Abstract
When psychostimulant drugs like amphetamine are administered repeatedly in the presence of a contextual stimulus complex, long-lasting associations form between the unconditioned effects of the drug and the contextual stimuli. Here we assessed the role played by the proline-directed serine/threonine kinase cyclin-dependent kinase 5 (Cdk5) in the nucleus accumbens (NAcc) on the expression of the conditioned locomotion normally observed when rats are returned to a context previously paired with amphetamine. Infusing the Cdk5 inhibitor roscovitine (40 nmol/0.5μl/side) into the NAcc 30-min before the test for conditioning significantly enhanced the conditioned locomotor response observed in rats previously administered amphetamine in the test environment. This effect was specific to the expression of a conditioned response as inhibiting Cdk5 produced no effect in control rats previously administered saline or previously administered amphetamine elsewhere. As inhibiting Cdk5 during exposure to amphetamine has been found to block the accrual of locomotor conditioning, the present results suggest distinct roles for NAcc Cdk5 in the induction and expression of excitatory conditioning by amphetamine.
Keywords: Cdk5, conditioning, learning, locomotion, psychostimulants, roscovitine
1. Report
Psychomotor stimulants like amphetamine acutely increase locomotor activity. When these drugs are administered repeatedly in the presence of a particular contextual stimulus complex, long-lasting associations form between the unconditioned effects of the drugs and the contextual stimuli. These associations then allow drug-paired stimuli to elicit drug-like excitatory conditioned responses as in the conditioned locomotion that is observed when rats are returned weeks to months later to the context in which they had previously been administered amphetamine [1]. Given the ubiquity of drug-related stimuli in a contextual stimulus complex previously associated with drug administration, conditioned enhancements in locomotion likely reflect increased approach and interaction with these stimuli [2], effects characteristic of drug seeking and craving [3]. Indeed, such drug-stimulus associations have been linked to addiction vulnerability and reinstatement in humans and animal models [4–6].
Some studies using pharmacological inhibitors have implicated actions of the proline-directed serine/threonine kinase cyclin-dependent kinase 5 (Cdk5) in different brain regions in various types of learning including fear conditioning (lateral septum and hippocampus [7]) and cocaine conditioned place preference (CPP; basolateral amygdala [BLA;8]). As the major subcortical forebrain projection field of mesolimbic dopamine neurons, the nucleus accumbens (NAcc) has also been found to be an important site of Cdk5 actions in drug conditioning. In an initial report, selective Cdk5 knock-out in the NAcc was found to produce decreased, increased, or no change in the acquisition of cocaine CPP depending on the training dose of cocaine used. This manipulation also spared the acquisition of instrumental responding [9]. However, those results are difficult to interpret as the Cdk5 knock-out manipulation used in that experiment spanned the development and expression phases of conditioning, making it impossible to determine whether the effects observed were due to actions of the Cdk5 knock-out during acquisition, testing, or a combination of the two. Indeed, different neuronal mechanisms appear to underlie the induction and expression of excitatory conditioning [10–11] and these may be differentially regulated by Cdk5. Recently, we reported that inhibiting Cdk5 signaling in the NAcc exclusively during exposure to amphetamine prevented the accrual of contextual locomotor conditioning by amphetamine, indicating that Cdk5 actions in the NAcc are necessary for the induction of excitatory contextual associative conditioning [12]. In the experiment reported here, we assessed the effect of inhibiting Cdk5 in the NAcc exclusively on the expression of contextual locomotor conditioning with amphetamine.
