Abstract
Prostaglandin E2 (PGE2) and several other prostaglandins synthesized by colon suppress the proliferative activity of colonic epithelium. However, bile salts stimulate colonic epithelial proliferation despite the actions of bile salts to enhance the release of arachidonate and consequent colonic synthesis of PGE2. The current study was conducted to assess whether bile salt-induced increases in colonic formation of arachidonate metabolites other than PGE2 were linked to the stimulation of the proliferative activity of colonic epithelium. Within 10 min of addition, deoxycholate markedly stimulated the in vitro release of [14C]arachidonate from prelabeled rat colon. When given in vivo by intracolonic instillation deoxycholate (10 mumol) increased colonic accumulation of immunoreactive prostaglandin E (PGE), thromboxane B2 (TXB2), and the lipoxygenase product 12-hydroxyeicosatetraenoic acid (12-HETE) by two to fourfold over control in 30 min. This effect of intracolonic deoxycholate was followed by a ninefold increase in mucosal ornithine decarboxylase activity (4 h), and a subsequent two to threefold increase in [3H]thymidine [( 3H]Thd) incorporation into DNA of either mucosal scrapings or isolated pools of proliferative colonic epithelial cells (24 h). Intracolonic instillation of indomethacin (50 mumol) suppressed to low or undetectable levels both basal colonic accumulation of PGE and TXB2 and the increases in each parameter induced by subsequent instillation of deoxycholate. By contrast, indomethacin enhanced accumulation of 12-HETE in both control colons and those subsequently exposed to deoxycholate. The increases in 12-HETE induced by indomethacin alone were correlated with stimulation of mucosal ornithine decarboxylase activity and [3H]Thd incorporation into mucosal DNA. Indomethacin also enhanced the increases in these parameters induced by deoxycholate. Intracolonic instillation of phenidone (25-100 mumol) suppressed accumulation of PGE, TXB2, and 12-HETE in control colons and the increases in these parameters induced by a subsequent instillation of deoxycholate. Phenidone alone did not alter mucosal ornithine decarboxylase activity or [3H]thymidine incorporation into mucosal DNA. However, phenidone suppressed or abolished increases in these parameters induced by a subsequent instillation of deoxycholate. 4-(2-[IH-imidazol-1-yl]ethoxy) benzoic acid hydrochloride UK 37,248, which selectively reduced colonic TXB2 to undetectable levels without altering PGE or 12-HETE, had no effect on control or deoxycholate-induced increases in mucosal ornithine decarboxylase activity or [3H]Thd incorporation into DNA. Neither indomethacin nor phenidone altered the increases in [(14)C]arachidonate release induced in vitro by deoxycholate. Chenodeoxycholate and cholate also stimulated [(14)C]arachidonate release from colon in vitro within 10 min, and increased colonic 12-HETE (30 min) and mucosal ornithine decarboxylase activity (4 h) upon intracolonic installation. Prior installation of phenidone inhibited the increases in both 12-HETE and ornithine decarboxylase activity induced by these bile salts. The results support a role for bile salt-induced increases in colonic accumulation of lipoxygenase products, as reflected by 12-HETE, in the subsequent stimulation of the proliferative activity of colonic epithelium.
