Abstract
Background
Hepatic artery lymph node (HALN) metastasis in pancreatic adenocarcinoma reportedly confers a survival disadvantage. This has led some authors to propose it as an indicator against pancreaticoduodenectomy (PD).
Methods
Consecutive patients who underwent PD during 2002–2012 were identified from the University of Louisville prospective hepatopancreaticobiliary database. Overall survival (OS) and disease-free survival (DFS) were estimated using Kaplan–Meier analysis. The log-rank test and multivariate Cox proportional hazards regression were used in further analyses.
Results
A total of 420 patients underwent PD during the period of study, of whom 197 had lymph node (LN) metastasis. Among these, 41 (20.8%) patients had disease-positive HALNs. The HALN was the only site of LN metastasis in only three of the 247 patients (1.2%). Median follow-up was 18.5 months (interquartile range: 4.1–28.2 months). Median OS and DFS were 22.7 months [95% confidence interval (CI) 19.0–26.3] and 12.6 months (95% CI 10.2–15.2). There was no significant difference in median OS between HALN-positive patients (18.4 months, 95% CI 12.3–24.0) and HALN-negative patients (19.7 months, 95% CI 16.7–22.6) (P = 0.659). On multivariate analysis, the hazard ratio (HR) of death was highest among patients with an LN ratio of >0.2 (HR 1.2, 95% CI 1.1–1.29; P = 0.012) followed by those with poorly differentiated histology (HR 1.09, 95% CI 1.04–1.11; P = 0.029).
Conclusions
In pancreatic adenocarcinoma patients with LN disease, survival after PD is comparable regardless of HALN status. Therefore, HALN-positive disease should not preclude the performance of PD.
Introduction
Operative resection and adjuvant chemotherapy remain the only effective course of therapy for pancreatic cancer although actual 5-year survival rates after successful resection and optimal medical treatment are only 12–18%.1 Even with improvements in perioperative care, pancreaticoduodenectomy (PD) is associated with morbidity and mortality rates of 24% and 2%, respectively.
The ability to identify a subset of patients in whom surgery does not confer a survival advantage would spare these patients the morbidity of a non-therapeutic procedure. Various authors have sought to identify clinicopathological prognostic markers in pancreatic cancer. The most important prognostic factors in patients who have undergone surgery with curative intent for pancreatic head adenocarcinoma are the presence of regional lymph node (LN) metastases and the LN ratio.2–4
Resection of the hepatic artery LN (HALN) or LN8a [Japan Pancreas Society (JPS) staging] is often performed routinely during PD.5 This LN is located along the common hepatic artery near the take-off of the gastroduodenal artery (GDA). Removal of this LN not only gives better access to and visualization of the GDA, but also facilitates the location of a suprapancreatic portal vein. In patients with pancreatic head malignancy, according to the JPS classification, the HALN is a second-echelon LN. In a study by Kayahara et al., metastatic disease to the 8a LN (HALN) occurred in 9.7% of patients.6 Several studies have reported a significantly worse prognosis in patients with HALN metastasis.7–9 There have been reports that involvement of the HALN represents a survival disadvantage similar to that implied by liver metastases or peritoneal disease in patients with pancreatic head cancers.8 In light of these data, some centres perform routine frozen-section analysis of this node and will not perform a PD if this node is positive for metastasis. However, there is still uncertainty about the prognostic significance of metastasis to the HALN. The aim of this study was to assess the prognostic relevance of the HALN and to assess whether its status should be used as a criterion for surgical selection.
Materials and methods
A review of the single-institution prospective patient hepatopancreatobiliary database at the University of Louisville was performed. Consecutive patients who underwent PD for pancreatic adenocarcinoma during 2002–2012 were included in this study. Patients were excluded if the data available for them were incomplete, if final pathology showed non-pancreatic adenocarcinoma and if they were aged <18 years. Institutional review board approval was obtained.
A disease-positive LN was defined by the presence of metastasis on routine haematoxylin and eosin staining. A positive resection margin was defined by the finding of tumour cells within ≤1 mm of the final resection margin. The patient's LN ratio was defined according to the total number of positive LNs divided by the total number of LNs harvested. The HALN or LN8a was required to have been clearly identified intraoperatively by the primary surgeon and sent as a separate specimen for pathological investigation.
