Abstract
Introduction
Liver transplantation (LT) is a treatment option in select patients with hepatocellular carcinoma (HCC). The aim of the present study was to compare survival in Stage I or II HCC patients undergoing either liver transplant (LT) or a liver resection (LR).
Method
The study is a retrospective analysis of the National Cancer Data Base (1998–2011). In total, 148 882 patients with liver cancer were identified, of which 5-year survival data (1998–2006) were available for 64 227 patients. Patients were stratified by the American Joint Committee on Cancer (AJCC) clinical stage I and II. Kaplan–Meier curves and log-rank tests were used for statistical analysis.
Results
3340 HCC patients met analysis criteria. Among stage I HCC, 860 had LT and 871 had LR. Among stage II HCC, 833 had LT and 776 LR. In stage I patients the median survival for LT and LR were 127.9 and 56.7 months, respectively, (P < 0.0001) and in stage II patients the median survival was 110.8 and 42.8 months (P < 0.0001). Unlike LT patients, LR patients with Stage I HCC had a longer median survival compared with Stage II patients (P = 0.0002).
Conclusion
Liver transplantation offers a survival advantage compared with a liver resection among patients with Stage I and II HCC. LT is the best surgical treatment for early stage (I/II) HCC in patients with advanced fibrosis or cirrhosis, whereas LR provides equivalent outcomes to LT in patients without advanced fibrosis and should be considered as the first surgical option.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary hepatic malignancy with over 30 000 cases diagnosed and 20 000 deaths per year in the United States.1 The incidence is on the rise with more patients being diagnosed with non-alcoholic fatty liver disease.2,3 Screening patients with underlying liver disease for HCC is variable; hence, patients often present with advanced disease at the time of diagnosis, which may preclude surgical treatment.4,5 The majority of patients develop HCC in the background setting of cirrhosis, especially in regions (western countries) where alcohol consumption and chronic hepatitis C serve as the primary aetiology of liver disease.2 This is different from the population developing HCC in eastern countries where Hepatitis B is the most common cause.
The prognosis of patients with HCC depends not only on the stage of the disease, but also on liver function at the time of diagnosis. Long-term disease-free survival can be achieved by surgically removing the tumour. The treatment for early stage disease in patients with preserved liver function is a liver resection (LR).7–14 With the advent of liver transplantation (LT) and effective immunosuppression, unresectable candidates can also be effectively treated.15–17 The treatment of choice for patients with advanced cirrhosis and HCC within the Milan criteria is LT; however, owing to the national shortage of organs, LT remains limited and is only an available option for a select group of patients.18
Several previous studies have compared LR with LT for HCC to determine the relative benefits and risks of each procedure.19–24 The largest study published to date was a meta-analysis by Zheng et al., that analysed 62 studies comparing LT and LR for HCC. Overall, these data reported increased survival and lower recurrence rates for LT compared with LR.25 Similarly, several single-centre retrospective studies conclude LT should be considered as the primary treatment of HCC for those patients that fall within the Milan criteria26,27 Conversely, studies by Facciuto et al. report survival rates for HCC patients are similar after LR and LT.28
Multiple patient- and tumour-related factors dictate the optimal treatment option for patients with HCC, including patient general health, extent of liver fibrosis/cirrhosis and tumour location/number/size. As a result, few studies address comparative outcomes for early stage HCC patients undergoing LT compared with LR. Early stage HCC patients [stratified by The American Joint Committee on Cancer (AJCC) Clinical Stage I and II – Table 1]29,30 are more likely to meet the Milan criteria and are therefore more likely to be eligible for LT. In order to compare long-term outcomes for LT compared with LR in Stage I and Stage II HCC patients, we sought permission to access medical information contained in the National Cancer Data Base (NCDB) via the American College of Surgeons.
Table 1.
