Abstract
Aberrant collagen production can lead to many diseases such as fibrosis. Current methods of collagen detection are insensitive, time consuming and laborious. We have developed a rapid, sensitive assay using chemiluminescence-based reporter cell system. Stable 3T3/NIH-SMAD-luciferase cells were generated for detection of collagen expression through TGF-β signaling, a major fibrogenic pathway. We demonstrated that these reporter cells could be used as a rapid screening tool for detection of SMAD-dependent collagen production with higher sensitivity than existing assays. Flexibility of this cell-based assay in different detection platforms makes it attractive for high throughput screening of potential fibrogenic agents and drug candidates.
Keywords: luciferase, reporter cells, TGF-β, collagen, fibrosis
The extracellular spaces in tissues which play important roles in cell signaling and tissue homeostasis are filled with organized extracellular matrix (ECM) composed of fibrous proteins, adhesion molecules, proteoglycans, and different types of proteinases (1). Collagens are the major fibrous proteins in the ECM. Collagen production and deposition in the ECM is highly regulated and abnormal regulation of this essential protein can lead to a variety of diseases such as fibrosis, sclerosis, and metastasis (2, 3). Over the years, various collagen assays have been developed to study collagen production and associated diseases. However, most of these assays generally overestimate collagen content due to interference of non-collageneous proteins and lack of specificity of detection which is commonly associated with most biochemical assays such as Sirius red and hydroxyproline assays (4, 5). Some antibody-based assays such as Western blotting provide excellent specificity but are laborious and time-consuming, and therefore are not suitable as a screening tool (6). In this study, we explored the use of cell-based assays to overcome the shortcomings of traditional assays. By using living cells, analysis of proteins can be performed under their native environment. In combination with reporter gene technique, the cell-based assay provides a versatile and quantitative assay to study cell function and gene expression. Among the various reporter genes, the firefly luciferase has become a predominant reporter due to its great sensitivity, wide dynamic range, and short assay time (7). In the luciferase assay, a genetic construct consisting of luciferase gene under the control of a promoter of the gene of interest is transfected into cells. Upon the promoter stimulation, the luciferase gene is activated and expresses into a luciferase enzyme. The enzyme activity is detected by adding appropriate luciferase substrates and measuring the luminescent products. The signal is quantitative and indicates enzyme activity and expression.
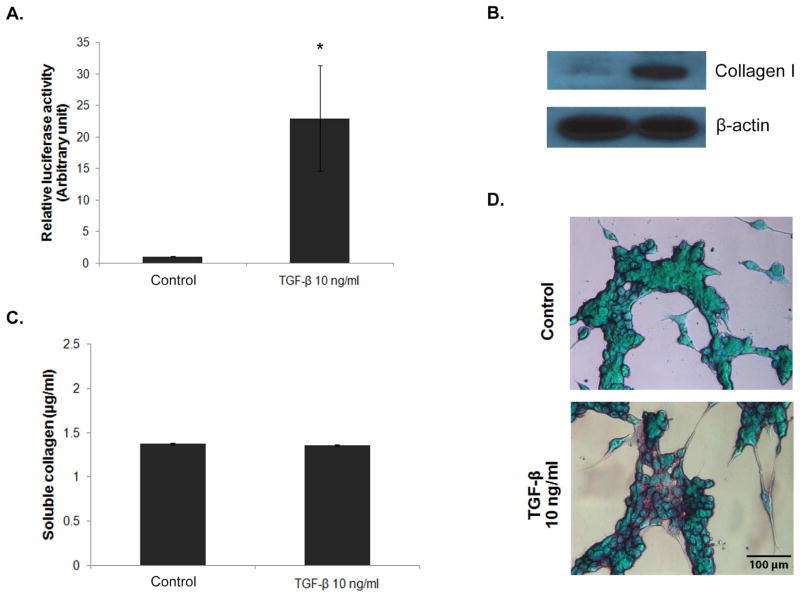
In this study, we generated luciferase reporter cells to study collagen production in fibroblasts following their stimulation with transforming growth factor (TGF)-β. TGF-β is a major fibrogenic cytokine known to be involved in the pathogenesis of several diseases by inducing excessive collagen production through the SMAD signaling pathway (8, 9). We used lentiviral particles expressing the firefly luciferase gene under the control of CMV promoter and tandem repeats of SMAD transcriptional response element (TRE) (SABiosciences, Valencia, CA, USA) to transduce 3T3/NIH fibroblast cells (American Type Culture Collection, Manassas, VA). Briefly, 1×105 cells were seeded into 6-well plate and incubated overnight at 37°C in a humidified 5% CO2 incubator. On the next day, completed medium was replaced