Patients were divided into four distinct subsets based on the presence of metastases to the HALN or peripancreatic LNs (PPLNs). These four groups represented patients who were, respectively: (i) LN disease-negative (HALN–/PPLN–); (ii) HALN+/PPLN–; (iii) HALN+/PPLN+, and (iv) HALN–/PPLN+. Overall survival (OS) and disease-free survival (DFS) were defined as survival in months from the date of surgery. Overall survival was calculated from surgery until the date of the last follow-up or death. Similarly, DFS was calculated from the date of surgery to the date of the first recurrence or the last recurrence-free follow-up. Statistical analysis was performed using IBM® SPSS® Statistics for Windows Version 21 (IBM Corp., Armonk, NY, USA). Categorical variables were analysed using the chi-squared and Fisher's exact tests; numeric variables were analysed using the independent-samples t-test. Kaplan–Meier curves were constructed and the log-rank test was used to evaluate significant differences among clinical and pathological variables. Variables that achieved a P-value of <0.2 were entered into a Cox proportional hazards regression model for final multivariate analysis. A P-value of ≤0.05 was considered to indicate statistical significance.
Results
During the study period, 420 patients underwent PD for pancreatic adenocarcinoma. Data on the intraoperative identification and separate pathological evaluation of the HALN were available for 247 (58.8%) of these patients. Of the 247 patients, LN metastasis was seen in 197 (79.8%) patients; the remaining 50 (20.2%) patients had no evidence of LN disease. In three (1.2%) patients the HALN was the only site of metastasis. The median number of LNs examined per specimen was 22 [interquartile range (IQR): 15–27]. The median number of positive LNs was 4.0 (IQR: 1.0–5.5) and the median size of LN metastases was 11 mm (IQR: 5.5–18.0 mm). A total of 38 patients, representing 15.4% of the total cohort and 19.3% of LN disease-positive patients demonstrated positive findings in both the HALN and PPLNs (HALN+/PPLN+). A total of 156 patients representing 63.2% of the total cohort and 79.1% of LN disease-positive patients showed positive findings for metastasis in PPLNs but not in the HALN (HALN−/PPLN+).
Table 1 shows a comparison of the two groups represented by HALN+ and HALN− patients. These groups were comparable except that the HALN+/PPLN+ group had a significantly higher number of disease-positive LNs, higher LN ratio and greater lymphovascular invasion (LVI).
Table 1.
Clinicopathologic variables of patients with lymph node metastases: comparison of patients with (HALN+) and without (HALN−) hepatic artery lymph node (HALN) metastatic disease
| HALN– (n = 156) | HALN+ (n = 41) | P-value | ||
|---|---|---|---|---|
| Lymph nodes retrieved, median (range) | 21 (5–58) | 20 (5–41) | 0.290 | |
| Lymph nodes positive, median (range) | 3 (1–24) | 6 (1–22) | 0.012 | |
| Lymph node ratio, median (range) | 0.14 (0.02–0.90) | 0.20 (0.30–0.57 | 0.001 | |
| Size of metastasis, mm, median (range) | 11 (2–26) | 13 (3–25) | NA | |
| Tumour stage, n (%) | T1 | 3 (2.4%) | 0 | 0.528 |
| T2 | 7 (5.7%) | 3 (9.1%) | ||
| T3 | 113 (91.9%) | 30 (90.9%) | ||
| Differentiation, n (%) | Good | 11 (7.4%) | 3 (7.3%) | 0.470 |
| Moderate | 71 (47.7%) | 17 (41.5%) | ||
| Poor | 21 (14.1%) | 10 (24.4%) | ||
| Perineural invasion, n (%) | 85 (77.3%) | 27 (87.1%) | 0.317 | |
| Lymphovascular invasion, n (%) | 62 (57.4%) | 26 (81.3%) | 0.021a | |
| Vascular invasion, n (%) | 10 (6.7%) | 5 (12.2%) | 0.323 | |
| Positive margin, n (%) | 21 (14.1%) | 8 (19.5%) | 0.462 | |
| Adjuvant chemotherapy, n (%) | 56 (60.8%) | 17 (56.6%) | 0.160 | |
Significant at P ≤ 0.05.
NA, not applicable.
The median follow-up for this study was 18.5 months (IQR: 4.1–28.2 months). Survival analysis showed that median OS in the entire cohort was 22.7 months (IQR: 11.8–40.4 months, 95% CI 19.0–26.3). A total of 32 patients were lost from follow-up and 114 patients died over the follow-up period. Patients with LN disease had a median OS of 19.5 months (IQR: 11.0–33.0 months, 95% CI 15.4–23.6). Lymph node disease-negative patients had a better median survival of 40.2 months (IQR: 26.1–63.0 months, 95% CI 29.2–76.2); this difference was statistically significant (P < 0.001). Among patients with node-positive disease (Fig. 1), the HALN+/PPLN+ group had a median OS of 16.9 months (IQR: 10.0–26.0 months, 95% CI 11.7–25.9), whereas the HALN−/PPLN+ group had a median OS of 20.5 months (IQR: 11.8–33.0 months, 95% CI 16.0–24.0) (P = 0.659).