AJCC clinical staging (5th and 6th edition) for Stage I & II HCC
| AJCC clinical staging 5th edition (1998–2002) | AJCC clinical staging 6th edition (2003–2006) |
|---|---|
| Stage I – T1 N0 M0 | Stage I – T1 N0 M0 |
| Stage II – T2 N0 M0 | Stage II – T2 N0 M0 |
| T1 – Solitary tumour 2 cm or less in greatest dimension without vascular invasion | T1 – Solitary tumour without vascular invasion |
| T2 – Solitary tumour 2 cm or less in greatest dimension with vascular invasion or multiple tumors limited to 1 lobe (none < 2 cm without vascular invasion), or a solitary tumor > 2 cm without vascular invasion | T2 – Solitary tumour with vascular invasion or multiple tumours none > 5 cm |
Instituted in 1989 the NCDB is a nationwide oncology outcomes database from more than 1500 commission-accredited cancer programmes in the United States and Puerto Rico.31 It is a joint programme of the Commission on Cancer (CoC), the American College of Surgeons and the American Cancer Society. Approximately 70% of all newly diagnosed cancers are captured at the centre and submitted to the NCDB. In all, the NCDB now contains ≈29 million records from hospital cancer registries across the United States and Puerto Rico. The key components of the database include patient demographics, cancer diagnostics, clinical and pathological staging, treatment details (surgery, radiation, chemotherapy and palliative) and survival. In addition, socio-economic information and insurance information is also captured.
Patients and methods
Data acquisition and patient selection
Access to the NCDB database was obtained after submitting an online proposal requesting participant user files (http://ncdbpuf.facs.org/) Patients with primary HCC were identified using codes 8170–8175 according to the International Classification of Diseases for Oncology.36 This approach provided data on 149 020 patients diagnosed with liver cancer between 1998–2011. We restricted the cohort to 1998–2006 to allow us to obtain accurate 5-year survival data from time of diagnosis. (Fig. 1). A total of 3340 patients with clinical stage I and II HCC were included in the final case cohort. The pre-operative (clinical) staging was used as the decision to resect versus transplant with early stage HCC was made based (largely) on the clinical staging of the disease. The AJCC clinical staging 5th edition was used from 1998–2002 and the 6th edition was used from 2003–2006. We used the information as recorded in the database to perform our study keeping the key changes between the 5th and 6th editions in mind (Table 1). All these patients had information for the subgroup analyses in the database except for tumour size (3145/3400).
Figure 1.
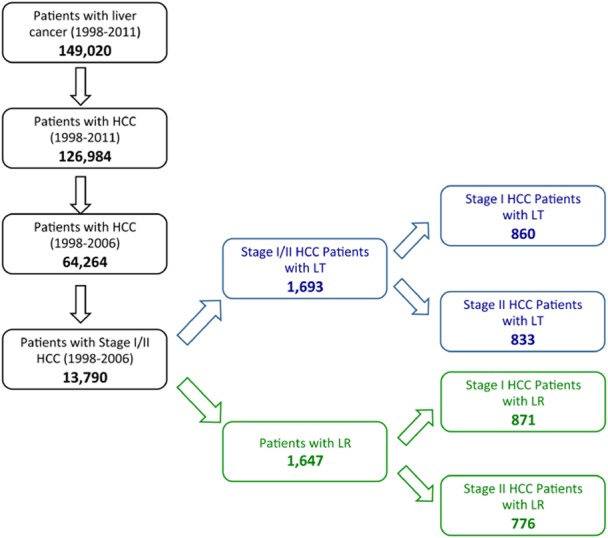
Flowchart representing selection of patient cohort for the study
Statistical analysis
Statistical analysis was performed using SAS Enterprise Guide®, V. 5.1. (SAS Institute, Inc., Cary, NC, USA). Descriptive statistics, including counts, percentages, means and standard deviations, were calculated for patient-specific demographics. Survival was calculated in months from date of diagnosis to date of death, or date of last contact. Overall survival was derived using Kaplan–Meier estimates and the log-rank test was used to analyse statistical significance of Kaplan–Meier estimates. Cox's proportional hazard models were used for univariate and multivariate analyses of time-to-event data and multivariate Cox's proportional hazard models adjusted for procedure, stage, tumour size, age and gender. A two-tailed P < 0.05 was considered significant.
Results
Patient demographics
In total, 77 460 patients were diagnosed and recorded to the NCDB with liver cancer (1998–2006), of which 64 264 (83.0%) had HCC as their primary diagnosis (Fig. 1). Within this group of HCC patients 13 790 (21.5%) were diagnosed as Stage I or II, of who 3340 (24.2%) underwent either LR [n = 1647 (49.3%); n = 871 Stage I (50.3%), n = 776 Stage II (49.7%)] or LT [n = 1693 (50.7%); n = 860 Stage I; n = 833 Stage II (49.3)] (Fig. 1). Within the Stage I/II HCC population undergoing LT or LR males outnumbered females by approximately 2:1 [2338 (70.0%)/1002 (30.0%)] with a comparable ratio of males and females undergoing LR compared with LT (1123 versus 1215, LR versus LT; males; 524 versus 478, LR versus LT; females). The mean age of LT patients was significantly lower than LR patients [62.8 ± 12.9 (LR) versus 55.7 ± 7.8 (LT)]. Demographic details of the case cohort are provided in Table 2.
