Abstract
Considerably diminished quality of life (QoL) is observed in patients with visual field defects after lesions affecting the visual pathway. But little is known to what extent vision-and health-related QoL impairments are associated with psychological distress. In 24 patients with chronic visual field defects (mean age=56.17±12.36) the National Eye Institute-visual functioning questionnaire (NEI-VFQ) for vision-related QoL, the Short Form Health Survey-36 (SF-36) for generic QoL and the revised Symptom-Checklist (SCL-90-R) were administered. Cases with clinically relevant SCL-90-R symptoms were defined. Demographic, QoL and visual field parameters were correlated with SCL-90-R scales. About 40% of the investigated patients met the criteria for the definition of psychiatric caseness. 8/12 NEI-VFQ scales correlated significantly with SCL-90-R phobic anxiety (r-range −0.41 to −0.64, P<0.05), 5/12 NEI-VFQ scales correlated with SCL-90-R interpersonal sensitivity (−0.43 to −0.50), and 3/12 with SCL-90-R depression (−0.51 to −0.57) and obsessive-compulsiveness (−0.41 to −0.43). In contrast, only 1/8 SF-36 scales correlated significantly with SCL-90-R depression, phobic anxiety and interpersonal sensitivity (−0.41 to −0.54). No substantial correlations were observed between visual field parameters and SCL-90-R scales. Significant correlations of SCL-90-R with NEI-VFQ but not with SF-36 suggest that self-rated psychological distress is the result of diminished vision-related QoL as a consequence of visual field loss. The extent of visual field loss itself did not influence the rating of psychological distress directly, since SCL-90-R symptoms were only reported when diminished vision-related QoL was present. Patients with reduced vision-related QoL due to persisting visual field defects should therefore be offered additional neuropsychological rehabilitation and supportive psychotherapeutic interventions even years after the lesion.
Key words: neuropsychological testing, phobic anxiety, psychological distress, Symptom-Checklist-90-revised, visual field defect, cerebral damage.
Introduction
Visual field defects after lesions to the visual pathway lead to impairments in activities of daily life such as reading, driving, or overall orientation and may therefore have severe impact on the patients' well-being and quality of life (QoL).1 Objective functional impairments of vision which may cause diminished vision-related QoL are measurable with the National Eye Institute visual functioning questionnaire (NEI-VFQ).2,3 Patients with visual field loss often show psychological distress which may be related to the vision impairment, but this is not sufficiently captured by the mental health subscale of NEI-VFQ. Since there is no questionnaire available that specifically targets psychological distress in the visually impaired, in the present study the Symptom-Checklist-90-revised (SCL-90-R) was applied to adequately measure mental distress.4
In studies which focus on the impact of vision impairment on subjective vision-related QoL, the NEI-VFQ is often used as a valid and reliable instrument.2 This questionnaire was originally designed for the assessment in ophthalmologic diseases.3 More recently, some studies focused on the assessment of vision-related QoL in patients with visual field loss after cerebral damage.5–7 Here, it was demonstrated that diminished vision-related QoL was significantly related to the extent of the visual field loss.7 However, no study to date assessed the subjective perception of psychological symptoms by a dedicated symptom check list, such as the SCL-90-R, to assess self-rated distress in patients with visual field loss. We hypothesized an association of SCL-90-R results with visual field loss as well as with vision-related QoL but not with general health-related QoL.
Many studies measured self-estimated disabilities after stroke using generic questionnaires of general health-related QoL.8–10 However, none of these studies considered visual field defects as a comorbidity with possible influence on the patients' QoL and psychological distress, although it is known that visual field loss occurs in as many as 10% of stroke patients.11
Additionally, a number of studies have shown that the incidence of neuropsychiatric conditions is high in post-stroke patients. Research focuses on poststroke depression,12–14 apathy and anxiety.13,15–17 Post-stroke depression is associated with greater cognitive impairment,18 increased mortality,19 and poorer recovery of activities of daily living skills.20 Again, none of these studies considered the possible effect of comorbid visual field defects.
