Abstract
Objectives
Repeat transarterial chemoembolization (TACE) is a common intervention performed for hepatocellular carcinoma (HCC). The aim of this study was to identify predictors of the need for repeat TACE.
Methods
Between 2008 and 2012, data on patient and tumour variables were collected for 262 patients treated with a first TACE procedure for HCC. The decision to perform repeat TACE procedures was made at the completion of the first TACE or after follow-up imaging demonstrated the subtotal treatment of HCC tumours.
Results
Repeat TACE was performed in 67 of 262 (25.6%) patients. Necrosis of HCC, measured after the first TACE, was lower in patients who subsequently received repeat TACE (P = 0.042). On multivariable analysis, total tumour diameter of >5 cm [odds ratio (OR) 2.76, 95% confidence interval (CI) 1.45–5.25; P = 0.002] and increasing age (OR 1.04/year, 95% CI 1.00–1.07; P = 0.030) were predictive of the need for repeat TACE. Measures of liver function and TACE approach (selective versus non-selective) were not predictive of repeat TACE. Median survival did not differ significantly between patients who did (median survival: 21.1 months) and did not (median survival: 26.1 months) receive a repeat TACE procedure (P = 0.574).
Conclusions
The requirement for repeat TACE is associated with older age, increased HCC tumour burden and subtotal TACE-induced HCC necrosis. Importantly, repeat TACE was not associated with reduced survival.
Introduction
Hepatocellular carcinoma (HCC) is a common primary liver malignancy in the USA and worldwide, and is the leading cause of death among patients with cirrhosis.1 Although HCC is the sixth most common neoplasm in the world, its dismal prognosis makes it the third leading cause of cancer-related mortality and responsible for approximately 600 000 deaths per year.2 The incidence of HCC in the USA has tripled in the past two decades and 5-year survival rates remain as low as 10–15%.3
The most widely utilized staging system for HCC is the Barcelona Clinic Liver Cancer (BCLC) staging system.4,5 This system distinguishes five tumour stages and links the stages to different treatment options. It is estimated that 70% of patients with HCC are diagnosed at intermediate (BCLC stage B) to advanced (BCLC stage C) tumour stages, at which treatment options are limited.4,5 Numerous studies and reviews have attempted to determine the best strategy for treating patients with intermediate-stage HCC (BCLC stage B and C) who are not candidates for potentially curative therapies.6–9 For patients with large (>5 cm) or multifocal HCC without macrovascular invasion or extrahepatic metastasis, transarterial chemoembolization (TACE) is the recommended treatment modality based on high-quality randomized clinical trials,10,11 and a meta-analysis of randomized controlled trials.12 Current practice guidelines from the American Association for the Study of Liver Disease (AASLD) recommend TACE as a first-line non-curative therapy for non-surgical patients with large or multifocal HCC who do not have vascular invasion or extrahepatic spread.13,14 An alternative locoregional therapy for this patient population is radioembolization.15,16
The administration of TACE is common. An analysis of the Surveillance, Epidemiology and End Results (SEER) database reported that TACE is the single most common oncologic intervention for HCC in the USA.17 Furthermore, a review of the Scientific Registry of Transplant Recipients (SRTR) data on liver transplantation for HCC demonstrates that TACE is the most common bridging therapy and is offered to >70% of waitlisted HCC patients in the USA.18
One common problem facing clinicians is subtotal HCC tumour necrosis (partial response) following a single TACE treatment. These patients are most commonly treated with repeat TACE interventions, although the efficacy of repeat TACE is unclear. Furthermore, there is a need to better define patient populations that may benefit from additional TACE procedures.
The primary aim of this study was to determine independent risk factors associated with repeat TACE treatment in patients with intermediate- to advanced-stage HCC at a single university-based hospital. The secondary aim was to measure the efficacy of repeat TACE.
Materials and methods
Ethical approval for this study was obtained from the University of Alabama (UAB) Institutional Review Board (protocol no. X100310006). A retrospective chart review was performed for all patients receiving TACE at UAB between 2008 and 2012.