Male Sprague-Dawley rats weighing 250–275g on arrival from Harlan (Madison, WI) were housed individually in a reverse cycle room (12-h light/12-h dark, lights on at 8pm). Food and water were freely available at all times. After 4–5 days of acclimation, rats were anesthetized with ketamine (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and stereotaxically implanted bilaterally with chronic indwelling guide cannula angled at 10° and aimed at the NAcc shell (A/P, +3.4; M/L, ± 0.8; DV, −7.5mm from bregma and skull). The NAcc shell was targeted because it has been shown to mediate context-induced drug seeking [13] and is uniquely innervated by the ventral hippocampus [14], a structure known to possess contextual information [15]. Guide cannulae were fixed in place with dental cement anchored by six screws securely fastened to the skull. Obturators (28 gauge; 0.32 mm, o.d.) were positioned in the guide cannulae to protrude 1mm beyond the guide tips. Rats were afforded a 10–14 day recovery period in their home cages before the start of all experimental procedures. All surgical procedures were conducted using aseptic techniques according to an approved Institutional Animal Care and Use Committee protocol.
The experiment consisted of three phases: drug exposure, withdrawal, and testing for conditioning. In the drug exposure and testing phases, locomotion was measured using a bank of 8 open fields (43.2 X 43.2 X 30.5 cm; Med Associates, St. Albans, VT) constructed with acrylic walls, wire flooring, and a Plexiglas top. A horizontal 16 X 16 grid of infrared sensors positioned 3.5cm above the wire floor was used to detect ambulatory counts. These were recorded by Med Associate’s software (SOF-811).
The drug exposure phase consisted of four 3-day blocks. Injections were administered on the first two days of each block, the first immediately before placing rats in the open field and the second in the home cage. On the third day, rats were left undisturbed in the home cage. Rats in one group (Paired) were administered amphetamine (1.5 mg/kg, i.p.) in (paired with) the open field and saline (1.0 ml/kg, i.p.) in the home cage. Rats in a second group (Unpaired) were administered saline in the open field and amphetamine in the home cage (unpaired with the open field). Rats in a final group (Control) were administered saline in both environments. Thus, during the drug exposure phase, rats in all groups were equally exposed to the open fields but subjected to different amphetamine-open field pairings. Locomotion was recorded in the open fields for 2-h. S(+)-amphetamine sulfate (Sigma-Aldrich Inc., Saint Louis, MO) was dissolved in sterile saline. The dose [12] refers to the weight of the salt.
Following the 12 days of exposure (4 X 3-day blocks), rats were afforded a 1-week withdrawal period during which they were left undisturbed in their home cages. Rats in each of the conditioning groups were then randomly assigned to two subgroups that determined whether they received an infusion into the NAcc of vehicle (Veh) or the Cdk5 inhibitor (R)-roscovitine (Ros) prior to the test for conditioning. Six different groups were thus tested on the conditioning test: Paired-Veh, Paired-Ros, Unpaired-Veh, Unpaired-Ros, Control-Veh, and Control-Ros. On the test for conditioned locomotion, rats were administered a systemic saline injection (1.0 ml/kg, i.p.) preceded 30-min earlier by a bilateral infusion into the NAcc of Veh (0.5μl/side) or Ros (40 nmol/0.5μl/side) and their locomotor activity was assessed for 1-hr in the open fields. Ros (Enzo Life Sciences Inc., Plymouth Meeting, PA) was dissolved in 1XPBS/50% DMSO vehicle. The dose of Ros tested was selected because it has been used by others to study the effects of Cdk5 inhibition in the NAcc (see [16] and references therein). We also recently showed that when infused into the NAcc during drug exposure, this dose of Ros prevents the induction of locomotor conditioning by amphetamine [12]. The use of the same dose in the present study assessing the effects of inhibiting NAcc Cdk5 on the expression of locomotor conditioning will thus allow the direct comparison of the results obtained to those obtained in our recent study [12] as well as those reported by others (see [16]).