Full text
PDF


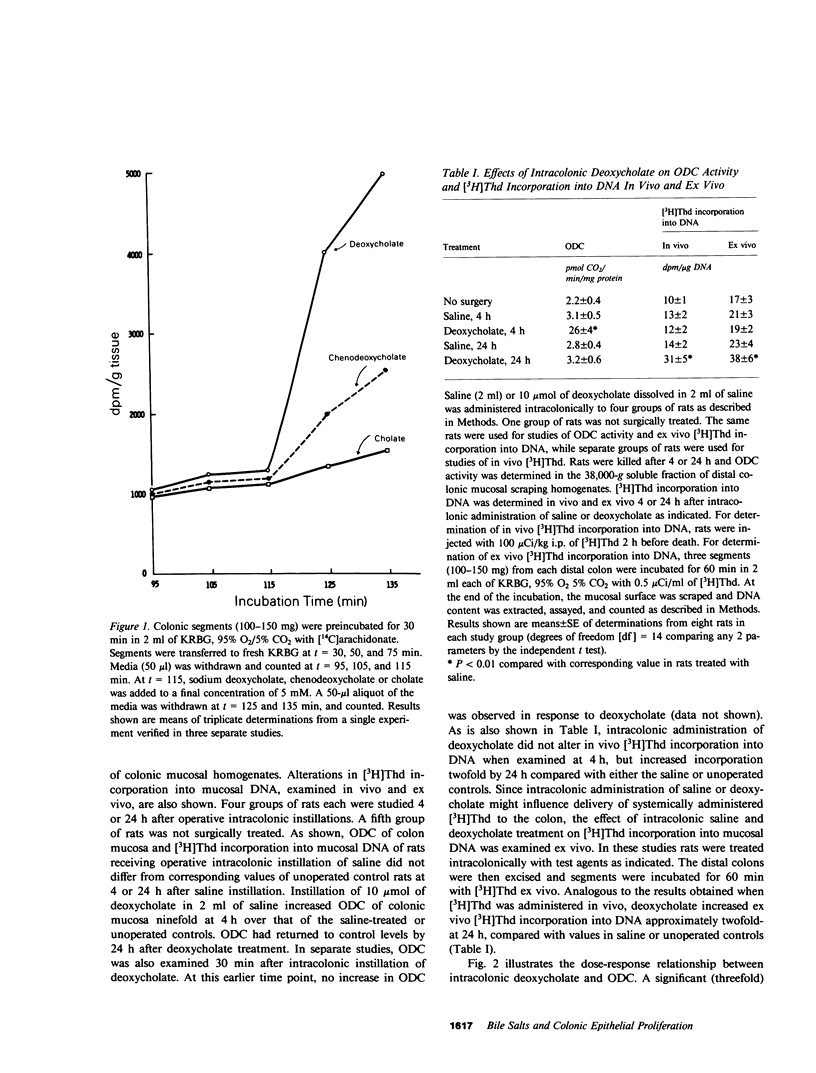
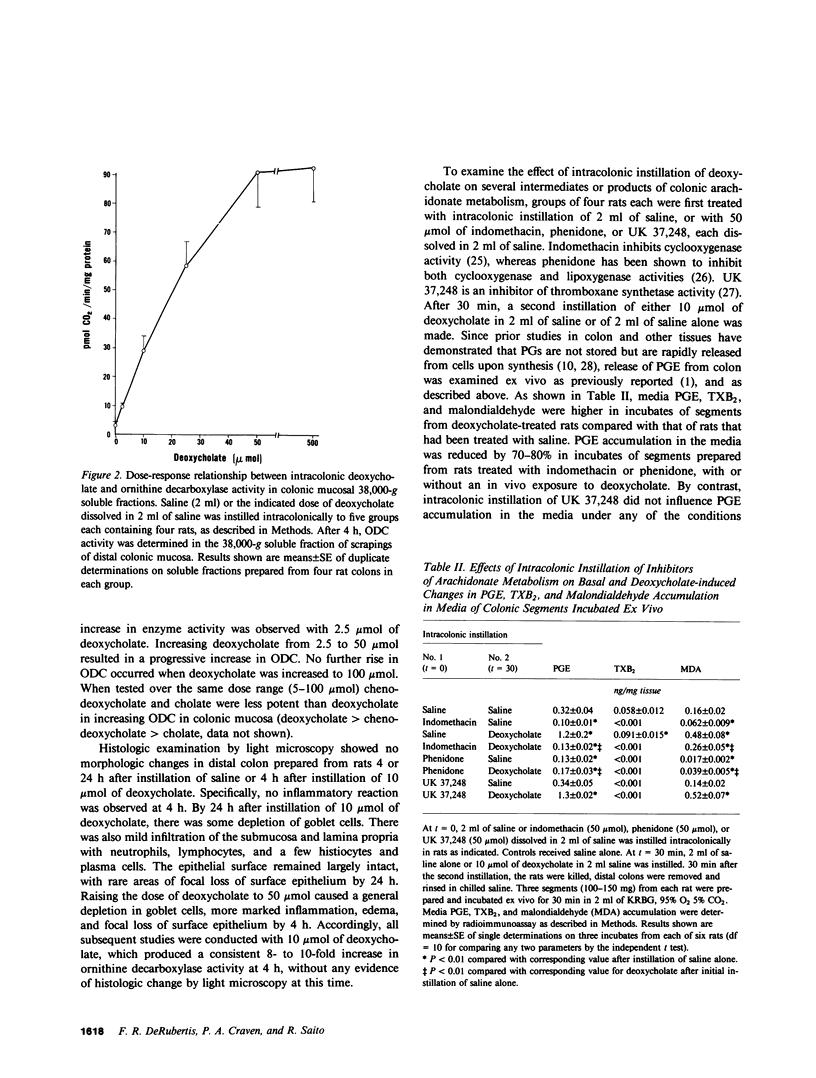


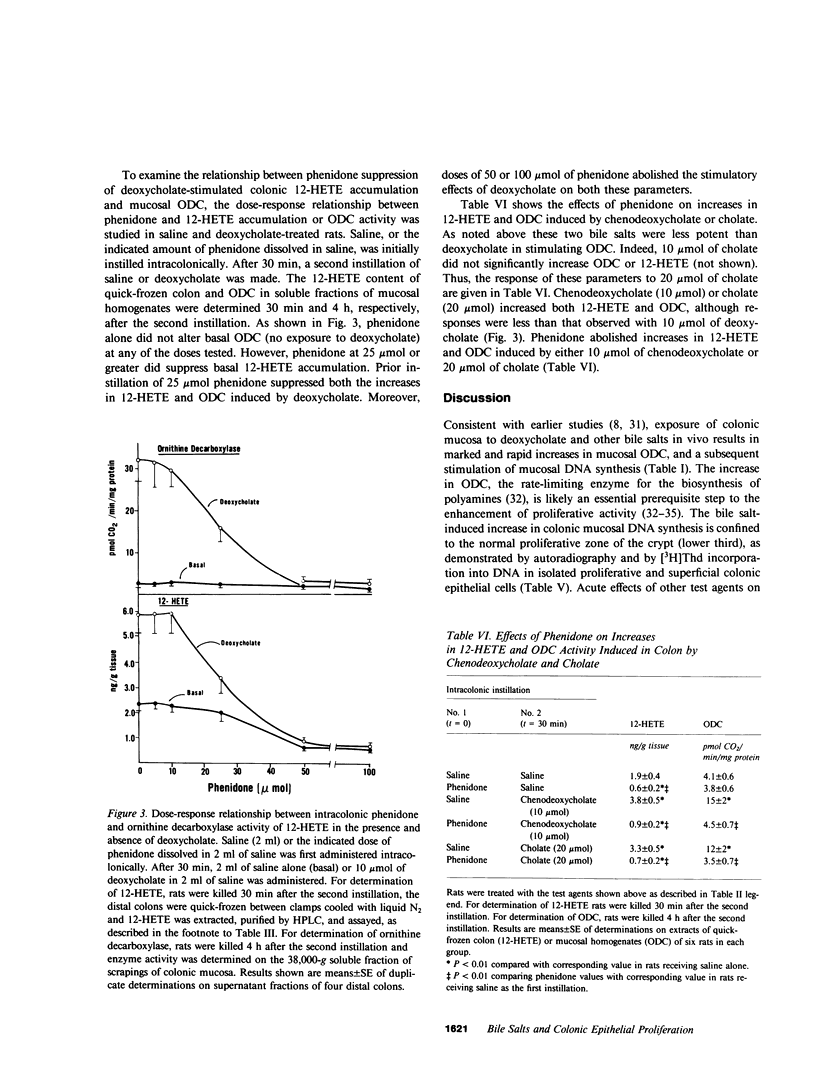



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers D. H., Philpott G. W. Control of deoxyribonucleic acid synthesis in normal rabbit colonic mucosa. Gastroenterology. 1975 Oct;69(4):951–959. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell G. J., Flower R. J. 1-phenyl-3-pyrazolidone: an inhibitor of cyclo-oxygenase and lipoxygenase pathways in lung and platelets. Prostaglandins. 1978 Sep;16(3):417–425. doi: 10.1016/0090-6980(78)90220-4. [DOI] [PubMed] [Google Scholar]
- Boeynaems J. M., Oates J. A., Hubbard W. C. Preparation and characterization of hydroperoxy-eicosatetraenoic acids (HPETEs). Prostaglandins. 1980 Jan;19(1):87–97. doi: 10.1016/0090-6980(80)90156-2. [DOI] [PubMed] [Google Scholar]
- Bull A. W., Marnett L. J., Dawe E. J., Nigro N. D. Stimulation of deoxythymidine incorporation in the colon of rats treated intrarectally with bile acids and fats. Carcinogenesis. 1983;4(2):207–210. doi: 10.1093/carcin/4.2.207. [DOI] [PubMed] [Google Scholar]
- Craven P. A., Briggs R., DeRubertis F. R. Calcium-dependent action of osmolality on adenosine 3',5'-monophosphate accumulation in rat renal inner medulla: evidence for a relationship to calcium-responsive arachidonate release and prostaglandin synthesis. J Clin Invest. 1980 Feb;65(2):529–542. doi: 10.1172/JCI109697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Cyclic nucleotide metabolism in rat colonic epithelial cells with different proliferative activities. Biochim Biophys Acta. 1981 Aug 17;676(2):155–169. doi: 10.1016/0304-4165(81)90183-5. [DOI] [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Patterns of prostaglandin synthesis and degradation in isolated superficial and proliferative colonic epithelial cells compared to residual colon. Prostaglandins. 1983 Oct;26(4):583–604. doi: 10.1016/0090-6980(83)90196-x. [DOI] [PubMed] [Google Scholar]
