Abstract
How does the human brain absorb information and turn it into skills of its own in psychotherapy? In an attempt to answer this question, the authors will review the intricacies of processing channels in psychotherapy and propose the term transprocessing (as in transduction and processing combined) for the underlying mechanisms. Through transprocessing the brain processes multimodal memories and creates reparative solutions in the course of psychotherapy. Transprocessing is proposed as a stage-sequenced mechanism of deconstruction of engrained patterns of response. Through psychotherapy, emotional-cognitive reintegration and its consolidation is accomplished. This process is mediated by cellular and neural plasticity changes.
Key words: transprocessing and neuroprocessing, neurobiology of psychotherapy, subcortical-cortical mid-line networks, self-related processing, cognitive-emotional link, neuropsychoanalysis, psychoanalysis
Background
Contemporary research has generated a series of contributions meant to establish a neurobiological framework for human change in psychotherapy.1,2 This paper starts from the hypothesis that human survival has as its purpose not only personal but also social viability. Evolutionarily, social viability is as important as personal existence. For this to be accomplished effectively, an initial aim of psychotherapy is to tune down negative affects and strengthen positive ones. Toning down of the panic/anxiety signaling is necessary. Panic limits social affiliation, secure attachments, confidence, and ultimately, procreation and reproduction.
Human healing and change engages a large variety of neurobiological mechanisms that involve the processing of complex memories – multimodal memories (MM), comprised of affective, cognitive and behavioral components. The authors propose the term transprocessing (from transduction and processing) to refer to neurobiological changes induced by environmental influences which are incorporated as new memory functions. Based on previous contributions and on updated functional neuroanatomy, the authors propose that new neural networks occur predominantly in anatomical areas where implicit emotional and explicit cognitive information intersect. A description of transprocessing along vertical, horizontal and molecular (transduction) neural networks will be proposed. The particular role of the subcortico-cortico midline system (SCMS) in the constitution of the Self, its relationship to primary process emotions or protoemotions will be discussed.3 Even though mental processing like transprocessing and healing are anatomically ubiquitous, there are brain networks that serve special roles as initiators of mental processing, learning and psychological healing.
Unlike natural processing, transprocessing is targeting the reconstruction of complex multimodal memories, developed primarily for defensive purposes, which have become crystallized and thereby associated with chronically dysfunctional behaviors. Mechanisms of processing and repair will be examined both from a bottom-up and top-down perspective (referring to the hierarchy of different brain structures).4 We will conclude with the proposal of three phases of transprocessing: i) evaluation; ii) acquisition and self-reference; and iii) reconsolidation.
Socrates: «You declared just now that you yourself cannot even explain what knowing is. How did you learn the verb to know as a child?»
Plato: «Evidently, I assimilated it from hearing it used around me».
Socrates: «Then it was by automatic action that you gained control of it».
Plato: «No… Well, perhaps I see what you mean. I grew accustomed to hearing it in certain contexts, and thus came to be able to use it myself in those contexts, in a more or less automatic fashion».
Socrates: «Much as you use language now – without having to reflect on each word?
Plato: «Yes, exactly».
An imaginary dialogue between Socrates and Plato by Douglas Hofstadter (2007).5
Introduction and terms
Change is a fundamental attribute for the human evolutionary potential. It must include an internalization of the ever-changing living environment and forms of assimilation of experiences, like memory and long-term adaptive changes in behavior. Some contemporary views have touched on all past interactions as permanently encoded human experiences that define one’s identity,5 thus conferring a new meaning to personal history. Healing in psychotherapy has been the subject of a large body of literature, but less has been written about brain processing in psychotherapy and its relationship to human change. This paper explores the multiple facets of brain changes activated by psychotherapy. Neuroanatomical considerations of brain regions where explicit and implicit memory mechanisms intersect, will be reviewed as crucial to the development of new reparative memory constructs. The dimension of time as a determining factor in human change will be emphasized. Specifically, time is seen as a bridging element between the reactive, instantaneous functional brain response, and the lasting changes of neural connectivity and functional remapping. Such remapping of the brain mirror lasting experiential change.
The term psychotherapy will be used to refer to a psychodynamic, interpretation-based therapeutic interaction that promotes change. The term transprocessing (TP) denotes a broad therapeutic event. It includes the roots of trans from transduction, the biological term that refers to cellular changes induced by environmental influences, and the term processing, common to the brain activities related to incorporation of information. TP as used here will refer to the changes that occur in psychotherapy: a deconstruction of target memories, which include cognition, affects and behaviors followed by an ensuing adoption of more adaptive constructs. Such a change includes a fundamental learning process (an acquisition and enrichment). Transprocessing is not reconsolidation as described by Nader and Duvarci,6 a process of reworking and reinterpretation of memories. Specifically, through reconsolidation, each time a memory is recalled, it is changed according to the context in which it is being recalled. While TP does include elements of reconsolidation, in TP, the newly acquired memory pool and related behaviors override, but do not erase, the old neurotic self. Multimodal memories (MM) are complex memories, through which one may hold, experience and/or evoke cognitive, affective and behavioral information concomitantly in mind. Thus through MM, one is able to remember an event, re-experience an emotion and evoke and/or act on one’s own behavior, which was previously acquired. MM are highly associative and therefore may activate a variety of brain regions. Traumatic memories are a form of MM that are activated by reminders of past adverse events. Neurotic Symptoms are multimodal memories. The term neurosis has been used interchangeably for the designation of mild psychiatric symptoms of anxiety and depression, for distortions as they transpire in everyday life and for a specific psychological profile for individuals who tend to overreact to environmental stressors. Neurosis is linked to the generation of some psychiatric symptoms. Psychiatric symptoms do not originate in predictable anatomical locations,7,8 but genetic and epigenetic factors participate in the development of reactions to environmental stress.9 While in this contribution we will not concern ourselves with symptoms per se, nevertheless neurosis, psychiatric symptoms and maladaptive reactions have certain autonomous nervous manifestation as a common denominator. This denominator points to the large extent of connectivity between the CNS and the ANS.
Examples of the CNS-ANS anatomic link
The amygdala and the ANS. Through its central nucleus, the amydala is linked to the dorsal motor nucleus of the vagus in the medulla.10 By means of tracing studies of neurons, with horseradish peroxidase, the neurons from the amygdala and the dorsomedial medulla were found to extend into the forebrain, the sublenticular region of the substantia innominata, under the globus pallidus and internal capsule and into the lateral part of the bed nucleus of the stria terminalis (BNST), forming a continuum and uniting these structures. Thus, areas responsible for somatic symptoms link to neuronal substrate of emotions. Schore pointed to the right lateralization of the connection between the cortical circuits of social and emotional processing and the autonomic nervous system.11-14 It is noteworthy that an older term for ANS is the Involuntary Nervous System, which more precisely denotes its function. The ANS is not autonomous but highly interactive with the CNS.15 Within the symptomatology of psychiatric disorders, somatic symptoms occur involuntarily, but are linked to mental content (e.g., panic attacks, hyperarousal in PTSD) albeit not at all autonomous.
Porges’ proposed polyvagal theory views autonomic control as a result of three separate systems:16,17 i) the myelinated vagus, which acts under circumstances of low arousal and calm; ii) the sympathetic, which steps in as a second line of response in stress exposure; iii) the unmyelinated vagus, which has a predominate survival function. Therefore, the ANS is highly linked to response patterns of an individual in adverse life circumstances. A dominance of the (myelinated) vagus nerve will maintain a high vagal tone, which is associated with increased attention and increased recognition memory, which further influences the cognitive component of anxiety. This high vagal tone is a state that psychotherapy aims to achieve.18 Autonomic activity is crucial in the body’s social engagement mechanisms, as it controls gaze, middle ear musculature (selective sound amplification) and involuntary vocalization.19 Furthermore, attachment research has also pointed to a transmission from mother to child of fear response and dissociation in attachment of disorganized type.20 Both organized, e.g., secure-autonomous, and insecure, e.g., dismissive, preoccupied and disorganized/disoriented states of mind in parents, with respect to attachment, predicted the same type of attachment in their children.21-23 All these constitute accumulating evidence for a multilink evolutionary and adaptive, mechanism of intergenerational transmission of responses to the environment in which the ANS along with implicit memory and language have particularly poignant contributions.24
The neurological model of transprocessing
The idea that mental dysfunction is associated with a miswiring process is not new.25-30 Yet, psychological processing, long thought of as part of a healing mechanism, is commonly referred to as understanding and internalizing.31 Formed memories of either external events or of internal experiences are split up, reorganized and interpreted within a personal context or an autobiographical narrative. Commonalities in the process of psychological recovery and healing across different schools of thought have been outlined,32 and an integrative function of healing during psychotherapy has been proposed.33 Given strong social evolutionary pre-programming of humans, multimodal memories that develop interactively, in psychotherapy, carry a heavy weight in defining a changing individual. Such memories are likely to heavily impact an individual’s future interpretation of the external world or Weltanschaung. Neurologically, transprocessing is a structured form of processing. Processing occurs at the crossing of specialized pathways where signals of different intensity and dimensions intersect. Anatomically these pathways will be described according to vertical, horizontal and cellular brain distribution (Figure 1).
Figure 1.
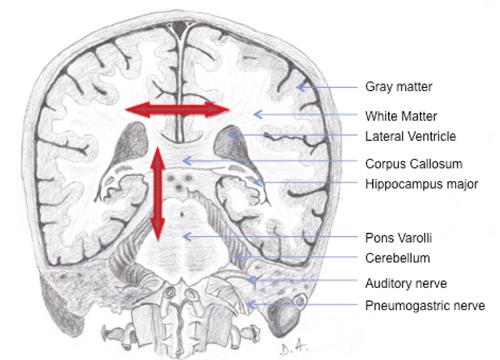
Transverse vertical section of the brain, through the fore part of the foramen magnum, looked at from the front.
Vertical processing systems
Vertical processing system includes: i) self-Related Processing and the Subcortical-Cortical Midline System (SCMS); ii) re-entrance; iii) prefrontal-subcortical circuitry and mood regulation.
The subcortical-cortical midline system and the Self
A large body of literature on the Self and Consciousness has recently emerged.7,34 Leonardo da Vinci referred to senso commune as the center of all senses including the center of the soul.35 While an in-depth analysis of different schools of thought is beyond our proposed scope, several contributions are noteworthy. Panksepp and Northoff have referred to self-related processing (SRP) as a specific and evolutionary-directed mode of interaction between organism and the environment.36 Through SRP, environmental stimuli are related to organismic needs, and the higher cortical areas are at the basis of epigenetic changes for interactive behaviors necessary for survival. All these are accomplished by a set of midline structures that start in the brain stem, the reticular activating system (RAS) and are interconnected with higher brain structures in the subcortical and cortical areas, referred to as the subcortical-cortical midline system (SCMS). These include theperiaqueductal gray (PAG), an extremely rich connected brain structure,37 the superior culiculi (SC), the bed nucleus of the stria terminalis (BNST), ventral tegmental area (VTA), the mesencephalic locomotor regions, preoptic areas, the hypothalamus and the dorsomedial thalamus,3,38 which accomplish the integrative bodily functions and the convergence of basic emotional systems to form the proposed bodily self or proto-self.37,39-46
Panskepp and Watt suggested that a number of affective states constitute the instinctual building block of basic affective responses necessary for survival.3,47 They include: SEEKING (refers to appetitive desires, motivation); FEAR (generates a pure form of trepidation without a specific object which animals can associate with many circumstances and helps the avoidance of danger); RAGE (facilitates the need to protect and defend); LUST (associated with sexual functions); CARE (includes the maternal instinct); PANIC (activated in young mammals when lost accompanied by a crying out for care. The crying out triggers care by the parent); PLAY (exposes young animals to the social systems of their species and provides an initiation of skills by adult animals, necessary to function in the world).
Panksepp’s vast contribution and the emergence of Affective Neuroscience points to the primary process emotions that originate in the lower brain structures.43-45,48-51 These structures constitute the lower segment of the neural substrate of the core self and are of a particular significance in providing a bottom-up loop for some basic affective processes with evolutionary significance. The neural basis of the basic emotions has been proposed by Panksepp: SEEKING = nucleus accumbens, VTA, mesolimbic and mesocortical outputs, the lateral hypothalamus, PAG. RAGE = the medial amygdala to BNST, medial hypothalamus to PAG. FEAR = Central to lateral amygdala to medial hypothalamus and dorsal PAG. LUST = Corticomedial amygdala, BNST, preoptic hypothalamus, VHM, PAG. CARE = anterior cingulate, BNST, preoptic area, VTA, PAG. PANIC = anterior cingulate, BNST and preoptic area, dorsomedial thalamus, PAG. Play = dorsomedial diencephalons, parafascial area, PAG.