Table 2.
Demographics table
| Resection Stage 1 | Resection Stage 2 | Transplant Stage 1 | Transplant Stage 2 | ||
|---|---|---|---|---|---|
| n | 871 | 776 | 860 | 833 | |
| Mean age (years) | 63.0 | 62.7 | 55.6 | 55.8 | |
| Gender | Male | 597 | 526 | 651 | 664 |
| Female | 274 | 250 | 209 | 169 | |
| Tumour size | < = 2 cm | 133 | 57 | 349 | 216 |
| >2 cm | 676 | 679 | 464 | 571 | |
| Unknown | 62 | 40 | 47 | 46 | |
| Fibrosis | None – moderate | 85 | 37 | 38 | 22 |
| Severe – cirrhosis | 48 | 22 | 130 | 139 | |
| Missing | 738 | 717 | 692 | 672 | |
| AFP | Elevated | 227 | 124 | 258 | 267 |
| Normal | 172 | 56 | 115 | 93 | |
| Borderline | 1 | 1 | 1 | 2 | |
| Missing | 471 | 595 | 486 | 471 | |
AFP, alpha fetoprotein.
Survival data
For patients diagnosed with Stage I or Stage II HCC the median survival for those undergoing LT was significantly better than LR [127.9 (107.9–180) and 56.7 (51.8–65.1) months, respectively, for Stage I, P < 0.0001, and 110.8 (103.7–150.5) and 42.8 (38.3–48.9) months, respectively, for Stage II, P < 0.0001, Fig. 2]. Unlike LT patients, LR patients with Stage I HCC had a longer median survival compared with Stage II patients (P = 0.0002).
Figure 2.
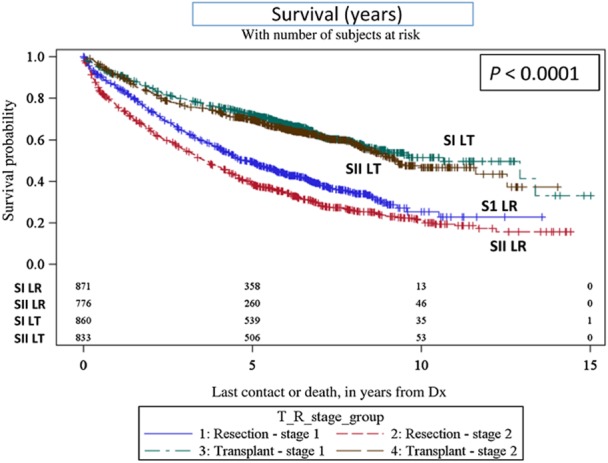
Kaplan–Meier survival curves – overall survival (transplant versus resection). SI LT, Stage 1 liver transplant; SII LT, Stage 2 liver transplant; S1 LR, Stage 1 liver resection; and SII LR, Stage 2 liver resection
To further analyse independent risk factors we employed a Cox model to include age, gender, size of tumour and clinical stage. Females had a 18% decreased risk of mortality compared with males when undergoing a LT or LR in the adjusted model (Table 3 ). This remained true when the Cox model was applied to resection alone (P = 0.0006) but there was no difference in mortality between males and females when the Cox Model was applied to the transplant group alone (P = 0.5775).
Table 3.
Cox model (transplant and resection)
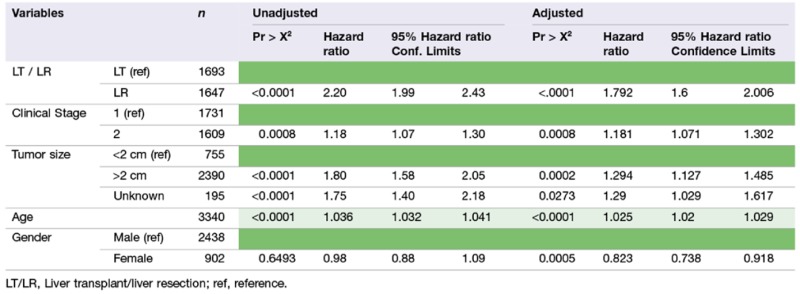 |
Subgroup analysis
Chemotherapy
This variable is documented only as ‘single’ agent or ‘multiagent’ chemotherapy in the database. All patients who had undergone transarterial chemoembolization (TACE) were also grouped in this category. To date, patients who had single or multi drug chemotherapy for Stage I HCC had similar survival outcomes if they underwent LT or LR (median survival for LR = >150, LT = 155.04, P = 0.671, Fig. 3a). However, patients who underwent LR for Stage I HCC with chemotherapy had a better survival than those without chemotherapy (P < 0.0004, Fig. 3b). There was no significant difference in survival for either Stage I or II HCC patients who received chemotherapy in the LT group.