with antibiotics-free medium containing viral particles at Multiplicity of Infection (MOI) of 10 and incubated for 20 hrs in the incubator. After the incubation, the medium containing lentiviral particles was removed and replaced by completed fresh medium. Fibroblasts were used since they are the primary cell type responsible for the production of collagen in the body. Selection of the transduced cells was performed in DMEM medium supplemented with 10% fetal calf serum (FCS) in the presence of 2 μg/ml puromycin. Collagen production and SMAD signaling activity of the reporter cells in the presence or absence of TGF-β1 were evaluated by various techniques, including chemiluminescence, Western blotting, and Sirius red-based colorimetry. Figure 1A shows that treatment of the reporter cells with TGF-β1 strongly enhanced their luciferase activity as compared to non-treated control. Western blot analysis of collagen content in the treated cells shows a substantial increase in collagen type I protein expression as compared to non-treated control (Figure 1B). However, Picro-Sirius red assay shows indistinguishable levels of soluble collagen in the treated and control cells (Figure 1C) due to the limited sensitivity of the assay (10). Visualization of collagen proteins in the cells by sirius red/fast green staining shows the induction of collagen (purple stained) by TGF-β1 in the treated cells (Figure 1D). These results indicate the responsiveness of the reporter cells to TGF-β stimulation and the potential utility of luciferase assay to monitor SMAD-dependent collagen production. As compared to other collagen assays which normally take hours to days to complete, the luciferase assay takes a few minutes to complete with great sensitivity, demonstrating key advantages of the assay.
Figure 1.
TGF-β-induced SMAD pathway and collagen production. Stable 3T3/NIH-SMAD-luciferase cells were generated from lentiviral transduction followed by puromycin selection. Cells were seeded into a 6-well plate at the density of 100,000 cells/well and incubated overnight. After a pre-incubation period of 6 hrs in serum-free medium, the cells were treated with 10 ng/ml of TGF-β1 for 16 hrs and analyzed for SMAD signaling activity and collagen production by (A) luciferase assay, (B) Western blot assay, (C) Picro-Sirius red assay, and (D) Sirius red/fast green staining. * = significant difference from control with P<0.05. Wild-type 3T3/NIH cells were used as a negative control for luciferase assay. Values obtained from the wild-type cells were subtracted from the test values before calculating relative luciferase activity.
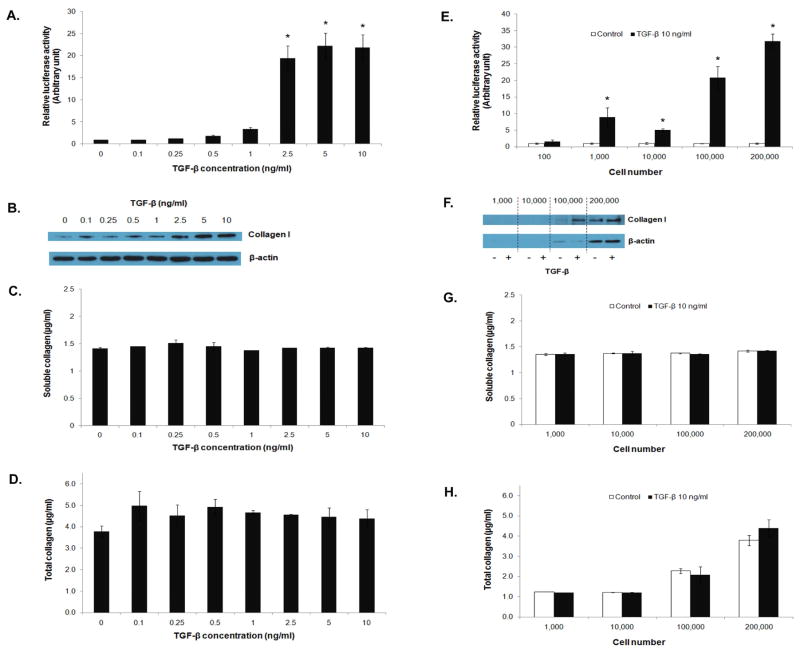
We further investigated the sensitivity of luciferase assay in comparison to Western blot and Picro-Sirius red assays in detecting collagen expression. The reporter cells at the seeding density of 100,000 cells/well were treated with different concentrations of TGF-β1 (0.1 to 10 ng/ml), and collagen content and luciferase activity were examined. Figure 2A and B show a dose-dependent increase in luciferase activity and collagen production in the treated cells. Luciferase and Western blot assays gave comparable results, i.e. significant induction of luciferase and collagen was observed at the TGF-β1 concentration of about 2.5 ng/ml and higher. Analysis of collagen content by Picro-Sirius red assay shows no significant changes in the level of soluble collagen but a slight increase in total collagen as determined by using cell supernatants and whole cell lysates, respectively (Figure 2C and D). These results indicate the relative insensitivity of Picro-Sirius red assay as compared to Western blot and luciferase assays.