Northoff et al. observed on brain imaging that emotional dimensions and self-relatedness were modulated into opposite directions in the DMPFC and the ventral striatum, while the neural activity in subcortical regions (tecturm, right amygdala, hypothalamus) for self-relatedness and emotions was regulated into the same direction.52
All environmental stimuli, through self-related processing (SRP) are being evaluated implicitly or explicitly for their possible value for the survival of the organism.38 This evaluation is accomplished in both as action and as affect (Figure 2).2,3,44,45
Figure 2.
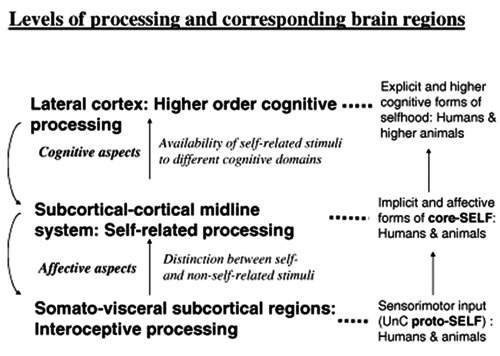
A summary of the different levels of processing of the self in association with possible neural and psychological substrate. (Reproduced with permission: Panksepp & Northoff, 2008).
The higher brain structures associated with functions of the Self include the medial prefrontal cortex (MPFC), the dorsomedial prefrontal cortex (DMPFC), the supra-genual area of the anterior cingulate, and the precuneus.53
In summary, what Panksepp and Northoff have proposed as the Core-Self,36 refers to a network of midline structures with its lower segment, the reticular activating system (RAS), described above linked to interoceptive states and raw emotions and its higher brain structures, the subcortical-cortical midline structures (SCMS), which provide affective-cognitive integration. Together, these structures of the Core Self make Self Related Processing (SRP) a very specific mode of interaction between organism and environments. From an evolutionary viewpoint, SRP allows for an interaction in which stimuli from the environment are related to organismic needs, and through the higher midline structures, provide the basis for epigenetic emergence of higher forms of selfhood across different individuals within a species. It has been proposed that the SRP and the Core Self are highly evolutionary trans-species domains. In psychotherapy, the operation of the human cortex mirrors and modulates proto-emotions that originate in the PAG. Human socialization can be maintained thanks to cortical inhibition of protoemotions. Neurosis routinely includes a compromising of such modulatory power with both states of unleashing of some and states of blocking of other emotions. Seen from outside, neurotic emotions seem idiosyncratic and defensive (primitive defenses). As we will propose below, target memories are constructs that interfere with the regulatory effect of higher brain functions. Psychotherapy, by way of transprocessing, deconstructs the target memories that dysregulate the cortical modulatory functions. This paradigm shift is often accompanied by a release of emotions that seem unusually intense. As proposed below, in subsequent stages of evaluation, self-reference and reconsolidation, transprocessing constructs proper networks that restore and maintain self-related processing (SRP) necessary for individual survival and affect regulation necessary for group and social survival.36 Of all proto-emotions, PLAY seems particularly significant as access to the know how of early development.54
Re-entrance
The term re-entrance is used in the description of the cortico-subcortical parallel reentrant circuits of the brain, a set of anatomical regions which provide an interactive functional link between cortex and subcortical region. Current knowledge about memory function makes it very obvious that implicit, non-declarative memory shapes and influences explicit, declarative memory, and thus decisions and actions. Re-entrance is a function based on a newer anatomical organization. Lennart Heimer’s group reported that the piriform (olfactory) cortex and the neighboring cortex of the amygdaloid complex (together the piriform-amygdaloid cortex) send the majority of their projections to the basal ganglia and terminate in the ventral striatum rather than in the anterior-lateral hypothalamus as was widely accepted previously.55-58 A lesion in the olfactory tubercles would result in a massive degeneration in the substantia innominata, whose precise role in the function of the striatum and the amygdala was believed to be minor. By using the retrograde lesion technique, it was demonstrated that these areas and the rest of the hemispheres project into the accumbens and the striatal areas of the basal ganglia of the forebrain which projects back to the different areas of the cortex: hence the designation re-entrant circuits (Figure 3).
Figure 3.
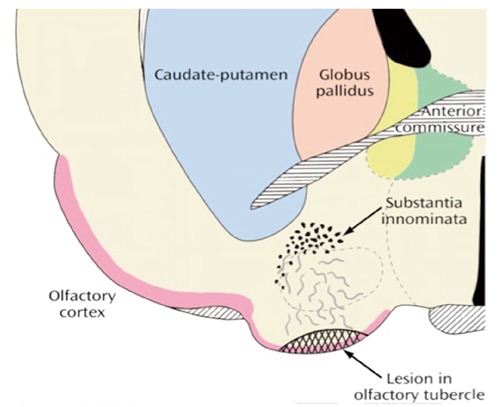
Pattern of degeneration in the Medial Forebrain Bundle in a rat olfactory tubercle and the corresponding terminal degeneration in the substantia innominata, two days later. (Reproduced with permission: Heimer & Wilson, 1975. Copyright Raven Press, New York).
The anatomical areas in question are the basal ganglia (caudate, putamen and globus pallidus), the adjacent, interspersed white matter extrapyramidal structures, as well as the extensions of nearby regions, e.g., the substantia innominata and the extended amygdala.
The extended amygdala
Currently, the entire region of the amygdaloid system in the medial temporal lobe is viewed as a network of numerous nuclei with different functions. In its classical description, the amygdala contains two major components: i) the central-medial nuclei, closely connected to the striatum, and ii) the cortical and basal nuclei, which are associated with the cortex. Of this division, the central-medial nuclei extend projections that reach the bed nucleus of the stria terminalis, a part of the reticular activating system (RAS). In its entirety, the extended amygdala is a ring of neurons that encircle the internal capsule and the basal ganglia. It is subdivided into the central extended amygdala and the medial extended amygdala reaching to the stria terminalis and the bed nucleus of the stria terminalis. These are medially located brain structures.55 The extended amygdala is continuous with the shell of the accumbens. Together these two structures form neuronal circuits which connect to the orbito-frontal cortex and the medial temporal lobe.
The striatal-pallidal system
This includes the basal ganglia (caudat, globus pallidum, putamen and the white matter capsules), which form functional units. The ventral striatal pallidal system is especially involved with behavioral functions. Its anterior part, the nucleus accumbens, has a high dopamine neuron content, and thus its importance in reward and motivation. The dorsal striatum and pallidum, and their cortico-subcortical connections, are charged mainly with motor functions.
The substantia innominata
The substantia innominata is a ventral extension of the pallidal complex of the basal ganglia, which is closely linked to the two major subcortical structures: the striatal pallidal complex and the extended amygdala, which appear like two large diagonally-oriented sub-cortical bands. The ventral part of the substantia innominata is called subcomissural substantia innominata. The posterior substantia innominata is also referred to as the sublenticular substantia innominata. This latter portion is mainly occupied by elements of the extended amygdala. Heimer et al.56 pointed to the improper name of this region, substantia innominata, which was considered the equivalent of the geographical designation of terra incognita, or uncharted territory. However, in this region the basal nucleus of Meynert is a collection of cholinergic and non-cholinergic cells, which are corticopetal and thalamopetal. They stretch from the septum diagonal band to the caudal globus pallidus. Of further importance is the abundance in this area of neurons congregated as the so-called interface (small-celled) island, which are rich in neuro-active substances. They may constitute reserve cells for remodeling and raw material for the adaptive structural reworking of the brain.
There seems to be considerable individual variability of the cortical-subcortical circuits achieved by brain plasticity, e.g., neurogenesis and rapid dendritic spine rewiring, which is sensitive to biochemical changes.59 There is also a large variety of neurotransmitter neurons which distribute into different areas during development, according to set patterns of migration.60,61 This entire region of the medial forebrain bundle (MFB) has a major role in emotion regulation as it interposes itself between the lower midline structures like the PAG and the higher thalamic and cortical structures. Electrical stimulation of MFB during neurosurgery has produced striking behavioral changes, including hypomania.62
The pathway system of re-entrance allows for the possibilities of return and mirroring of subcortical neural activity into the cortex and vice versa. This vertical organization of processing allows for a cortico-subcortical reshaping mechanism by which implicit and unconscious memories and behavioral patterns are projected into explicit memories for personal biasing. Thus, past and very past experiences that can no longer be retrieved as conscious memories, give a personal slant to new mental material. Here a distinction between unconscious and implicit is in place. Psychoanalysis views the unconscious as the largest portion of memory. In comparison, only a small portion of memories are conscious. Unlike the psychoanalytical unconscious, implicit memories are not conscious but are easily accessible to one’s consciousness. For instance, one can drive an automobile without thinking of each step in driving, but the driving skill is accessible to conscious thinking and can be explained to others. On the other hand, information stored in the psychoanalytical unconscious are not directly accessible to consciousness but rather manifest in an indirect, symbolic manner.
Freud outlined the unconscious as a vast system of memory traces and associations that are not readily accessible to consciousness. The largest part of the mind that includes the conscious, which is more limited in size. However, elements of the unconscious do enter the conscious mind through slips of the tongue, dreams (the royal road to the unconscious), memory blanks and different unintentional acts. Through psychotherapy, one can gain insight into some aspects of the unconscious.63
Prefrontal-subcortical circuitry and mood control
Of importance here are the two major regions of the prefrontal cortex: the orbital medial prefrontal cortex (OMPFC) and the lateral prefrontal cortex (LPFC), and their connections to the striatum and thalamic nuclei. Between them, these structures create a dense network of circuits that account largely for mood regulation, viscero-somatic manifestations of emotions and cognitive-emotional responses to the environment, all of particular significance in psychotherapy. An excellent and most detailed up-to-date description of this area of research can be found in Price and Drevets,64 which is summarized here.
The orbital medial prefrontal cortex
The orbital medial prefrontal cortex is subdivided into: i) The orbital prefrontal network; ii) the medial prefrontal network (Figure 4).
Figure 4.
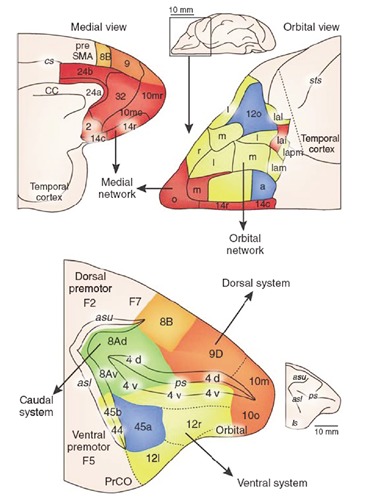
Orbitomedial prefrontal cortex and lateral prefrontal cortex. (Reproduced with permission: Price & Drevets, 2010).
The orbital network
The orbital network contains many corticocortico interactions, which connect areas specialized in olfactory, gustatory, visual (in the inferior temporal lobe) and somatic sensory (the insula and the frontal operculum) functions, an important role in food stimulation. There are no connections to the auditory system. In addition, the orbital network with its multimodal functions has the ability to assess the value of stimuli and to use reward as a guide for behavior.65-67 Significantly, at the completion of psychotherapy, a significant change in the right orbito-frontal cortex and its subcortical connections has been demonstrated by means of PET scanning.68
The medial network
The medial network (Figure 4) is connected to the limbic system, including the amygdala, the visceral control areas of the hypothalamus and the PAG,69-71 and thus, provides visceral modulation in relation to emotions. The medial network is also connected to the rostal part of the superior temporal gyrus (STG) and dorsal bank of the superior temporal sulcus (STS), the anterior and posterior cingulate cortex and the entorhinal and parahippocampal cortex.66 It is part of the default midline system that is active on brain imaging during resting states but becomes deactivated during new mental activity.64,72-74
The lateral prefrontal cortex
The lateral prefrontal cortex (LPFC) is subdivided into: i) the dorsal prefrontal system; ii) the ventral prefrontal system; and iii) the caudal prefrontal system (Figure 2).