Figure 1.
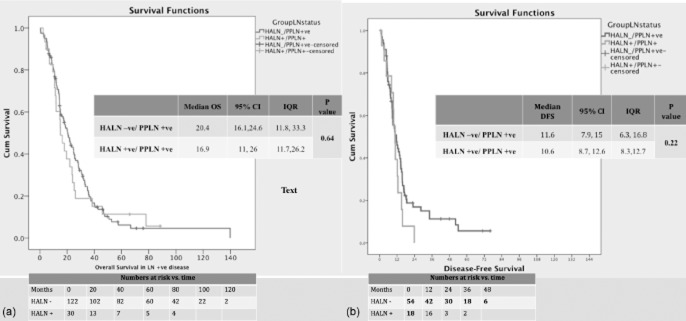
Kaplan–Meier curves for survival in patients (n = 197) with node-positive disease for (a) overall survival (OS) and (b) disease-free survival (DFS). HALN, hepatic artery lymph node; PPLN, peripancreatic lymph node(s); 95% CI, 95% confidence interval; IQR, interquartile range
Median DFS in the entire cohort was 12.6 months (IQR: 7.4–33.8 months, 95% CI 10.2–15.2). Patients with disease-positive LNs had a lower median DFS of 10.8 months (IQR: 8.4–13.3 months, 95% CI 6.9–15.3) (P < 0.001). Among patients with disease-positive LNs (Fig. 1), the HALN+/PPLN+ group had a median DFS of 10.6 months (IQR: 8.3–12.7 months, 95% CI 8.4–12.6) which was comparable with that in the HALN−/PPLN+ group (median DFS: 11.6 months, IQR: 6.3–16.8 months, 95% CI 7.9–15.0) (P = 0.219).
Univariate and multivariate survival analyses of patients with LN-positive disease are shown in Table 2. Margin status, perineural or lymphovascular invasion, T-stage and adjuvant chemotherapy had no effect on survival. An LN ratio of >0.2 (P = 0.001), poorly differentiated histology (P = 0.043) and vascular invasion (P = 0.026) were associated with significantly shorter OS on univariate analysis. These variables along with LVI (P = 0.196) were then entered into a multivariate Cox proportional hazards model. On multivariate analysis (Table 2), only LN ratio [hazard ratio (HR) 1.13, 95% CI 1.00–1.23; P = 0.012] and poorly differentiated histology (HR 1.09, 95% CI 1.02–1.30; P = 0.029) were significant predictors of worse OS.
Table 2.
Factors affecting survival
| Variable | Patients, n (%) | Univariate analysis | Multivariate analysis |
||
|---|---|---|---|---|---|
| P-value | Hazard function | 95.0% CI | P-value | ||
| HALN + | 41 (20.8%) | 0.659 | |||
| LN ratio >0.2 | 82 (41.6%) | 0.001a | 1.129 | 1.0–1.2 | 0.012b |
| Well differentiated | 14 (10.5%) | 0.043a | 0.680 | 0.34–1.1 | 0.077 |
| Moderately differentiated | 88 (66.2%) | Reference group | |||
| Poorly differentiated | 31 (23.3%) | 1.090 | 1.02–1.33 | 0.029b | |
| Vascular invasion | 15 (7.9%) | 0.026a | 1.060 | 0.5–2.1 | 0.139 |
| Microscopically positive margins | 29 (14.7%) | 0.700 | |||
| Perineural invasion | 112 (79.4%) | 0.800 | |||
| Lymphovascular invasion | 88 (62.8%) | 0.200 | |||
Significant on univariate analysis (P ≤ 0.05).
Significant on multivariate analysis (P ≤ 0.05).
95% CI, 95% confidence interval; HALN, hepatic artery lymph node; LN, lymph node.
Similar results were found in an analysis of DFS: an LN ratio of >0.2 (P = 0.002) and a positive resection margin (P = 0.044) were associated with shorter DFS on univariate analysis. These variables along with poorly differentiated histology (P = 0.072) were then entered into a multivariate Cox proportional hazards model. On multivariate analysis only, LN ratio (HR 2.3, 95% CI 1.2–4.3; P = 0.013) was a significant predictor of shorter DFS.