Woessner and Caplan argued that since items of the SCL-90-R also cover general consequences of stroke, answers of neurological patients have to be interpreted in the context of the underlying disease.21 Thus, 14 items of the SCL-90-R, mainly items of the obsessive-compulsive, hostility and depression scale, reflect common neurological symptoms following traumatic brain injuries that impede clinical interpretation.22
Nevertheless, the SCL-90-R is a useful instrument in cerebrally damaged patients. Kaplan and Miner used SCL-90-R to test 17 patients with brain tumors and concluded that the SCL-90-R is an appropriate indicator of emotional distress and somatic effects of structural brain injury.23 Linn, Allen and Willer tested 60 patients and their partners with the scales anxiety and depression six years after traumatic brain injury:24 70% suffered from depression and 50% showed anxiety symptoms while also their partners demonstrated significant affective symptoms (73% depression, 55% anxiety). Baker et al. also tested patients after traumatic brain injuries with SCL-90-R and stated that this patient group can be analysed with this self-assessment inventory.25 McCleary et al. tested 105 patients six months after traumatic brain injury and found that 42% reported depressive symptoms.26 Finally, Baune et al. reported in a large population-based study that comorbid affective disorders in medical conditions severely impact health-related QoL.27
In visually impaired patients with lesions different from stroke, neuropsychiatric conditions were observed, too. For instance, in patients with diabetic retinopathy levels of depression and anxiety were clinically significant. 28,29 Moreover, declining vision in diabetes was found to be associated with substantially reduced QoL.28 Symptom severity and emotional distress due to vision loss were related with self-reported disability in persons with low vision.30 Psychological distress and depression were also common in patients with refractive error,31 myopia,32,33 and amblyopia.34 Biofeedback-visual training in myopic patients was found to improve the psychological mental state and subjective vision abilities, but not visual acuity as determined by objective computer tests.32,33 Reimer et al. reported clinically relevant distress measured with the SCL-90-R in every second patient before plaque radio-therapy.35 Vision- and health-related QoL were impaired in these patients and further reduced after radiotherapy. Therefore, QoL data should be regularly assessed in neuroophthalmic patients to provide psychosocial treatments for patients at risk for low QoL and high distress.35
Self-reported psychological distress was reported to be more appropriate to predict subjective QoL than clinician-rated symptom severity.36 In the present study we therefore assessed subjective psychopathological symptoms with the SCL-90-R in cerebrally damaged patients with visual field defects. Additionally, vision- and health-related QoL was measured to allow for correlation analyses of QoL data with self-rated psychological distress measured by the SCL-90-R.
Materials and Methods
All questionnaires were self-administered. Data was collected as part of a cross-sectional study which included 24 brain-damaged patients with visual field loss due to several reasons (Table 1). Patients were investigated at a late follow-up of a neuropsychological visual field training and had documented visual field loss after cerebral damage indicated by standard perimetry and campimetry. Verbal intelligence was measured with a German verbal intelligence test. Best corrected near and distance visual acuity was measured monocularly using Landolt-ring test charts.
Table 1. Description of patient sample.
| Age [years (M ± SD)] | 56.17 (±12.36) |
| Sex | |
| Male | 17 (70.8%) |
| Female | 7 (29.2%) |
| Lesion age [months (M ± SD)] | 27.58 (±36.38) |
| Etiology | |
| Hemorrhagic infarction | 11 |
| Ischemic infarction | 6 |
| Optic atrophy after craniocerebral injuries | 4 |
| Tumor | 2 |
| Traumatic encephalitis | 1 |
| Type of visual field defect | |
| Complete hemianopia | 12 |
| Incomplete hemianopia | 2 |
| Quadrantanopia | 6 |
| Scotoma | 1 |
| Diffuse loss | 3 |
| Comorbidities | |
| Cardiovascular conditions | 6 |
| Cancer | 3 |
| Allergy | 2 |
| Rheumatism | 1 |
| Visual acuity | |
| Near visual acuity | 0.54 (±0.31) |
| Distance visual acuity | 0.67 (±0.32) |
| Verbal IQ (M ± SD) | 118 (±15) |
M, mean age; SD, standard deviation.
The mean time from lesion onset to data acquisition was 2.0±2.7 years (mean age ± standard deviation). In 13 cases the lesion was older than twelve months. In the whole sample the visual field defect was assessed with a computer campimetric method and standard automated perimetry with the Rodenstock Perimat 206.37 Figure 1 shows the distribution of detected stimuli and the proportions of relative and absolute defects in perimetric measurements for both eyes. All patients were treated according to the ethical standards of the Declaration of Helsinki (1964).
Figure 1.
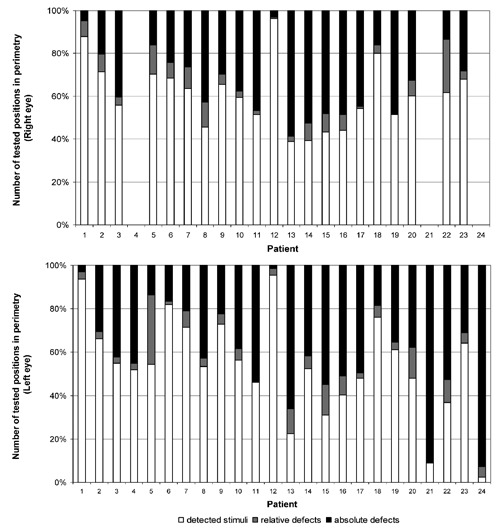
Distribution of detected stimuli and proportions of relative and absolute defects in perimetric measurements for the right and left eye of 24 patients with visual field loss. Due to full blindness, perimetric measurements were not carried out with the right eye in patients 4, 21 and 24.