The dorsal prefrontal system connects with the medial network of the OMPFC and to the hypothalamus and PAG, and therefore seems to be implicated in visceral and emotional functions. The ventral prefrontal system is believed to participate in non-food sensory functions, while the caudal prefrontal system and area 45 respond to combination of faces and auditory stimulations.64,75-77 Three types of projections of the frontal cortex will be mentioned here for their particular significance in mood regulation.64
Projections to the hypothalamus and the brain stem. Particularly, the medial network of the OMPFC projects to the hypothalamus and the PAG,78-83 which mediate visceral reactions to emotional stimuli.84,85 Damasio has in fact proposed a somatic marker hypothesis referring to somatic/body reactions that accompany emotions as warning signals for situations that ought to be avoided.84
The cortico-striato-thalamo-cortical circuits have four different subdivisions which include the medial dorsal thalamic nuclei (MD).86 The MD have two segments: the medial segment or medial MD and the lateral segment or the lateral MD. Medial Segment of Medial Dorsal Thalamic Nuclei (Medial MD).The medial MD receive subcortical input from the amygdala, the auditory cortex, and other limbic areas.87 However, given the direct cortical projections (the amygdala, the primary olfactory cortex entorhinal, the perirhinal, the parahippocampal cortex and the subiculum of the hippocampus), all send axonal projections to the OMPFC and to the medial MD.69,61,88 Non-thalamic input to the cortex from this limbic structure carries more detailed information than the connections that go through the MD nuclei of the thalamus.87-89 Thus, MD receive the limbic system connections which are excitatory (glutaminergic) and inhibitory (GABA-ergic) inputs from the ventral pallidum and rostral globus pallidus,90,91 which are part of the larger cortico-striato-pallido-thalamic loop, involving the OMPFC. Behavioral implicatios: the convergence of opposing neurotransmitter functional systems suggests a modulation of the reciprocal thalamo-cortical interactions between the OMPFC and MD. The limbic inputs are dominant, but there are also ongoing thalamo-cortical and cortico-thalamic interactions allowing for consistent behavior. When pallidal inputs become dominant, ongoing behavior is interrupted promoting a switch to another behavior. In rats lesions of the ventral striatum, pallidum, MD or OMPFC have been shown to cause perseveration, e.g., an inability to switch away from tasks that were previously rewarding and are no longer rewarding.92-95 Such an inability to switch is likely to have very broad implications in psychotherapy, as it is a component in many dysfunctional manifestations, including the inability of letting go of upsetting events, negative moods, depressive thoughts and traumatic events in a large number of psychiatric patients (not just in PTSD).
The lateral segment of the medio-dorsal thalamic nucleus (Lateral MD), receives most of the inputs from the brain stem including the superior colliculus, the vestibular nuclei, as well as the reticular formation.87 From the lateral MD they are further relayed to the frontal eye field and related areas in the caudal LPFC. Lateral MD receive GABA-ergic pallidal-like fibers from the parts reticulara of the substantia nigra.96,97 However, brainstem areas do not project directly to the cortex. Lateral MD serve as a relay to the cortex.
Prefrontal projections to the striatum. The OMPFC projects to the rostral, ventromedial part of the striatum.98 The subgenual part of the medial prefrontal cortex, also known as area 25, projects to the shell of the nucleus accumbens. The midline intralaminar nuclei of the thalamus, which include the para-ventricular thalamic nuclei (PVT), have a particular role in the response to chronic stress. Behavioral implications: lesions of the PVT block the habituation response to chronic stress in rats. In human brain imaging studies on neuroendocrine stress from chronic hypoglycemia, an activation of the medial prefrontal cortex, the PAG and the midline thalamic nuclei has been observed as well.8,99-105
Circuit-specific neuropsychiatric syndromes have been described.106-108 For the interested reader, a more comprehensive review of the cortical and fronto-subcortical circuits and their role in neuro-psychiatry can be found in several studies.108-111
The horizontal processing systems
Interhemispheric horizontal processing
In inter-hemispheric horizontal processing, the anatomical substrate, the corpus callosum and the anterior comissures represent white matter structures of an extraordinary functional capacity. This is supported by a huge network of fibers. The numbers of fibers comprising the corpus callosum and the anterior comissures are larger then the sum of all fibers ascending to or descending from the cerebral hemispheres.112 It is not the white matter per se that accomplishes processing but rather the potential for hemispheric synchrony through the fiber network, which conveys such an important role to the corpus callosum and the comissures. Historically, a developmental right to left shift in hemispheric control and dominance in learning has been recognized.113 Within the learning process, a dynamic shift from task-naïve to task-experienced recognition,114,115 and a change in locus of control in hemispheric dominance in the process of cognitive skill development have been identified.116
One prototypical example of horizontal-lateral processing is that of traumatic memories in trauma recovery.
A variety of psychiatric disorders have been linked to trauma exposure, but mood and anxiety disorders are the most common.117 Brain imaging techniques currently also provide a window into both anatomical and functional connectivity during emotional-cognitive tasks, recall and symptom activation in trauma patients.7 Yet neurologically, as explored by neuroimaging, it is not possible to pinpoint to a single neural network characteristic for memories accrued during trauma.8
These contributions support a horizontal type of processing. During a traumatic event, the release of high levels of catecholamines (with their memory consolidating properties) leads to a rapid fixation of traumatic memories and characteristic activation of the right hemisphere on brain imaging.118 Traumatic memories have features of right hemispheric manifestations: they are non-verbal memories and are accompanied by somatic symptoms. In an early experiment, patients with traumatic memories showed a right hemispheric dominance and right hemispheric activation during re-experiencing of traumatic memories triggered by means of script driven techniques.119 Following treatment with eye movement desensitization reprocessing (EMDR) psychotherapy, the left anterior cingulate became activated, detecting discrepancies and conflict.120,121 In this case, the anterior cingulate may facilitate the resolution of conflict and discrepancies regarding the trauma.122,123 By means of the inter-hemispherical (transcallosal) transfer, information reaches the speech center, which exerts its role in awareness formation. Speech, through words designs meaning to objects. It decreases emotional charge by diminishing the incomprehensible and unpredictable aspects of the environment. It also provides awareness of place and time, and therefore an understanding that the memories of the traumas are not occurring in the present but are events of the past. This in itself has a decathecting and emotionally modulating effect.124 As was recognized by Freud,125 language has multiple mental modalities (including visual, auditory, kinesthetic, and motor) with complex implications. Loss of any speech function usually affects thought process. For instance, patients with Wernicke’s aphasia who have loss of the auditory modality of language may describe an inability to think.126 On the other hand, psychotherapies focused on language and writing facilitate processing and mastery of the environment, as empirically demonstrated by the writing therapy experience of Pennebacker.127-130
The language brain is closely involved in the processing of new information during psychotherapy. Comprehension of language activates Herschl’s gyrus or the bilateral superior temporal gyrus (the primary auditory cortex) for initial phonological processing, the bilateral posterior inferior and middle temporal gyri for semantic processing and the left posterior frontal lobe and the temporoparietal area for mapping sounds into articulatory representations.131,132 Speech produced in accord with the therapist’s interpretation would recruit the areas of speech production: the left middle temporal gyrus activates in selection of phrases and lemmata. The left posterior middle temporal gyrus, the posterior superior temporal gyrus activate for phonological code retrieval. The left posterior inferior frontal gyrus is activated for syllabification. The bilateral sensorimotor and supplementary motor areas is activated for articulation. Finally, the bilateral superior temporal gyrus is activated for self-monitoring of speech.133 Over the past years, an extensive literature on lexical tone and pitch has emerged pointing to varied bilateral activations in frontal and temporal areas.134-143 When adding the posterior superior occipital areas activated for visualization and comprehension of writing, asemi-circle around the brain is activated by such a complex function as verbal communication. One can observe that speech in an interactional context involves extended bilateral areas that create a beltway of communication around the entire brain and not just limited to the classical speech anatomy of Broca and Wernicke. This beltway around the brain further supports the anatomical basis of speech as a main agent of processing and conscious emergence of both new (from exterior) and previously stored (from semantic and even operational memory storage) information. This recontextualization of present and past is the mainstay of transprocessing during psychotherapy.
In the same vein, the split-brain experience has demonstrated that in the absence of adequate inter-hemispheric anatomical pathways, processing is impaired, and awareness for emotions is limited. In alexythymia, a concept introduced by Sifneos for patients that are poor responders to psychoanalytical treatment, and likened to a functional commisurotomy, patients have a poor ability to fantasize, report no or few dreams, present somatization, and have difficulties finding adequate words for the descriptions of feelings.144
Alexythymia is a concept first introduced by psychoanalysts and later became the subject of interest in general psychiatry and psychosomatic medicine.145 It has offered a neuroanatomical understanding for poor integration of emotions and somatic expression in some individuals.145 It was first linked to trauma in veterans with PTSD as a protective mechanisms that helps evade the pain of trauma.146,147 This functional feature after trauma and its similarities to the split brain makes it an important contribution in the understanding of brain processing of emotions.
The significant role of the inter-hemispheric white matter in facilitating emotional processing and awareness is also suggested by findings related to childhood trauma often associated with a protective block in awareness and a deficit in emotional processing. Such trauma is associated with a reduction of the inter-hemispheric white matter as follows: 17% reduction compared to controls, 11% reduction compared to psychiatric patients and 15-18% reduction in the specific segments of the corpus callosum region. Of particular significance is the fact that sexual abuse in girls is the strongest associated with a reduced corpus callosum.148,149 Such anatomical reduction may stem from a lack of use of inter-hemispheric white matter from a partial closing of inter-hemispheric connectivity channels during periods of brain development.
Alexythymia has also been associated with limited creativity.145 Conversely, creativity has been associated with inter-hemispheric synchrony.150 Trauma may also induce alexythymia in some trauma survivors.147 On the other hand, transcendental meditation, has been shown to enhance creativity, well-being and access to emotions.151,152 Transcendental meditation is also known to induce hemispheric functional symmetry on EEG,151 a state opposite to alexythymia or a split-brain condition. Regarding the split-brain (secondary to commisurotomy), it would be erroneous to assume that repression and alexythymia are due to a malfunctioning of the corpus callosum per se.153 Instead, it is a lack of hemispheric connectivity from discrepancies in hemispheric signals that lead secondarily to a functional midline split. In this sense, alexythymia, like repression and posttraumatic stress are accompanied by hemispheric dyssyncrony and right hemispheric dominance on EEG. In summary, under normal conditions, brain activity is characterized by relative hemispheric synchrony with a slight left dominance, probably due to speech areas activity. States like alexythymia, repression, lack of creative expression and posttraumatic stress disorder are accompanied by right hemispheric dominance. States like, psychotherapy, meditation, EMDR and creative expression states are accompanied by enhanced brain synchrony.
Intrahemispheric, longitudinal horizontal processing
Intrahemispheric horizontal processing occurs within the ventro-dorsal and lateral axes of the same hemisphere and includes a coordination process between multimodal sensory and sensory-motor areas. The brain develops multiple routes of anatomic connectivity that facilitate interactions between the projection areas of the five senses. Large white matter structures like the inferior occipito-frontal fasciculus and the inferior longitudinal fasciculus provide a sizeable information highway.
Speech center in emotional and cognitive processing
Given the particular importance of the speech center in emotional and cognitive processing, one recent experiment demonstrates the involvement of left inferior occipital and longitudinal fasciculi in the facilitation of speech functions. By using intraoperative electrical stimulation in 12 patients who underwent resections for low-grade gliomas under local anesthesia, Mandonnet et al.154 induced semantic paraphasias in seven patients by stimulating the inferior occpito-frontal fascicle and non-semantic paraphasias by stimulating the nearby arcuate fascicle. The resection of a part of the inferior longitudinal fasciculus resulted in transient speech abnormalities only. To clarify, the left subcortical white matter network that subserves language semantics consists of the left inferior occipito-frontal fasciculus, the left inferior longitudinal fasciculus and the uncinat fasciculus, which connects the anterior temporal pole to the frontobasal area. The author’s findings suggest that the semantic ventral stream is constituted by two parallel pathways within the left dominant temporal lobe: a direct pathway, which is crucial in language semantic processing; an indirect pathway, which includes the inferior longitudinal fasciculus, which is not indispensable since its resection results in a speech deficit that is transient. Evolutionarily, such an extended network may suggest extensive functional reserve available in recovery processes.
