Abstract
A multidisciplinary Tier 3 weight management service in primary care recruited patients with a body mass index ≥40 kg·m−2, or 30 kg·m−2 with obesity-related co-morbidity to a 1-year programme. A cohort of 230 participants was recruited and evaluated using the National Obesity Observatory Standard Evaluation Framework. The primary outcome was weight loss of at least 5% of baseline weight at 12 months. Diet was assessed using the two-item food frequency questionnaire, activity using the General Practice Physical Activity questionnaire and quality of life using the EuroQol-5D-5L questionnaire. A focus group explored the participants' experiences. Baseline mean weight was 124.4 kg and mean body mass index was 44.1 kg·m−2. A total of 102 participants achieved 5% weight loss at 12 months. The mean weight loss was 10.2 kg among the 117 participants who completed the 12-month programme. Baseline observation carried forward analysis gave a mean weight loss of 5.9 kg at 12 months. Fruit and vegetable intake, activity level and quality of life all improved. The dropout rate was 14.3% at 6 months and 45.1% at 1 year. Focus group participants described high levels of satisfaction. It was possible to deliver a Tier 3 weight management service for obese patients with complex co-morbidity in a primary care setting with a full multidisciplinary team, which obtained good health outcomes compared with existing services.
Keywords: Adult, obesity, primary care, Tier 3
Background
Obesity is an increasing problem in the UK, where the obesity rate is the highest in Europe 1. The Department of Health's ‘Call to action on obesity’ in England 2 set a target to reduce the prevalence of adult obesity by 2020, and acknowledged the need to provide clinical services for obese adults in addition to public health preventative interventions. A recent review of effective UK weight management services for adults 3 suggested that self-referral to commercial agencies was a reasonable first step. The review also cited evidence that a primary care-based weight management service, the Counterweight programme 4, delivered by trained practice nurses or dietitians was effective in maintaining weight loss of over 5 kg for up to 2 years in 30–40% of attenders. It highlighted the lack of evidence of effectiveness of services for severely obese individuals (body mass index [BMI] ≥35 kg·m−2) with 12 months or longer follow-up, and suggested that complicated or resistant obesity should be referred to a secondary care-based service.
Patients with a BMI of at least 35 kg·m−2 and complex co-morbidity may be suitable for medical interventions including pharmacotherapy, low-energy liquid diets (LELDs) and bariatric surgery, which has been shown to be highly cost-effective for appropriately selected individuals. The National Health Service (NHS) Commissioning Board issued guidance on clinical commissioning for complex and specialized obesity surgery 5, which includes a four tier model for managing obesity, similar to the National Obesity Forum model (Fig. 1). The British Obesity and Metabolic Surgery Society sponsored a National Institute for Clinical Excellence (NICE) accredited process, run by the Royal College of Surgeons to provide a commissioning guide for weight assessment and management clinics which gives detailed guidance on Tier 3 and Tier 4 services, and a pertinent section on evaluation recommending the National Obesity Observatory Standard Evaluation Framework 6. Primary care is arguably an ideal place to provide weight management services, as most health service contact with obese people occurs there 2,7,8, and people may be more responsive to advice when it is linked to health-related issues 9. The National Service Frameworks for both coronary heart disease 10 and diabetes 11, and NICE guidelines 12–16 emphasize the role of primary care in management of obesity.
Figure 1.
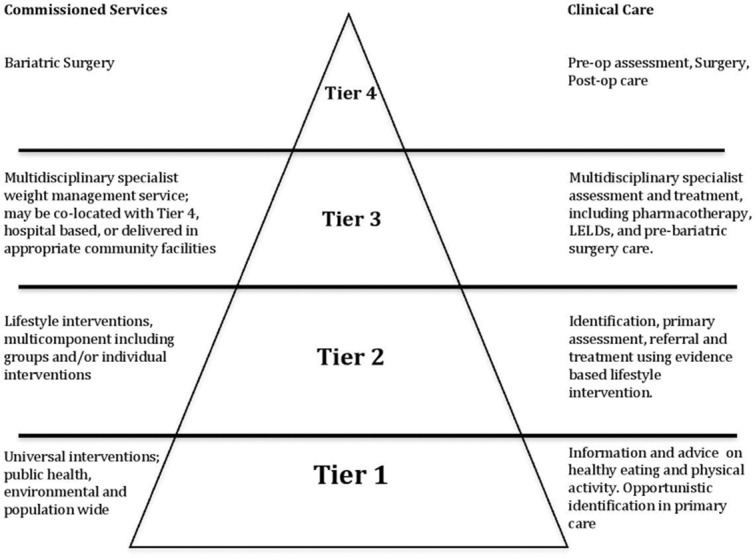
Tiered model of services.
NHS Norfolk Primary Care Trust and the North Norfolk Clinical Commissioning Group (NNCCG) identified obesity as a priority in 2011. A review of the Norfolk obesity care pathway identified a lack of Tier 3 specialist weight management services in the county. North Norfolk is a rural area with an elderly population, and poor public transport, and the NNCCG wished to provide an accessible service. In 2011, the NHS East of England Innovation Fund awarded funds to the Fakenham medical practice to develop a Tier 3 multidisciplinary team service in primary care.
Description of the service
The Fakenham weight management service (FWMS) provides Tier 3 services. It was developed from an existing service at the Fakenham medical practice, which had won the National Obesity Forum award for excellence in weight management in 2010. It was modelled on the Rotherham Institute of Obesity (RIO) service 17, following NICE guidelines 12, and the National Obesity Forum toolkit 18. FWMS aimed to deliver evidence-based interventions including medical assessment, motivational interviewing to support behaviour change 19–21, dietary and activity advice, psychological therapies, drug therapy with orlistat, medically supervised LELDs 6,12,22 and assessment for suitability for bariatric surgery using the NHS East of England criteria (aged 18–60 years, BMI ≥40 kg·m−2, with either diabetes or severe obstructive sleep apnoea, and having undergone a 6-month intensive weight-loss programme).
The service aimed to facilitate weight loss by implementing progressive and sustainable lifestyle changes, based on individually agreed goals over a 1-year programme. This was delivered by individual monthly appointments with obesity specialist nurses (OSNs) using a dietary diary as a basis for agreeing specific changes, accompanied by a structured educational programme (Fig. 2). Participants were considered for pharmacotherapy, LELDs or bariatric surgery if clinically appropriate. Psychological therapies offered included cognitive behaviour therapy, neurolinguistic programming, emotional freedom therapy, clinical hypnosis, solution focused therapy, transactional analysis, psychodynamic therapy and cognitive analytical therapy.
Figure 2.