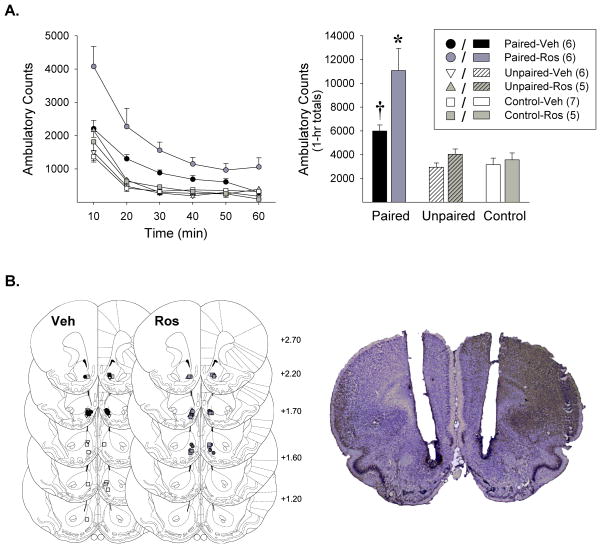
After the conditioning test, rats were deeply anesthetized with ketamine and xylazine and subjected to intracardiac perfusion with 0.9% saline followed by 10% formalin. Brains were then harvested, stored in 10% formalin, and 40 μm coronal slices taken with a cryostat. Brain slices were mounted onto gelatin-coated slides and subsequently stained with a cresyl violet solution in order to verify cannula tip placements within the NAcc shell. Only rats with bilateral cannula tips placed correctly within the NAcc shell were included in the behavioral analyses (Figure 2B). Of the 50 rats tested, 15 rats failed to meet this histological criterion and were thus excluded.
Figure 2. Microinjection of the cdk5 inhibitor roscovitine (Ros) into the NAcc exclusively on the test for conditioning enhances the expression of amphetamine-induced conditioned locomotion.
One week after exposure to amphetamine or saline in the open fields, rats were administered NAcc vehicle or Ros followed 30-min later by a systemic saline injection and returned to the open fields where their locomotor activity was assessed for 60-min. (A) Locomotor activity observed on the test for conditioning. Data are shown as (left) group mean ambulatory counts (± SEM) over the 1-h of testing and as (right) 1-h group mean totals (+ SEM). *, p<0.05, relative to all other groups. †, p<0.05, relative to NAcc-Veh treated Unpaired and Control groups. (B) Line drawings depicting location of microinjection cannula tips in the NAcc shell for rats included in the data analyses (left). Numbers indicate mm from bregma. The photomicrograph to the right shows a representative cresyl violet stained brain section with bilateral cannula tracks targeting the NAcc shell. n=5–7/group.
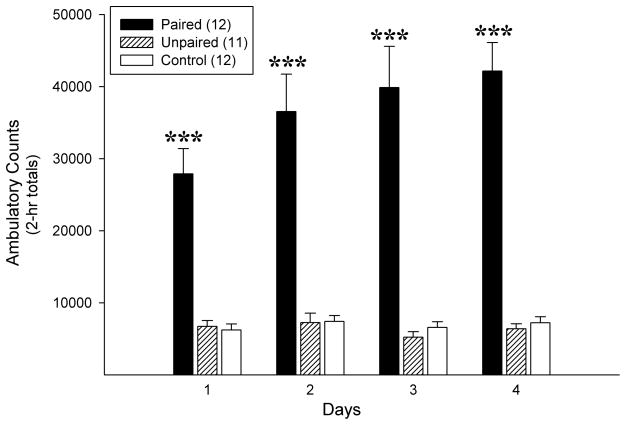
As expected, Paired rats administered amphetamine in the open fields displayed greater locomotor activation throughout the drug exposure phase compared to Unpaired and Control rats administered saline (Figure 1). This was true whether rats were subsequently designated Ros or Veh, as Ros was not administered during this phase. The one-way between one-way within repeated measures ANOVA conducted on the 2-h total ambulatory counts obtained for the 3 conditioning groups on each of the 4 exposure days revealed significant effects of conditioning [F2,32=43.87, p<0.001], injection day [F3,96=7.75, p<0.001], and a significant conditioning X injection day interaction [F6,96=7.72, p<0.001]. Post-hoc LSD analyses confirmed that Paired rats showed elevated ambulatory counts compared to the other two groups on all 4 exposure days (p<0.001) and that these counts increased progressively over days (p<0.001). No statistically significant differences were detected between the Unpaired and Control conditions nor did ambulatory counts change significantly over days in these groups.