Catani et al.,155 by using diffusion-tension MRI-obtained tractography of the human brain, point out the discrepancy between the classical description of the inferior longitudinal fasciculus (ILF) (as fibers connecting the occipital and anterior temporal cortex), and results of DT MRI. DT shows that the connections of the two areas are indirect: the occipito-temporal fibers are U-shaped association fibers. ILF includes: i) a major associative connection provided by a fiber bundle which are consistent with the classical ILF, connecting occipital and anterior temporal lobes; ii) ILF is distinct from the optic radiations and from U-shaped fibers connecting adjacent gyri; iii) ILF arises in extrastriate visual association areas; iv) ILF also projects to lateral and medial anterior temporal regions. The latter mediates the fast transfer of visual signals to anterior temporal areas and provides neuromodulatory back-projections from the amygdala to early visual areas.
Seen in the context of the earlier described complexity of language integration over large areas bilaterally within the language beltway, the white matter network provides an extensive association between the language brain and the deeper brain structures involved in modulation, including structures of the cortical-subcortical-midline structures (CSMS).
Anterior/motor brain structures
Anterior/motor brain structures have a major role in emotional processing and control and the constancy of personality, a fact supported by the major affective and personality disruptions in individuals with frontal lobe damage with lesions comparable in size with posterior sensory brain areas.3,156-158 Further, animal studies have also shown a direct cerebellar input from numerous anatomical structures: the prefrontal cortex, the posterior parietal cortex, the superior temporal cortex, the cingulum, the parahippocampus and the hypothalamus. This implies a significant role of the cerebellum in affect and cognition,159 which is accomplished through the dense network of white matter fasciculi. Allen et al.160 demonstrated functional connectivity between cerebellar-parietal and cerebellar-prefrontal areas, that is, a coherence between different cerebellar structures and brain regions involved in cognitive/affective processing. In particular, a significant correlation of signals between the dentate nucleus of the cerebellum and frontal and parietal regions, in particular the dorsolateral prefrontal cortex, was found. The authors also demonstrated correlations of signals between the dentate nucleus of the cerebellum and other cerebellar, thalamic, limbic and striatal areas, all participants in emotional processing.
Anatomical variants
White matter diffusion through diffusion-tension imaging, can reveal individual anatomical variants that participate in processing throughout brain development. This is an example of the anatomical principal that neurons that fire together wire together.161 In a study by Huang et al.162 the developmental schedule of different white and gray matter structures was studied in a 19-week fetus, at birth and at 5-6 years of age. The authors reported some structures to develop earlier, during fetal development, while others lag behind in development. In the fetal brain, the cerebellar peduncles and the medial lemnisci are smaller, and the descending tracts are larger compared to the brain of the newborn and the brain of the 5/6-year-old child. Major white matter structures, like the inferior longitudinal fasciculus and the inferior fronto-occipital fasciculus, are rather undeveloped in the 19-week-old fetus, suggesting a more prominent function in higher cognitive and emotional processing after birth. In the 19-week fetus, the cingulum and the fornix are well developed, while the corpus callosum is developed mainly in the frontal portion, suggesting a progressive development of the corpus callosum from the anterior toward the posterior region.
These preliminary findings confirm a biological principle of economic development that allows for a gradual introduction of gray and white matter structures: a gross neuroendocrine system is significantly developed at birth to sustain the impact of birth stress on the infant,163 early motor functions and emotional interactions, while the massive long-distant white matter structures (like the longitudinal fasciculus) mature later in connection with the development of evolved cognitive emotional processing. The latter may also show greater experience-dependent individual variability.
Neuro-plasticity systems
This refers to experiential remapping of brain projections and pathway rerouting. There is significant experiential learning and memory reworking that occurs during the course of psychotherapy. These are akin to long-term exposure to novel social situations, and the implicated adaptation triggers learning processes.164 Experiential learning results in the remapping of brain projection.165 The neural substrate of experiential learning includes: i) dendrite rebranching; ii) hippocampal learning and neurogenesis; iii) synaptic processes.
Dendritic rebranching
Dendritic rebranching is at the base for remapping of brain projections. The dendritic tree has been demonstrated to exhibit rapid and early (within minutes) directional changes as a response to modifications in the immediate biochemical environment. Early dendritic arborization is an elaborate process that occurs during development, which is in part influenced by activity. The specificity of dendritic connections at maturity is determined both early in development, by biochemical laminar cues and by later neuronal activity.166-170
Hippocampal learning
The hippocampus mediates episodic memory, the retaining of information within a context of time and place. A series of papers have covered the more intimate functioning of different hippocampal regions.171-176 The hippocampus is one of the main sites of neuogenesis.
During psychotherapy, specific steps in memory function include the participation of the hippocampal areas CA1 and CA3: auto-association which participates in error correction; cued sequence recall and comparison with information from the cortex; transitive inference, the correct identification of different items based on prior learning. More recently a large body of literature on the difference between memory consolidation and reconsolidation has emerged.177,178
Neurogenesis
Neurogenesis is among the most commonly cited discoveries that have fundamentally changed the understanding of memory and learning.179-184 Neurogenesis is keenly sensitive to environmental changes (Table 1) and also constitutes an important component of brain plasticity.185 However, only some experiences have been associated with activation of neurogenesis.186-189
Table 1.
Adult neurogenesis.
| Positive regulators | Negative regulators |
|---|---|
| Estrogen | Persistent emotional stress |
| Testosterone | Adrenal steroids like cortisol |
| A low stress environment | Sleep depravation |
| Sleep | Alcohol abuse |
| Physical activity | Excitatory inputs mediated by N-methyl-D-aspartate (NMDA) |
| SSRI’s | Limited learning opportunities and a relatively deprived environment |
| Mood stabilizing psychotropics/antiepiletics | Environmental complexity and intellectually enriched environments |
| Intellectual activity and learning | |
Psychotherapy creates new multimodal memories through simultaneous activation of cognitive, emotional and behavioral (motivational) experiences. In this regard, psychotherapy resembles early childhood learning when neurogenesis is particularly active. Like in early childhood when learning is interwoven with the pool of early neuron formation leading to a sculpting effect of the developing brain,148 psychotherapy may create a partial reframing of brain connectivity.190 Such new connectivity may create associations with implicit value similar to memories of early infancy that are not accessible yet continue to implicitly shape actions and personality.153 In parallel, they have the potential for neuronal repair.191
Synaptic processes
The synapse provides an extremely wide range of neural activity modulation which participates in learning and implicitly in human change.192,193
Synaptic plasticity
Regarding the messenger system, each synapse contains two type of receptors: inotropic and metabotropic receptors. An inotropic receptor is activated by neurotransmitter molecules which are primary messengers. They open a channel that creates a brief communication between the extracellular and the intracellular environment. Metabotropic receptors secrete enzymes which create the second messengers. Second messengers regulate the activity of the postsynaptic membrane according to functional needs, by either increasing the electrical potential and/or increasing the number of receptors. Thus, a potential of great variability in synaptic response and long-term potential changes is created and provides the plasticity associated with memory and learning.194
The processes of forming, strengthening and collateral pruning of synapses, further constitutes the basis of associability. Synaptic plasticity provides the ability of neural networks to combine information from new representations, which can later be retrieved.195 Synaptic plasticity has also been examined in regards to memory traces, drives and defense mechanisms in the context of psychoanalytical growth of an individual and in endurance of long-term memory.196 Thus, Si et al.,197 summarizing previous contributions,198,199 hypothesized that cytoplasma polyadenylation element binding protein (CPEB), a marker of mRNA activity in the protein synthesis of the neuron, mediates long-term memories. In the absence of CPEB, no memory was formed in the synapse of the aplaysia. The amino acid sequence of CPEB includes repetition in glutamine. Its structure provides an unusual resiliency for long-term survival that is necessary for long-term memory functions. Its amino acid sequence was compared to that of a prion,200 hence its ability to survive the long-term variation in the biochemical environment of the brain. Recent contribution further clarify the links between synaptic/molecular processes and aspects of long-term memory.172,201
Long-term potentiation
Memory is initially unstable, and over the course of about 30 minutes it becomes increasingly resistant to interference. This is explained by the fact that long term potentiation (LTP) is accompanied by changes in dendritic spine probably due to cytoskeletal changes. The process is accompanied by the polymerization of the protein actin in spine heads. The new cytoskeletal organization, and thus the new spine morphology, characterizes the initial phase of LTP consolidation. A variety of proteins and signaling cascades modulate LTP.202
LTP induces protein synthesis, synaptic reshaping and spine modification in the hippocampus. Patterned electrical stimulation results in postsynaptic depolarization and LTP, which is not automatically induced by the patterns of electrical stimulation through prolongation of NMDA activity.203 A variety of molecules have been shown to modulate LTP. Ampakines increase excitatory monosynaptic responses in the brain. They improve communication in complex networks and thus facilitate LTP and induce the expression of neurotrophic factors.204 Ampakines elevate BDNF in the rat hippocampus and BDNF promotes long-term potentiation-related cytoskeletal changes in the adult hippocampus.205,206
In conclusion, the adult makes use of an extraordinary network of fibers and synapses, some permanent and parallel, others neuroplastic and experience dependent, which participate in reinterpretation of complex memories, not unlike the systems proposed by Freud and later by Pribram.124,207
Transduction
Transduction refers to a biological process at the cellular level by which external stimuli produce cellular changes which result in a change in the cell’s functional output. For instance, if repeated stimulation of a specific cell results in the change of a secreted enzyme, that process is a form of transduction. Most of the time, external events result in a modification of effector genes, which results in changes in protein synthesis, which in turn modifies cell functions. An example here is the manner in which exposure to external stress leads to a signal change in the effector genes of the hypothalamus, which leads to the increased output of CRF, a neurohormone. CRF travels through the hypothalamo-pituitary axis to the pituitary gland and triggers, through positive feedback, the secretion of ACTH. In turn, ACTH will stimulate the adrenal glands into the increased production and release of cortisol, into the bloodstream. Stress and exposure to trauma is transduced into the effector genes of the neurons and could, in the long run, result in sizable changes in metabolism but also in clear bodily changes (obesity, diabetes). By means of transduction, retinal changes in the cones and rod neurons translate their functions into neuronal activity. These are ATP-energy dependent mechanisms.208,209 All other senses have similar transduction systems by which the auditory, gustatory, olfactory and tactile environment are transduced into virtual models of the outside world at the cortical level.209,210 All long-term cellular changes induced by external events like psychotherapy include transduction. Since psychotherapy includes a furthering of individual development,211 all processes of development and maturation may be activated duringpsychotherapy: neuropeptide activation (oxytocin, vasopressin, substance P, endogenous opioids), and neurotransmitter activation in bonding and attachment, neuromirroring during empathic processes andstrong right lateralized representations as in early attachment.12,13,212-216
The transprocessing model and the brain
Humans have the natural ability to interpret and reframe awareness of the present moment in the context of past experiences, while at the same time, retrieved memories, the remembering of the past, is incessantly remodeled in the context of present states of mind. This reworking of memories by means of reconsolidation stays at the foundation of one’s capabilities for change. In contrast, the proposed mechanism of transprocessing occurs in psychotherapy and thus carries with it, directly guided transformation. Besides the inclusion of reconsolidation, transprocessing contains a controlled processing of the past and a targeted learning process with acquisition of new adaptive abilities and often a new world view. Thus, transprocessing (referring to both processing and transduction) in psychotherapy is part of a universal human biological potential of adaptive change and survival. We are proposing the following stages of transprocessing.
Stage I: the evaluation phase
Through its white matter bundles, information is constantly captured from the periphery and channeled according to each sensory modality or motor functions to specialized areas of the cortex. In the course of a psychotherapy session, the new information signals intersect signals of pre-existing implicit memories. Behavioral patterns and complex memories are multimodal memories. The preexisting personal behavioral patterns of implicit memories define an individual before the onset of psychotherapy and have their origins in an individual’s ontogeny. Simultaneously, from the brain stem, continuously generated activation, representing proto-emotions are produced as a lifeline that maintains a basic drive to live. These proto-emotions are PANIC, FEAR, CARE, PLAY LUST, etc. and are generated in the PAG and other areas of the reticular activation system. Bailey & Davis (1942, 1943) demonstrated that lesions of these areas cause slow death in experimental animals pointing to their vital significance.217,218 These neural signals generated as a continuous lifeline reach higher sub-cortical and cortical areas, including areas specialized in different sensory modalities which have direct access to stored implicit memories. Thus, an intersection between the proto-emotions, from the ancient reticular activating system and implicit memories (stemming from prior experiences) occurs. This intersection provides a personal context to the proto-emotions. In this evaluation phase of transprocessing, a conscious comparison at different levels occurs between internally generated signals of protoemotions perceived as needs and the explicit memory pool, including memory about one’s own behavioral patterns. Under circumstances of rest, the cortical subcortical midline system (CSMS), associated with functions of the self, is likely to provide a top down control over proto-emotions through its known default state of activation seen on brain imaging during rest.36 The top down regulating of protoemotions has a significant social function, promoting delayed personal gratifications and thus communal cooperation and survival.