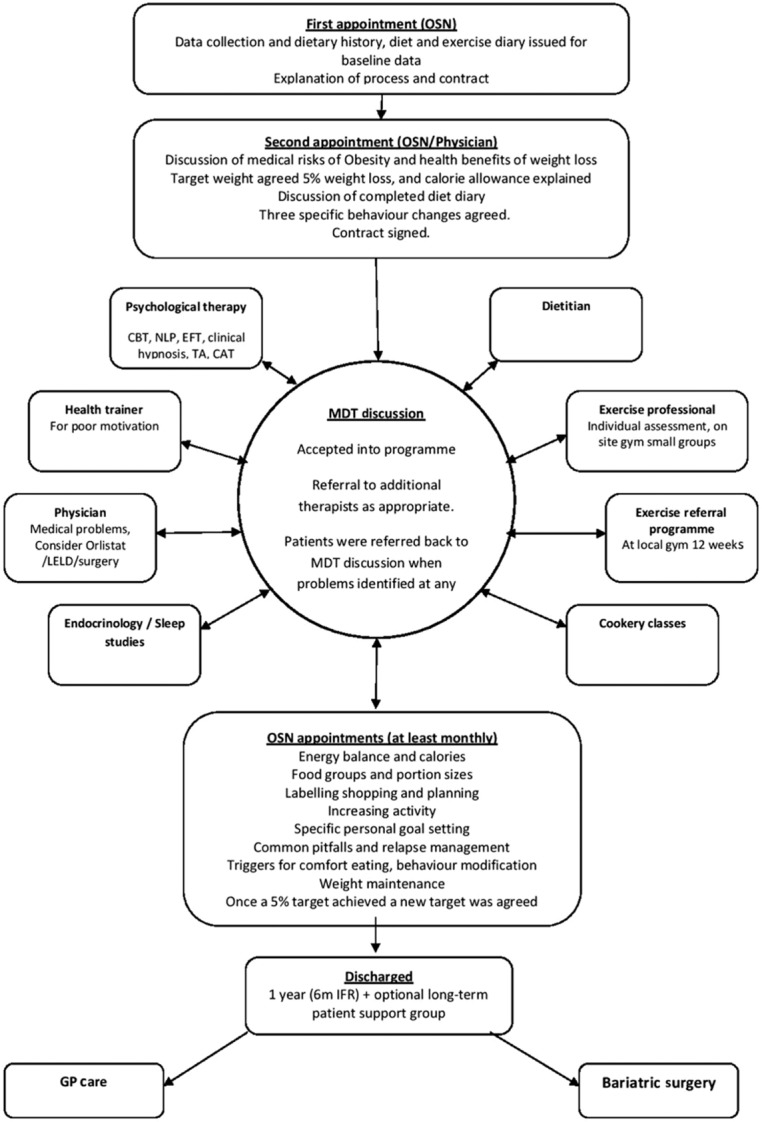
Model of care FWMS.
In addition to the 1 year service commissioned by NNCCG, neighbouring Clinical Commissioning Groups (CCGs) also referred 17 patients via the individual funding request (IFR) mechanism. These IFR patients met the same entry criteria, and were given the same intervention but over a 6-month period. The number of visits ranged from nine to 15 visits for the IFR programme over 6 months compared with a range of 10–15 visits for the 1-year programme.
The programme was provided by a lead general practitioner with additional training as a bariatric physician (specialist certificate of obesity professional education), OSNs, dietitian, psychological therapist, exercise professional, health trainer and supported by a consultant endocrinologist and public health consultant. The OSNs were trained in motivational interviewing techniques 19–21, and the psychological therapist had extensive experience in working with clients with obesity and eating disorders. The clinical core steering group also included two patient representatives from the weight management programme, and a representative from the Fakenham Patient Participation Group who was responsible for checking that the patient information literature was easy to understand.
The primary clinical outcome measure for evaluation of the service was 5% weight loss at 12 months, as this has been shown to produce definite health benefits 23–26, with the aim to achieve this in 50% of participants at 6 months.
Referrals were accepted from General Practitioners (GPs) or practice nurses. Entry criteria were: age over 18 years, BMI ≥40 kg·m−2, or BMI ≥30 kg·m−2 with obesity-related co-morbidity and/or waist circumference ≥102 cm in men or ≥88 cm in women. Exclusion criteria were: pregnancy, severe eating disorder, poor motivation identified by a motivational questionnaire 18 or failure to respond to an invitation to contact the service.
All participants were asked to sign a contract, which committed them to attending regularly, and allowed the FWMS to contact their own GP for up to 2 years after discharge to obtain data on weight maintenance. The programme involved an initial information-gathering interview coupled with motivational assessment (Fig. 2). The second visit included a medical assessment, followed by a discussion on the health risks of obesity and the health benefits of losing weight. The daily energy requirement of each participant was calculated using the Schofield equation, and a 600 kcal daily deficit was recommended, aiming for a weight loss of 0.5–1 kg a week. An initial 5% weight loss goal was agreed, and this was modified once they reached this target to aim for a further progressive 5% weight loss targets. A number of those assessed declined to enrol after the assessment visits, and a few patients were deemed medically unsuitable for a primary care-based service and were referred to the local endocrinology service (Fig. 3).
Figure 3.
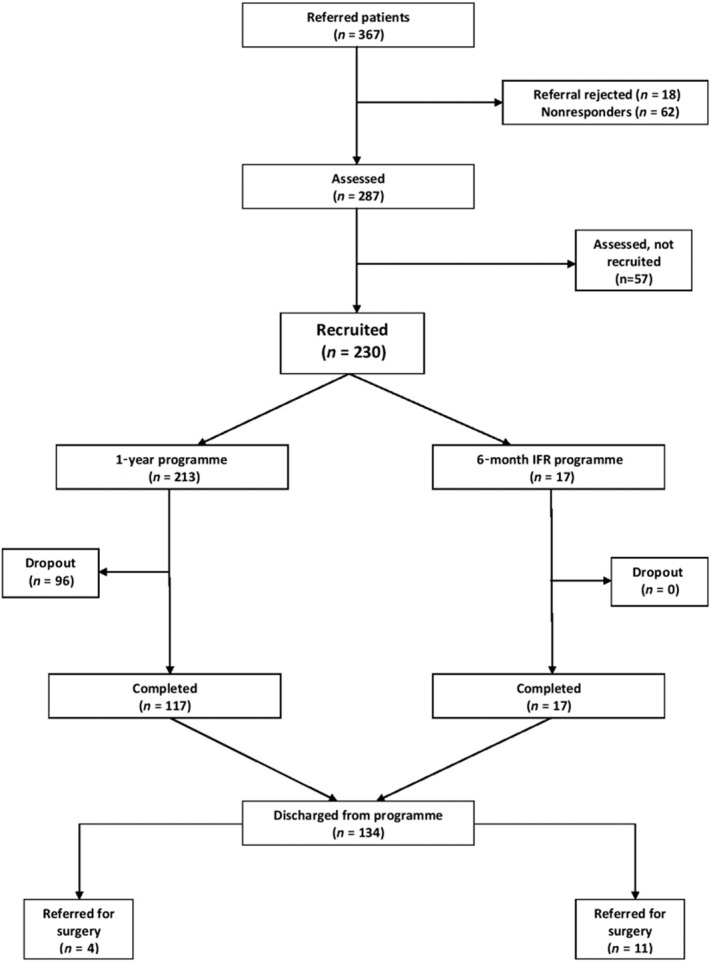
Patient flow diagram.
