: 10.1136/gut.52.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schroeder BO, et al. Reduction of disulphide bonds unmasks potent antimicrobial activity of human beta-defensin 1. Nature. 2011;469(7330):419–23. doi: 10.1038/nature09674. [DOI] [PubMed] [Google Scholar]
- 87.Kinnebrew MA, et al. Bacterial flagellin stimulates Toll-like receptor 5-dependent defense against vancomycin-resistant Enterococcus infection. J Infect Dis. 2010;201(4):534–43. doi: 10.1086/650203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Salzman NH, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11(1):76–83. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meyer-Hoffert U, et al. Secreted enteric antimicrobial activity localises to the mucus surface layer. Gut. 2008;57(6):764–71. doi: 10.1136/gut.2007.141481. [DOI] [PubMed] [Google Scholar]
- 90.Philpott A, Winton DJ. Lineage selection and plasticity in the intestinal crypt. Curr Opin Cell Biol. 2014;31C:39–45. doi: 10.1016/j.ceb.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hamer HM, et al. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27(2):104–19. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 92.Maslowski KM, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282–6. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smith PM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–73. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blad CC, Tang C, Offermanns S. G protein-coupled receptors for energy metabolites as new therapeutic targets. Nat Rev Drug Discov. 2012;11(8):603–19. doi: 10.1038/nrd3777. [DOI] [PubMed] [Google Scholar]
- 95.Galvez J, Rodriguez-Cabezas ME, Zarzuelo A. Effects of dietary fiber on inflammatory bowel disease. Mol Nutr Food Res. 2005;49(6):601–8. doi: 10.1002/mnfr.200500013. [DOI] [PubMed] [Google Scholar]
- 96.Pedron T, et al. A crypt-specific core microbiota resides in the mouse colon. MBio. 2012;3(3) doi: 10.1128/mBio.00116-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Buchon N, et al. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5(2):200–11. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 98.Jones RM, et al. Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species. EMBO J. 2013;32(23):3017–28. doi: 10.1038/emboj.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guo L, et al. PGRP-SC2 Promotes Gut Immune Homeostasis to Limit Commensal Dysbiosis and Extend Lifespan. Cell. 2014;156(1-2):109–22. doi: 10.1016/j.cell.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Van der Sluis M, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131(1):117–29. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 101.Kandori H, et al. Histochemical, lectin-histochemical and morphometrical characteristics of intestinal goblet cells of germfree and conventional mice. Exp Anim. 1996;45(2):155–60. doi: 10.1538/expanim.45.155. [DOI] [PubMed] [Google Scholar]
- 102.Enss ML, et al. Changes in colonic mucins of germfree rats in response to the introduction of a “normal” rat microbial flora. Rat colonic mucin. J Exp Anim Sci. 1992;35(3):110–9. [PubMed] [Google Scholar]
- 103.Langille MG, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31(9):814–21. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Le Chatelier E, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–6. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 105.Qin J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 107.Miyoshi H, et al. Wnt5a potentiates TGF-beta signaling to promote colonic crypt regeneration after tissue injury. Science. 2012;338(6103):108–13. doi: 10.1126/science.1223821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cammarota G, Ianiro G, Gasbarrini A. Fecal Microbiota Transplantation for the Treatment of Clostridium difficile Infection: A Systematic Review. J Clin Gastroenterol. 2014 doi: 10.1097/MCG.0000000000000046. [DOI] [PubMed] [Google Scholar]