- Craven P. A., Saito R., DeRubertis F. R. Role of local prostaglandin synthesis in the modulation of proliferative activity of rat colonic epithelium. J Clin Invest. 1983 Oct;72(4):1365–1375. doi: 10.1172/JCI111093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRubertis F. R., Craven P. A. Early alterations in rat colonic mucosal cyclic nucleotide metabolism and protein kinase activity induced by 1,2-dimethylhydrazine. Cancer Res. 1980 Dec;40(12):4589–4598. [PubMed] [Google Scholar]
- Deschner E. E., Cohen B. I., Raicht R. F. Acute and chronic effect of dietary cholic acid on colonic epithelial cell proliferation. Digestion. 1981;21(6):290–296. doi: 10.1159/000198579. [DOI] [PubMed] [Google Scholar]
- Deschner E. E., Raicht R. F. Influence of bile on kinetic behavior of colonic epithelial cells of the rat. Digestion. 1979;19(5):322–327. doi: 10.1159/000198379. [DOI] [PubMed] [Google Scholar]
- Dix T. A., Marnett L. J. Metabolism of polycyclic aromatic hydrocarbon derivatives to ultimate carcinogens during lipid peroxidation. Science. 1983 Jul 1;221(4605):77–79. doi: 10.1126/science.6304879. [DOI] [PubMed] [Google Scholar]
- Emerit I., Cerutti P. A. Tumour promoter phorbol-12-myristate-13-acetate induces chromosomal damage via indirect action. Nature. 1981 Sep 10;293(5828):144–146. doi: 10.1038/293144a0. [DOI] [PubMed] [Google Scholar]
- Flower R. J. Drugs which inhibit prostaglandin biosynthesis. Pharmacol Rev. 1974 Mar;26(1):33–67. [PubMed] [Google Scholar]
- Harvey J., Parish H., Ho P. P., Boot J. R., Dawson W. The preferential inhibition of 5-lipoxygenase product formation by benoxaprofen. J Pharm Pharmacol. 1983 Jan;35(1):44–45. doi: 10.1111/j.2042-7158.1983.tb04262.x. [DOI] [PubMed] [Google Scholar]
- Heby O. Role of polyamines in the control of cell proliferation and differentiation. Differentiation. 1981;19(1):1–20. doi: 10.1111/j.1432-0436.1981.tb01123.x. [DOI] [PubMed] [Google Scholar]
- Hofmann S. L., Majerus P. W. Identification and properties of two distinct phosphatidylinositol-specific phospholipase C enzymes from sheep seminal vesicular glands. J Biol Chem. 1982 Jun 10;257(11):6461–6469. [PubMed] [Google Scholar]