Neurotic states, in particular emotional symptoms, may represent in some clinical cases an escape of one or several of the protoemotions that exceed or escape the barrier of emotional regulation provided by the CSMS and probable other subcortical and cortical structures. The uncontrolled release of PANIC combined with blocked CARE/NURTURANCE may dominate in depression and anxiety disorders. Unmodulated SEEKING, LUST and PLAY may dominate, at least in part, manic episodes.
Stage II: the acquisition and self-reference phase
In an awake individual, information from the external world is sent to the thalamic nuclei and the amygdala and is further analyzed for discrepancies by the anterior cingulate. These pathways have been largely elucidated in the study of danger response and trauma.193 Neural inputs from the five senses representing external events are initially emotion-neutral but are assigned an emotional valence by the OMPFC.193 The assigned valence of threat may determine the degree of amygdala firing in the fight flight response.
During a psychotherapy session, different ego states are evoked: at rest, during free associations, the Benson relaxation response dominates with its hemispheric synchrony.219-221 At the same time, the language brain is stimulated in its entirety providing a language beltway of activation that extends into different segments of the temporo-frontal, parietal and occipital areas, bilaterally (during phonological and semantic stimulation, phonological code retrieval, articulation, self-monitoring of speech, lexical tone, pitch, articulation and concomitant visualization of expressed material).222 Interactive use of language (like during psychotherapy) creates a bilateral fronto-temporo-occipital shell of activation which may replace the default activation state of the cortical subcortical midline structures (CSMS) during mental rest. This process may also explain the role of language in mood and behavioral regulation. Contrary to the classical description of the left hemispheric language areas (in right-handedness) recent studies revealed a very extensive brain activation during language use amounting to a language belt of brain activation that surrounds the horizontal convexity of the brain.131-143
Newly acquired insights are also multimodal memories that can be stored through new dendritic and synaptic modifications as well as reconfiguration of the white matter.165 Through such neuroplastic changes, associations are expanded. Conversely, the loss of white matter from vascular lesions, visualized on MRI as white matter hyperintensity, resulted in loss of mood regulation as seen in syndromes of vascular depression.223
The newly formed memories and states of mind may act as extinguishers by overriding older, maladaptive multimodal memories (extinction is not erasing).224,225 The old information is still stored and could be reactivated during states of regression, a fact demonstrated by clinical experience.163 The emerging self-awareness in psychotherapy, a THIRD person meaning or observing ego quality is also stored as a new multimodal memory. Psychotherapy is not a linear process. The back and forth interactive process may be punctuated by regressive states which routinely release old emotions from time-trapped multimodal memories. With time and repetition, cytoskeletal modifications and transduction at the cellular level eventually consolidate the changes.202,209 At the point where one is able to maintain such multimodal memories and their newer meanings within the working memory, one learning cycle has been completed. This is a new memory acquisition, distinct from reconsolidation.171
In this stage, awareness or the know how about neurotic experiences is transformed from a third person experiential memory, which is an objectified experience, (It did happen there to me, this is me) into a first person experience (Me). This is a major transfer process viewed as a change in aspects of consciousness.
The ME experience of ownership or self-reference is backed by new midline structure activation.52 Through the continuous Me (or first-person) experience in psychotherapy, such structural activations become more routinely accessed. This reinforcement process eventually becomes the main established activation, the basis of a newly acquired aspect of the self. Psychological awareness of new aspects of the self is correlated with language brain activation.123 However, from a neurobiological perspective, perception of ownership or self-reference is likely to include a multi-stage mechanism. It may include the participation of the mirroring system of the patient rather then the therapist, as ownership/self-reference may emulate the developmental stages of the self during infancy. Given that the mirror neurons are a system of specialized cells, psychotherapy like normal self-development, by way of practicing, may lead to further specialization and differentiation of these cells. The inter-subjective nature of psychotherapy is a vehicle for ownership/self-reference of one’s past Self and the transformation into a new, present Self.
Stage III: the re-consolidation phase
New experiences are reworked through reconsolidation,4 but the modified connectivity of the self-related midline structures (the CSMS) become the new global point of reference for future learning and association. New implicit memories and their associations may, through re-entrance,111 influence explicit memories and the decision-making process. Changes produced by psychotherapy become new skills. Most likely, both new memories and reconsolidation,6,171,201 participate in this process. Like many highly practiced skills, as their execution becomes automatic, the brain projections undergo a mutation from occupying initially a larger, predominantly cortical projection, to a limited participation of mainly subcortical and cerebellar structures. Such a shift has been demonstrated in instances where skills were practiced at length.226 Schore hypothesized over a decade ago about the occurrence of literal anatomical pathway changes throughout the psychotherapeutic process.11,13,227 These in turn become backed by intracellular processes referred to here as transduction. Transduction is followed by changes in cellular functions. In the long run, epigenetics may further stabilize the qualitative transformation achieved through long-term psychotherapy. For instance, epigenetic changes secondary to an individual’s experience have been reported in prolonged trauma and in the intrafamilial and intergenerational transfer of trauma.228,229 Vice-versa, in long-term psychotherapy, a reverse process to traumatic changes may be achieved by epigenetics. Ultimately, new, modified cell functions and neural networks are necessary to back long-term behavioral and personality changes characteristic for a modified self through long-term psychotherapy.
Summary and future directions of inquiry
We have reviewed a large body of literature that suggests the participation of numerous anatomical structures at play in cognitive-affective-behavioral changes achieved in psychotherapy. In the future, research of transprocessing will have to map individual timing of processing and define early (the functional) and intermediate stages in new network formation. In the not so far future, the co-administration of very targeted molecules and/or stem cell repopulation of certain circuits (techniques that are already considered in the treatment of some neurological disorders) could create a new enhanced neuro-psychotherapeutic technique. This would replace the currently largely non-specific co-administration of psychotherapy and medications. Then, a future classification into professional Neuropsychotherapy (or analysis) and Enhanced Neuropsychotherapy would cover the treatment of the entire spectrum of psychiatric and psychological afflictions. Such progress could provide psychotherapy a prominent status among developing brain treatments in neuroscience.
Acknowledgements
This paper is dedicated to the late Lennart Heimer, who inspired many of us to keep our curiosity about the mysteries of the brain. Much gratitude is also extended to Georg Northoff and Jaak Panskepp for their encouragement and suggestions. Thanks also go to Laura Weissman and Jonathan Penley for assistance with the manuscript. The authors also thank Dr. Daniela Alexandru for assistance with editing the paper and drawing Figure 1.
References
- 1.Beitman B, Blinder BJ, Thase M, et al., Integrating psychotherapy and pharmacotherapy. Dissolving the mind brain barrier. New York: W. Norton and Co.; 2004. [Google Scholar]
- 2.Gerber A, Protopopescu X.Bridging the gap between neuroscientific and psycho-dynamic models in child and adolescent psychiatry. Child Adolesc Psychiatric Clin N Am. 2013;22:1-31 [DOI] [PubMed] [Google Scholar]
- 3.Panksepp J.The periconscious substrates of consciousness: affective states and the evolutionary origins of the SELF. J Consciousness Stud. 1998;5:566-82. [Google Scholar]
- 4.Northoff G, Duncan NW, Hayes DJ.The brain and its resting state activity--experimental and methodological implications. Prog Neurobiol. 2010;92:593-600. [DOI] [PubMed] [Google Scholar]
- 5.Hofstadter DR.I am a strange loop. New York: Basic Books; 2007. [Google Scholar]
- 6.Duvarci S, Nader K.Characterization of fear memory reconsolidation in the hippocampus. J Neurosci. 2004;24:9269-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hull AM.Neuroimaging findings in post-traumatic stress disorder. Systematic review. Br J Psychiatry. 2002;181:102-10. [PubMed] [Google Scholar]
- 8.Schacter DL, Buckner RL.On the relations among priming, conscious recollection, and intentional retrieval: evidence from neuroimaging research. Neurobiol Learn Mem. 1998;70:284. [DOI] [PubMed] [Google Scholar]
- 9.Hariri AR, Mattay VS, Tessitore A, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400-3 [DOI] [PubMed] [Google Scholar]
- 10.Schwaber JS, Kapp BS, Higgins G.The origin and extend of direct amygdala projections to the region of the dorsal motor nucleus of the vagus and the nucleus of the solitary tract. Neurosci Lett. 1980;20:15-20. [DOI] [PubMed] [Google Scholar]
- 11.Schore AN.Affect regulation and the origin of the self: the neurobiology of emotional development. Mahwah: Erlbaum; 1994. [Google Scholar]
- 12.Schore AN.The experience-dependent maturation of a regulatory system in the orbital prefrontal cortex and the origin of developmental psychopathology. Dev Psychopathol. 1996;8:59-87. [Google Scholar]
- 13.Schore AN.Early organization of the nonlinear right brain in the development of a predisposition to psychiatric disorders. Dev Psychopathol. 1997;9:595-631. [DOI] [PubMed] [Google Scholar]
- 14.Schore AN.Affect dysregulation and disorders of the self. New York: WW Norton; 2003. [Google Scholar]
- 15.Gloor P.Autonomic functions of the diencephalon; a summary of the experimental work of Prof. W. R. Hess. AMA Arch Neurol Psychiatry. 1954;71:773-90. [DOI] [PubMed] [Google Scholar]
- 16.Porges SW.Vagal tone: a physiologic marker of stress vulnerability. Pediatrics. 1992;90:498-504. [PubMed] [Google Scholar]
- 17.Porges SW.The polyvagal perspective. Biol Psychol. 2007;74:116-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynnmeyer SA, Porges SW.Recognition memory and cardiac vagal tone in 6-month-old infants. Infant Behav Dev. 1986;9:43-56. [Google Scholar]
- 19.Porges S.Reciprocal influences between body and brain in the perception and expression of affect: a polyvagal perspective. Fosha D, Siegel D, Solomon M, The healing power of emotions. New York: WW Norton; 2009. [Google Scholar]
- 20.Main M.The organized categories of infant, child, and adult attachment: flexible vs. inflexible attention under attachment-related stress. J Am Psychoanal Assoc. 2000;48:1055-96. [DOI] [PubMed] [Google Scholar]