Once enrolled, participants attended regular monthly OSN consultations, using dietary diaries and a structured but flexible individual diet and activity education programme. The areas covered are described in Fig. 2. All participants were screened for obstructive sleep apnoea using the Epworth score, neck circumference and clinical history, and were referred for sleep studies as appropriate.
Weight was measured using specialist digital bariatric scales which were regularly calibrated. Baseline blood tests were fasting glucose and lipids, liver function and thyroid function, and HbA1c if diabetic.
Participants with complex medical problems were all seen by the physician either at the second assessment visit or subsequently. The physician's role was to assess and manage complex comorbidities, and to detect others such as endocrine problems, depression or sleep apnoea. The physician communicated with the participants' own GP or the secondary care specialists when medically appropriate. A medical opinion on fitness to exercise was also required for some participants. Orlistat was prescribed and monitored by FWMS, but all other medications were prescribed by participants' own GP. The FWMS physician was also involved in the assessment and monitoring of those on a LELD, in accordance with an agreed protocol with local endocrinologists.
Every participant was discussed initially at the weekly multidisciplinary team meeting, and appropriate referrals made to other team members. Any pre-bariatric surgery participants saw the physician and psychological therapist on at least two occasions, and all other participants were referred from the multidisciplinary team meetings to the physician or psychological therapist as required.
As part of the programme, nine patients used LELDs, and 36 patients were prescribed orlistat. Four patients were referred to the endocrinologist for investigation, and eight were referred for investigation of possible obstructive sleep apnoea.
The exercise professional provided both individual and small group sessions at the on-site gym, and there was also a 12-week exercise referral scheme using local gyms. The gym was unavailable till January 2012, 5 months after the start of the programme. A post-discharge patient-led support group was set up in 2012 and met at monthly intervals with open access for all those attending the programme.
The participants were discharged if they failed to attend twice with any of the therapists.
Aims
The service was evaluated using the National Obesity Observatory Standard Evaluation Framework (NOO SEF) 27–29. The primary outcome measure was >5% weight loss at 1 year, with the aim to reach this target within 6 months in 50% of the participants.
Methods
In 2009, England's National Obesity Observatory set out a list of essential and desirable criteria that should be collected as part of any evaluation of a weight management intervention to standardize core information, to encourage the use of evidence-based tools and to allow comparisons between different interventions. Its value was recognized by the Department of Health and highlighted in its ‘call to action on obesity’ 2, and in commissioning guidelines 5,6.
This service evaluation had a cohort design, following all 230 participants enrolled sequentially from August 2011 to August 2012 (Fig. 3), and describing changes in their weight and other health indicators, as defined in the NOO SEF 27–29. At baseline, years of education was recorded as a socioeconomic indicator. Nineteen years of education or above corresponds to first degree or National Vocational Qualification (NVQ) level 4 and 5, which is associated with higher socioeconomic status 27. The following data on each participant was recorded at baseline, 3, 6, 9 and 12 months: height, weight, BMI, waist circumference, dietary intake, (two-item food frequency questionnaire 30,31, physical activity levels [General Practice Physical Activity Questionnaire (GPPAQ) 32 ] and quality of life (EQ-5D-5L questionnaire 33).
The two-item food frequency questionnaire was chosen as fruit and vegetable intake has been shown to be a good proxy marker for a healthy diet 29–31. The GPPAQ was chosen as it is widely used in general practice and is recognized and recommended by NICE 12,16, with the proviso that it is not a direct measure of activity, and that it may not be the most sensitive instrument to measure change of activity in those with severe limitations on increasing activity. The EQ-5D-5L 33 measures impact of health status of five domains of the quality of life, has been validated for an obese population, and is short and easy to use. The visual analogue scale (EQ-VAS) of perceived health status is simple to present to a non-specialist audience and was favoured by the patient participants on the clinical core group.
The primary outcome was weight loss of at least 5% of initial weight at 1 year. Secondary outcomes were changes in quality of life, quality of diet, physical activity, blood pressure, and HbA1c in diabetic patients. Smoking status, alcohol consumption, newly identified co-morbidities and referrals were also recorded.
Two hundred and thirty participants were recruited sequentially (Fig. 3). Two hundred and thirteen were recruited to the year programme and 17 IFR patients were admitted for a 6-month intensive programme. The IFR patients were included in the whole cohort analysis as they represent the type of patients commonly referred to Tier 3 services nationally, and they received the full intervention, over a shorter time (Fig. 3). The IFR group differed slightly in their baseline characteristics with a mean BMI 49.9 kg·m−2 (whole cohort 44.1 kg·m−2), and the IFR group mean age was 43.6 years (mean age whole cohort 52.7 years).
At the 1-year point, none of the patients referred for bariatric procedures had been operated on. By 18 months and 2 years, two IFR patients had undergone bariatric surgery, but their post-surgical weights were not included in the analysis to prevent favourably skewing the weight-loss data.
Completers were defined as those who had completed the full 1-year programme during the period of the evaluation, which excluded the 17 IFR patients. The 17 IFR participants all completed their 6-month programme, and 1-year weight data was collected on them where possible. The IFR 1-year and later follow-up weights were included in the analysis of all with weights recorded, and baseline observation carried forward (BOCF) analysis (Table 1). Dropouts were defined as those who had failed to attend two appointments without explanations, or who declined to attend monthly and been discharged. All dropouts were contacted by letter or phone call to confirm their discontinuation.
Table 1.