The validated German 39-item version of the NEI-VFQ was administered for assessment of vision-related QoL2 and the German Health Survey Short Form-36 (SF-36) for the collection of data concerning general health-related QoL based on the experiences during the last four weeks.38 The German SCL-90-R revised version was assessed for measuring the subjective perception of psychological symptoms assessing the degree of self-rated distress over a time period of four weeks.4 Cases with clinically relevant psychological symptoms were defined according to T-criteria.4 Demographic, QoL (NEI-VFQ and SF-36) and visual field parameters were correlated with SCL-90-R scales using the Spearman coefficient. NEI-VFQ and SF-36 results were compared by univariate analysis of variance between patients meeting the SCL-90-R case criteria and those who did not. Statistical analyses were carried out with SPSS 15.0.
Results
Vision- and health-related quality of life results of patients with visual field loss: National Eye Institute-Visual Functioning Questionnaire and German Health Survey Short Form-36
The mean values of the investigated patients were 20 points or more below those of a reference group in the following NEI-VFQ subscales:39 general health (M=48.33, SD=20,27), general vision (M=54.79, SD= 17.59), near vision (M=60.24, SD=23.57), distance vision (M=68.23, SD=24.66), social functioning (M=66.67, SD=26.47), mental health (M=64.17, SD=24.35), role difficulties (M=57.42, SD=21.86), dependency (M=69.53, SD=32.52), driving (M=44.05, SD=33.56) and peripheral vision (M=54.17, SD=30.10). A difference of more than 10 points worse than the reference group was detected for the subscale color vision (M=83.33, SD=27.25) and no difference was observed for ocular pain (M=90.10, SD=12.76).
Compared to reference data presented in the manual of the German SF-36,38 patients showed comparable SF-36 mean values for 6/8 subscales with no difference to the control persons in the scales: physical functioning (M=73.33, SD=20.94), role limitations (physical) (M=67.71, SD=38.64), general health perceptions (M=55.83, SD=22.83), vitality (M=55.00, SD=14.07), role limitations (emotional) (M=86.11, SD=27.66), and emotional well-being (M=63.33, SD=6.09). More than 50 points below the reference group were found for the subscale bodily pain (M=13.33, SD=20.57) and more than 30 points for social functioning (M=47.92, SD=9.52).
Symptom-Checklist-90-revised case definitions in patients with visual field loss
Patients were categorized as case if the T-values of the global severity index (GSI) or of at least two SCL-90-R subscales were ≥63 (see Hessel et al. for reference population results).40 Ten patients (41.7%) were categorized as cases. Mean SCL-90-R results of the investigated sample are shown in Table 2. In the GSI eight patients reached results of T≥60. Four patients showed T-values ≥60 for the positive symptom distress index and T-values ≥60 for the positive symptom total were observed in eight patients.
Table 2. Mean T-values and case definitions of Symptom-Checklist-90-revised subscales.
| SCL-90-R subscales | T(M ± SD) | Cases with T ≥ 60 |
|---|---|---|
| Somatization | 50.92±8.79 | 4 |
| Obsessive-compulsiveness | 56.46±11.49 | 12 |
| Interpersonal sensitivity | 55.21±11.74 | 10 |
| Depression | 55.08±12.51 | 8 |
| Anxiety | 53.63±9.44 | 5 |
| Anger-hostility | 52.71±12.11 | 7 |
| Phobic anxiety | 59.83±9.41 | 13 |
| Paranoid ideation | 51.88±11.28 | 5 |
| Psychoticism | 53.33±9.58 | 6 |
| GSI | 55.13±11.47 | 8 |
| PSDI | 52.71±8.99 | 4 |
| PST | 55.04±11.13 | 8 |
SCL-90-R, Symptom-Checklist-90-revised; M, mean; SD, standard deviation; GSI, global severity index; PSDI, positive symptom distress index; PST, total number of positive symptoms.
Group comparison of quality of life questionnaire results according to Symptom-Checklist-90-revised case definitions
Figure 2 shows NEI-VFQ mean values of patients according to the categorization in cases and no cases and F-values for group comparisons. Apparently cases had descriptively lower NEI-VFQ results in all subscales. However, only mental health and role difficulties scores significantly differed between groups. Figure 3 shows SF-36 mean values of patients according to the categorization in cases and no cases and F-values for group comparisons. Except for subscales bodily pain, vitality, social functioning and emotional well-being, cases showed lower scores. However, only physical functioning and role limitations (emotional) showed significant group differences (Figure 3).
Figure 2.
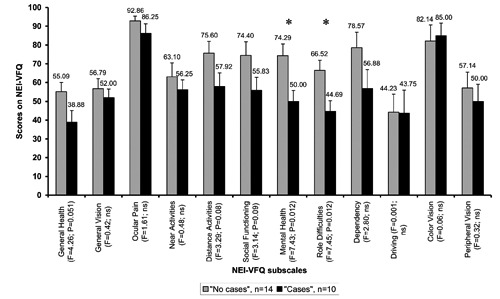
Mean National Eye Institute-visual functioning questionnaire values of patients with visual field loss categorized as cases and no cases. Patients were categorized as case if the T value of the global severity index was ≥63 or if at least two Symptom-Checklist-90-revised subscales showed values ≥63. F-values correspond to between group comparisons.
Figure 3.