Figure 1. Locomotor activity in the open fields during the drug exposure phase.
Locomotion was significantly greater in Paired rats administered amphetamine compared to Unpaired and Control rats administered saline in the open fields. Data are shown as 2-h group mean total ambulatory counts (+ SEM). ***, p<0.001, relative to the Unpaired and Control groups at the indicated day. n=11–12/group.
On the test for conditioning conducted 1 week later, rats that had been exposed to amphetamine Paired with the open fields showed, as expected, a greater locomotor response when injected with saline and returned to the open fields than rats that were previously exposed to the same number of amphetamine injections but Unpaired with the open fields or Control rats previously exposed to saline. Interestingly, this conditioned locomotor response was enhanced in Paired rats administered NAcc-Ros 30-min prior to the test. No effects of NAcc-Ros were detected in Unpaired or Control rats (Figure 2A). The two-way between ANOVA conducted on the 1-h total ambulatory counts obtained on this test, with conditioning (3) and Ros (2) groups as the between factors, showed significant effects of conditioning [F2,29=21.47, p<0.001] and Ros [F1,29=8.72, p<0.01], as well as a significant conditioning X Ros interaction [F2,29=3.99, p<0.05]. Post-hoc LSD comparisons revealed that NAcc-Veh injected Paired rats showed the expected conditioned locomotor response compared to equally NAcc-Veh injected Unpaired and Control rats (p<0.05). The conditioned locomotor response observed in NAcc-Ros injected Paired rats was also revealed to be significantly greater than that of all other groups (p<0.05–0.001). No other statistically significant differences between groups were detected.
In the present experiment, pharmacologically inhibiting Cdk5 in the NAcc with Ros exclusively on the test for conditioning enhanced the conditioned locomotor response observed in Paired rats. This effect was specific to the expression of the excitatory conditioned response as NAcc-Ros produced no significant effects in Unpaired and Control rats.
The present results suggest that NAcc Cdk5 can normally exert inhibitory constraints on the expression of conditioned locomotion previously established by repeated amphetamine-open-field pairings. Such inhibitory constraints were previously proposed to account for the ability of Cdk5 inhibition in the NAcc to enhance the expression of cocaine-induced locomotor sensitization and incentive to self-administer the drug [16]. In these studies, evidence for a potential underlying mechanism was obtained by which repeated exposure to cocaine increased striatal levels of Cdk5 mRNA, leading to increased Cdk5 phosphorylation of DARPP-32 at Thr 75, and thereby decreasing phosphorylation by PKA of DARPP-32 at Thr 34. This pathway results in less protein phosphatase (PP1) inhibition and decreased phosphorylation of proteins responsible for psychostimulant induced locomotion [16–17]. Thus, inhibiting Cdk5 in the NAcc would lead to increased PP1 inhibition, greater phosphorylation of target proteins, and greater locomotor output. Such a sequence of neuronal events could potentially also provide a mechanism for the present results showing that inhibiting NAcc Cdk5 enhances the expression of conditioned locomotion. However, the signaling pathway by which a drug-paired stimulus complex might recruit NAcc Cdk5 to constrain the expression of conditioned locomotion remains to be determined. Interestingly, increased Cdk5 activity and levels of the Cdk5 activator p35 have been observed in the BLA of rats displaying a CPP for a cocaine associated context on a drug-free test, although in that study, infusion into the BLA of a dose of β-butyrolactone known to inhibit Cdk5 blocked rather than enhanced the expression of the CPP (see [8] and references therein). These findings together with those reported here clearly highlight important differences in the effects of Cdk5 in the NAcc and the BLA on the expression of excitatory conditioned drug effects. It will be important to determine whether drug-paired stimuli can alter Cdk5 activity in the NAcc and delineate how downstream signaling is affected in this site.
