Abstract
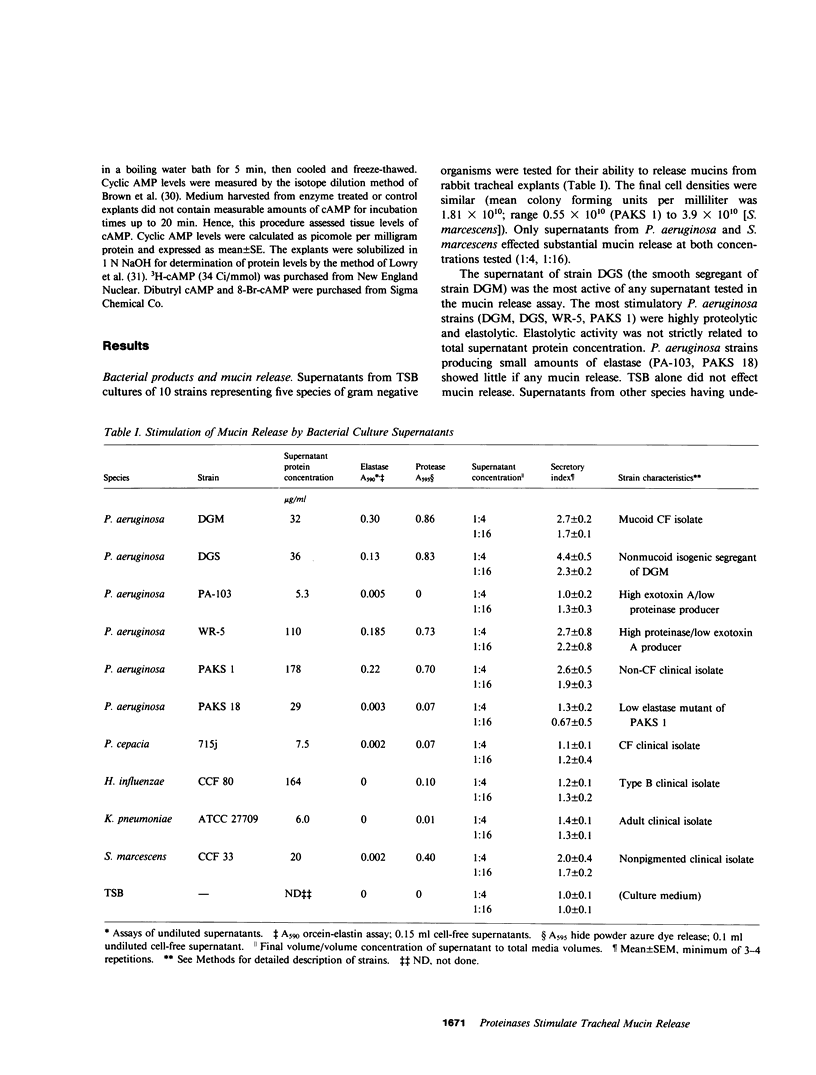
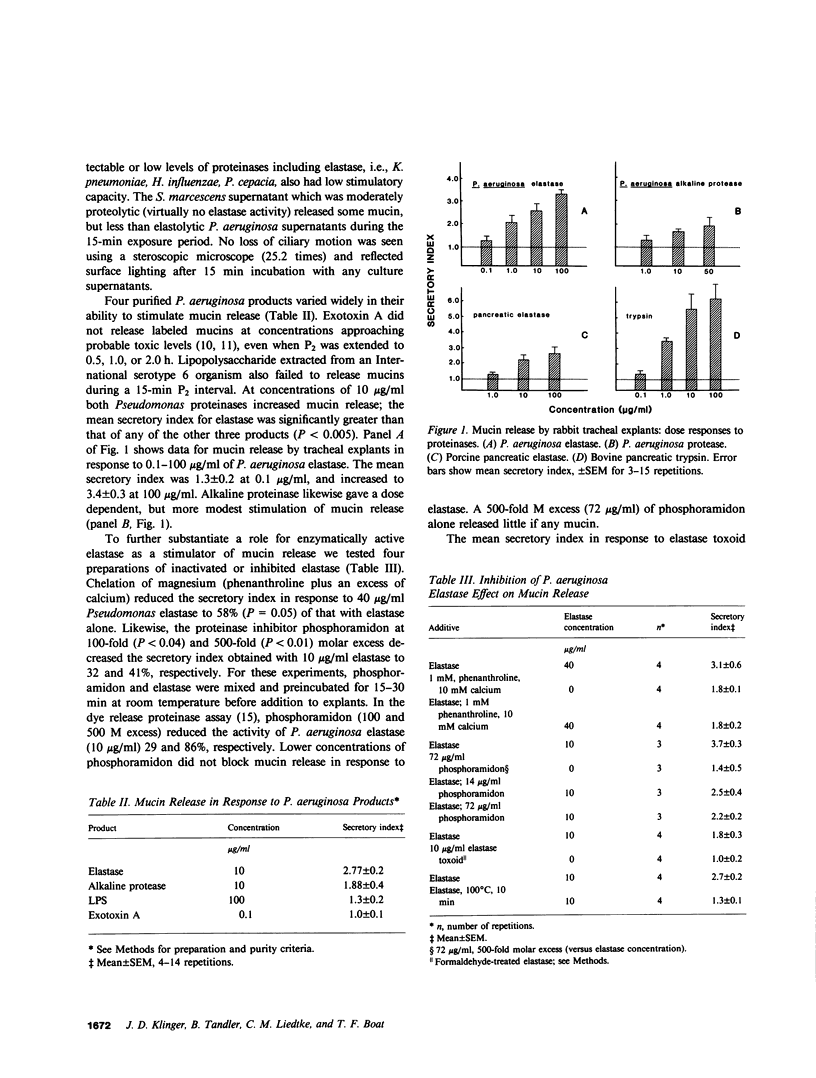
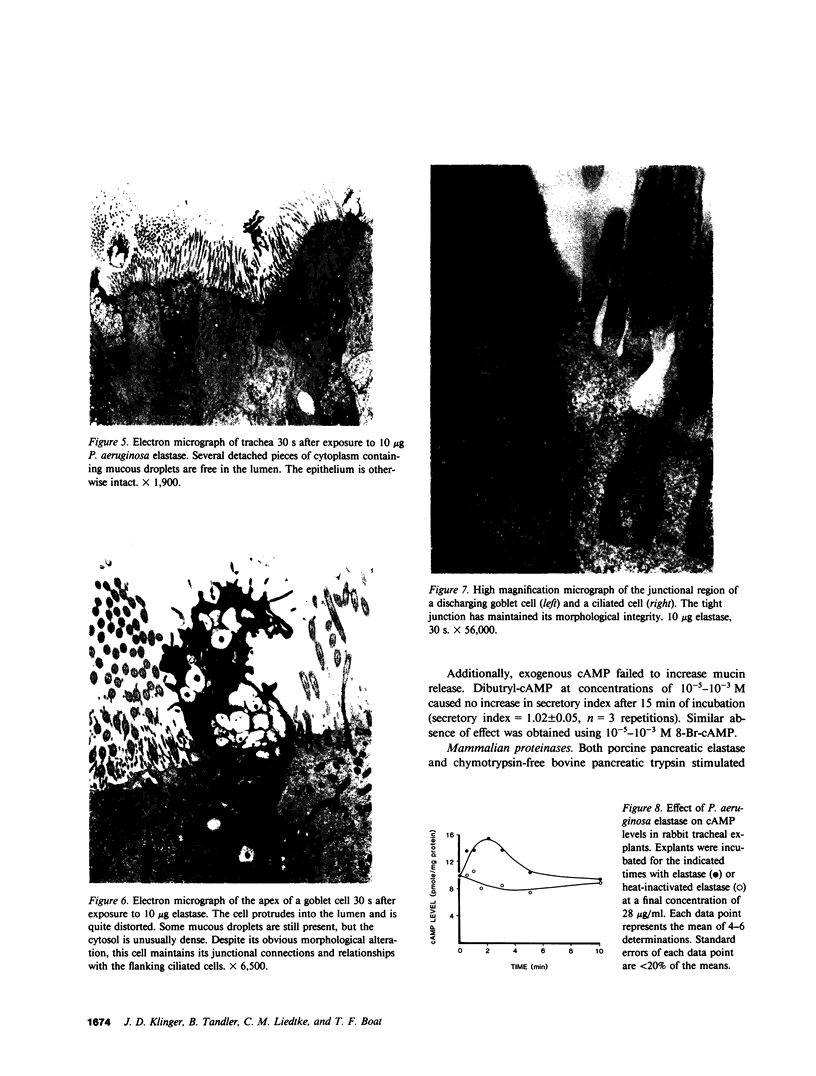
We have determined the potential of exoproducts from pathogenic bacteria to stimulate the release of high molecular weight mucins from goblet cells of airway epithelium in a rabbit tracheal explant system. Culture supernatants from proteolytic strains of Pseudomonas aeruginosa and Serratia marcescens, but not supernatants from a number of non-proteolytic strains, released mucins from goblet cells. Highly purified elastase and alkaline proteinase from P. aeruginosa stimulated goblet cell mucin release in a dose-dependent fashion. Lipopolysaccharide, exotoxin A, and alginate of P. aeruginosa did not possess mucin release properties. Proteolytic activity was required for mucin release by P. aeruginosa elastase, but such release in goblet cells was not mediated by cyclic AMP. Morphologic studies suggested rapid release of mucins from goblet cells was response to elastase by a process resembling apocrine secretion. Several nonbacterial proteinases mimicked the effect of Pseudomonas proteases. These studies provide support for the hypothesis that bacterial and other play a role in the pathogenesis of mucus hypersecretion in acute and chronic lung infections.