Figure 3.
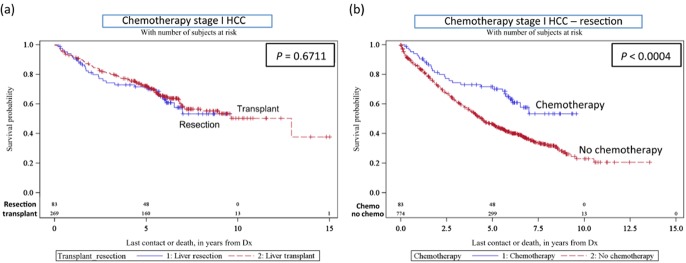
(a) Kaplan–Meier survival curves – chemotherapy for stage I hepatocellular carcinoma (HCC) (transplant versus resection); (b) effect of chemotherapy for stage I HCC (resection)
Tumour size
Data regarding tumour size (<2 versus >2 cm) were available for 3145 patients (1545 LR and 1600 LT patients). In cases with multiple tumours, tumour size was defined as the tumour with the largest diameter. We included tumour size as well as staging in the model to see if tumour size alone was associated with survival. The clinical staging includes tumour size plus vascular invasion and multiplicity. We chose to dichotomize tumour size by less than or greater than 2 cm because 2 cm is the minimum size that qualifies for transplant. The primary tumour alone was taken into consideration in determining size as the largest dimension of the primary tumour was what was recorded in the data base. In this study we deliberately opted to use both clinical stage and tumour size in the Cox model as we had an interest in establishing whether tumour size alone was a significant factor in outcome when comparing patients receiving a transplant compared with a resection.
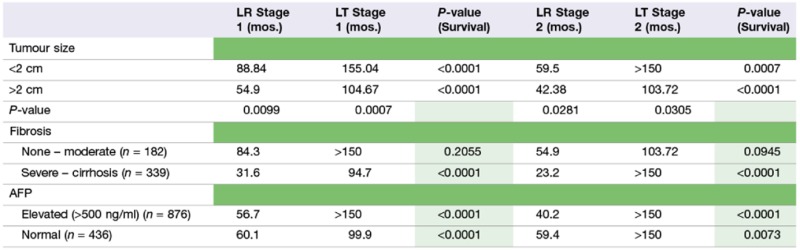
Irrespective of tumour size, patients undergoing LT for Stage I or II HCC had an improved survival compared with those undergoing LR. However, patients with smaller tumours (<2 cm) had significantly improved outcomes compared with tumours >2 cm, independent of whether the patient underwent LT or LR (Table 4).
Table 4.
Median survival (months; mos.) based on tumour size, stage of fibrosis and alpha-fetoprotein (AFP) level (transplant (LT) versus resection (LR))
 |
Fibrosis (based on the Ishak Score)
From 2004 on, the NCDB database started including data for degree of fibrosis (none to moderate) (Ishak score 0–4; AJCC F0) and severe to cirrhosis (Ishak score 5–6; AJCC F2). For our analysis this provides fibrosis data for 192 (out of 900) LR patients and 329 (out of 1071) LT patients between 2004–2006 (i.e. data for which 5-year survival is also available). As might be expected, more liver resections were performed in patients with Ishak scores between 0–4 (122 LR versus 60 LT), whereas more transplants were performed in patients with Ishak scores of 5–6 (269 LT versus 70 LR). There was no significant difference in survival between patients with Ishak scores between 0–4 (none to moderate fibrosis) undergoing LT compared with LR based on disease stage (P = 0.2055 for stage I, P = 0.0945 for stage II, Table 2; Fig. 4). Conversely, a significant difference in survival was identified between patients with Stage I and II HCC undergoing LT compared with LR when the Ishak scores were between 5 and 6 (i.e. severe fibrosis or cirrhosis) (P < 0.0001 for stage I, P < 0.0001 for stage II; Table 4; Fig. 4).
Figure 4.