In addition to inhibiting Cdk5, Ros has also been shown to produce other effects with lower potency, including slowing of the deactivation of N-type calcium channels [18] and increasing dopamine overflow in vitro [19], effects that could also enhance behavioral responding to psychostimulants [20–21]. In these cases as well, however, it remains to be determined by which signaling pathway these effects might have selectively modulated the expression of conditioned locomotion in the present experiment as no detectible effects of NAcc-Ros were observed in Unpaired and Control rats.
Interestingly, the enhancement of the conditioned locomotion expressed in the present experiment when NAcc-Ros was administered exclusively on the test for conditioning differs remarkably from what we observed previously when NAcc-Ros was administered exclusively during the amphetamine-open-field pairings and inhibited the development of conditioned locomotion [12]. Together, these findings clearly indicate different roles for NAcc Cdk5 in the induction and expression of excitatory conditioning by amphetamine that likely reflect different actions of the enzyme. As with sensitization [21], different neuronal mechanisms have been proposed to underlie the development and expression of excitatory conditioning [10–11]. The present results suggest that these are modulated differently by Cdk5. This enzyme may contribute to the induction of excitatory drug conditioning by regulating spine maturity [22] and the capacity of spines for rapid morphological change upon the presentation of drug-paired stimuli [12,23]. Importantly, these different actions of Cdk5 require that caution be exercised when interpreting the results of a number of recent reports using knock-out, knock-down or transgenic mouse preparations to manipulate Cdk5 signaling [9,24,25] as these manipulations spanned the induction and expression phases of conditioning and thus could not distinguish between the two.
HIGHLIGHTS.
The formation of drug-context associations contributes to addiction
Inhibiting NAcc Cdk5 enhances the expression of amphetamine-induced conditioning
This effect differs from its known ability to block the induction of conditioning
NAcc Cdk5 plays distinct roles in the induction and expression of drug conditioning
Acknowledgments
Research in this report was funded by National Institutes of Health grants R01 DA09397 (PV), T32 DA07255 (BFS), and F31 DA030021-01A1 (BFS). This work was also partially funded by the Chicago Biomedical Consortium with support from The Searle Funds at The Chicago Community Trust (BFS).
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stewart J, Vezina P. Conditioning and behavioral sensitization. In: Kalivas PW, Barnes CD, editors. Sensitization in the nervous system. Caldwell, NJ: Telford Press; 1988. pp. 207–224. [Google Scholar]
- 2.Vezina P, Stewart J. Conditioned locomotion and place preference elicited by tactile cues paired exclusively with morphine in an open field. Psychopharmacology. 1987;91:375–80. doi: 10.1007/BF00518195. [DOI] [PubMed] [Google Scholar]
- 3.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 4.Vezina P, Leyton M. Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology. 2009;56:160–168. doi: 10.1016/j.neuropharm.2008.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leyton M, Vezina P. Striatal ups and downs: Their roles in vulnerability to addictions in humans. Neurosci Biobeh Rev. 2013;37:1999–2014. doi: 10.1016/j.neubiorev.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leyton M, Vezina P. Dopamine ups and downs in vulnerability to addictions: A neurodevelopmental model. TIPS. 2014;35:268–276. doi: 10.1016/j.tips.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer A, Sananbenesi F, Schrick C, Spiess J, Radulovic J. Cyclin-dependent kinase 5 is required for associative learning. J Neurosci. 