- 21.Main M, Hesse E.Parents’ unresolved traumatic experiences are related to intants’ disorganized attachmentstatus: is frightened and/or frightening parental behavior the linking mechanism? Greenberg MT, Cicchetti D, Cummings EM, Attachment in the preschool years: theorie, research and intervention. Chicago: Chicago University Press; 1990. pp 161-182. [Google Scholar]
- 22.Main M, Hesse E.Disorganized/disoriented behavior in the strange situation, lapses in the monitoring of reasoning and discourse during the parents’ adult attachment interview, and dissociative states. Amamniti M, Stern D, Attachment and psychoanalysis. Rome: Guis, Lateza e Figli; 1992. pp 86-140. [Google Scholar]
- 23.Hesse E, Main M.Disorganized infant, child, and adult attachment: collapse in behavioral and attentional strategies. J Am Psychoanal Assoc. 2000;48:1097-127. [DOI] [PubMed] [Google Scholar]
- 24.Novac A, Hubert-Schneider S.Acquired vulnerability: comorbidity in a patient population of adult offspring of holocaust survivors. Am J Foren Psychiatry. 1998;19:45-58. [Google Scholar]
- 25.Akbarian S, Bunney WE, Jr, Potkin SG, et al. Altered distribution of nicotinamideadenine dinucleotide phosphatediaphorase cells in frontal lobe of schizophrenics implies disturbances of cortical development. Arch Gen Psychiatry 1993;50;169-77. [DOI] [PubMed] [Google Scholar]
- 26.Akbarian S, Viñuela A, Kim JJ, et al. Distorted distribution of nicotinamideadenine dinucleotide phosphatediaphorase neurons in temporal lobe of schizophrenics implies anomalous cortical development. Arch Gen Psychiatry. 1993;50:178-87. [DOI] [PubMed] [Google Scholar]
- 27.Akbarian S, Kim JJ, Potkin SG, et al. Maldistribution of interstitial neurons in prefrontal white matter of the brains of schizophrenic patients. Arch Gen Psychiatry. 1996;53:425-36. [DOI] [PubMed] [Google Scholar]
- 28.Ramachandran VS, Hirstein W.Three laws of Qualia: what neurology tells us about the biological functions of consciousness. J Consciousness Stud. 1997;4:429-57. [Google Scholar]
- 29.Ramachandran VS.A brief tour of human consciousness. From impostor poodles to purple numbers. New York: PI Press; 2004. [Google Scholar]
- 30.Peled A.Brain profiling and clinical neuroscience. Med Hypotheses. 2006;67:941-6. [DOI] [PubMed] [Google Scholar]
- 31.Weiner H.Some comments on the transduction of experience by the brain: implications for our understanding of the relationship of mind to body. Psychosom Med. 1972;34:355-80. [DOI] [PubMed] [Google Scholar]
- 32.Fosha D.Positive affects and the transformation of suffering into florishing. Bushell WC, Olivo EL, Theise ND, Longevity, regeneration and optimal health: integrating eastern and western perspectives. Hoboken: Wiley-Blackwell; 2009 [Google Scholar]
- 33.Siegel D.Mindsight. The new science of personal transformation. New York: Bantam Books; 2010. [Google Scholar]
- 34.Blackmore S.Conversation on consciousness: what the best minds think about the brain, free will and what it means to be human. New York: Oxford University Press; 2006. [Google Scholar]
- 35.Cianchi M.Leonardo: the anatomy. Florence, Milan: Giunti Editore S.p.A.; 1998. [Google Scholar]
- 36.Panksepp J, Northoff G.The trans-speces core Self: the emergence of active cultural and neuro-ecologic agents through self-related processing within subcortical-cortical midline networks. Conscious Cogn. 2009;18:193-215. [DOI] [PubMed] [Google Scholar]
- 37.Strehler BL.Where is the self? A neuroanatomical theory of consciousness. Synapse. 1991;7:44-91. [DOI] [PubMed] [Google Scholar]
- 38.Holstege GR, Bandler R, Saper CB.The emotional motor system. Holstege G, Bandler R, Saper CB, Progress in brain research. Vol 170. The emotional motor system. Amsterdam: Elsevier; 2010. pp 3-6. [DOI] [PubMed] [Google Scholar]
- 39.Damasio AR.The feeling of what happens: body and emotion in the making of consciousness. New York: Harcourt Brace; 1999. [Google Scholar]
- 40.Parvizi J, Damasio A.Consciousness and the brain stem. Cognition. 2001;79:135-60. [DOI] [PubMed] [Google Scholar]
- 41.Craig AD.How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655-66. [DOI] [PubMed] [Google Scholar]
- 42.Craig AD.Interoception: Tthe sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500-5. [DOI] [PubMed] [Google Scholar]
- 43.Panksepp J.At the interface of affective, behavioural and cognitive neuroscience: decoding the emotional feelings of the brain. Brain Cogn. 2003;52:4-14. [DOI] [PubMed] [Google Scholar]
- 44.Panksepp J.Affective consciousness: core emotional feelings in animals and humans. Conscious Cogn. 2005;14:19-69. [DOI] [PubMed] [Google Scholar]
- 45.Panksepp J.On the embodied neural nature of core emotional affects. J Conscious Stud. 2005;12:161-87. [Google Scholar]
- 46.Denton D.The primordial emotions: the dawning of consciousness. New York: Oxford University Press; 2006. [Google Scholar]
- 47.Watt D.Consciousness and emotions: review of Jaak Panksepp’s affective neuroscience. J Consciousness Stud. 1999;6:191-200. [Google Scholar]
- 48.Panksepp J.Towards a general psychobiological theory of emotions. Behav Brain Sci. 1982;5:407-67. [Google Scholar]
- 49.Panksepp J, Psychoneurology of fear: evolutionary perspectives and the role of animal models in understanding human anxiety. Handbook of Anxiety. Amsterdam: Elsevier; 1990. [Google Scholar]
- 50.Panksepp J, Burgdorf J.Laughing rats and the evolutionary antecedents of human joy? Physiol Behav. 2003;79:533-47. [DOI] [PubMed] [Google Scholar]
- 51.Panksepp J, Zellner M.Towards a neurobiologically based unified theory of aggression. Int Rev Soc Psychol. 2004;17:37-61. [Google Scholar]
- 52.Northoff G, Schneider F, Rotte M, et al. Differential parametric modulation of self-relatedness and emotions in different brain regions. Hum Brain Mapp. 2009;30:369-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Northoff G, Bermpohl F.Cortical midline structures of the self. Trends Cogn Sci. 2004;8:102-7. [DOI] [PubMed] [Google Scholar]
- 54.Brown S.Play: how it shapes the brain, opens the imagination, and invigorates the soul. New York: Penguin; 2009. [Google Scholar]
- 55.Alheid GF, Heimer L.New perspectives in basal forebrain organization of spatial relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1-39. [DOI] [PubMed] [Google Scholar]
- 56.Heimer L, Harlan RE, Alheid GF, et al. (). Substantia innominata: a notion which impedes clinical-anatomical correlations in neuropsychiatric disorders. Neuroscience. 1997;76:957-1006. [DOI] [PubMed] [Google Scholar]
- 57.Heimer L.A new anatomical framework for neuropsychiatric disorders and drug abuse. Am J Psychiatry. 2003;160:1726-39. [DOI] [PubMed] [Google Scholar]
- 58.Heimer L, Wilson RD.The subcortical projections of allocortex: similarities in the neuronal associations of the hippocampus, the piriform cortex and the neocortex. Santini M, Golgi centiennial symposium proceedings. New York: Raven Press; 1975. pp 177-193. [Google Scholar]
- 59.Wong RO, Ghosh A.Activity-dependant regulation of dendritic growth and patterning. Nat Neurosci. 2002;3:803-12. [DOI] [PubMed] [Google Scholar]
- 60.Fallon JH, Koziell DA, Moore RY.Catecholamine innervation of the basal forebrain. II Amygdala, suprarhinal cortex and endorhinal cortex. J Comp Neurol. 1978;180:509-32. [DOI] [PubMed] [Google Scholar]
- 61.Fallon JH, Ciofi P.Distribution of monoamines within the amygdala. Aggleton JP, The Amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley-Liss; 1992. pp 97-114. [Google Scholar]
- 62.Coenen VA, Honey CR, Hurwitz T, et al. Medial forebrain bundle stimulation as a pathophysiological mechanism for hypomania in subthalamic nucleus deep brain stimulation for Parkinson’s Disease. Neurosurgery 2009;64;1106-115. [DOI] [PubMed] [Google Scholar]
- 63.Freud S.The interpretation of dreams. 1900. [Google Scholar]
- 64.Price JL, Drevets WC.Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carmichael ST, Price JL.Sensory and premotor connections of the orbital and medial prefrontal cortex. J Comp Neurol. 1995;363:642-64. [DOI] [PubMed] [Google Scholar]
- 66.Saleem KS, Kondo H, Price JL.Complimentary circuits connecting the orbital and medial prefrontal networks with the temporal insular and opercular cortex in the macaque monkey. J Comp Neurol 2008:506:659-93. [DOI] [PubMed] [Google Scholar]
- 67.Rudebeck PH, Murray EA.Amygdala and orbitofrontal cortex lesions differentially influence choices during object reversal learning. J Neurosci. 2008;28:8338-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schwartz JM, Stoessel PW, Baxter LR, et al. Systematic cerebral glucose metabolic rate changes after successful behavior modification treatment of obsessive-compulsive disorder. Arch Gen Psychiatry. 1996;53:109-13. [DOI] [PubMed] [Google Scholar]
- 69.Carmichael ST, Price JL.Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363:615-41. [DOI] [PubMed] [Google Scholar]
- 70.Carmichael ST, Price JL.Connectional networks within the orbital and medialprefrontal cortex of macaque monkeys. J Comp Neurol. 1996;371:179-207. [DOI] [PubMed] [Google Scholar]
- 71.Kondo H, Saleem KS, Price JL.Differential connections of the perirhinal andparahippocampal cortex with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol. 2005;493:479-509. [DOI] [PubMed] [Google Scholar]
- 72.Miller B, Saleem KS, Price JL.Cingulate cortex connections with prefrontalcortex circuits in monkeys. Program No. 465.11. Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2008. [Google Scholar]
- 73.Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Raichle ME, Snyder AZ.A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083-99. [DOI] [PubMed] [Google Scholar]
- 75.Corbetta M, Kincade JM, Shulman GL.Neural systems for visual orienting and their relationships to spatial working memory. J Cogn Neurosci. 2002;14:508-23. [DOI] [PubMed] [Google Scholar]
- 76.Romanski LM.Representation and integration of auditory and visual stimuli in the primate ventral lateral prefrontal cortex. Cereb Cortex. 2007;17:i61-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsao DY, Schweers N, Moeller S, Freiwald WA.Patches of face-selectivecortex in the macaque frontal lobe. Nat Neurosci. 2008;11:877-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ongur D, An X, Price JL.Prefrontal cortical projections to the hypothalamusin macaque monkeys. J Comp Neurol. 1998;401:480-505. [PubMed] [Google Scholar]
- 79.Ongur D, Drevets WC, Price JL.Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.An X, Bandler R, Ongur D, Price JL.Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J Comp Neurol. 1998;401:455-79. [PubMed] [Google Scholar]
- 81.Rempel-Clower NL, Barbas H.Topographic organization of connections between the hypothalamus and prefrontal cortex in the rhesus monkey. J Comp Neurol. 1998;398:393-419. [DOI] [PubMed] [Google Scholar]
- 82.Freedman LJ, Insel TR, Smith Y.Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. J Comp Neurol. 2000;421:172-88. [PubMed] [Google Scholar]
- 83.Barbas H, Saha S, Rempel-Clower N, Ghashghaei T.Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci. 2003;4:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Damasio AR, Tranel D, Damasio H.Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav Brain Res. 1990;41:81-94. [DOI] [PubMed] [Google Scholar]
- 85.Bechara A, Damasio H, Damasio AR.Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295-307. [DOI] [PubMed] [Google Scholar]
- 86.Ray JP, Price JL.The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1993;337:1-31. [DOI] [PubMed] [Google Scholar]
- 87.Russchen FT, Amaral DG, Price JL.The afferent input to the magnocellular division of the mediodorsal thalamic nucleus in the monkey Macaca fascicularis. J Comp Neurol. 1987;256:175-210. [DOI] [PubMed] [Google Scholar]
- 88.Carmichael ST, Clugnet MF, Price JL.Central olfactory connections in the macaque monkey. J Comp Neurol. 1994;346:403-34. [DOI] [PubMed] [Google Scholar]
- 89.Price JL.The olfactory system. Paxinos G, The human nervous system. 2nd ed San Diego: Academic Press; 2003. pp 1198-1212. [Google Scholar]