Weight changes over time for all participants with recorded weights, all who completed 1-year programme, and all with baseline observation carried forward
| Baseline | 3 months | 6 months | 9 months | 12 months | 18 months | 2 years | |
|---|---|---|---|---|---|---|---|
| Eligible for programme (n) | 230 | 230 | 230 | 230 | 230 | 157 | 84 |
| All with weight recorded n* (%) | 230 | 218 (95) | 195 (85) | 149 (65) | 170 (74) | 96 (61) | 45 (54) |
| Mean weight kg (SD) | 124.4 | 120.8 | 118.2 | 116.4 | 115.8 | 112.9 | 112.2 |
| (27.3) | (27.0) P < 0.001 | (27.4) P < 0.001 | (27.2) P < 0.001 | (26.0) P < 0.001 | (24.9) P < 0.001 | (24.8) P = 0.001 | |
| Mean weight change from baseline kg (SD) | – | −3.6 | −5.8 | −8.0 | −8.1 | −7.1 | −5.0 |
| (3.9) | (5.6) | (7.0) | (8.2) | (9.5) | (9.8) | ||
| Mean weight change from baseline % (SD) | – | −3.0 | −4.7 | −6.5 | −6.4 | −5.2 | −4.3 |
| (2.9) | (4.3) | (5.5) | (6.1) | (7.9) | (8.2) | ||
| ≥ 5% weight loss from baseline n (%) | – | 55 | 86 | 88 | 102 | 46 | 20 |
| (25.2) | (44.1) | (59.1) | (60) | (47.9) | (44.4) | ||
| ≥ 10% weight loss from baseline n (%) | – | 8 | 24 | 30 | 37 | 26 | 9 |
| (3.6) | (12.3) | (20.1) | (21.8) | (27.1) | (20) | ||
| All who completed 1-year programme (117/213) n (%) | 117 | 117 | 117 | 117 | 117 (51) | 58 (37) | 29 (35) |
| Mean weight kg (SD) | 125.6 | 120.8 | 118.4 | 116.6 | 115.5 | 113.5 | 113.6 |
| (29.6) | (29.2) p < 0.001 | (28.8) P < 0.001 | (28.5) P < 0.001 | (27.8) P < 0.001 | (25.6) P < 0.001 | (26.5) P = 0.006 | |
| Mean weight change from baseline kg (SD) | – | −4.6 | −5.8 | −8.9 | −10.2 | −9.6 | −5.9 |
| (4.0) | (4.3) | (7.2) | (8.1) | (12.8) | (10.7) | ||
| Mean weight change from baseline % (SD) | – | −3.7 | −5.7 | −7.1 | −8.0 | −7.1 | −5.1 |
| (3.0) | (4.4) | (5.5) | (6.0) | (9.0) | (9.1) | ||
| ≥ 5% weight loss from baseline n (%) | – | 40 | 63 | 77 | 85 | 35 | 13 |
| (34.2%) | (53.8%) | (65.8%) | (72.6%) | (60.3%) | (44.8%) | ||
| ≥ 10% weight loss from baseline n (%) | – | 7 (6%) |
19 (16.2%) |
27 (23.1%) |
32 (27%) |
20 (34.5%) |
6 (20.7%) |
| Baseline observation carried forward* | 230 | 230 | 230 | 230 | 230 | 157 | 84 |
| Mean weight kg (SD) | 124.4 (27.3) |
120.8 | 119.3 | 119.1 | 118.4 | 118.0 | 117.1 |
| (26.8) P < 0.001 | (26.6) P < 0.001 | (26.8) P < 0.001 | (26.4) P < 0.001 | (25.8) P < 0.001 | (25.6) P = 0.095 | ||
| Mean weight change from baseline kg (SD) | – | −3.4 | −4.9 | −5.5 | −5.9 | −4.7 | −2.6 |
| (3.9) | (5.6) | (6.8) | (7.8) | (9.7) | (7.4) | ||
| Mean weight change from baseline % (SD) | – | −2.9 | −3.9 | −4.2 | −4.8 | −3.7 | −2.3 |
| (2.9) | (4.3) | (5.4) | (5.9) | (6.6) | (6.3) | ||
| ≥5% weight loss from baseline n (%) | – | 55 | 86 | 88 | 102 | 46 | 20 |
| (23.9) | (37.4) | (38.3) | (44.3) | (29.3) | (23.9) | ||
| ≥10% weight loss from baseline n (%) | – | 8 | 24 | 30 | 37 | 26 | 9 |
| (3.5) | (10.4) | (13) | (16.1) | (16.6) | (10.7) |
Includes both 1-year programme and 6-month IFR programme.
SD, standard deviation.
Follow-up weights post-discharge were obtained from GP records, or self- reported in response to a written request (n = 2). Weights were recorded within a month of the expected attendance date while on the programme, and for follow-up after discharge a weight within 90 d was accepted for 18 months and 2-year data.
Statistical analysis
Continuous values in the text and tables are presented as mean (standard deviation; SD) and categorical data as percentage (n =), unless otherwise stated. Differences between pre- and post-programme measurements were compared by paired sample t-tests. Therefore, for each comparison, we only compared those with observations at both time points. Independent sample t-tests or Pearson χ2 tests were used to compare characteristics between those recruited to the programme and those who were assessed but not recruited. Data were analysed on an intention-to-treat basis, with the baseline weight available carried forward when a final weight was not available (BOCF group). Statistical analyses were performed using SPSS version 18.0 for Windows (SPSS Inc., Chicago, IL, USA), and the criterion for statistical significance was P < 0.05.
A focus group was held to explore the experiences of participants in the service. Participants were recruited by invitations sent to 50 current participants, purposely chosen to include participants from different geographic areas, and who had seen the full range of therapists within the project.
Ethics committee approval was not required as the NHS Norfolk research governance team had advised this was a service evaluation as defined by the National Research Ethics Service website.
Results
Participant characteristics
In the study period, 1 August 2011 to 1 August 2012, 367 people were referred to FWMS; 18 referrals were rejected (outside the age or BMI range, requests for post-bariatric surgery follow-up, or poorly controlled diabetics who required endocrinology care). Sixty-two people declined to attend and 57 attended for assessment but were not recruited. Two hundred and thirty participants were recruited (Fig. 3).
The 57 who only attended for assessment did not differ from recruited participants in terms of gender (21% male vs. 30% male respectively; P = 0.227) or baseline weight (mean 117 kg vs. mean 124 kg; P = 0.096) or BMI (42.6 kg·m−2 vs. 44.1 kg·m−2; P = 0.229). However, there was a significant difference in age with those recruited significantly older than those assessed but not recruited (52.7 years vs. 45.2 years, P = 0.001). There was also a significant difference in EQ-VAS score with higher scores (i.e. better self-reported quality of life) for those recruited (55.7) than those assessed but not recruited (48.6) for the programme (P = 0.031).
Of the 230 recruited participants, two-thirds of participants had morbid obesity (BMI of ≥40 kg·m−2) (Table 2). The commonest co-morbid conditions were hypertension, depression and diabetes. Seventy per cent of participants were female, and 82% had less than 19 years of education, an indicator of lower socioeconomic status. Participants included non-English speakers, people with learning disability, and physically handicapped participants who were expected to be less likely to attend a group intervention. Of the 230 participants recruited, 229 (99.6%) attended 3-month follow-up, of whom 197 (85.6%) attended 6-month follow-up, and 117 (54.9%) completed the programme at 12 months.
Table 2.