Patient population
Patients were diagnosed with HCC according to the AASLD criteria. The decision to offer TACE to patients with HCC was made by a multidisciplinary liver tumour board at UAB that included medical oncologists, surgeons, hepatologists and interventional radiologists. Patient candidacy for TACE was guided by established AASLD practice guidelines.13,14 Transarterial chemoembolization was administered as previously described.19 The decision to perform additional TACE procedures was made either at the completion of the first TACE because the HCC tumour(s) was considered by the interventional radiologist to have been subtotally treated (referred to as ‘planned repeat TACE’) or after follow-up imaging suggested the subtotal treatment of lesions or the appearance of new lesions (referred to as ‘unplanned repeat TACE’).
A list of all patients treated with TACE as an initial oncologic therapy was generated from the UAB interventional radiology procedures electronic database. Patients submitted to TACE were excluded if they had a non-HCC tumour type. Patients with recurrent HCC following liver resection or transplantation were also excluded. In addition, patients with HCC tumours that had been treated previously with another locoregional therapy such as radiofrequency ablation or external beam radiotherapy were excluded. Patients taking chemotherapeutic agents before and after the procedure were not excluded.
Radiographic measurements
Diagnosis of HCC
Hepatocellular carcinoma was diagnosed according to the presence of an arterially enhancing lesion of 1–2 cm in size with portal venous washout and pseudocapsule formation on delayed-phase imaging, or an arterially enhancing lesion of >2 cm in size with portal venous washout or pseudocapsule formation on delayed-phase imaging.20,21
Tumour necrosis in HCC
Tumour response was assessed according to mRECIST (modified response evaluation criteria in solid tumours) as previously described.19,22 Response data are presented for the largest lesion only, herein referred to as the index lesion. There are four categories of tumour response according to mRECIST, which indicate, respectively: complete response; partial response; stable disease, and progressive disease.23 Because TACE most commonly results in a partial response as defined by mRECIST19 and because the partial response category includes a large range of response (30–99%), mRECIST partial response is presented in three subcategories according to data granularity: 30–60%; 60–90%, and 90–99%.
Sarcopoenia
Psoas cross-sectional area was used as a surrogate marker of fragility.24 The area of the right and left psoas muscle was measured at the level of the fourth lumbar vertebral body and a region of interest was drawn around the borders of the psoas muscle. The enclosed region was then used to calculate the cross-sectional area of the psoas muscle.
Cirrhosis
A caudate to right lobe ratio of >0.9,25,26 nodular transformation of the liver, and sequela of portal venous hypertension27 were used as radiographic indicators of cirrhosis.
Data analysis
Data on patient demographics, clinical history, laboratory findings and HCC tumour characteristics were collected. To allow for the use of common statistical procedures, the analysis was restricted to examination of the index HCC tumour, which was defined as the largest tumour (the use of more than one tumour per patient in the analysis would have violated the common assumption of independent data observations). When the data followed a normal distribution, a two-sample t-test was used to compare group means. When the normality of the data distribution was questionable, the non-parametric Wilcoxon rank sum test was used to compare group distributions. The primary analytic approach for dichotomous variables utilized chi-squared analysis. Kaplan–Meier curves were constructed to evaluate patient survival. Survival probabilities were analysed using the log-rank test. For all inferences, the probability of a type I error (α) was set to 0.05. All analyses were conducted using sas Version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Results
Patient demographics
Data for a total of 262 patients treated with either single or multiple TACE(s) as locoregional therapy for HCC were reviewed. A single TACE procedure was performed in 194 (74.3%) and multiple TACE procedures were performed in 67 (25.7%) patients. The median time between TACE procedures was 1.83 months [interquartile range (IQR) 1.31–4.34 months]. The repeat TACE group included 53 (79.1%) patients who underwent two TACE procedures, 12 (17.9%) patients who underwent three TACE procedures, and two (3.0%) patients who underwent four TACE procedures. Figure 1 depicts the distribution of HCC patients treated with TACE stratified by unifocal versus multifocal disease, TACE vehicle [lipiodol versus drug-eluting beads (DEBs)], and the delivery of single versus multiple TACE treatments.