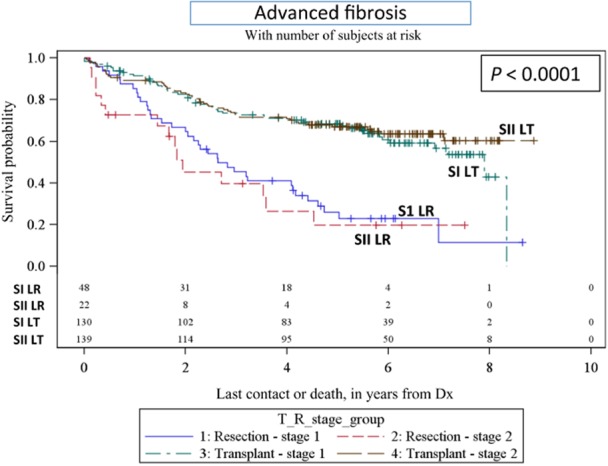
Kaplan–Meier survival curves – advanced fibrosis (transplant versus resection) SI LT, Stage 1 liver transplant; SII LT, Stage 2 liver transplant; S1 LR, Stage 1 liver resection; SII LR, Stage 2 liver resection
Alpha fetoprotein (AFP)
Alpha fetoprotein (AFP) data were available for 1312 patients with Stage I or II HCC, of whom 579 underwent a resection and 733 received a transplant. Patients who underwent LT for Stage I or II HCC had a better overall survival than patients who had LR independent of AFP values. Normal AFP (P < 0.0001; Stage I HCC, and P = 0.0073; stage II HCC) and elevated AFP (>500 ng/ml) (P < 0.0001; Stage I HCC, and P < 0.01 for Stage II HCC) (Table 4).
Discussion
Hepatocellular carcinoma is one of the leading causes of cancer-related mortality in the world.1 The aim of this study was to analyse outcomes of patients treated for early stage HCC (Stage I/II) by LT or LR using data stored in the NCDB. Overall, the median survival for LT recipients was significantly better compared with LR patients for both stage I and II HCC (127.9 (107.9–180) compared with 56.7 (51.8–65.1) months (Stage I), 110.8 (103.7–150.5) compared with 42.8 (38.3–48.9) months (Stage II)). This is consistent with the meta-analysis performed by Zheng et al. that reported LT recipients had significantly better overall survival, disease-free survival and lower recurrence rates than LR patients for HCC.25
The majority of studies that compare LT with LR for HCC are single-centre studies. For example, Baccarani et al. analysed patients with HCC, on an intention-to-treat basis, and confirmed transplantation was superior to LR in terms of overall survival and disease-free survival.9 Similarly, Lee et al. reported patients with HCC and Childs A or B cirrhosis showed recurrence-free survival to be significantly greater after LT compared with LR over a 10-year period.26 While our study further confirms these data, this study is unique in that it uses data acquired from over 1500 Commission on Cancer accredited centres. This approach probably accounts for variability in outcomes between centres to provide a more accurate representation of long-term outcomes on a national scale. We were also able to look at extent of fibrosis, tumour size, AFP levels and chemotherapy to determine each variable's effect as an independent risk factor for survival.
In a subset analysis of patients from 2004–2006 in which the fibrosis score was recorded, these data demonstrate that patients with advanced fibrosis (n = 339) (Ishak Score 5–6) and HCC derived a survival benefit from LT compared with LR (94.7 versus 31.6 months, Stage I HCC, and >150 versus 23.2 months, Stage II HCC)(Table 2), whereas patients with Ishak Scores 0–4 (n = 182) and HCC did equally well with LR as they did with transplant (P = 0.2055 for Stage I HCC; P = 0.0945 for Stage II HCC) (Table 2). These data suggest that this patient population would benefit equally from a LR as first choice of therapy. The fibrosis score was included in the NCDB data set starting in 2004; therefore, further analysis of the effect of the fibrosis score on survival will need to be assessed in the later time period from 2006–2011. Interestingly although, our analysis revealed LT recipients had an overall better survival than LR patients independent of either tumor size or AFP levels (Table 2). While limited data were available regarding the use of chemotherapy as part of the treatment in the study groups, our analyses did demonstrate that patients with Stage I HCC who underwent a LR in conjunction with chemotherapy had a longer survival than those undergoing LR alone. However, it should noted that the information contained within this database meant it was not possible to ascertain the time of chemotherapy relative to other parameters for these patient groups and the fact that all patients in the study group who received TACE (most likely all of them) were grouped into the chemotherapy section.