2002;22:3700–3707. doi: 10.1523/JNEUROSCI.22-09-03700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li F, Xue Y, Wang J, Fang Q, Li Y, Zhu W, et al. Basolateral amygdala cdk5 activity mediates consolidation and reconsolidation of memories for cocaine cues. J Neurosci. 2010;30:10351–10359. doi: 10.1523/JNEUROSCI.2112-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benavides DR, Quinn JJ, Zhong P, Hawasli AH, DiLeone RJ, Kansy JW, et al. Cdk5 modulates cocaine reward, motivation, and striatal neuron excitability. J Neurosci. 2007;27:12967–12976. doi: 10.1523/JNEUROSCI.4061-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banasikowski TJ, Bespalov A, Drescher K, Behl B, Unger L, Haupt A, et al. Double dissociation of the effects of haloperidol and the dopamine D3 receptor antagonist ABT-127 on acquisition vs. expression of cocaine-conditioned activity in rats. J Pharmacol Exp Ther. 2010;335:506–515. doi: 10.1124/jpet.110.171348. [DOI] [PubMed] [Google Scholar]
- 11.Cervo L, Samanin R. Effects of dopaminergic and glutamatergic receptor antagonists on the acquisition and expression of cocaine conditioned place preference. Brain Res. 1995;673:242–250. doi: 10.1016/0006-8993(94)01420-m. [DOI] [PubMed] [Google Scholar]
- 12.Singer BF, Neugebauer NM, Forneris J, Rodvelt KR, Li D, Bubula N, Vezina P. Locomotor conditioning by amphetamine requires cyclin-dependent kinase 5 signaling in the nucleus accumbens. Neuropharmacology. 2014;85:243–252. doi: 10.1016/j.neuropharm.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cruz FC, Rabin KR, Leao RM, Goldart EM, Bossert JM, Shaham Y, et al. Role of nucleus accumbens shell neuronal ensembles in context-induced reinstatement of cocaine-seeking. J Neurosci. 2014;34:7437–7446. doi: 10.1523/JNEUROSCI.0238-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moses SN, Sutherland RJ, McDonald RJ. Differential involvement of amygdala and hippocampus in responding to novel objects and contexts. Brain Res. 2002;58:517–527. doi: 10.1016/s0361-9230(02)00820-1. [DOI] [PubMed] [Google Scholar]
- 16.Taylor JR, Lynch WJ, Sanchez H, Olausson P, Nestler EJ, Bibb JA. Inhibition of Cdk5 in the nucleus accumbens enhances the locomotor-activating and incentive-motivational effects of cocaine. PNAS. 2007;104:4147–4152. doi: 10.1073/pnas.0610288104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, et al. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signaling in neurons. Nature. 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- 18.Buraei Z, Schofield G, Elmslie KS. Roscovitine differentially affects CaV2 and Kv channels by binding to the open state. Neuropharmacology. 2007;52:883–894. doi: 10.1016/j.neuropharm.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Price DA, Sorkin A, Zahniser NR. Cyclin-dependent kinase 5 inhibitors: Inhibition of dopamine transporter activity. Mol Pharm. 2009;76:812–823. doi: 10.1124/mol.109.056978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierce RC, Quick EA, Reeder DC, Morgan ZR, Kalivas PW. Calcium-mediated second messengers modulate the expression of behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1998;286:1171–1176. [PubMed] [Google Scholar]
- 21.Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Xin X, Wang Y, Ma X, Rompolas P, Keutmann HT, Mains RE, et al. Regulation of Kalirin by Cdk5. J Cell Sci. 2008;121:2601–2611. doi: 10.1242/jcs.016089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013;77:867–872. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer A, Sananbenesi F, Pang PT, Lu B, Tsai L-H. Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron. 2005;48:825–838. doi: 10.1016/j.neuron.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 25.Hawasli AH, Benavides DR, Nguyen C, Kansy JW, Hayashi K, Chambon P, et al. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nature Neurosci. 2007;10:880–886. doi: 10.1038/nn1914. [DOI] [PMC free article] [PubMed] [Google Scholar]