Figure 1.
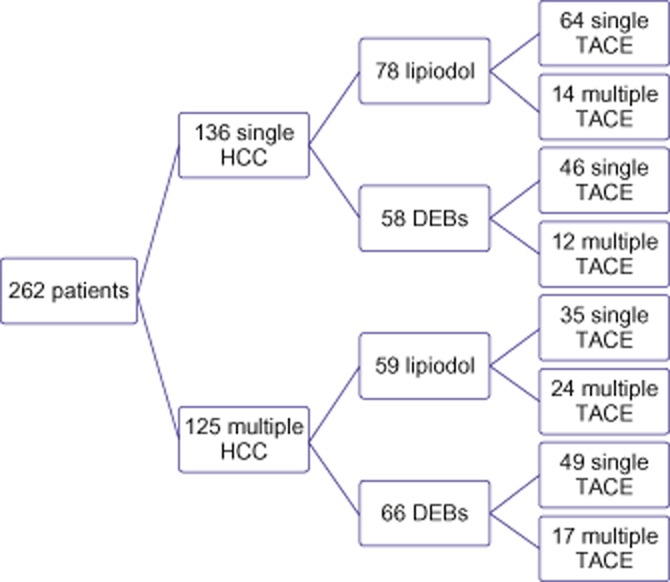
Flow chart depicting the distribution of unifocal and multifocal hepatocellular carcinoma (HCC), the use of lipiodol versus drug-eluting beads (DEBs), and the administration of one versus more than one transarterial chemoembolization (TACE) procedure
Patients in the repeat TACE group were older and less likely to have hepatitis C. All patients had underlying liver disease, although 11.4% of those in the single TACE group and 6.1% of those in the multiple TACE group did not have cirrhosis appreciable by laboratory or radiology criteria (Table 1).
Table 1.
Baseline demographics in 262 patients with hepatocellular carcinoma treated with transarterial chemoembolization (TACE)
| Variable | Single TACE group |
Multiple TACE group |
P-value |
|---|---|---|---|
| (n = 195) | (n = 67) | ||
| Age, years, mean ± SD | 61.1 ± 9.2 | 64.6 ± 9.4 | 0.009 |
| Male, n (%) | 141 (72.3%) | 54 (80.6%) | 0.179 |
| Race, n (%) | 0.875 | ||
| Black | 39 (20.2%) | 15 (23.1%) | |
| White | 147 (76.2%) | 48 (73.9%) | |
| Aetiology of liver diseasea, n (%) | |||
| Alcohol | 47 (24.2%) | 17 (25.4%) | 0.851 |
| HBV | 12 (6.2%) | 6 (9.0%) | 0.413 |
| HCV | 109 (56.2%) | 27 (40.3%) | 0.025 |
| NASH | 42 (21.5%) | 12 (17.9%) | 0.526 |
| Pre-TACE laboratory values | |||
| Laboratory MELD score | 11.1 ± 3.8 | 11.3 ± 3.9 | 0.659 |
| α-fetoprotein, ng/ml, median (IQR) | 16.1 (5.69–155.00) | 29.1 (9.54–227.00) | 0.268 |
| Δ Sarcopoenia | −5.5 ± 9.0 | −5.2 ± 8.7 | 0.853 |
| Tumour characteristics | |||
| Number of tumours, median (percentiles 25–75) | 1.0 (1.00–2.00) | 2.0 (1.00–3.00) | 0.023 |
| Size of largest tumour, cm, mean ± SD | 3.6 ± 2.5 | 4.6 ± 2.7 | 0.028 |
| Total tumour diameterb, cm, mean ± SD | 5.0 ± 3.9 | 6.7 ± 3.7 | 0.003 |
| Type of TACE, n (%) | |||
| Lipiodol | 100 (51.3%) | 38 (56.7%) | 0.442 |
| Drug-eluting beads | 92 (47.2%) | 29 (43.3%) | 0.581 |
| Non-selective (lobar) TACE | 13 (6.7%) | 5 (7.5%) | 0.840 |
| Post-TACE interventionsc, n (%) | |||
| Ablation | 34 (17.4%) | 16 (23.9%) | 0.247 |
| Radiation therapy | 39 (20.0%) | 11 (16.4%) | 0.519 |
| Liver transplantation | 40 (20.5%) | 5 (7.5%) | 0.015 |
Some patients had liver disease of more than one aetiology.
