A new syndrome of paraganglioma-somatostatinoma-polycythemia caused by somatic gain-of-function HIF2A mutations has been recently described.1,2 All patients presented with high erythropoietin. We report additional newly identified ocular manifestations of this syndrome in 4 HIF2A patients (Supplementary Table 1). Patients with this syndrome shared unique eye lesions clustered with new presentations of their tumors. IRB approval was received for this study. A 9-year-old female was referred for evaluation of an abdominal paraganglioma with polycythemia. On admission, we confirmed ocular abnormalities (Figure 1). A 32-year-old female with a Marfanoid habitus and paraganglioma-somatostatinomapolycythemia was previously reported in our initial study.1 A follow-up exam in 2013 revealed ocular abnormalities (Supplementary Figure 1). A 15-year-old male with a Marfanoid habitus was evaluated for the presence of right adrenal and pelvic paragangliomas associated with polycythemia from birth. At age 3 years, he was reported to have ocular abnormalities (Supplementary Figure 2). A 39-year-old female, who developed polycythemia at age 2, was referred to us for recurrent/multiple abdominal paragangliomas and a peripancreatic neuroendocrine tumor. Her eye exam was found to be abnormal (Supplementary Figure 3). The common finding in all these cases included bilateral dilated capillaries and fibrosis overlying the optic disc in both eyes. Two patients had exudation secondary to the optic disc abnormalities that resulted in macular edema and retinal hard exudate with visual acuity decrease, requiring anti-vascular endothelial growth factor agents. One patient developed peripheral retinal neovascularization that were flat, and some had dilated arterioles and venules. All patients had gain-of-function mutations in the EPAS1 gene (encoding HIF-2A peptide) detected in tumors but not in leukocyte DNA.
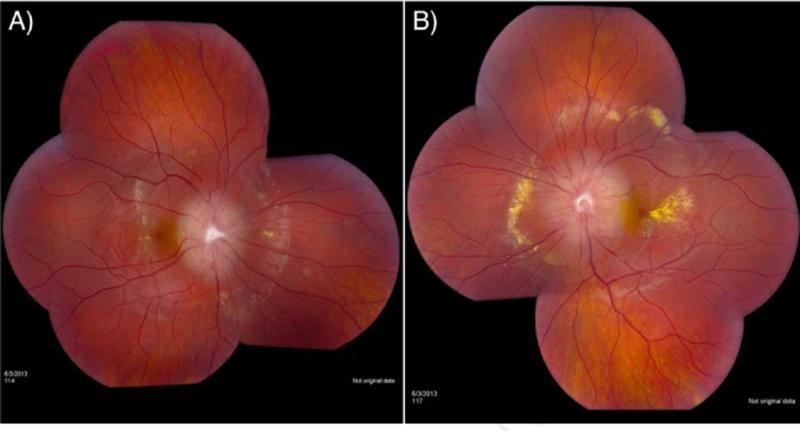
Figure 1.
A 9-year-old female was incidentally diagnosed at 18 months with bilateral disc edema, including dilated capillaries and fibrosis. She was asymptomatic until 8 years of age when she experienced bilateral macular edema with retinal hard exudates. She was treated with intravitreal injections of anti-vascular endothelial growth factor (VEGF), bevacizumab (Avastin®). At an NIH exam in June 2013, her visual acuity was 20/25 and 20/30 in the right and left eyes, respectively. She had considerably elevated optic discs bilaterally with evidence of fibrosis and dilated capillaries. Marked intraretinal hard exudate was also observed around the optic disc as well as in the macular area. No other lesions were seen in the retinal periphery. Panel 1A shows the retinal hard exudate surrounding the elevated right optic disc and the macular edema with hard exudate in the fovea. Marked exudative changes in the left macula and around her left optic disc are seen in Panel 1B, demonstrating the bilateral nature of this condition.
Paraganglioma-somatostatinoma-polycythemia is a new syndrome, and the unique eye lesions clustered with the syndrome suggest that somatic gain-of-function HIF2A mutations play an important role in the pathogenesis of these eye lesions. Hypoxia-inducible factor (HIF) signaling is one of the critical regulation pathways for cells under hypoxia/pseudohypoxia to activate cellular responses. HIFs are composed of HIF-β and one of the three HIF-α (HIF-1α, HIF-2α, and HIF-3α) subunits.3 HIF-2α is expressed preferentially in neural crest cells, the endothelium, kidney, heart, lung, and gastrointestinal epithelium, and HIF-2α is the principal regulator of erythropoietin production. In humans, the retina develops from neural crest cells, through evagination from the diencephalon, and the lens originates from the surface of the ectoderm.4 HIF-2α alteration in a transgenic mouse model exhibits marked retinopathy.5 In addition, erythropoietin is known as a potent angiogenic stimulus in the retina. Therefore, a HIF2A mutation, along with high erythropoietin levels, may affect the eye, especially the retina, resulting in the ocular abnormalities among patients.
In addition to the HIF2A mutation, the genes affecting the HIF signaling pathway such as von Hippel-Lindau, succinate dehydrogenase, and prolyl hydroxylase can have mutations found to be associated with neural crest cell tumors, paragangliomas/pheochromocytomas. Dysregulation of the HIF signaling pathway initiates an activation cascade of genes, many of them participating in angiogenesis, abnormal apoptosis, cell migration, and development, processes that can lead to tumorigenesis and may affect tissue-specific development of various organs or structures (as indicated by the presence of a Marfanoid habitus and brain abnormalities), including the eye and retina.3,5
As of the time of this report, over 20 patients have been described presenting with HIF2A mutations associated with paraganglioma and polycythemia. Our series of cases (4 out of 7 examined cases) with somatic HIF2A mutations clearly document the existence of an additional phenotype of this syndrome, i.e. ocular abnormalities in addition to paraganglioma, somatostatinoma, and polycythemia. Ophthalmologists and physicians of various subspecialties should recognize this new clinical entity. Referral to an ophthalmologist for screening of these patients for retinal abnormalities is advised.
Supplementary Material
Acknowledgements
This research was supported, in part, by the Intramural Research Program of the NIH, Eunice Kennedy Shriver NICHD, NINDS, and NEI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
KP and EYC examined patients, collected clinical data, wrote, reviewed and revised the manuscript.
MBA examined patients, collected clinical data and reviewed and revised the manuscript.
ASP and MWW examined the male patient, collected clinical data, and reviewed and revised the manuscript.
JAY and VP collected clinical data and reviewed and revised the manuscript.
F.R.L. performed genetic studies on Patient 3, reviewed and revised the manuscript.
ZZ and CY performed genetic studies, wrote, reviewed and revised the manuscript.
Written consent to publish was obtained.
REFERENCES
- 1.Zhuang Z, Yang C, Lorenzo F, Merino M, Fojo T, Kebebew E, et al. Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. N Engl J Med. 2012;367(10):922–30. doi: 10.1056/NEJMoa1205119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pacak K, Jochmanova I, Prodanov T, Yang C, Merino MJ, Fojo T, et al. New syndrome of paraganglioma and somatostatinoma associated with polycythemia. J Clin Oncol. 2013;31(13):1690–8. doi: 10.1200/JCO.2012.47.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001;17:255–96. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- 5.Ding K, Scortegagna M, Seaman R, Birch DG, Garcia JA. Retinal disease in mice lacking hypoxia-inducible transcription factor-2alpha. Invest Ophthalmol Vis Sci. 2005;46(3):1010–6. doi: 10.1167/iovs.04-0788. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.