Discussion
The presence of nodal metastases has been shown to be an independent prognostic factor in pancreatic adenocarcinoma.3,4,7 Unfortunately, as with most other independent prognostic markers in resectable disease, this can only be assessed at the time of surgery. Accurately identifying those patients in whom PD would be futile in terms of their subsequent survival would be advantageous. The HALN is an anatomically constant, easily accessible node, which is routinely harvested at a revocable point during PD. Therefore, there has been significant interest in evaluating this node as a potential intraoperative prognostic marker.
Previous studies demonstrate that metastasis to the HALN occurs in 9.7–24.0% of patients.6,7 The current study found a rate of 16.6%, which was in concordance with those reported previously. The location of pancreatic node-positive disease has been shown to be a significant prognostic marker for survival: patients with metastases to the superior mesenteric artery and anterior pancreatic nodes fare poorly compared with those with peripancreatic nodal metastasis.10 However, another study reported no survival difference between patients with first- and second-echelon LN disease.11
Subsequently, a study by Maithel et al.8 evaluated 49 patients with HALN metastasis and found a significant reduction in survival, similar to that in patients with carcinomatosis or liver metastases. Cordera et al.9 evaluated 55 patients of whom 10 had HALN metastases; these 10 patients had poorer OS and no longterm survival. Similar results were seen in two other studies, which showed that HALN disease conferred a survival disadvantage.7,12
At the study institution, the HALN is routinely harvested during PD by most surgeons and is separately examined by histopathology. As expected, a majority (79.8%) of the patients in the present series were found to have LN metastasis, which is in line with the ranges described in previous reports. Isolated HALN metastasis in this study was extremely rare (1.2%), which is consistent with prior studies and lends credence to the supposition that the HALN is unlikely to represent a first-echelon node for metastasis.6 Among the patients with node-positive disease, 79.3% did not have HALN metastasis and only 19.3% had HALN metastasis in the presence of PPLN metastasis. As various studies have clearly established that LN metastasis is associated with worse OS, the present study concentrated on only patients with node-positive disease.3,4,7 As Table 1 shows, patients in the HALN+/PPLN+ group had a significantly higher rate of nodal metastasis with respect to number of positive LNs, as well as the LN ratio.
The median OS of 23 months in the whole cohort is comparable with rates reported in the current literature. Patients with node-negative disease did significantly better. Although OS was reduced in patients with HALN metastases, the difference in OS between these and other patients with node-positive disease was not statistically significant. Based on the results of the current study, the present authors do not agree that HALN status should be used as a criterion for selection for PD. This conclusion differs from those of some previous studies. The current study is distinct in its large number of HALN+ patients (n = 41; numbers in previous studies range from 10 to 237–9) and lengthy follow-up period. Some of the prior studies included a mix of histologies,7,8 which makes the interpretation of survival analyses difficult. In the one comparable study with an exclusively adenocarcinoma-associated histology, patient numbers were low (HALN+ disease in 10 of 55 patients) and showed a trend towards worse OS.9 The study by LaFemina et al.12 included fewer patients with HALN+ disease than this study (23 of 147 patients), looked at only pancreatic adenocarcinomas and found significantly worse OS and DFS in these patients. In the current study, OS in HALN+ patients was 16.9 months, which is higher than OS reported at the Memorial Sloan–Kettering and Fox Chase Cancer Centers.9,12
Previous studies have used the LN ratio as a surrogate for a greater burden of LN disease (LN ratio of >0.2) and have shown poorer prognoses in patients with higher ratios.13–15 The current study confirmed the LN ratio as an independent prognostic predictor of poorer OS, as was a poorly differentiated histology. This study also validates the role of poorly differentiated histology as an independent prognostic marker for a poor prognosis with regard to OS, a finding similar to those reported in the literature.4
In the current study, patients with HALN metastases had a significantly higher tumour burden. Therefore, the authors believe that HALN disease should be used as a surrogate not only for a higher tumour burden, but also for a worse tumour biology. However, larger studies are required to further define the significance of disease in this node. The suggested role for HALN metastasis (or positive nodal disease) may refer to its preoperative evaluation with endoscopic ultrasound or laparoscopic staging. As this node is positive in 10–24% of patients (16.6% in this study), a subset of patients with a higher disease burden and poor tumour biology can be identified and selected for neoadjuvant chemotherapy.
There are some limitations to this study. Data on HALN status were unavailable for a significant portion of patients submitted to PD (173 of 420 patients). This primarily represents a practice preference of some surgeons at this institution; however, the exclusion of these patients may have contributed to some degree of selection bias. In addition, this study included a larger proportion of node-positive patients (79.8%) compared with other reports in the literature.