Sum of axial diameters of three largest lesions.
Some patients had more than one post-TACE intervention.
HBV, hepatitis B virus; HCV, hepatitis C virus; IQR, interquartile range; MELD, Model for End-stage Liver Disease (range: 6–40); NASH, non-alcoholic steatohepatitis; SD, standard deviation.
Planned repeat TACE versus unplanned repeat TACE
Planned repeat TACE procedures were performed in 20 of 67 (29.9%) HCC patients. The previous HCC tumour was retreated in 10 patients, different HCC tumours were treated in four patients, and a combination of previous and different HCC tumours were treated in the remaining six patients. The control of HCC tumours was achieved in nine of 20 (45.0%) patients, in three of whom control was achieved by TACE alone. In the other six, control was achieved by repeat TACE plus additional potentially curative therapies. Complete response, as defined by mRECIST, was not achieved in six patients submitted to more than one TACE procedure. The remaining five patients received more than one TACE treatment, but were non-compliant with follow-up recommendations.
Unplanned repeat TACE procedures were performed in the remaining 47 of the 67 (70.1%) HCC patients in the repeat TACE group. The previous HCC tumour was retreated in 30 patients, different HCC tumours were treated in 10 patients, and a combination of previous and different HCC tumours were treated in the remaining seven patients. Tumour control was achieved in 16 of 47 (34.0%) patients, in seven by TACE alone, and in the remainder by repeat TACE plus additional potentially curative therapies. Tumour control was not achieved in 30 patients who received more than one TACE treatment. The remaining patient was treated with more than one TACE procedure but was non-compliant with follow-up recommendations.
Tumour necrosis in HCC
Hepatocellular carcinoma tumour necrosis was measured in all patients following the first TACE procedure (Fig. 2). There were significant differences in the distribution of HCC necrosis between patients treated with one and those treated with more than one TACE procedure (P = 0.042).
Figure 2.
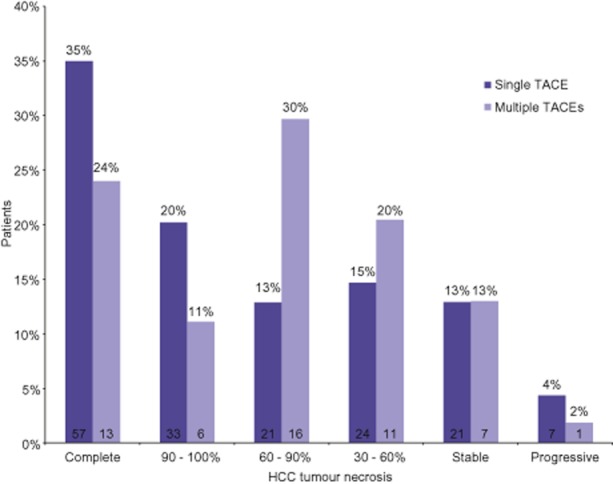
Necrosis of hepatocellular carcinoma (HCC) tumour(s) following first transarterial chemoembolization (TACE) procedure, quantified according to mRECIST. Data above each bar show the percentages of patients meeting the respective mRECIST response; data within each bar show the number of patients used to calculate the percentage response. Patients who received one TACE procedure experienced better HCC tumour necrosis than patients treated with more than one TACE procedure (P = 0.042)
Predictors of repeat TACE
Univariate and subsequent multivariate analyses for risk factors for repeat TACE are shown in Table 2. A sensitivity analysis was performed to see if predictors of repeat TACE varied based upon whether the TACE was planned or unplanned. These analyses demonstrated that total tumour diameter was the dominant predictor of planned repeat TACE, whereas age was the dominant predictor of unplanned repeat TACE.