Over the past decade the number of patients transplanted for HCC has increased significantly and studies indicate there has been a relative shift away from LR towards LT for HCC patients.32 Current expert opinion indicates LT is the best treatment for patients with early stage HCC because, not only does the surgery remove the tumour, but it also removes the organ that harbours potentially recurrent foci.33 A recent review by Earl and Chapman concluded that it is vital to consider tumour size, multifocality, medical comorbidity and geographical factors that affect the waiting list time and organ availability when considering patients with early stage HCC for transplant versus a resection.34
The NCDB provides information on a large number of patients with HCC and outcomes reported from these data are representative of the combined outcomes of multiple centres across the United States; however, this data set is not without significant limitations. For example, while we were able to analyse outcomes in a subset of patients based on degree of fibrosis based on pathology reports, we were not able to examine clinically relevant pre-operative indications of liver dysfunction (ascites and esophageal varices) or pre-operative laboratory values/composite scores [prothrombin time/international normalized ratio, bilirubin, creatinine, model for end-stage liver disease (MELD) score and Child–Turcotte–Pugh score]. This is likely to be of particular significance as liver function is a key determinant in the clinical decision-making process to decide between a liver resection or transplantation. AJCC pathological staging was not available on all patients. Hence for patients with stage II disease, given the variability in definition and staging system it is possible that there were patients who were transplanted but who were found to have vascular invasion in the explant post-transplantation. Similarly, other data was either absent completely (e.g. disease recurrence from which disease-free survival could be derived), limited in availability (e.g. chemotherapy data, the use of loco-regional therapy as bridging therapy toward transplant), or has only recently been included in the data base (e.g. information from which a MELD score can be calculated has only been included since 2010).35 Similarly, some data points were very loosely defined and not amenable to subset analysis. For example, the ‘chemotherapy’ classification provided no data regarding timing, route of administration, type of agent used or number of treatments given. Finally, details on medical comorbidity were extremely limited and thus could not be included in our analyses. Certainly, patient comorbidities are also a key determinant in the clinical decision-making process when assessing for liver resection and transplantation.
The use of thermal and non-thermal ablation technologies as both definitive treatment for HCC and as bridging therapy prior to transplant has been repeatedly demonstrated to improve survival.37–39 The lack of data related to the use of this therapy is a limitation of the NCDB, and imposes a possible selection bias as the distribution of use of ablation between resection and transplant is likely not uniform. Future data collection by the NCDB would benefit from the addition of this information.
Conclusions
This study represents the largest retrospective analysis of patients with early stage HCC undergoing either LT or resection LRin the United States. Based on our analyses, LT is the best surgical treatment for early stage (I/II) HCC in patients with advanced fibrosis or cirrhosis (Ishak Score 5–6). Conversely LR provides equivalent outcomes to LT in patients with mild-to-moderate fibrosis (Ishak Score 0–4) and should be considered as the first surgical option. However, neither tumor size nor patient AFP level impacted the overall survival between LT and LR patients. Currently, systems that incorporate measures of liver function are the preferred staging system for HCC. Thus, while the NCDB provides a powerful platform for large, multi-centre data set analyses, including measurements of liver function and outcomes from other treatment approaches would further allow us to define the preferential surgical procedure for those patients diagnosed with early Stage HCC.
Conflicts of interest
None declared.