Baseline characteristics
| Characteristic | Number | Mean (SD)/% | Range |
|---|---|---|---|
| Age (years) | 230 | 52.7 (13.6) | 18–76 |
| Female | 161 | (70%) | |
| Weight (kg) | 230 | 124.4 (27.3) | 72.4–262.5 |
| BMI (kg·m–2) | 230 | 44.1 (7.8) | 30.2–78.6 |
| BMI category | |||
| ● <35 kg·m−2 | 29 | 12.6% | |
| ● ≥35 kg·m−2 | 46 | 20% | |
| ● ≥40 kg·m−2 | 155 | 67.4% | |
| Waist circumference (cm) | 219* | 127.7 (16.3) | 85–182 |
| Diabetes | 73 | 31.7% | |
| Impaired fasting glycaemia | 1 | 0.43% | |
| IHD | 27 | 11.7% | |
| Hypertension | 88 | 38.3% | |
| Sleep apnoea | 27 | 11.7% | |
| Depression | 72 | 31.3% | |
| Years of education | |||
| ● ≤15 years | 60 | 30% | |
| ● 15–19 years | 104 | 52% | |
| ● ≥19 years | 36 | 18% |
Waist circumference not measured if extreme obesity and measurements are unreliable.
BMI, body mass index; IHD, Ischaemic heart disease; SD, standard deviation.
Weight loss
Weight values were recorded on 170 patients in total at the 1-year point; completers, IFR follow-up data, plus weights on those who had dropped out were obtained from medical records (Table 1).
There were significant reductions in body weight at each data collection point.
The percentages of the 170 participants with weight recorded at 12 months who had 5% or greater weight loss were 25.2, 44.1, 59.1 and 60.0% at 3, 6, 9 and 12 months, respectively. Of the 117 participants who completed the year programme (‘completers’), weight losses >5% were 34.2, 53.8, 65.8 and 72.6% at 3, 6, 9 and 12 months. The mean percentage weight loss at 1 year was –6.4% (SD 6.1) in the cohort with weights recorded, and –8% (SD 6.0) in completers.
In a subanalysis of patients with BMI ≥40 kg·m−2, weight loss was significantly different to baseline at 3 months (mean weight loss −4.3 [SD 4.2] n = 152), 6 months (mean weight loss −6.9 [SD 5.9] n = 134), 9 months (mean weight loss −9.0 [SD 7.4] n = 102) and 12 months (mean weight loss −9.3 [SD 8.7] n = 116).
All weight data available from 1 August 2011 to 31 December 2013 were analysed. One hundred and fifty-seven participants were 18 months from initial recruitment, and 84 were 2 years or more from initial recruitment at the data analysis point (December 2013). Eighteen-month data were available for 96 participants, whose mean weight loss at 18 months was −7.1% (SD 9.5), and of whom 47.9% maintained a loss of at least 5% of their initial body weight. At 2 years, data were available for 45 participants, and mean weight loss was −5 kg with 44.4% maintaining the 5% weight loss (Table 1).
An additional analysis, using the BOCF method to impute missing values, showed statistically significant weigh losses from baseline at 1 year and 18 months. At 2 years, the absolute numbers were small, weight loss was statistically significant in the whole cohort with weight recorded and completers, but not using BOCF (Table 1).
Secondary outcomes
Participants reported increases in fruit and vegetable consumption and levels of physical activity (Table 3). Mean physical activity index scores were calculated with higher scores indicating less activity. Physical activity scores were reduced significantly at each 3-month data collection point indicating that participants became more active. Quality of life increased significantly at each measurement point (indicated by decreased EQ-5D-5L scores). The EQ-VAS scores increased significantly over the course of the intervention reflecting participant's perception of better health (Table 3). Significant reductions in blood pressure and HbA1c values in diabetics were also seen between baseline and 12-month follow-up (Table 3). The secondary outcome data at 1 year was from the completer group only as the questionnaires were not posted to patients who had dropped out.
Table 3.
Secondary outcomes
| Baseline | 3 months | 6 months | 9 months | 12 months | |
|---|---|---|---|---|---|
| n Mean (SD) | n Mean (SD) | n Mean (SD) | n Mean (SD) | n Mean (SD) | |
| Waist circumference (cm) | 219 128 (16.2) | 191 124 (16.1) P < 0.001 | 147 120 (15.8) P < 0.001 | 113 118 (15.3) P < 0.001 | 105 118 (15.4) P < 0.001 |
| Body mass index (kg·m–2) | 230 44.1 (7.8) | 218 42.7 (7.7) P < 0.001 | 195 41.8 (7.7) P < 0.001 | 149 41.2 (7.8) P < 0.001 | 170 41.0 (7.6) P < 0.001 |
| Systolic blood pressure (mmHg) | 228 131 (13.7) | – | – | – | 122 122 (11.2) P < 0.001 |
| Diastolic blood pressure (mmHg) | 228 76.0 (9.3) | – | – | – | 122 71.3 (7.5) P < 0.001 |
| HbA1c (mmol·mol–1) | 70 57.8 (15.3) | 39 54.5 (14.5) P = 0.001 | 39 53.5 (15.0) P = 0.009 | 22 51.0 (11.0) P = 0.113 | 60 53.7 (14.1) P = 0.006 |
| Fruit and vegetable intake (portions per day) | 228 4.2 (2.1) | 202 5.6 (1.7) P < 0.001 | 162 5.8 (1.7) P < 0.001 | 119 51.0 (11.0) P = 0.113 | 104 6.4 (1.7) P < 0.001 |
| Physical activity index* | 228 3.4 (1.0) | 210 2.9 (1.2) P < 0.001 | 169 2.7 (1.2) P < 0.001 | 126 2.7 (1.2) P < 0.001 | 111 2.8 (1.2) P < 0.001 |
| EQ-5D-5L | 203 2.0 (0.8) | 165 1.8 (0.7) P < 0.001 | 131 1.8 (0.8) P < 0.001 | 93 1.7 (0.7) P < 0.001 | 81 1.7 (0.7) P < 0.001 |
| EQ-VAS | 202 55.6 (21.7) | 173 63.0 (19.4) P < 0.001 | 134 65.9 (21.7) P < 0.001 | 92 69.9 (19.0) P < 0.001 | 81 73.7 (18.6) P < 0.001 |
Mean physical activity index score calculated as the average of 1 = active, 2 = inactive, 3 = moderately inactive and 4 = inactive, therefore a higher mean score indicates participants are more inactive.
EQ-VAS, Euroqual visual analogue scale; SD, standard deviation.
The service also identified additional pathology in 48 patients: two with hypothyroidism, three with hypercholesterolaemia (total cholesterol >6.5 mmol·L−1), 32 with abnormal liver function, seven with diabetes mellitus, two with obstructive sleep apnoea, one with hypertension (blood pressure >160/100 mmHg−1 on three occasions and requiring antihypertensive medication) and one with impaired fasting glucose. The incidence of new diabetes was higher than expected which may reflect the higher incidence in Norfolk, or the age and BMI range of participants. The incidence of obstructive sleep apnoea is lower than expected, and may reflect the screening tools used to detect it, using oximetry on all the participants might increase detection rates.
Focus group
The group was attended by three men and nine women. The group did not include anyone who had seen the psychologist, or dropped out of the programme. The experiences of attenders were very positive. The main reasons for attending the service were personal health issues:
‘I had breast cancer and heard it was weight related’.
‘I was told I was at risk of becoming diabetic’.