Table 2.
Univariate and multivariable analyses of predictors of repeat transarterial chemoembolization (TACE)
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| More than one tumour | 1.92 (1.08–3.40) | 0.026 | ||
| HCC tumour size >3 cm | 2.52 (1.20–5.30) | 0.015 | ||
| Total tumour diameter >5 cm | 2.82 (1.52–5.27) | 0.001 | 2.76 (1.45–5.25) | 0.002 |
| Central position (vs. peripheral) | 1.09 (0.54–2.22) | 0.806 | ||
| α-fetoprotein >400 ng/ml | 0.89 (0.41–1.94) | 0.764 | ||
| Age (per year) | 1.04 (1.01–1.08) | 0.008 | 1.04 (1.00–1.07) | 0.030 |
| Child–Pugh class A (vs. B/C) | 1.13 (0.65–1.97) | 0.671 | ||
| MELD score (per point) | 1.02 (0.95–1.09) | 0.653 | ||
| Platelet count >100 | 0.89 (0.51–1.55) | 0.676 | ||
| Sarcopoenia | 1.00 (0.97–1.04) | 0.854 | ||
| Lipiodol (vs. DEBs) | 0.80 (0.46–1.41) | 0.442 | ||
| Selective TACE (vs. non-selective) | 1.12 (0.38–3.26) | 0.840 | ||
95% CI, 95% confidence interval; DEBs, drug-eluting beads; HCC, hepatocellular carcinoma; MELD, Model for End-stage Liver Disease (range: 6–40); OR, odds ratio.
Survival
Median survival did not differ significantly between patients who did (median survival: 21.1 months) and did not (median survival: 26.1 months) receive repeat TACE procedures (P = 0.574) (Fig. 3). Median survival decreased in both groups when censored for liver transplantation, although the differences in survival remained non-significant (one TACE: 19.5 months; more than one TACE: 20.1 months; P = 0.612).
Figure 3.
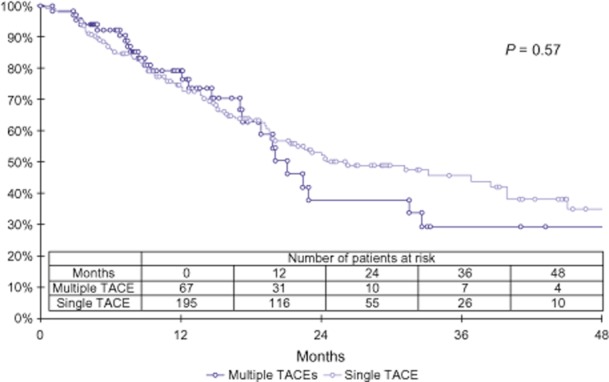
Kaplan–Meier survival curves comparing length of survival using the log-rank test. Median survival did not differ significantly between patients treated with one (26.1 months) and those treated with more than one (21.1 months) transarterial chemoembolization (TACE) procedure (P = 0.704)
Discussion
The most clinically significant findings demonstrated in this study are that advancing age and increasing total tumour burden are associated with need for repeat chemoembolization for intermediate to advanced HCC. Patients submitted to repeat TACE were more likely to experience inferior HCC tumour necrosis after the first TACE treatment. Importantly, survival did not differ between patients who received one TACE and those who received more than one TACE. This equivalent survival suggests that efforts to obtain more complete HCC tumour necrosis by using repeat TACE treatments do not result in increased mortality and may improve overall survival.
The association between advancing age and repeat TACE probably reflects a negative patient selection bias. Older patients are often not considered candidates for other more invasive and potentially curative locoregional or surgical interventions (such as liver transplantation). Transarterial chemoembolization is an ideal cross-sectional intervention which allows for the observation of older HCC patients over time to establish how they tolerate the first TACE before decisions about repeat TACE are made.