References
- 1.American Cancer Society. Cancer Facts and Figures 2013. Atlanta, GA: American Cancer Society; 2013. [Google Scholar]
- 2.El-Serag HB. Epidemiology of hepatocellular carcinoma in USA. Hepatol Res. 2007;37(Suppl. S):88–94. doi: 10.1111/j.1872-034X.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 3.Hucke F, Sieghart W, Schöniger-Hekele M, Peck-Radosavljevic M, Müller C. Clinical characteristics of patients with hepatocellular carcinoma in Austria – is there a need for a structured screening program? Wien Klin Wochenschr. 2011;123:542–551. doi: 10.1007/s00508-011-0033-9. [DOI] [PubMed] [Google Scholar]
- 4.Davila JA, Henderson LM, Kramer JR, Richardson P, Duan Z, Kanwal F, et al. Utilization of Surveillance for Hepatocellular Carcinoma among HCV-infected Veterans in the United States. Ann Intern Med. 2011;154:85–93. doi: 10.7326/0003-4819-154-2-201101180-00006. [DOI] [PubMed] [Google Scholar]
- 5.Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, El-Serag HB. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology. 2010;52:132–141. doi: 10.1002/hep.23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou XD, Tang ZY, Yang BH, Lin ZY, Ma ZC, Ye SL, et al. Experience of 1000 patients who underwent hepatectomy for small hepatocellular carcinoma. Cancer. 2001;91:1479–1486. doi: 10.1002/1097-0142(20010415)91:8<1479::aid-cncr1155>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 7.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373–382. doi: 10.1097/00000658-200203000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherqui D, Laurent A, Tayar C, Chang S, Van Nhieu JT, Loriau J, et al. Laparoscopic liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: midterm results and perspectives. Ann Surg. 2006;243:499–506. doi: 10.1097/01.sla.0000206017.29651.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baccarani U, Isola M, Adani GL, Benzoni E, Avellini C, Lorenzin D, et al. Superiority of transplantation vs resection for the treatment of small hepatocellular carcinoma. Transpl Int. 2008;21:247–254. doi: 10.1111/j.1432-2277.2007.00597.x. [DOI] [PubMed] [Google Scholar]
- 10.Jarnagin W, Chapman WC, Curley S, D'Angelica M, Rosen C, Dixon E, et al. Surgical treatment of hepatocellular carcinoma: expert consensus statement. HPB (Oxford) 2010;12:302–310. doi: 10.1111/j.1477-2574.2010.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Figueras J, Jaurrieta E, Valls C, Ramos E, Serrano T, Rafecas A, et al. Resection or transplantation for hepatocellular carcinoma in cirrhotic patients: outcomes based on indicated treatment strategy. J Am Coll Surg. 2000;190:580–587. doi: 10.1016/s1072-7515(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 12.Margarit C, Escartin A, Castells L, Vargas V, Allende E, Bilbao I. Resection for hepatocellular carcinoma is a good option in Child-Turcotte- Pugh class A patients with cirrhosis who are eligible for liver transplantation. Liver Transpl. 2005;11:1242–1251. doi: 10.1002/lt.20398. [DOI] [PubMed] [Google Scholar]
- 13.Michel J, Suc B, Montpeyroux F, Hachemanne S, Blanc P, Domergue J, et al. Liver resection or transplantation for hepatocellular carcinoma? Retrospective analysis of 215 patients with cirrhosis. J Hepatol. 1997;26:1274–1280. doi: 10.1016/s0168-8278(97)80462-x. [DOI] [PubMed] [Google Scholar]
- 14.Moon DB, Lee SG, Hwang S. Liver transplantation for hepatocellular carcinoma: single nodule with Child-Pugh class A sized less than 3 cm. Dig Dis. 2007;25:320–328. doi: 10.1159/000106912. [DOI] [PubMed] [Google Scholar]
- 15.Yao FY, Bass NM, Nikolai B, Davern TJ, Kerlan R, Wu V, et al. Liver transplantation for hepatocellular carcinoma: analysis of survival according to the intention-to-treat principle and dropout from the waiting list. Liver Transpl. 2002;8:873–883. doi: 10.1053/jlts.2002.34923. [DOI] [PubMed] [Google Scholar]
- 16.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 17.Majno P, Mentha G, Mazzaferro V. Resection, transplantation, either, or both? Other pieces of the puzzle. Liver Transpl. 2005;11:1177–1180. doi: 10.1002/lt.20495. [DOI] [PubMed] [Google Scholar]
- 18.Bismuth H, Chiche L, Adam R, Castaing D, Diamond T, Dennison A. Liver resection vs transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;218:145–151. doi: 10.1097/00000658-199308000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection vs transplantation. Hepatology. 1999;30:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 20.Bellavance EC, Lumpkins KM, Mentha G, Marques HP, Capussotti L, Pulitano C, et al. Surgical management of early-stage hepatocellular carcinoma: resection or transplantation? J Gastrointestinal Surg. 2008;12:1699–1708. doi: 10.1007/s11605-008-0652-2. [DOI] [PubMed] [Google Scholar]