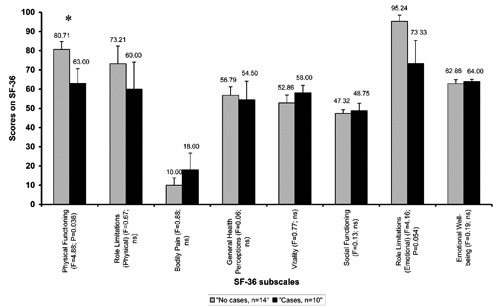
Mean Short Form Health Survey-36 values of patients with visual field loss categorized as cases and no cases. Patients were categorized as case if the T value of the global severity index was ≥63 or if at least two Symptom-Checklist-90-revised subscales showed values ≥63. F-values correspond to between group comparisons.
Correlation analyses between vision impairment, visual field and demographic variables with National Eye Institute-visual functioning questionnaire and Symptom-Checklist-90-revised
Visual acuity
Distance and near visual acuity (always best corrected) significantly correlated with some of the NEI-VFQ subscales. Distance visual acuity showed correlations with 6/12 NEI-VFQ-subscales (r-range from 0.44 to 0.66, all P<0.05) and with the composite score (r=0.50, P<0.05). Near visual acuity correlated significantly with NEI-VFQ scale near activities (r=0.44, P<0.05), and SCL-90-R somatization (r=0.45, P<0.05). Correlations are shown in detail in Table 3.
Table 3. Spearman correlations between variables of visual impairment and demography, with National Eye Institute-visual functioning questionnaire and Symptom-Checklist-90-revised subscales.
| Visual acuity (best corrected) | Number of detected stimuli in computer campimetry (%) | Absolute defects in perimetry (%) | Detected stimuli in perimetry (%) | Verbal intelligence | Age | ||
|---|---|---|---|---|---|---|---|
| Distance | Near | ||||||
| National Eye Institute-visual functioning questionnaire (NEI-VFQ) | |||||||
| Composite score | 0.50* | 0.21 | 0.66*** | −0.76*** | 0.67*** | 0.36 | −0.06 |
| 1. General health | −0.13 | −0.30 | 0.18 | −0.19 | 0.05 | 0.30 | −0.22 |
| 2. General vision | 0.58** | 0.27 | 0.43* | −0.58** | 0.58** | 0.16 | 0.15 |
| 3. Ocular pain | −0-30 | −0.40 | 0.09 | 0.04 | 0.04 | −0.13 | −0.35 |
| 4. Near activities | 0.69*** | 0.44* | 0.48* | −0.65** | 0.68*** | 0.29 | −0.06 |
| 5. Distance activities | 0.61** | 0.35 | 0.55** | −0.71*** | 0.66*** | 0.40 | 0.09 |
| 6. Social functioning | 0.50* | 0.23 | 0.56** | −0.79*** | 0.66*** | 0.42* | −0.04 |
| 7. Mental health | 0.31 | 0.20 | 0.68*** | −0.61** | 0.51* | 0.46* | 0.02 |
| 8. Role difficulties | 0.45* | 0.28 | 0.65** | −0.73*** | 0.65*** | 0.39 | −0.04 |
| 9. Dependency | 0.38 | 0.24 | 0.58** | −0.59** | 0.45* | 0.42* | −0.03 |
| 10. Driving | 0.24 | −0.05 | 0.45* | −0.72*** | 0.65** | 0.01 | 0.03 |
| 11. Colour vision | 0.24 | 0.11 | 0.26 | −0.49* | 0.44* | 0.21 | −0.10 |
| 12. Peripheral vision | 0.44* | 0.15 | 0.61** | −0.65** | 0.61** | 0.35 | 0.08 |
| Symptom-Checklist-90-revised (SCL-90-R) | |||||||
| 1. Somatization | 0.32 | 0.45* | −0.16 | 0.03 | −0.04 | −0.47* | −0.05 |
| 2. Obsessive-compulsiveness | 0.26 | 0.33 | −0.41* | 0.33 | −0.29 | −0.28 | −0.25 |
| 3. Interpers. sensitivity | 0.07 | 0.15 | −0.28 | 0.35 | −0.23 | −0.42* | −0.05 |
| 4. Depression | 0.01 | −0.06 | −0.36 | 0.34 | −0.26 | −0.37 | −0.01 |
| 5. Anxiety | 0.24 | 0.29 | −0.05 | −0.01 | 0.07 | −0.01 | −0.14 |
| 6. Anger-hostility | 0.04 | −0.003 | −0.12 | 0.19 | −0.16 | −0.18 | 0.04 |
| 7. Phobic anxiety | −0.14 | −0.21 | −0.32 | 0.36 | −0.27 | −0.46* | 0.01 |
| 8. Paranoid ideation | 0.28 | 0.24 | −0.33 | 0.10 | −0.01 | −0.15 | −0.22 |
| 9. Psychoticism | 0.01 | 0.04 | −0.08 | 0.27 | −0.17 | −0.10 | 0.02 |
| 10. Global Severity Index (GSI) | 0.14 | 0.11 | −0.28 | 0.24 | −0.17 | −0.37 | −0.10 |
P <0.05;
P< 0.01;
P<0.001.