The association between increasing total tumour diameter and repeat TACE is predictable. A finite amount of chemotherapy and embolic material is administered in each TACE and thus a greater tumour volume might be expected to require additional TACE procedures to achieve adequate HCC tumour necrosis. This concept is demonstrated nicely in Fig. 2, which shows that a lower overall percentage of HCC necrosis following the first TACE procedure was observed in patients who subsequently underwent additional TACE procedures. The mRECIST protocol defines HCC necrosis as the percentage of non-enhancing tumour of tumour volume.23 Even with an equivalent volume of tumour necrosis, it is readily apparent how a larger denominator would result in a lower percentage of HCC necrosis. In addition, a larger total tumour diameter was correlated with the presence of both multifocal tumours and bilobar tumours, each of which would be common indications for repeat TACE.
Few other studies in the literature have examined the efficacy of repeat TACE procedures. Two recent studies conducted in Austria report a scoring system that predicts risk factors for poor survival following repeat TACE procedures.28,29 The Assessment for Retreatment with TACE (‘ART’) score is derived from clinical measurements obtained after the initial TACE treatment, and includes transaminasaemia (reflecting an increase in the level of aspartate aminotransferase of >25%), worsening liver function (increase in Child–Pugh score), and the absence of a radiologic tumour response to initial TACE as independent negative prognostic factors for overall survival following a second TACE.28,29 Prospective studies of liver function following TACE demonstrate that significant transaminasaemia and significant liver dysfunction are rare,22,30,31 which limits the applicability of the ART score.
As with all retrospective studies, the present report has several limitations. The first of these refers to patient selection bias, which derives from the fact that older patients are preferentially considered for repeat TACE rather than other locoregional or surgical therapeutic options. Secondly, the risk factors for repeat TACE identified in this study are fairly intuitive and these published findings may not significantly impact on current TACE practice. The limited generalizability of all single-centre TACE studies represents another limitation and reflects significant inter-centre variation in patient candidacy, TACE vehicle (lipiodol or DEBs and type of DEBs), and technical approaches (lobar, selective, sub-selective). This variation is further highlighted by the fact that the frequency of re-TACE in the current series was much lower than that in most published studies. Regardless of these limitations, this is one of the few studies to identify pre-TACE risk factors for repeat TACE and the fact that the study participants represent an HCC population spanning a period of only 4.5 years suggests its relevancy to current practice.
In conclusion, advanced age and increasing total tumour diameter were identified as independent risk factors for repeat TACE. Patients undergoing repeat TACE experienced significantly lower HCC tumour necrosis following the initial TACE. Survival did not differ significantly between HCC patients submitted to one TACE and those submitted to more than one TACE procedure. Specific analysis of repeat TACE recipients demonstrates that repeat TACE is an efficacious intervention that should be considered for patients in whom subtotal HCC tumour necrosis is achieved following the first TACE procedure.
Conflicts of interest
None declared.
References
- 1.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 2.Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15(Suppl. 4):5–13. doi: 10.1634/theoncologist.2010-S4-05. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 4.Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 6.Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, et al. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37:212–220. doi: 10.1016/j.ctrv.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Lencioni R, Chen XP, Dagher L, Venook AP. Treatment of intermediate/advanced hepatocellular carcinoma in the clinic: how can outcomes be improved? Oncologist. 2010;15(Suppl. 4):42–52. doi: 10.1634/theoncologist.2010-S4-42. [DOI] [PubMed] [Google Scholar]