- Jänne J., Pösö H., Raina A. Polyamines in rapid growth and cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):241–293. doi: 10.1016/0304-419x(78)90015-x. [DOI] [PubMed] [Google Scholar]
- Kingsnorth A. N., King W. W., Diekema K. A., McCann P. P., Ross J. S., Malt R. A. Inhibition of ornithine decarboxylase with 2-difluoromethylornithine: reduced incidence of dimethylhydrazine-induced colon tumors in mice. Cancer Res. 1983 Jun;43(6):2545–2549. [PubMed] [Google Scholar]
- Knapp H. R., Oelz O., Sweetman B. J., Oates J. A. Synthesis and metabolism of prostaglandins E2, F2alpha and D2 by the rat gastrointestinal tract. Stimulation by a hypertonic environment in vitro. Prostaglandins. 1978 May;15(5):751–757. doi: 10.1016/0090-6980(78)90141-7. [DOI] [PubMed] [Google Scholar]
- Koga S., Kaibara N., Takeda R. Effect of bile acids on 1,2-dimethylhydrazine-induced colon cancer in rats. Cancer. 1982 Aug 1;50(3):543–547. doi: 10.1002/1097-0142(19820801)50:3<543::aid-cncr2820500326>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marnett L. J., Bienkowski M. J., Pagels W. R. Oxygen 18 investigation of the prostaglandin synthetase-dependent co-oxidation of diphenylisobenzofuran. J Biol Chem. 1979 Jun 25;254(12):5077–5082. [PubMed] [Google Scholar]
- Marnett L. J., Siedlik P. H., Fung L. W. Oxidation of phenidone and BW755C by prostaglandin endoperoxide synthetase. J Biol Chem. 1982 Jun 25;257(12):6957–6964. [PubMed] [Google Scholar]
- Marx J. L. Do tumor promoters affect DNA after all? Science. 1983 Jan 14;219(4581):158–159. doi: 10.1126/science.6849126. [DOI] [PubMed] [Google Scholar]
- Matsushima M., Bryan G. T. Early induction of mouse urinary bladder ornithine decarboxylase activity by rodent vesical carcinogens. Cancer Res. 1980 Jun;40(6):1897–1901. [PubMed] [Google Scholar]
- Nakadate T., Yamamoto S., Ishii M., Kato R. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced epidermal ornithine decarboxylase activity by lipoxygenase inhibitors: possible role of product(s) of lipoxygenase pathway. Carcinogenesis. 1982;3(12):1411–1414. doi: 10.1093/carcin/3.12.1411. [DOI] [PubMed] [Google Scholar]