Visual field
The intact visual field (measured as the number of correctly detected stimuli in % in computer campimetry) showed significant correlations with 9/12 NEI-VFQ subscales (r-range from 0.43 to 0.68, all P<0.05) and with the composite score (r=0.66, P<0.001). The absolute defect of the visual field, i.e. the number of missed stimuli in the perimetric visual field test in %, correlated with 10/12 NEI-VFQ subscales (r-range from −0.49 to −0.79, P<0.05) and with the composite score (r=−0.76, P<0.001). As expected, the number of detected stimuli in perimetry (in %) also showed significant correlations with 10/12 NEI-VFQ-subscales (r-range from 0.44 to 0.68, P<0.05) and with the composite score (r=0.67, P<0.001).
A significant correlation with visual field variables was observed only for the SCL-90-R subscale obsessive-compulsiveness (r=−0.41, P<0.05) (Table 3).
Verbal intelligence and age
Verbal intelligence correlated significantly with NEI-VFQ subscales social functioning, mental health and dependency (r-range 0.42 to 0.46, P<0.05) and with SCL-90-R subscales somatization, interpersonal sensitivity and phobic anxiety (r-range −0.42 to −0.47, P<0.05). The patients' age was not significantly correlated related with NEI-VFQ or SCL-90-R subscales (Table 3).
Correlation analyses between vision- and health-related quality of life data and Symptom-Checklist-90-revised
There were a number of moderate correlations between vision-related QoL data as measured with the NEI-VFQ and SCL-90-R subscales (Table 4). Also the GSI was related to general health (r=−0.42) and mental health (r=−0.44).
Table 4. Spearman correlations of subjective vision-related (National Eye Institute-visual functioning questionnaire) and health-related quality of life (Short Form Health Survey-36) with Symptom-Checklist-90-revised results.
| Somatization | Obsessive-compul- siveness | Interpers. sensitivity | Depression | Anxiety | Anger-Hostility | Phobic anxiety | Paranoid ideation | Psychoticism | Global severity Index | |
|---|---|---|---|---|---|---|---|---|---|---|
| National Eye Institute-visual functioning questionnaire (NEI-VFQ) | ||||||||||
| Composite score | 0.01 | −0.25 | −0.34 | −0.40 | 0.10 | −0.26 | −0.51* | −0.09 | −0.18 | −0.25 |
| 1. General health | −0.37 | −0.43* | −0.47 * | −0.37 | −0.17 | −0.28 | −0.41* | −0.23 | −0.39 | −0.42* |
| 2. General vision | 0.20 | −0.09 | −0.17 | −0.12 | 0.16 | −0.14 | −0.30 | −0.02 | −0.05 | −0.02 |
| 3. Ocular pain | −0.34 | −0.29 | −0.18 | −0.17 | −0.59** | −0.21 | 0.02 | −0.26 | −0.40 | −0.31 |
| 4. Near activities | 0.07 | −0.09 | −0.08 | −0.21 | 0.23 | −0.15 | −0.46* | 0.12 | −0.00 | −0.12 |
| 5. Distance activities | 0.07 | −0.10 | −0.28 | −0.30 | 0.20 | −0.27 | −0.48* | −0.06 | −0.08 | −0.19 |
| 6. Social functioning | 0.01 | −0.16 | −0.43* | −0.38 | 0.06 | −0.34 | −0.55** | −0.17 | −0.27 | −0.25 |
| 7. Mental health | −0.19 | −0.41* | −0.43* | −0.57** | 0.03 | −0.34 | −0.64*** | −0.21 | −0.21 | −0.44* |
| 8. Role difficulties | −0.07 | −0.4* | −0.43* | −0.51* | 0.07 | −0.33 | −0.64*** | −0.11 | −0.24 | −0.38 |
| 9. Dependency | −0.11 | −0.26 | −0.50* | −0.56** | 0.01 | −0.41* | −0.62*** | −0.26 | −0.25 | −0.39 |
| 10. Driving | 0.35 | −0.14 | 0.02 | 0.06 | 0.19 | 0.17 | 0.05 | 0.15 | 0.02 | 0.18 |
| 11. Colour vision | −0.06 | −0.04 | −0.13 | −0.19 | 0.09 | −0.04 | −0.22 | 0.13 | −0.10 | 0.02 |
| 12. Peripheral vision | 0.14 | −0.03 | −0.13 | −0.17 | 0.22 | −0.08 | −0.15 | −0.02 | 0.06 | −0.02 |
| Short Form Health Survey-36 (SF-36) | ||||||||||
| 1. Physical functioning | −0.07 | −0.10 | −0.25 | −0.24 | 0.06 | −0.15 | −0.49* | 0.11 | −0.24 | −0.23 |
| 2. Role limitations due to physical problems | −0.06 | −0.15 | −0.11 | −0.24 | 0.19 | −0.00 | −0.18 | 0.27 | −0.06 | −0.05 |
| 3. Bodily pain | 0.15 | −0.16 | 0.04 | 0.03 | −0.08 | −0.00 | 0.22 | −0.02 | −0.12 | 0.03 |
| 4. General health perceptions | −0.06 | 0.07 | 0.02 | 0.12 | −0.22 | 0.01 | 0.27 | −0.01 | −0.14 | 0.05 |
| 5. Vitality | 0.28 | 0.13 | 0.18 | 0.30 | −0.05 | 0.20 | 0.20 | 0.26 | 0.13 | 0.32 |
| 6. Social functioning | 0.02 | 0.06 | 0.04 | −0.03 | −0.02 | −0.15 | −0.23 | −0.04 | 0.09 | −0.11 |
| 7. Role limitations due to emotional problems | −0.38 | −0.32 | −0.41* | −0.54** | −0.18 | −0.35 | −0.22 | −0.39 | −0.26 | −0.42* |
| 8. Emotional well-being | −0.18 | −0.30 | 0.03 | 0.00 | −0.32 | 0.1 | −0.05 | 0.17 | −0.13 | −0.08 |
| Physical component score | 0.23 | −0.01 | 0.06 | 0.00 | 0.29 | 0.13 | 0.00 | 0.37 | −0.02 | 0.12 |
| Mental component score | −0.26 | −0.11 | −0.11 | −0.16 | −0.38 | −0.12 | 0.03 | −0.23 | −0.03 | −0.17 |
P<0.05;
P<0.01;
P<0.001.