- 8.Liapi E, Geschwind JF. Intra-arterial therapies for hepatocellular carcinoma: where do we stand? Ann Surg Oncol. 2010;17:1234–1246. doi: 10.1245/s10434-010-0977-4. [DOI] [PubMed] [Google Scholar]
- 9.Vogl TJ, Naguib NN, Nour-Eldin NE, Rao P, Emami AH, Zangos S, et al. Review on transarterial chemoembolization in hepatocellular carcinoma: palliative, combined, neoadjuvant, bridging, and symptomatic indications. Eur J Radiol. 2009;72:505–516. doi: 10.1016/j.ejrad.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, et al. Arterial embolization or chemoembolization versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomized controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 11.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 13.Bruix J, Sherman M. American Association for the Study of Liver Disease. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruix J, Sherman M Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 15.Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Carr BI. Hepatic arterial 90Yttrium glass microspheres (Therasphere) for unresectable hepatocellular carcinoma: interim safety and survival data on 65 patients. Liver Transplant. 2004;10(2 Suppl. 1):107–110. doi: 10.1002/lt.20036. [DOI] [PubMed] [Google Scholar]
- 17.Shah SA, Smith JK, Li Y, Ng SC, Carroll JE, Tseng JF. Underutilization of therapy for hepatocellular carcinoma in the Medicare population. Cancer. 2011;117:1019–1026. doi: 10.1002/cncr.25683. [DOI] [PubMed] [Google Scholar]
- 18.Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver transplantation in the United States, 1999–2008. Am J Transplant. 2010;10(4 Pt 2):1003–1019. doi: 10.1111/j.1600-6143.2010.03037.x. [DOI] [PubMed] [Google Scholar]
- 19.Bryant MK, Dorn DP, Zarzour J, Smith JK, Redden DT, Saddekni S, et al. Computed tomography predictors of hepatocellular carcinoma tumour necrosis after chemoembolization. HPB. 2013;16:327–335. doi: 10.1111/hpb.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wald C, Russo MW, Heimbach JK, Hussain HK, Pomfret EA, Bruix J. New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma. Radiology. 2013;266:376–382. doi: 10.1148/radiol.12121698. [DOI] [PubMed] [Google Scholar]
- 21.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 22.Dorn DP, Bryant MK, Zarzour J, Smith JK, Redden DT, Saddekni S, et al. Chemoembolization outcomes for hepatocellular carcinoma in cirrhotic patients with compromised liver function. HPB. 2013;16:648–655. doi: 10.1111/hpb.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 24.Englesbe MJ, Terjimanian MN, Lee JS, Sheetz KH, Harbaugh CM, Hussain A, et al. Morphometric age and surgical risk. J Am Coll Surg. 2013;216:976–985. doi: 10.1016/j.jamcollsurg.2013.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Awaya H, Mitchell DG, Kamishima T, Holland G, Ito K, Matsumoto T. Cirrhosis: modified caudate–right lobe ratio. Radiology. 2002;224:769–774. doi: 10.1148/radiol.2243011495. [DOI] [PubMed] [Google Scholar]
- 26.Lee JK, Sagel SR, Stanley RJ. Computed Body Tomography with MRI Correlation. 4th edn. Philadelphia, PA: Lippincott, Williams & Wilkins; 2003. [Google Scholar]
- 27.Ito K, Mitchell DG, Siegelman ES. Cirrhosis: MR imaging features. Magn Reson Imaging Clin N Am. 2002;10:75–92. doi: 10.1016/s1064-9689(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 28.Sieghart W, Hucke F, Pinter M, Graziadei I, Vogel W, Muller C, et al. The ART of decision making: retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology. 2013;57:2261–2273. doi: 10.1002/hep.26256. [DOI] [PubMed] [Google Scholar]
- 29.Hucke F, Sieghart W, Pinter M, Graziadei I, Vogel W, Muller C, et al. The ART-strategy: sequential assessment of the ART score predicts outcome of patients with hepatocellular carcinoma re-treated with TACE. J Hepatol. 2014;60:118–126. doi: 10.1016/j.jhep.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 30.Jaeger HJ, Mehring UM, Castaneda F, Hasse F, Blumhardt G, Loehlein D, et al. Sequential transarterial chemoembolization for unresectable advanced hepatocellular carcinoma. Cardiovasc Intervent Radiol. 1996;19:388–396. doi: 10.1007/BF02577625. [DOI] [PubMed] [Google Scholar]
- 31.Sacco R, Bertini M, Petruzzi P, Bertoni M, Bargellini I, Bresci G, et al. Clinical impact of selective transarterial chemoembolization on hepatocellular carcinoma: a cohort study. World J Gastroenterol. 2009;15:1843–1848. doi: 10.3748/wjg.15.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]