The role of medical staff in raising the issue of obesity was important in motivating people to join the project.
The NHS brand appeared to add credibility to the service;
‘I saw the word obesity on my medical screen…I was shocked’.
‘It was medically directed…more confident’.
‘Commercial weight loss scheme not interested in my medical problems’.
The OSNs were described as very important in supporting motivation.
‘individual-private and tailored’.
The exercise scheme was highly valued in its own right, and not just as a tool to achieve weight loss.
‘When I first started I could hardly walk – only 50 yards – now I can walk 300–400 yards…if this project has done nothing else it has helped me to walk’.
Individual appointments were valued, to allow personal and confidential discussion, but there was an interest in setting up a patient run support group to provide long-term support and motivation. This idea was subsequently developed and a patient run long-term support group was set up for those discharged from the project. The venue of the service was important with most people being prepared to travel a maximum of 10–15 miles. The time taken to attend appointments, the difficulty of travel in a rural area and the cost of travel were all raised as minor barriers to attending. However, cost was the least important factor, and indeed some participants stated that they would be prepared to pay for the service.
Discussion
The FWMS has demonstrated that a Tier 3 service can be provided for complex obese patients by a specialist team in a primary care setting, and was associated with a progressive and substantial reduction in weight at 1 year. The weight losses achieved of 5% or more of initial weight could deliver improved health outcomes 20–23. There was no control group but control groups in randomized controlled trials of weight-loss interventions have shown much smaller or no reductions in weight without treatment 34,35,44, suggesting that these reported changes were at least partly the effects of FWMS.
Although the aim of 5% of initial weight being lost by 50% of participants at 6 months was not quite achieved (44.1% whole cohort, 53.8% completers) at 6 months, within 1 year 60% of the whole cohort or 72.6% completers had achieved this target. This compares with a 5% loss at 1 year in of 47% of patients in the specialist multidisciplinary Canadian UETRO service 36 (Table 4), a group with very similar baseline BMI and interventions.
Table 4.
Comparative data multidisciplinary weight management services and primary care-based individual programme
| FWMS | Clyde 37 | Walsall 34 | RIO 38 | Counterweight 4 | Counterweight 39 | UETRO 36 | |
|---|---|---|---|---|---|---|---|
| Programme summary | Tier 3 primary care-based MDT. Based on NICE (12 months) and NoF Toolkit (18 months). 1-year programme | Whole system MDT based and community venues 16 weeks. Phase 1 diet/exercise/psychological support | Tier 3 medical secondary care MDT completers audit (6 months) | Tier 3 medical MDT based in primary care (6 months). Based on NoF Toolkit (18 months) and NICE (12 months) completers | Delivered by practice nurses/dieticians in 56 GP practices. Individual/group sessions lifestyle modification. 1-year programme | Implementation of Counterweight programme in Scotland over 13 boards in comparison to original research paper | Canadian Academic outpatient MDT; individual appointments 6 weekly nurse, dietician, and endocrinologist, plus educational groups |
| Published (year) | 2014 | 2011 | 2013 | 2013 | 2008 | 2012 | 2009 |
| Baseline data | |||||||
| n | 230 | 2976 | 96 | 3325 | 1906 | 6715 | 115 |
| Age (mean) | 52.7 | Men 47.5, women 44.6 | 50 | – | 49.4 | 53 | 46 |
| Sex (% female) | 70 | 72.4 | 76 | – | 77 | 74.3 | 84 |
| Socioeconomic status | 82% < 19 years of education | Majority of patients from most deprived areas | – | – | 67.6% in deprived or intermediate deprived area | – | – |
| Initial weight kg (mean) | 124.4 | 134.4 | – | 101.2 | – | 118.4 | |
| Initial BMI kg·m–2 | 44.1 | 88.6% BMI >35 | 50.1 | – | 37.1 | 37 | 44.7 |
| % with diabetes mellitus | 31.7 | 17.3 | 45 | – | 13.5 | – | 23 |
| 3-month weight change (mean ) | −3.6 kg | – | – | – | −3.34 kg | – | – |
| 6-month weight change (mean) | −5.8 kg | – | – | – | −4.2 kg | – | −2.8 kg |
| 6 months % change (mean) | 4.9 | – | 6.4 | 7.7 | − | – | – |
| 5% loss or more at 6 months | 44.1% whole cohort (48.3% completers) | (35.5%) completers lost over 5 kg at 16 weeks (13.6% whole cohort) | 57.9 (completers) | 786 (72% of completers) | 38% | – | – |
| 12-month weight change (mean) | −8.1 kg all with weight recorded (−10 kg completers) | – | – | – | −3.0 kg (attenders 642/1906) | – | −6.6 kg |
| % with >5% weight loss at 1 year | 60% (72% completers) | – | – | – | 30.7% (attenders) | 35.2% (attenders) | 47% |
| 2-year weight change (if applicable)mean | −5.0 kg (−5.9 kg completers) | – | – | – | −2.3 kg (attenders) | – | – |
| Dropout at 6 months (if applicable) | 14.3% | 62.5% | – | 51% | – | 63% | – |
| Dropout at 1 year (if applicable) | 41.7% | – | – | – | 55% | 72% | 37% |
BMI, body mass index; GP, general practitioner; MDT, multidisciplinary team.
The 6-month 50% target was challenging with a group of patients who had mostly tried, and failed with previous interventions, with a very high baseline mean BMI (44.1 kg·m−2) and multiple co-morbidities. Two other services published results for 5% loss at 6 months in completers only, (Table 4) RIO 72% 38 and Walsall 57.9% 34, but it was not possible to correct for missing values or dropouts with the available published information, which makes comparison difficult.
The mean weight loss of 10 kg in completers, 5.9 kg using BOCF analysis and 8.1 kg at 1 year in all those with weight recorded, compared favourably with the data available from other services (Table 4). The secondary outcomes all showed statistically significant improvements, with improvement in health-related quality of life and diabetic control being the most clinically relevant. The mean drop in systolic blood pressure over the year was comparable with the effect of prescribing an additional antihypertensive medication. The dietary and activity questionnaires have limitations discussed in the NOO SEF 29, and in particular increase in activity was limited in some participants by extreme obesity and physical disability.
In comparison with the limited published data on Tier 3 services in the UK, FWMS had a relatively low dropout rate of 45.1% at 1 year. The 6-month rate of 14.3% dropout vs. 51% at RIO 38, and 62.5% Clyde 37 was particularly striking. The FWMS dropout figures may reflect the convenience of a primary-care setting, as well as the design of the service, with a dedicated administrator, and a choice of appointment times.