- 90.Churchill L, Zahm DS, Kalivas PW.The mediodorsal nucleus of the thalamus in rats-I. Forebrain gabaergic innervation. Neuroscience. 1996;70:93-102. [DOI] [PubMed] [Google Scholar]
- 91.Kuroda M, Price JL.Synaptic organization of projections from basal forebrain structures to the mediodorsal thalamic nucleus of the rat. J Comp Neurol. 1991;303:513-33. [DOI] [PubMed] [Google Scholar]
- 92.Roberts AC, Robbins TW, Everitt BJ, et al. The effects of excitotoxic lesions of the basal forebrain on the acquisition retention and serial reversal of visual discriminations in marmosets. Neuroscience. 1990;34:311-29. [DOI] [PubMed] [Google Scholar]
- 93.McBride SA, Slotnick B.The olfactory thalamocortical system and odor reversal learning examined using an asymmetrical lesion paradigm in rats. Behav Neurosci. 1997;111:1273-84. [DOI] [PubMed] [Google Scholar]
- 94.Ferry AT, Lu X-CM, Price JL.Effects of excitotoxic lesions in the ventral striatopallidal-thalamic pathway on odor reversal learning to extinguish an incorrect response. Exp Brain Res. 2000;131:320-35. [DOI] [PubMed] [Google Scholar]
- 95.Kazama A, Bachevalier J.Selective aspiration or neurotoxic lesions of orbital frontal areas 11 and 13 spared monkeys’ performance on the object discrimination reversal task. J Neurosci. 2009;29:2794-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ilinsky IA, Jouandet ML, Goldman-Rakic PS.Organization of the nigrothalamocortical system in the rhesus monkey. J Comp Neurol. 1985;236:315-30. [DOI] [PubMed] [Google Scholar]
- 97.Kuroda M, Price JL.Ultrastructure and synaptic organization of axon terminals from brainstem structures to the mediodorsal thalamic nucleus of the rat. J Comp Neurol. 1991;313:539-52. [DOI] [PubMed] [Google Scholar]
- 98.Ferry AT, Ongur D, An X, Price JL.Prefrontal cortical projections to the striatum in macaque monkeys: evidence for an organization related to prefrontal networks. J Comp Neurol. 2000;425:447-70. [DOI] [PubMed] [Google Scholar]
- 99.Hsu DT, Price JL.Midline and intralaminar thalamic connections with theorbital and medial prefrontal networks in macaque monkeys. J Comp Neurol. 2007;504:89-111. [DOI] [PubMed] [Google Scholar]
- 100.Hsu DT, Price JL.Paraventricular thalamic nucleus: subcortical connections and innervation by serotonin, orexin, and corticotropin-releasing hormone in macaque monkeys. J Comp Neurol. 2009;512:825-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Spencer SJ, Fox JC, Day TA.Thalamic paraventricular nucleus lesions facilitate central amygdala neuronal responses to acute psychological stress. Brain Res. 2004;997:234-7. [DOI] [PubMed] [Google Scholar]
- 102.Bhatnagar S, Huber R, Nowak N, Trotter P.Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. J Neuroendocrinol. 2002;14:403-10. [DOI] [PubMed] [Google Scholar]
- 103.Jaferi A, Bhatnagar S.Corticosterone can act at the posterior paraventricular thalamus to inhibit hypothalamic-pituitary-adrenal activity in animals that habituate to repeated stress. Endocrinology. 2006;147:4917-30. [DOI] [PubMed] [Google Scholar]
- 104.Cryer PE.Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2004;350:2272-9. [DOI] [PubMed] [Google Scholar]
- 105.Arbelaez AM, Powers WJ, Videen TO, et al. Attenuation of counterregulatory responses to recurrent hypoglycemia by active thalamic inhibition: a mechanism for hypoglycemia-associated autonomic failure. Diabetes. 2008;57:470-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cummings JL, Mendez MF.Secondary mania with focal cerebrovascular lesions. Am J Psychiatry. 1984;141:1084-7. [DOI] [PubMed] [Google Scholar]
- 107.Cummings JL.Clinical neuropsychiatry. New York: Grune & Stratton Inc; 1985. [Google Scholar]
- 108.Cummings JL.Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873-80. [DOI] [PubMed] [Google Scholar]
- 109.Salloway SP, Malloy PF, Duffy JD.The frontal lobes and neuropsychiatric illness. Washington DC: American Psychiatric Publishing; 2001. [Google Scholar]
- 110.Kaplan GB, Hammer RP., Jr.Brain circuitry and signaling in psychiatry: basic science and clinical implications. Washington DC: American Psychiatric Publishing; 2002. [Google Scholar]
- 111.Heimer L, Van Hoesen GW, Trimble M, Zahm DS.Anatomy of neuropsychiatry: The new anatomy of the basal forebrain and its implications for neuropsychiatric illness. Amsterdam: Elsevier; 2008. [Google Scholar]
- 112.McCulloch WS, Garol HW.Cortical origin and distribution of corpus callosum and anterior commisure in the monkey (Macaca mulata). J Neurophysiol. 1941;4:555-63. [Google Scholar]
- 113.Goldberg E, Podell K, Lovell M.Lateralization of frontal lobe functions and cognitive novelty. J Neuropsychiatry Clin Neurosci. 1994;6:371-8. [DOI] [PubMed] [Google Scholar]
- 114.Bever TG, Chiarello K.Cerebral dominance in musicians and non-musicians. Science. 1974;185:537-9. [DOI] [PubMed] [Google Scholar]
- 115.Johnson PR.Dichotically stimulated ear differences in musicians and non-musicians. Cortex. 1977;13:385-9. [DOI] [PubMed] [Google Scholar]
- 116.Brown J, Jaffe J.Hypothesis on cerebral sominance. Neuropsychologia. 1975;13: 107-10. [DOI] [PubMed] [Google Scholar]
- 117.McGrath TR, Brooker AE.Combat stress reaction: a concept in evolution. Mil Med. 1985;150:186-90. [PubMed] [Google Scholar]
- 118.McGaugh J.Preserving the presence of the past. Hormonal influence on memory storage. Am Psychologists. 1983;39:161-73. [DOI] [PubMed] [Google Scholar]
- 119.Pitman RK, Orr SP.Psychophysiologic testing of posttraumatic stress disorder: forensic psychiatric applications. Bull Acad Psychiatry Law. 1993;21:37-52. [PubMed] [Google Scholar]
- 120.Carter CS, MacDonald A, III, Ross LL, Stenger VA.Anterior cingulated cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry. 2001;158:1423-8. [DOI] [PubMed] [Google Scholar]
- 121.Jackson PL, Melzhoff AN, Decety J.How do we perceive pain in others? A window into the neural process involved in empathy. Neuroimage. 2005;24:771-9. [DOI] [PubMed] [Google Scholar]
- 122.Levin P, Lazrove S, van der Kolk B.What psychological testing and neuroimaging tell us about the treatment of posttraumatic stress disorder by eye movement desensitization and reprocessing. J Anxiety Disord. 1999;13:159-72. [DOI] [PubMed] [Google Scholar]
- 123.Frewen P, Lane RD, Neufeld RW, et al. Neural correlates of levels of emotional awareness during trauma script-imagery in posttraumatic stress disorder. Psychosom Med. 2008;70:27-31. [DOI] [PubMed] [Google Scholar]
- 124.Freud S.Project for scientific psychology. 1895 [Google Scholar]
- 125.Freud S.On aphasia: a critical study. 1891. [Google Scholar]
- 126.Kaplan-Solmes K, Solmes M.Clinical studies in neuropsychoanalysis: introduction to a depth neuropsychology. NY, London: Karnac; 2002. [Google Scholar]
- 127.Pennebacker J.Confession, inhibition and disease. Beerkowitz L, Advances in experimental social psychology. Vol 22 New York: Academic Press; 1989. pp. 211-244. [Google Scholar]
- 128.Pennebacker J.Opening up: the healing power of confiding in others. New York: Morrow; 1990. [Google Scholar]
- 129.Pennebacker J.Putting stress into words: health, linguistic, and therapeutic implication. Behav Res Ther. 1993;31:536-48. [DOI] [PubMed] [Google Scholar]
- 130.Berry DS, Pennebacker JW.Nonverbal and verbal emotions. Psychother Psychosom. 1993;59:11-9. [DOI] [PubMed] [Google Scholar]
- 131.Hickok G, Poppel D.Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67-99. [DOI] [PubMed] [Google Scholar]
- 132.Hickok G, Poppel D.The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393-402. [DOI] [PubMed] [Google Scholar]
- 133.Indefrey P, Levelt WJM.The spacial and temporal signatures of word production components. Cognition. 2004;92:101-44. [DOI] [PubMed] [Google Scholar]
- 134.Wong PC, Parsons LM, Martinez M, Diehl RL.The role of the insular cortex in pitch pattern perception: the effect of linguistic contexts. J Neurosci. 2004;24:1953-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gandour J, Wong D, Hutchins G.Pitch processing in the human brain is influenced by language experience. Neuroreport. 1998;9:2115-9. [DOI] [PubMed] [Google Scholar]
- 136.Gandour J, Wong D, Hsieh L, et al. Crosslinguistic PET study of tone perception. J Cogn Neurosci. 2000;14:207-22. [DOI] [PubMed] [Google Scholar]
- 137.Gandour J, Wong D, Lowe M, et al. A cross-linguistic fMRI study of spectral and temporal cues underlying phonological processing. J Cogn Neurosci. 2002;14: 1076-87. [DOI] [PubMed] [Google Scholar]
- 138.Gandour J, Dzemidzic M, Wong D, et al. Temporal integration of speech prosody is shaped by language experience: an fMRI study. Brain Lang. 2003;84:318-36. [DOI] [PubMed] [Google Scholar]
- 139.Klein D, Zatorre RJ, Milner B, Zhao V.A cross-linguistic PET study of ton perception in Mandarin Chinese and English speakers. Neuroimage. 2001;13:646-53. [DOI] [PubMed] [Google Scholar]
- 140.Wong PCM, Perrachione TK, Parrish TB.Neural characteristics of successful and less successful speech and word learning in adults. Hum Brain Mapp. 2007;28:995-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xu Y, Gandour J, Talavage T, et al. Activation of the left planum temporale in pitch processing is shaped by language experience. Hum Brain Mapp. 2006;27:173-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zatorre RJ, Evans AC, Meyer E, Gjedde A.Lateralization of phonetic and and pitch discrimination in speech processing. Science. 1992;265:846-9. [DOI] [PubMed] [Google Scholar]
- 143.Zatorre RJ, Evans AC, Meyer E.Neural mechanisms underlying melodic perception and memory for pitch. J Neurosci. 1994;14:1908-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Apfel RJ, Sifneos PE.Alexithymia: concept and measurement. Psychother Psychosom. 1979;32:180-90. [DOI] [PubMed] [Google Scholar]
- 145.Sifneos PE.Alexithymia and its relationship to hemispheric specialization, affect and creativity. Psychiatr Clin North Am. 1988;11:287-301. [PubMed] [Google Scholar]
- 146.Shipko S, Alvarez WA, Noviello N.Toward a teleological model of alexithymia. Psychother Psychosom. 1983;39:122-6. [DOI] [PubMed] [Google Scholar]
- 147.Krystal JH, Giller EL, Cicchetti DV.Assessment of alexithymia in posttraumatic stress disorder and somatic illness: introduction of a reliable measure. Psychosom Med. 1986;48:84-94. [DOI] [PubMed] [Google Scholar]
- 148.Teicher MH, Dumont NL, Ito Y, et al. Childhood neglect is associated with reduced corpus callosum area. Biol Psychiatry. 2004;56:80-5. [DOI] [PubMed] [Google Scholar]
- 149.Teicher MH, Samson JA, Sheu YS, et al. Hurtful words: association of exposure to peer verbal abuse with elevated psychiatric symptom scores and corpus callosum abnormalities. Am J Psychiatry. 2010;167:1464-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hoppe KD.Hemispheric specialization and creativity. Psychiatr Clin North Am. 1988;11:303-15. [PubMed] [Google Scholar]
- 151.Gaylord C.The effects of transcendental meditation technique and progressive muscle relaxation on EEG coherence, stress reactivity, and mental health in black adults. Intern J Neurosci. 1989;46:77-86. [DOI] [PubMed] [Google Scholar]
- 152.Wallace RK, Benson H, Wilson AF.A wakeful hypometabolic state. Am J Physiol. 1971;221:795-9. [DOI] [PubMed] [Google Scholar]
- 153.Solmes M, Turnbull O.The brain and the inner world: an introduction to the neuroscience of subjective experience. London: Karnac; 2002. [Google Scholar]
- 154.Mandonnet E, Nouet A, Gatignol P, et al. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain. 2007;130:623-9. [DOI] [PubMed] [Google Scholar]
- 155.Catani M, Jones D, Donato R, Fytche DH.Occipito-temporal connections in the human brain. Brain. 2003;126:2093-107. [DOI] [PubMed] [Google Scholar]
- 156.MacLean PD.The triune brain. New York: Plenum Press; 1990. [Google Scholar]
- 157.Damasio AR.Descartes’ error. New York: G.P. Putnam and Sons; 1994. [Google Scholar]