Figure 2.
Comparison of detection sensitivity of various collagen assays. 3T3/NIH-SMAD-luciferase cells were seeded into a 6-well plate at the density of 100,000 cells/well and treated with TGF-β1 at different concentrations ranging from 0.1 to 10 ng/ml for 16 hrs. SMAD signaling activity and collagen type I expression were determined by (A) luciferase assay and (B) Western blot assay. Soluble and total collagen content was measured by Picro-Sirius red assay (C and D). Cell number-dependent detection sensitivity was demonstrated by treating the reporter cells at different cell numbers with 10 ng/ml of TGF-β1 and measuring luciferase activity and collagen content (E to H). * = significant difference from control with P<0.05.
Next, we determined the sensitivity of various assays by determining the minimum number of cells required for the assay. Cells at different cell numbers (100 – 200,000) were exposed to TGF-β1 (10 ng/ml) and analyzed for luciferase activity and collagen content. Figure 2E–H show that the luciferase assay requires the least number of cells for the detection, i.e. 100–1,000 cells (Figure 2E). In contrast, a 10-fold increase in the cell number (~100,000 cells) is needed for the Western blot assay (Figure 2F). The Picro-Sirius red assay was insensitive for the detection of soluble collagen regardless of the cell number (Figure 2G). For total collagen detection, the assay was operational at the high cell number of 200,000 (Figure 2H).
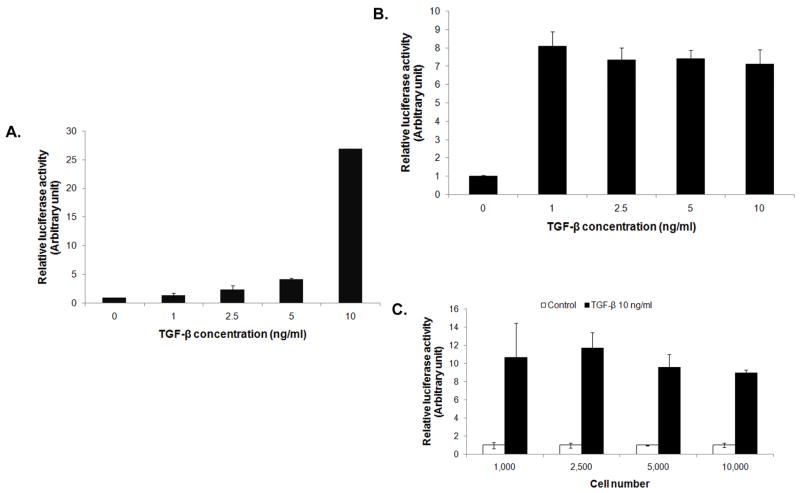
The potential usefulness of the luciferase assay was further evaluated in different detection platforms using microfluidic device and multi-well plates. In these experiments, the reporter cells were introduced into a single-channel microfluidic device or a 96-well plate, and their luciferase activity in response to TGF-β stimulation was determined using GloMax® 20/20 luminometer (Promega, Madison, WI) and FLUOstar OPTIMA (BMG GmbH, Ortenberg, Germany), respectively. In the microfluidic device with 0.45 cm2 growth area, the luciferase activity of the reporter cells was dose dependently increased by the TGF-β1 stimulation (Figure 3A). In the 96-well plate, the luciferase response to TGF-β was observed at the low concentration of TGF-β1 of 1 ng/ml (Figure 3B) and at the low cell number of 1,000 cells/well (Figure 3C). Increasing the TGF-β1 concentration or cell number, however, did not increase the luciferase activity due to limited dynamic range of the measuring device. Nonetheless, these results indicate the sensitivity of the reporter cell assay and its usefulness for high-throughput screening.