Those with poor motivation may have been deterred from enrolment at the assessment stage, and by being asked to sign a contract to attend regularly. The mean age of those assessed but not recruited was younger than those who attended. This may reflect competing priorities of family or employment, but needs to be explored further. The numbers of participants followed-up at 18 months and 2 years are small, and longer-term follow-up data are required but the results are encouraging. It is hoped that the addition of the patient-led long-term support group in 2012 will aid maintenance of weight loss. Although the NOO SEF is designed to facilitate evaluation and data comparison, it is not appropriate to compare the FWMS results with Tier 2 services 45, as the FWMS participants represent complex or resistant obesity, and many had tried and failed to lose weight or maintain weight loss prior to recruitment using Tier 2 services. The FWMS participants had a mean BMI of 44.1 kg·m−2 which is much higher than the Counterweight 4 (mean BMI 37.1 kg m−2) or Lighten Up 40 trials (mean BMI 33.1 kg·m−2 GP subgroup).
Tier 3 services may need additional outcome measures as absolute or percentage weight loss may not reflect all the appropriate clinical goals, such as appropriate treatment before referral to bariatric surgery, improved diabetes control or detection of undiagnosed co-morbidities such as obstructive sleep apnoea 6. An agreed co-morbidity scoring system, such as the Edmonson Obesity Scoring System 41, might be useful used to describe the populations attending a service, and allow more accurate comparisons between services.
The FWMS was less expensive than secondary care-based Tier 3 services. It cost between £900 and £1250 per year for each patient including access to the whole range of therapists. However, it was more costly than Tier 2 services such as commercial weight-loss groups (£45–£60 for 12 weeks), or the Counterweight intervention (£100 per patient per annum).
The service was evaluated from its initial set-up, which might lead to an underestimate of its eventual effectiveness. The first 6 months included extensive additional staff training, and the gym was unavailable till January 2012. The patient follow-on support group was available from mid-2012 onwards. Less orlistat was prescribed than expected as there was a national shortage of the drug during this period. The availability of bariatric surgery was limited by the NHS East of England guidelines, which were more restrictive than NICE guidelines. Only nine patients were started on LELDs during this period, but the numbers are now increasing as evidence emerges of their safety and efficacy in primary care 42,43.
In view of the increasing prevalence of obesity, there is need for more research on the cost-effectiveness of weight management services for complex obese patients in a both primary and secondary care 6. Further research is needed to evaluate this programme in other locations, and with a control group. The FWMS is one model of how a Tier 3 service can be delivered in a primary-care setting, and achieve comparable results to published interventions in similar populations.
Acknowledgments
BK and NS provided public health support in designing and submitting the innovation fund bid, reviewing the protocol and advising on data analysis. CAH and MC drew up the clinical protocol. CAH was the clinical lead and GPwSI Obesity who saw the participants and guarantees the data. AJ did the data analysis with assistance from KC. MB and NS advised on the study design and analysis of the results, and data presentation with assistance from KC. All authors were involved in writing the paper and had final approval of the submitted and published versions. We would like to acknowledge the help of all the endocrinologists at the Norfolk and Norwich University trust, especially Dr Swe Myint and Dr Jeremy Turner; Judy Henwood (Research Design Lead, Norfolk and Suffolk Primary and Community Care Research Office); Sharon Thompson Nurse practitioner; all the staff at RIO; Rebecca Champion (NNCCG); John Fraser CEO and the GP partners and staff at the Fakenham medical practice; Professor Nick Finer and IASO SCOPE team; Professor Anthony Leeds; and all the patient participants who have helped design the service and run the long-term follow-up group.
Funding was provided by the NHS East of England innovation fund, NHS Norfolk PCT, NNCCG and donations from patients at Fakenham medical practice.
Conflicts of Interest Statement
No conflicts of interest were declared within the submitted work. Dr Capehorn reports grants from Public Health Rotherham and Lighter Life, and personal fees from Novo Nordisk, MSD, BI/Lilly Alliance outside the submitted work. Cambridge Weight Plan donated product for use at RIO and FWMS outside the submitted work.
References
- 1.Organisation for economic cooperation and development (OECD) Obesity and The Economics of Prevention; Fit not Fat UK Key Facts. UK: OECD Publishing; 2010. WWW document]. URL http://www.oecd.org/health/health-systems/obesityandtheeconomicsofpreventionfitnotfat.htm (accessed March 2012) [Google Scholar]
- 2.Department of health and Department for Children, Schools and Families. Healthy weight, healthy lives; a toolkit for developing local strategies. 2009. WWW document]. URL https://www.gov.uk/government/publications/healthy-lives-healthy-people-a-call-to-action-on-obesity-in-england (accessed March 2011)
- 3.McCombie L, Lean ME, Haslam D Counterweight Research Group. Effective UK weight management services for adults. Clin Obes. 2012;2:96–102. doi: 10.1111/j.1758-8111.2012.00049.x. [DOI] [PubMed] [Google Scholar]
- 4.Counterweight Project Team. Evaluation of the Counterweight Programme for obesity management in primary care: a starting point for continuous improvement. Br J Gen Pract. 2008;58:548–554. doi: 10.3399/bjgp08X319710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NHS Commissioning board. Clinical commissioning policy: complex and specialised obesity surgery NHSCB/A05/P/a. 2013. WWW document]. URL http://www.england.nhs.uk/wp-content/uploads/2013/04/a05-p-a.pdf (accessed August 2013)
- 6.Royal College of Surgeons England and British Obesity and Metabolic Surgery Society Guidelines Development Group. Commissioning guide for weight assessment and management clinics (Tier 3). British Obesity and Metabolic surgery society (NICE accredited) 2014. WWW document]. URL http://www.bomss.org.uk/Commissioning-Guide-assessment-and-management-clinics-tier-3/ (accessed April 2014)
- 7.Department for Innovation, Universities and Skills. Foresight project. tackling obesities: future choices. 2007. WWW document]. URL http://www.bis.gov.uk/foresight/our-work/projects/published-projects/tackling-obesities (accessed March 2011)
- 8.National Audit Office. Tackling Obesity in England. 2001. WWW document]. URL http://www.nao.org.uk/report/tackling-obesity-in-england/ (accessed March 2011)
- 9.Wee C, Davis RB, Phillips RS. Stages of readiness to control weight and adopt weight control behaviour in primary care. J Gen Intern Med. 2005;20:410–415. doi: 10.1111/j.1525-1497.2005.0074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NSF group. National service framework CHD. WWW document]. URL http://www.nhs.uk/NHSEngland/NSF/Pages/Coronaryheartdisease.aspx (accessed March 2011)
- 11.NSF group. National service framework diabetes. WWW document]. URL http://www.nhs.uk/NHSEngland/NSF/Pages/Diabetes.aspx (accessed March 2011)