- 158.Damasio AR.Descartes’ error and the future of human life. Sci Am. 1994;271:144. [DOI] [PubMed] [Google Scholar]
- 159.Schmahmann JD.From movement to thought: anatomic substrates of the cerebellar contributions to cognitive processing. Hum Brain Mapp. 1996;4:174-98. [DOI] [PubMed] [Google Scholar]
- 160.Allen G, McColl R, Barnard H, et al. Magnetic resonance imaging of cerebellar-prefrontal and cerebellar-parietal functional connectivity. Neuroimage. 2005;28:39-48. [DOI] [PubMed] [Google Scholar]
- 161.Hebb D.The organization of behavior. New York: Wiley; 1949. [Google Scholar]
- 162.Huang H, Zhang J, Wakana S, et al. White and gray matter development in human fetal, newborn and pediatric brains. Neuroimage. 2006;33:27-38. [DOI] [PubMed] [Google Scholar]
- 163.Novac A.The pseudoborderline syndrome: a proposal based on case studies. J Nerv Ment Dis. 1986;174:84-91. [PubMed] [Google Scholar]
- 164.Blinder BJ.Psychodyanmic neurobiology. Beitman B, Blinder BJ, Thase M, et al., Integrating psychotherapy and pharmacotherapy. Dissolving the mind brain barrier. New York: W. Norton and Co; 2004. pp 161-180. [Google Scholar]
- 165.Kilgard MP, Merzenich MM.Cortical map organization enabled by nucleus basalis activity. Science. 1998;279:1714-8. [DOI] [PubMed] [Google Scholar]
- 166.Stacy R, Wong R.Developmental relationship between cholinergic amacrine cell processes and ganglion cell dendrites of the mouse retina. J Comp Neurol. 2003;456:154-66. [DOI] [PubMed] [Google Scholar]
- 167.Wong WT, Wong RO.Changing specificity of neurotransmitter regulation of rapid dendritic remodeling during synaptogenesis. Nat Neurosci. 2001;4:251-352. [DOI] [PubMed] [Google Scholar]
- 168.Wong RO, Ghosh A.Activity-dependant regulation of dendritic growth and patterning. Nat Neurosci. 2002;3:803-12. [DOI] [PubMed] [Google Scholar]
- 169.Lohmann C, Wong R.Regulation of dendritic growth and plasticity by local and global calcium dynamic. Cell Calcium. 2005;37:403-9. [DOI] [PubMed] [Google Scholar]
- 170.Lohmann C, Myhr KL, Wong RO.Transmitter-evoked local calcium release stabilizes developing dendrites. Nature. 2002;418:177-81. [DOI] [PubMed] [Google Scholar]
- 171.Alberini CM.Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 2005;28:51-6. [DOI] [PubMed] [Google Scholar]
- 172.Alberini CM.Long-term memories: the good, the bad, and the ugly. 2010. Available from: http://www.dana.org/Cerebrum/2010/Long-term_Memories__The_Good,_the_Bad,_and_the_Ugly/ [PMC free article] [PubMed] [Google Scholar]
- 173.Shors T, Miesegas G, Beylin A, et al. Neurogenesis in the adult involved in the formation of trace memories. Nature. 2001;15:314-317. [DOI] [PubMed] [Google Scholar]
- 174.Lisman JE.Hippocampus, II. In:Tamminga CA.Images in Neuroscience. Am J Psychiatry. 2005;162:239. [Google Scholar]
- 175.Greene RW.Hippocampus, V. In:Tamminga CA.Images in Neuroscience. Am J Psychiatry. 2005;162:856 [Google Scholar]
- 176.Heckers S, Titone D.Hippocampus, IV. In:Tamminga CA.Images in Neuroscience. Am J Psychiatry. 2005;162:663. [DOI] [PubMed] [Google Scholar]
- 177.Alberini CM, Milekic MH, Tronel S.Mechanisms of memory stabilization and de-stabilization. Cell Mol Life Sci. 2006;63:999-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tronel S, Milekic MH, Alberini CM.Linking new information to a reactivated memory requires consolidation and not reconsolidation mechanisms. PLoS Biol 2005:3:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Altman J.Are new neurons formed in the brain of adult mammals? Science. 1962;135:1127-8. [DOI] [PubMed] [Google Scholar]
- 180.Altman J, Das G.Autoradiography and histological evidence of post natal hippocampal neurogenesis in rats. J Comp Neuro. 1965;124:319-35. [DOI] [PubMed] [Google Scholar]
- 181.Gould E, Reeves A, Graziano M, Gross C.Neurogenesis in the neocortex of adult primates. Science. 1999;286:548-52. [DOI] [PubMed] [Google Scholar]
- 182.Gould E, Tanapat P, Rydel T, Hastimhs N.Regulation of hippocampal neurogenesis in adulthood. Biol Psychiatry. 2000;45:7134. [DOI] [PubMed] [Google Scholar]
- 183.Gould E, Vail N, Wagers M, Gross C.Adult generated hippocampal and neocortical neurons in Macaques have a transient existence. Proc Natl Acad Sci USA. 2001;98:10910-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gould E, Gross C.Neurogenesis in adult mammals some progress and problems. J Neurosci. 2002;22:619-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Doidge N.The brain that changes itself: Stories of personal triumph from the frontiers of brain science. London: Penguin Books; 2007. [Google Scholar]
- 186.Kornack DR, Rakic P.Cell proliferation without neurogenesis in adult primate cortex. Science. 2001;294:2127-30. [DOI] [PubMed] [Google Scholar]
- 187.Rakic P.Neurogenesis in adult primate neocortex: an evaluation of the evidence. Nat Ref Neurosci. 2002;3:65-71. [DOI] [PubMed] [Google Scholar]
- 188.Markakis EA, Palmer TD, Rudolph-Moore L, et al. Novel neuronal phenotypes from neural progenitor cells. J Neurosci. 2004;24:2886-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shors TJ, Townsend DA, Zhao M, et al. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Johnston M, Nishimura A, Harum K, et al. Sculpting the developing brain. Advances in pediatrics. Vol. 48 Maryland Heights: Mosby; 2001, pp 1-38. [PubMed] [Google Scholar]
- 191.Bao S, Chang EF, Davis JD, et al. Progressive degradation and subsequent refinement of acoustic representation in the adult auditory cortex. J Neurosci. 2003;23:10765-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bear MF, Connors BW, Paradiso MA.Neuroscience. Exploring the brain. Baltimore: Lippincott William & Wilkins; 2001. [Google Scholar]
- 193.LeDoux J.Synaptic self. London: Penguin; 2002. [Google Scholar]
- 194.Bliss TV, Collingridge GL, Morris RGM.Long-term potential: enhancing neuroscience for 30 years. Oxford: Oxford University Press; 2003. [Google Scholar]
- 195.Jeffery K, Reid I.Modifiable neuronal connections: an overview for psychiatrists. Am J Psychiatry. 1997;154:156-64. [DOI] [PubMed] [Google Scholar]
- 196.Ansermet F, Magistretti P.Biology of freedom: neural plasticity, experience, and the unconscious. London: Karnac; 2007. [Google Scholar]
- 197.Si K, Linquist S, Kandel E.A neuronal iso-form of aplysia CPEB has prion-like properties. Cell. 2003;115:879-91. [DOI] [PubMed] [Google Scholar]
- 198.Martin KC, Casadio A, Zhu H, et al. Synapse-specific, long-term facilitation of aplysia sensory to motor synapses: a function of local protein synthesis in memory storage. Cell. 1997;91:927-38. [DOI] [PubMed] [Google Scholar]
- 199.Wu L, Wells D, Tay J, et al. CPEB-mediated cytoplasmic polyadenylation and the regulation of the experience-dependant translation of alpha-CaMKII mRNA at synapses. Neuron. 1998;21:1129-39. [DOI] [PubMed] [Google Scholar]
- 200.Papassotiropoulos A, Wollmer MA, Aguzzi A, et al. The prion gene is associated with human long-term memory. Hum Mol Genet. 2005;14:2241-6. [DOI] [PubMed] [Google Scholar]
- 201.Alberini CM.Transcription factors in long-term memory and synaptic plasticity. Physiol Rev. 2009;89:121-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lynch G, Rex CS, Gall CM.LTP consolidation: substrates, explanatory power, and functional significance. Neuropharmacology. 2007;52:12-23. [DOI] [PubMed] [Google Scholar]
- 203.Larson J, Lynch G.Theta pattern stimulation and the induction of LTP: the sequence in which synapses are stimulated determines the degree to which they potentiate. Brain Res. 1989;489:49-58. [DOI] [PubMed] [Google Scholar]
- 204.Lynch G, Gall CM.Ampakines and the threefold path to cognitive enhancement. Trends Neurosci. 2006;29:554-62. [DOI] [PubMed] [Google Scholar]
- 205.Lauterborn JC, Truong GS, Baudry M, et al. Chronic elevation of brain-derived neurotrophic factor by ampakines. J Pharmacol Exp Ther. 2003;307:297-305. [DOI] [PubMed] [Google Scholar]
- 206.Rex CS, Lin CY, Kramár EA, et al. Brain-derived neurotrophic factor promotes long-term potentiation-related cytoskeletal changes in adult hippocampus. J Neurosci. 2007;27:3017-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pribram KH.Brain and perception. Hillsdale: Lawrence Erlbaum Associates; 1991. [Google Scholar]
- 208.Crick F, Koch C.A framework for consciousness. Nat Neurosci. 2003;6:119-26. [DOI] [PubMed] [Google Scholar]
- 209.Viamontes GI.Signal transduction in psychiatry. Psychiatr Ann. 2008;39:235-24. [Google Scholar]
- 210.Gomperts BD, Kramer IM, Tatham PE.Signal transduction. San Diego: Elsevier Academic Press; 2002. [Google Scholar]
- 211.Gedo J.Encore. J Am Psychoanal Assoc. 2005;43:384-92. [Google Scholar]
- 212.Rizzolatti G, Fadiga L, Fofassi L, Gallese V.Resonance behaviors and mirror neurons. Arch Italien Biol. 1999;137:85-100. [PubMed] [Google Scholar]
- 213.Wolf NS, Gales M, Shane E, Shane M.Mirror neurons, procedural learning, and the positive new experience; A developmental systems self psychology approach. J Am Acad Psychoanalysis. 2002;8:409-30. [DOI] [PubMed] [Google Scholar]
- 214.Williams JHG, Whiten A, Suddendorf T, Perrett DI.Imitation, mirror neurons and autism. Neurosci Behav Rev. 2001;25:287-95. [DOI] [PubMed] [Google Scholar]
- 215.Iacoboni M.Mirroring people. New York: Farrar, Strauss & Giroux; 2008. pp 106-130. [Google Scholar]
- 216.Watt D.Toward a neuroscience of empathy: integrating affective and cognitive perspectives. Neuropsychoanalysis. 2007;9:119-72. [Google Scholar]
- 217.Bailey P, Davis EW.Effects of lesions of the periaqueductal gray matter in the cat. Proc Soc Exp Biol Med. 1942;351:305-6. [Google Scholar]
- 218.Bailey P, Davis EW.Effects of lesions of the periaqueductal gray matter on the macacam mulata. J Neuropathol Exp Neurol. 1943;3:69-72. [Google Scholar]
- 219.Benson H, Herd JA, Morse WH, Kelleher RT.Behavioral induction of arterial hypertension and its reversal. Am J Physiol. 1969;217:30-4. [DOI] [PubMed] [Google Scholar]
- 220.Benson H, Klipper M.The relaxation response. New York: Harper Collins; 2000. [Google Scholar]
- 221.Dusek JA, Benson H.Mind-body medicine: a model of the comparative clinical impact of the acute stress and relaxation responses. Minn Med. 2009;92:47-50. [PMC free article] [PubMed] [Google Scholar]
- 222.Chen C, Xue G, Mei L, et al. Cultural neurolinguistics. Chiao JY, Progress in brain research. Vol 178. Cultural neuroscience: cultural influences on brain function. Amsterdam: Elsevier, 2009. pp 159-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sheline YI, Pieper CF, Barch DM, et al. Support for the vascular depression hypothesis in late life depression: results of a 2-site prospective antidepressant treatment trial. Arch Gen Psychiatry. 2010;67:277-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Quirk GJ.Memory for extinction of conditioned fear id long-lasting and persists following spontaneous recovery. Learn Mem. 2002;9:402-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Schiller D, Cain CK, Curley NG, et al. Evidence for recovery of fear following immediate extinction in rats and humans. Learn Mem. 2008;15:394-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Frewen P, Lane RD, Neufeld RW, et al. Neural correlates of levels of emotional awareness during trauma script-imagery in posttraumatic stress disorder. Psychosom Med. 2008;70:27-31. [DOI] [PubMed] [Google Scholar]
- 227.Lotze M, Scheler G, Tan HRM, et al. The musician’s brain: functional imaging of amateur and professionals during performance and imagery. Neuroimage. 2003;20:1817-29. [DOI] [PubMed] [Google Scholar]
- 228.Schore AN.Interdisciplinary developmental research as a source of clinical models. Moskowitz M, Monk C, Kaye C, Ellman S, The neurobiological and developmental basis for psychotherapeutic intervention. Northvale: Aronson; 1997. pp 171 [Google Scholar]
- 229.Yehuda R, Engel SM, Brand SR, et al. Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the World Trade Center attacks during pregnancy. J Clin Endocrinol Metab. 2008;90:4115-8. [DOI] [PubMed] [Google Scholar]