Correlations with SF-36 results for health-related QoL were low and in most cases not significant. Only the SF-36 subscale role limitations due to emotional problems showed significant correlations with SCL-90-R interpersonal sensitivity (r=−0.41), depression (r=−0.54; P<0.01), and the total score (r=−0.42). SF-36 physical functioning correlated with SCL-90-R phobic anxiety (r=−0.49).
Discussion
In the present study psychological distress was self-evaluated with the SCL-90-R in patients with visual field defects after cerebral damage. Since psychological distress seems to be a better predictor of reduced QoL than symptom severity,36 the relation between vision- and health-related
QoL measured with the NEI-VFQ and SF-36 with SCL-90-R subscales was investigated.
The observed relations between visual acuity, visual field parameters, and NEI-VFQ confirm that the NEI-VFQ is an appropriate instrument to assess vision-related QoL after visual field loss. The results support previous studies demonstrating that the extent of visual field loss is related to the extent of reduced vision-but not health-related QoL.5–7
We observed negative correlations between vision-related QoL and self-rated psychological distress as measured with the SCL-90-R. Thus, patients with higher vision-related QoL were less affected by psychopathological symptoms than patients with lower vision-related QoL. However, this relation was present for only some vision-related QoL dimensions. Thus, SCL-90-R phobic anxiety, interpersonal sensitivity, depression, and obsessive-compulsiveness were related to vision-related QoL.
Except for the SCL-90-R subscale somatisation that showed an unexpected positive correlation with near visual acuity, no relations between psychopathological distress and vision impairment parameters were observed. Thus, whereas vision-related QoL was associated with SCL-90-R results, the objective results of assessment of visual functioning were not. Although patients with visual field defects clearly reported diminished vision-related QoL, the extent of the visual field loss itself did not cause psychological distress in a direct way but mediated by vision-related QoL. This conclusion is supported by comparisons of patients that met case definition criteria of the SCL-90-R who showed consistently lower vision-related QoL than patients without clinically relevant psychological symptoms. With only a few exceptions reduced health-related QoL measured by the SF-36 was not related with SCL-90-R subscales. Also, SF-36 results did not correlate with vision parameters.
Verbal intelligence was positively correlated with NEI-VFQ social functioning, mental health and dependency, but negatively with SCL-90-R somatization, interpersonal sensitivity and phobic anxiety. This implies that persons with higher verbal abilities demonstrate higher NEI-VFQ scores, foremost in subscales that imply mental well-being and interaction with others. Moreover, patients with a higher verbal intelligence complained less frequently about somatization or anxieties. Thus, SCL-90-R results also seem to depend on intellectual abilities as reported earlier.41 Further studies should focus on replicating this finding in larger samples.
Conclusions
We finally conclude that brain-damaged patients with visual field loss resulting in reduced vision-related QoL are at greater risk to suffer from psychological distress or to develop psychopathological symptoms. Psychological distress was independent of the extent of visual field loss but related to diminished vision-related QoL. Because of the risk of suffering psychological distress, patients with persisting visual field defects should be offered supportive psychotherapeutic interventions in addition to neuropsychological rehabilitation even many years after the lesion.