- 12.National Institute of Health and Clinical Excellence NICE. London: 2006. CG43 obesity. WWW document]. URL http://www.nice.org.uk/nicemedia/pdf/CG43NICEGuideline.pdf (accessed March 2010) [Google Scholar]
- 13.National Institute of Health and Clinical Excellence NICE. PH 42 obesity-working with local communities. 2012. WWW document]. URL http://publications.nice.org.uk/obesity-working-with-local-communities-ph42 (accessed March 2013)
- 14.National Institute of Health and Clinical Excellence NICE. PH 35 Preventing type 2 diabetes-population and community interventions. 2011. WWW document]. URL http://publications.nice.org.uk/preventing-type-2-diabetes-population-and-community-level-interventions-ph35 (accessed January 2013)
- 15.National Institute of Health and Clinical Excellence NICE. PH 38 Preventing type 2 diabetes-risk identification and interventions for individuals at high risk. 2012. WWW document]. URL http://publications.nice.org.uk/preventing-type-2-diabetes-risk-identification-and-interventions-for-individuals-at-high-risk-ph38 (accessed January 2013)
- 16.National Institute of Health and Clinical Excellence NICE. PH 44 Physical activity: brief advice for adults in primary care. 2013. WWW document]. URL http://publications.nice.org.uk/physical-activity-brief-advice-for-adults-in-primary-care-ph44 (accessed September 2013)
- 17.Rotherham institute for obesity. WWW document]. URL http://www.rotherhaminstituteforobesity.co.uk (accessed March 2010)
- 18.National Obesity Forum. Obesity care pathway and toolkit. 2005. WWW document]. URL http://www.nationalobesityforum.org.uk/ (accessed August 2009)
- 19.Rollnick S, Heather N, Bell A. Negotiating behaviour change in medical settings: the development of brief motivational interviewing. J Ment Health. 1992;1:25–37. [Google Scholar]
- 20.Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23:325–334. doi: 10.1017/S1352465809005128. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong MJ, Mottershead TA, Ronksley PE, et al. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2011;12:709–723. doi: 10.1111/j.1467-789X.2011.00892.x. [DOI] [PubMed] [Google Scholar]
- 22.Tsigos C, Hainer V, Basdevant A, et al. Management of obesity in adults: European clinical practice guidelines. Obes Facts. 2008;1:106–116. doi: 10.1159/000126822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avenell A, Broom J, Brown TJ, et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess. 2004;21:1–458. doi: 10.3310/hta8210. [DOI] [PubMed] [Google Scholar]
- 24.Norris SL, Zhang X, Avenell A, et al. Long-term effectiveness of lifestyle and behavioural weight loss interventions in adults with type 2 diabetes: a metaanalysis. Am J Med. 2004;117:762–774. doi: 10.1016/j.amjmed.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 25.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tuomilehto J, Lindstrom J, Eriksson J, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2002;44:1343–1349. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 27.Roberts K, Cavill N, Rutter H. National obesity observatory standard evaluation framework. 2009. WWW document]. URL http;//www.noo.org.uk/core/frameworks/SEF (accessed March 2011)
- 28.Ellis LJ, Cavill N, Roberts K, Rutter H. Development of a standard evaluation framework for weight management interventions. Public Health. 2013;127:345–347. doi: 10.1016/j.puhe.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Richardson D, Cavill N, Ells L, Roberts K. Supplement: Measuring Diet and Physical Activity in Weight Management Interventions. Oxford: National Obesity Observatory; 2011. [Google Scholar]
- 30.Wardle J, Parmenter K, Waller J. Nutrition knowledge and food intake. Appetite. 2000;34:269–275. doi: 10.1006/appe.1999.0311. [DOI] [PubMed] [Google Scholar]
- 31.Cappuccio FP, Rink E, Perkins-Porras L, et al. Estimation of fruit and vegetable intake using a two-item dietary questionnaire: a potential tool for primary health care workers. Nutr Metab Cardiovasc Dis. 2003;13:12–19. doi: 10.1016/s0939-4753(03)80163-1. [DOI] [PubMed] [Google Scholar]
- 32.Wareham NJ, Jakes RW, Renni KL, et al. Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr. 2002;6:407–413. doi: 10.1079/PHN2002439. [DOI] [PubMed] [Google Scholar]
- 33.The EuroQol Group. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–20833. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 34.Kalmus E, Hartland AJ, Parkinson J, et al. Type 2 diabetes and mobility problems do not negatively impact on the success of multidisciplinary specialist medical weight management clinics, but depression might. Obes Facts. 2013;6(Suppl. 1):109. [Google Scholar]
- 35.Appel JA, Clark JM, Yeh HC, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 2011;365:1959–1968. doi: 10.1056/NEJMoa1108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamga-Ngande CN, Carpentier AC, Nadeau-Marcotte F, et al. Effectiveness of a multidisciplinary program for management of obesity: the unite d'Enseignement de Traitment et de recherché sur l'Obesite (UETRO) database study. Metab Syndr Relat Disord. 2009;4:297–304. doi: 10.1089/met.2008.0063. [DOI] [PubMed] [Google Scholar]
- 37.Morrison DS, Boyle S, Morrison S, et al. Evaluation of the first phase of a specialist weight management programme in the UK National Health Service: prospective cohort study. Public Health Nutr. 2012;15:22–38. doi: 10.1017/S1368980011001625. [DOI] [PubMed] [Google Scholar]
- 38.Senior L, Carter D, Capehorn M. Service evaluation of the Rotherham Institute for Obesity and comparison of 2010 and 2011 data. Obes Facts. 2013;6(Suppl. 1):116. [Google Scholar]
- 39.Counterweight Project Team. The implementation of the Counterweight Programme in Scotland, UK. Fam Pract. 2012;29:i139–i144. doi: 10.1093/fampra/cmr074. [DOI] [PubMed] [Google Scholar]
- 40.Jolly K, Lewis A, Beach J, et al. Comparison of range of commercial or primary care led weight reduction programmes with minimal intervention control for weight loss in obesity: lighten up randomised controlled trial. BMJ. 2011;343:1485–1492. doi: 10.1136/bmj.d6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma AM, Kushner RF. A proposed clinical staging system for obesity. Int J Obes. 2009;33:289–295. doi: 10.1038/ijo.2009.2. [DOI] [PubMed] [Google Scholar]
- 42.Lean MEJ, Brosnahan N, McLoone P, et al. Feasibility and indicative results from a 12-month low-energy liquid diet treatment and maintenance programme for severe obesity. Br J Gen Pract. 2013;63:e115–e124. doi: 10.3399/bjgp13X663073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lean MEJ, Bell-Higgs A, Brosnahan N, et al. 12-month weight-loss outcomes for the counterweight low energy liquid diet (LELD) and weight- loss maintenance programme delivered in primary care. Obes Facts. 2012;5(Suppl. 1):196. [Google Scholar]
- 44.Wadden TA, Volger S, Sarwer DB, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med. 2011;365:1969–1979. doi: 10.1056/NEJMoa1109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahern AL, Olson AD, Aston LM, Jebb SA. Weight Watchers on prescription: an observational study of weight change among adults referred to Weight Watchers by the NHS. BMC Public Health. 2011;11:434. doi: 10.1186/1471-2458-11-434. WWW document]. URL http://www.biomedcentral.com/1471-2458/11/434 (accessed December 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]