- Narisawa T., Magadia N. E., Weisburger J. H., Wynder E. L. Promoting effect of bile acids on colon carcinogenesis after intrarectal instillation of N-methyl-N'-nitro-N-nitrosoguanidine in rats. J Natl Cancer Inst. 1974 Oct;53(4):1093–1097. doi: 10.1093/jnci/53.4.1093. [DOI] [PubMed] [Google Scholar]
- O'Brien T. G. The induction of ornithine decarboxylase as an early, possibly obligatory, event in mouse skin carcinogenesis. Cancer Res. 1976 Jul;36(7 Pt 2):2644–2653. [PubMed] [Google Scholar]
- Porter N. A., Nixon J., Isaac R. Cyclic peroxides and the thiobarbituric assay. Biochim Biophys Acta. 1976 Sep 27;441(3):506–512. doi: 10.1016/0005-2760(76)90247-2. [DOI] [PubMed] [Google Scholar]
- Rampton D. S., Breuer N. F., Vaja S. G., Sladen G. E., Dowling R. H. Role of prostaglandins in bile salt-induced changes in rat colonic structure and function. Clin Sci (Lond) 1981 Nov;61(5):641–648. doi: 10.1042/cs0610641. [DOI] [PubMed] [Google Scholar]
- Reddy B. S., Watanabe K., Weisburger J. H., Wynder E. L. Promoting effect of bile acids in colon carcinogenesis in germ-free and conventional F344 rats. Cancer Res. 1977 Sep;37(9):3238–3242. [PubMed] [Google Scholar]
- Takano S., Matsushima M., Ertürk E., Bryan G. T. Early induction of rat colonic epithelial ornithine and S-adenosyl-L-methionine decarboxylase activities by N-methyl-N'-nitro-N-nitrosoguanidine or bile salts. Cancer Res. 1981 Feb;41(2):624–628. [PubMed] [Google Scholar]
- Tyler H. M., Saxton C. A., Parry M. J. Administration to man of UK-37,248-01, a selective inhibitor of thromboxane synthetase. Lancet. 1981 Mar 21;1(8221):629–632. doi: 10.1016/s0140-6736(81)91551-8. [DOI] [PubMed] [Google Scholar]
- Werner B., de Heer K., Mitschke H. Cholecystectomy and carcinoma of the colon. An experimental study. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1977;88(3):223–230. doi: 10.1007/BF00305360. [DOI] [PubMed] [Google Scholar]
- Wölbling R. H., Aehringhaus U., Peskar B. A., Morgenroth K., Peskar B. M. Release of slow-reacting substance of anaphylaxis and leukotriene C4-like immunoreactivity from guinea pig colonic tissue. Prostaglandins. 1983 Jun;25(6):809–822. doi: 10.1016/0090-6980(83)90005-9. [DOI] [PubMed] [Google Scholar]