- 21.Otto G. Is transplantation really superior to resection in the treatment of small hepatocellular carcinoma? Transplant Proc. 1997;29:489. doi: 10.1016/s0041-1345(96)00220-5. [DOI] [PubMed] [Google Scholar]
- 22.Sangro B, Herraiz M, Martinez-Gonzalez MA, Bilbao I, Herrero I, Beloqui O, et al. Prognosis of hepatocellular carcinoma in relation to treatment: a multivariate analysis of 178 patients from a single European institution. Surgery. 1998;124:575. [PubMed] [Google Scholar]
- 23.Weimann A, Schlitt HJ, Oldhafer KJ, Hoberg S, Tusch G, Raab R. Is liver transplantation superior to resection in early stage hepatocellular carcinoma? Transplant Proc. 1999;31:500. doi: 10.1016/s0041-1345(98)01727-8. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto J, Iwatsuki S, Kosuge T, Dvorchik I, Shimada K, Marsh JW, et al. Should hepatomas be treated with hepatic resection or transplantation? Cancer. 1999;86:1151. doi: 10.1002/(sici)1097-0142(19991001)86:7<1151::aid-cncr8>3.0.co;2-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng Z, Liang W, Milgrom DP, Zheng Z, Schroder PM, Kong NS, et al. Liver transplantation vs liver resection in the treatment of hepatocellular carcinoma: a meta-analysis of observational studies. Transplantation. 2014;97:227–234. doi: 10.1097/TP.0b013e3182a89383. [DOI] [PubMed] [Google Scholar]
- 26.Lee KK, Kim DG, Moon IS, Lee MD, Park JH. Liver transplantation vs liver resection for the treatment of hepatocellular carcinoma. J Surg Oncol. 2010;101:47–53. doi: 10.1002/jso.21415. [DOI] [PubMed] [Google Scholar]
- 27.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 28.Facciuto ME, Rochon C, Pandey M, Rodriguez-Davalos M, Samaniego S, Wolf DC, et al. Surgical dilemma: liver resection or liver transplantation for hepatocellular carcinoma and cirrhosis. Intention-to-treat analysis in patients with in and out with Milan criteria. HPB (Oxford) 2009;11:398–404. doi: 10.1111/j.1477-2574.2009.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleming ID, Cooper JS, Henson DE, Hutter RV, Kennedy BJ, Murphy GP, et al. AJCC Cancer Staging Manual. 5th edn. Philadelphia, PA: Lippincott-Raven; 1997. [Google Scholar]
- 30.Henderson JM, Sherman M, Tavill A, Abecassis M, Chejfec G, Gramlich T, et al. AHPBA/AJCC consensus conference on staging of hepatocellular carcinoma: consensus statement. HPB (Oxford) 2003;5:243–250. doi: 10.1080/13651820310015833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.American College of Surgeons. National cancer data base: public reports. Available at http://www.facs.org/cancer/ncdb/ (last accessed 18 June 2014)
- 32.Nathan H, Schulick RD, Choti MA, Pawlik TM, et al. Predictors of survival after resection of early hepatocellular carcinoma. Ann Surg. 2009;249:799–805. doi: 10.1097/SLA.0b013e3181a38eb5. [DOI] [PubMed] [Google Scholar]
- 33.Fan ST. Hepatocellular carcinoma – resection or transplant? Nat Rev Gastroenterol Hepatol. 2012;9:732–737. doi: 10.1038/nrgastro.2012.158. [DOI] [PubMed] [Google Scholar]
- 34.Earl TM, Chapman WC. Hepatocellular carcinoma: resection vs transplantation. Semin Liver Dis. 2013;33:282–292. doi: 10.1055/s-0033-1351783. [DOI] [PubMed] [Google Scholar]
- 35.Saab S, Wang V, Ibrahim AB, Durazo F, Han S, Farmer DG, et al. MELD score predicts 1-year patient survival post-orthotopic liver transplantation. Liver Transpl. 2003;9:473–476. doi: 10.1053/jlts.2003.50090. [DOI] [PubMed] [Google Scholar]
- 36.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al. International Classification of Diseases for Oncology (ICD-O) 2000. 3rd edn. Geneva: World Health Organisation.
- 37.Groeschl RT, Pilgrim CH, Hanna EM, Simo KA, Swan RZ, Sindram D, et al. Microwave ablation for hepatic malignancies: a multi-institutional analysis. Ann Surg. 2014;259:1195–2000. doi: 10.1097/SLA.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 38.Swan RZ, Sindram D, Martinie JB, Iannitti DA. Operative microwave ablation for hepatocellular carcinoma: complications, recurrence, and long-term outcomes. J Gastrointest Surg. 2013;17:719–729. doi: 10.1007/s11605-013-2164-y. [DOI] [PubMed] [Google Scholar]
- 39.Niemeyer DJ, Simo KA, Iannitti DA, McKillop IH. Ablation therapy for hepatocellular carcinoma: past, present and future perspectives. Hepatic Oncol. 2014;1:67–79. doi: 10.2217/hep.13.8. [DOI] [PMC free article] [PubMed] [Google Scholar]