Conclusions
The HALN is almost never the isolated site of disease. Metastasis in the HALN is associated with a higher burden of LN metastasis. However, among patients with node-positive pancreatic head adenocarcinoma, the presence of HALN-positive disease is not an independent adverse prognostic marker for OS and therefore metastasis to the HALN should not be considered as a criterion for exclusion from PD.
Conflicts of interest
None declared.
References
- 1.Ferrone CR, Brennan MF, Gonen M, Coit DG, Fong Y, Chung S, et al. Pancreatic adenocarcinoma: the actual 5-year survivors. J Gastrointest Surg. 2008;12:701–706. doi: 10.1007/s11605-007-0384-8. [DOI] [PubMed] [Google Scholar]
- 2.Lee SR, Kim HO, Son BH, Yoo CH, Shin JH. Prognostic factors associated with longterm survival and recurrence in pancreatic adenocarcinoma. Hepatogastroenterology. 2013;60:358–362. doi: 10.5754/hge12727. [DOI] [PubMed] [Google Scholar]
- 3.Breslin TM, Hess KR, Harbison DB, Jean ME, Cleary KR, Dackiw AP, et al. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Ann Surg Oncol. 2001;8:123–132. doi: 10.1007/s10434-001-0123-4. [DOI] [PubMed] [Google Scholar]
- 4.Brennan MF, Kattan MW, Klimstra D, Conlon K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. 2004;240:293–298. doi: 10.1097/01.sla.0000133125.85489.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawarada Y. New classification of pancreatic carcinoma – Japan Pancreas Society. Nihon Shokakibyo Gakkai Zasshi. 2003;100:974–980. [PubMed] [Google Scholar]
- 6.Kayahara M, Nagakawa T, Kobayashi H, Mori K, Nakano T, Kadoya N, et al. Lymphatic flow in carcinoma of the head of the pancreas. Cancer. 1992;70:2061–2066. doi: 10.1002/1097-0142(19921015)70:8<2061::aid-cncr2820700808>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 7.Connor S, Bosonnet L, Ghaneh P, Alexakis N, Hartley M, Campbell F, et al. Survival of patients with periampullary carcinoma is predicted by lymph node 8a but not by lymph node 16b1 status. Br J Surg. 2004;91:1592–1599. doi: 10.1002/bjs.4761. [DOI] [PubMed] [Google Scholar]
- 8.Maithel SK, Khalili K, Dixon E, Guindi M, Callery MP, Cattral MS, et al. Impact of regional lymph node evaluation in staging patients with periampullary tumours. Ann Surg Oncol. 2007;14:202–210. doi: 10.1245/s10434-006-9041-9. [DOI] [PubMed] [Google Scholar]
- 9.Cordera F, Arciero CA, Li T, Watson JC, Hoffman JP. Significance of common hepatic artery lymph node metastases during pancreaticoduodenectomy for pancreatic head adenocarcinoma. Ann Surg Oncol. 2007;14:2330–2336. doi: 10.1245/s10434-006-9339-7. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa O, Ohigashi H, Sasaki Y, Kabuto T, Furukawa H, Nakamori S, et al. Practical grouping of positive lymph nodes in pancreatic head cancer treated by an extended pancreatectomy. Surgery. 1997;121:244–249. doi: 10.1016/s0039-6060(97)90352-4. [DOI] [PubMed] [Google Scholar]
- 11.Pawlik TM, Abdalla EK, Barnett CC, Ahmad SA, Cleary KR, Vauthey JN, et al. Feasibility of a randomized trial of extended lymphadenectomy for pancreatic cancer. Arch Surg. 2005;140:584–589. doi: 10.1001/archsurg.140.6.584. discussion 589–591. [DOI] [PubMed] [Google Scholar]
- 12.LaFemina J, Chou JF, Gonen M, Rocha FG, Correa-Gallego C, Kingham TP, et al. Hepatic arterial nodal metastases in pancreatic cancer: is this the node of importance? J Gastrointest Surg. 2013;17:1092–1097. doi: 10.1007/s11605-012-2071-7. [DOI] [PubMed] [Google Scholar]
- 13.House MG, Gonen M, Jarnagin WR, D'Angelica M, DeMatteo RP, Fong Y, et al. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J Gastrointest Surg. 2007;11:1549–1555. doi: 10.1007/s11605-007-0243-7. [DOI] [PubMed] [Google Scholar]
- 14.Pawlik TM, Gleisner AL, Cameron JL, Winter JM, Assumpcao L, Lillemoe KD, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141:610–618. doi: 10.1016/j.surg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Berger AC, Watson JC, Ross EA, Hoffman JP. The metastatic/examined lymph node ratio is an important prognostic factor after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg. 2004;70:235–240. discussion 240. [PubMed] [Google Scholar]