Figure 3.
Adaptability of 3T3/NIH-SMAD-luciferase reporter cells to different detection platforms. (A) Reporter cells were introduced into a single-channel microfluidic device and their luciferase activity in response to different concentrations of TGF-β1 was examined after 16 hrs using GloMax® 20/20 luminometer. (B) Induction of luciferase activity of the reporter cells by TGF-β1 in a 96-well format was evaluated. Reporter cells (10,000 cells/well) were treated with different concentrations of TGF-β1 and analyzed for luciferase activity using FLUOstar® OPTIMA plate reader. (C) Cells at different seeding densities were treated with 10 ng/ml of TGF-β1 and analyzed for luciferase activity.
In conclusion, we demonstrated the potential utility of the luciferase reporter cell assay to detect SMAD-dependent collagen production. As compared to conventional collagen assays, this cell-based assay is more sensitive, requires less cell number and preparation time for the detection, and is adaptable to different detection platforms. The assay is suitable for high throughput screening of disease-causing agents or drug candidates that affect collagen synthesis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Raghow R. The role of extracellular matrix in post-inflammatory wound healing and fibrosis. FASEB J. 1994;8:823–831. doi: 10.1096/fasebj.8.11.8070631. [DOI] [PubMed] [Google Scholar]
- 2.Uitto J, Chu M-L. Regulation of collagen gene expression in human skin fibroblasts and its alterations in diseases. In: Olsen BR, Nimni ME, editors. Collagen. Boca Raton, FL: CRC Press; 1989. pp. 110–124. [Google Scholar]
- 3.Byers PH. Collagens: building blocks at the end of the development time. Clin Genet. 2000;58:270–279. doi: 10.1034/j.1399-0004.2000.580404.x. [DOI] [PubMed] [Google Scholar]
- 4.Walsh BJ, Thornton SC, Penny R, Breit SN. Microplate reader-based quantitation of collagens. Analytical Biochem. 1992;203:187–190. doi: 10.1016/0003-2697(92)90301-m. [DOI] [PubMed] [Google Scholar]
- 5.Xu Q, Norman JT, Shrivastav S, Lucio-Cazana J, Kopp JB. In vitro models of TGF-beta-induced fibrosis suitable for highthroughput screening of antifibrotic agents. Am J Physiology. 2007;293:F631–640. doi: 10.1152/ajprenal.00379.2006. [DOI] [PubMed] [Google Scholar]
- 6.Ramshaw JA, Werkmeister JA. Electrophoresis and electroblotting of native collagens. Analytical Biochem. 1988;168:82–87. doi: 10.1016/0003-2697(88)90013-9. [DOI] [PubMed] [Google Scholar]
- 7.Wood KV. The chemistry of bioluminescent reporter assays. Promega Notes. 1998;65:14–20. [Google Scholar]
- 8.Trojanowska M, LeRoy EC, Eckes B, Krieg T. Pathogenesis of fibrosis: type I collagen and the skin. J Mol Med. 1998;76:266–274. doi: 10.1007/s001090050216. [DOI] [PubMed] [Google Scholar]
- 9.Chen SJ, Yuan W, Mori Y, Levenson A, Trojanowska M, Varga J. Stimulation of Type I collagen transcription in human skin fibroblasts by TGF-β: involvement of Smad3. J Invest Dermatol. 1999;112:49–57. doi: 10.1046/j.1523-1747.1999.00477.x. [DOI] [PubMed] [Google Scholar]
- 10.Taskiran D, Taskiran E, Yercan H, Kutay FZ. Quantification of total collagen in rabbit tendon by the sirius red method. Tr J Med Sci. 1999;29:7–9. [Google Scholar]