References
- 1.Kerkhoff G. Restorative and compensatory therapy approaches in cerebral blindness - a review. Restor Neurol Neurosci. 1999;15:255–71. [PubMed] [Google Scholar]
- 2.Franke GH, Esser J, Voigtländer A, Mähner N. Der National Eye Institute Visual Function Questionnaire (NEI-VFQ) - Erste Ergebnisse zur psychometrischen Über-prüfung eines Verfahrens zur Erfassung der Lebensqualität bei Sehbeeinträ-chtigten. Zeitschrift für Medizinische Psychologie. 1998;7:178–84. [Google Scholar]
- 3.Mangione CM, Lee PP, Gutierrez PR, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050–8. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 4.Franke GH. Symptom-Checkliste von L.R. Derogatis - Deutsche Version (SCL-90-R) Göttingen: Beltz Test; 2002. [Google Scholar]
- 5.Papageorgiou E, Hardiess G, Schaeffel F, et al. Assessment of vision-related quality of life in patients with homonymous visual field defects. Graefes Arch Clin Exp Ophthalmol. 2007;245:1749–58. doi: 10.1007/s00417-007-0644-z. [DOI] [PubMed] [Google Scholar]
- 6.Gall C, Lucklum J, Sabel BA, Franke GH. Vision- and health- related quality of life in subjects with visual field loss after postchiasmatic lesions. Invest Ophthalmol Vis Sci. 2009;50:2765–7. doi: 10.1167/iovs.08-2519. [DOI] [PubMed] [Google Scholar]
- 7.Gall C, Franke GH, Sabel BA. Vision-related quality of life in first stroke patients with homonymous visual field defects. Health Qual Life Outcomes. 2010;8:33. doi: 10.1186/1477-7525-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ronning OM, Stavem K. Determinants of change in quality of life from 1 to 6 months following acute stroke. Cerebrovasc Dis. 2008;25:67–73. doi: 10.1159/000111524. [DOI] [PubMed] [Google Scholar]
- 9.Madden S, Hopman WM, Bagg S, et al. Functional status and health-related quality of life during inpatient stroke rehabilitation. Am J Phys Med Rehabil. 2006;85:831–41. doi: 10.1097/01.phm.0000240666.24142.f7. [DOI] [PubMed] [Google Scholar]
- 10.Naess H, Waje-Andreassen U, Thomassen L, et al. Health-related quality of life among young adults with ischemic stroke on long-term follow-up. Stroke. 2006;37:1232–6. doi: 10.1161/01.STR.0000217652.42273.02. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Kedar S, Lynn MJ, et al. Natural history of homonymous hemianopia. Neurology. 2006;66:901–5. doi: 10.1212/01.wnl.0000203338.54323.22. [DOI] [PubMed] [Google Scholar]
- 12.Hackett ML, Anderson CS. Predictors of depression after stroke: a systematic review of observational studies. Stroke. 2005;36:2296–301. doi: 10.1161/01.STR.0000183622.75135.a4. [DOI] [PubMed] [Google Scholar]
- 13.Starkstein SE, Manes F. Apathy and depression following stroke. CNS Spectr. 2000;5:43–50. doi: 10.1017/s1092852900012955. [DOI] [PubMed] [Google Scholar]
- 14.Bour A, Rasquin S, Limburg M, Verhey F. Depressive symptoms and executive functioning in stroke patients: a follow-up study. Int J Geriatr Psychiatry. 2011;26:679– 86. doi: 10.1002/gps.2581. [DOI] [PubMed] [Google Scholar]
- 15.Chemerinski E, Robinson RG. The neuropsychiatry of stroke. Psychosomatics. 2000;41:5–14. doi: 10.1016/S0033-3182(00)71168-6. [DOI] [PubMed] [Google Scholar]
- 16.Aström M. Generalized anxiety disorder in stroke patients. A 3-year longitudinal study. Stroke. 1996;27:270–5. doi: 10.1161/01.str.27.2.270. [DOI] [PubMed] [Google Scholar]
- 17.Shimoda K, Robinson RG. Effects of anxiety disorder on impairment and recovery from stroke. J Neuropsychiatry Clin Neurosci. 1998;10:34–40. doi: 10.1176/jnp.10.1.34. [DOI] [PubMed] [Google Scholar]
- 18.Robinson RG, Starr LB, Kubos KL, Price TR. A two-year longitudinal study of post-stroke mood disorders: findings during the initial evaluation. Stroke. 1983;14:736–41. doi: 10.1161/01.str.14.5.736. [DOI] [PubMed] [Google Scholar]
- 19.Gump BB, Matthews KA, Eberly LE, et al. Depressive symptoms and mortality in men: results from the multiple risk factor intervention trial. Stroke. 2005;36:98–102. doi: 10.1161/01.STR.0000149626.50127.d0. [DOI] [PubMed] [Google Scholar]
- 20.Chemerinski E, Robinson RG, Arndt S, Kosier JT. The effect of remission of post-stroke depression on activities of daily living in a double-blind randomized treatment study. J Nerv Ment Dis. 2001;189:421– 5. doi: 10.1097/00005053-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Woessner R, Caplan B. Emotional distress following stroke: Interpretive limitations of the SCL-90-R. Assessment. 1996;3:191–305. [Google Scholar]
- 22.Woessner R, Caplan B. Affective-disorders following mild-to-moderate brain injury. Interpretive hazards of the SCL-90-R. J Head Trauma Rehabil. 1995;10:78–89. [Google Scholar]
- 23.Kaplan CP, Miner ME. Does the SCL 90-R obsessive-compulsive dimension identify cognitive impairments? J Head Trauma Rehabil. 1998;13:94–101. doi: 10.1097/00001199-199806000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Linn RT, Allen K, Willer BS. Affective symptoms in the chronic stage of traumatic brain injury: a study of married couples. Brain Inj. 1994;8:135–47. doi: 10.3109/02699059409150965. [DOI] [PubMed] [Google Scholar]
- 25.Baker KA, Schmidt MF, Heinemann AW, et al. The validity of the Katz Adjustment Scale among people with traumatic brain injury. Rehab Psychol. 1998;43:30–40. [Google Scholar]
- 26.McCleary C, Satz P, Forney D, et al. Depression after traumatic brain injury as a function of Glasgow Outcome Score. J Clin Exp Neuropsychol. 1998;20:270–9. doi: 10.1076/jcen.20.2.270.1172. [DOI] [PubMed] [Google Scholar]
- 27.Baune BT, Caniato RN, Arolt V, Berger K. The effects of dysthymic disorder on health-related quality of life and disability days in persons with comorbid medical conditions in the general population. Psychother Psychosom. 2009;78:161–6. doi: 10.1159/000206870. [DOI] [PubMed] [Google Scholar]
- 28.Robertson N, Burden ML, Burden AC. Psychological morbidity and problems of daily living in people with visual loss and diabetes: do they differ from people without diabetes? Diabet Med. 2006;23:1110–6. doi: 10.1111/j.1464-5491.2006.01970.x. [DOI] [PubMed] [Google Scholar]
- 29.Cox DJ, Kiernan BD, Schroeder DB, Cowley M. Psychosocial sequelae of visual loss in diabetes. Diabetes Educ. 1998;24:481–4. doi: 10.1177/014572179802400406. [DOI] [PubMed] [Google Scholar]
- 30.Dreer LE, Elliott TR, Berry J, et al. Cognitive appraisals, distress and disability among persons in low vision rehabilitation. Br J Health Psychol. 2008;13:449–61. doi: 10.1348/135910707X209835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Owsley C, McGwin G, Jr, Scilley K, et al. Effect of refractive error correction on health-related quality of life and depression in older nursing home residents. Arch Ophthalmol. 2007;125:1471–7. doi: 10.1001/archopht.125.11.1471. [DOI] [PubMed] [Google Scholar]
- 32.Rupolo G, Angi M, Sabbadin E, et al. Treating myopia with acoustic biofeedback: a prospective study on the evolution of visual acuity and psychological distress. Psychosom Med. 1997;59:313–7. doi: 10.1097/00006842-199705000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Angi MR, Caucci S, Pilotto E, et al. Changes in myopia, visual acuity, and psychological distress after biofeedback visual training. Optom Vis Sci. 1996;73:35–42. doi: 10.1097/00006324-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Koklanis K, Abel LA, Aroni R. Psychosocial impact of amblyopia and its treatment: a multidisciplinary study. Clin Experiment Ophthalmol. 2006;34:743–50. doi: 10.1111/j.1442-9071.2006.01317.x. [DOI] [PubMed] [Google Scholar]
- 35.Reimer J, Voigtlaender-Fleiss A, Karow A, et al. The impact of diagnosis and plaque radiotherapy treatment of malignant choroidal melanoma on patients' quality of life. Psychooncology. 2006;15:1077–85. doi: 10.1002/pon.1046. [DOI] [PubMed] [Google Scholar]
- 36.Lasalvia A, Ruggeri M, Santolini N. Subjective quality of life: its relationship with clinician-rated and patient-rated psychopathology. The South-Verona outcome project 6. Psychother Psychosom. 2002;71:275–84. doi: 10.1159/000064809. [DOI] [PubMed] [Google Scholar]
- 37.Mueller I, Gall C, Kasten E, Sabel BA. Long-term learning of visual functions in patients after brain damage. Behav Brain Res. 2008;191:32–42. doi: 10.1016/j.bbr.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Bullinger M, Kirchberger I. Der SF-36 Fragebogen zum Gesundheitszustand - Handbuch für die deutschsprachige Fagebogen-Version. Göttingen: Hogrefe; 1998. [Google Scholar]
- 39.Mangione CM, Lee PP, Pitts J, et al. Psychometric properties of the national eye institute visual function questionnaire (NEI-VFQ). NEI-VFQ field test investigators. Arch Ophthalmol. 1998;116:1496–504. doi: 10.1001/archopht.116.11.1496. [DOI] [PubMed] [Google Scholar]
- 40.Hessel A, Schumacher J, Geyer M, Brähler E. Symptom-Checkliste SCL-90-R: Testth-eoretische Überprüfung und Normierung an einer bevölkerungsrepräsentativen Stichprobe. Diagnostica. 2001;47:27–39. [Google Scholar]
- 41.Iezzi T, Archibald Y, Barnett P, et al. Neurocognitive performance and emotional status in chronic pain patients. J Behav Med. 1999;22:205–16. doi: 10.1023/a:1018791622441. [DOI] [PubMed] [Google Scholar]