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler K. B., Hardwick D. H., Craighead J. E. Effect of cholera toxin on secretion of mucin by explants of guinea pig trachea. Lab Invest. 1981 Oct;45(4):372–377. [PubMed] [Google Scholar]
- Adler K. B., Winn W. C., Jr, Alberghini T. V., Craighead J. E. Stimulatory effect of Pseudomonas aeruginosa on mucin secretion by the respiratory epithelium. JAMA. 1983 Mar 25;249(12):1615–1617. [PubMed] [Google Scholar]
- BIERRING F. Electron microscopic observations on the mucus production in human and rat intestinal goblet cells. Acta Pathol Microbiol Scand. 1962;54:241–252. doi: 10.1111/j.1699-0463.1962.tb01752.x. [DOI] [PubMed] [Google Scholar]
- BJORKMAN N. Low magnification electron microscopy in histological work. Acta Morphol Neerl Scand. 1962;4:344–348. [PubMed] [Google Scholar]
- Baker A. P., Hillegass L. M., Holden D. A., Smith W. J. Effect of kallidin, substance P, and other basic polypeptides on the production of respiratory macromolecules. Am Rev Respir Dis. 1977 May;115(5):811–817. doi: 10.1164/arrd.1977.115.5.811. [DOI] [PubMed] [Google Scholar]
- Boat T. F., Kleinerman J. I. Human respiratory tract secretions. 2. Effect of cholinergic and adrenergic agents on in vitro release of protein and mucous glycoprotein. Chest. 1975 Feb;67(2 Suppl):32S–34S. doi: 10.1378/chest.67.2_supplement.32s. [DOI] [PubMed] [Google Scholar]
- Boat T. F., Polony I., Cheng P. W. Mucin release from rabbit tracheal epithelium in response to sera from normal and cystic fibrosis subjects. Pediatr Res. 1982 Sep;16(9):792–797. doi: 10.1203/00006450-198209000-00017. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown B. L., Ekins R. P., Albano J. D. Saturation assay for cyclic AMP using endogenous binding protein. Adv Cyclic Nucleotide Res. 1972;2:25–40. [PubMed] [Google Scholar]
- Cheng P. W., Sherman J. M., Boat T. F., Bruce M. Quantitation of radiolabeled mucous glycoproteins secreted by tracheal explants. Anal Biochem. 1981 Nov 1;117(2):301–306. doi: 10.1016/0003-2697(81)90782-x. [DOI] [PubMed] [Google Scholar]
- Christensen T. G., Korthy A. L., Snider G. L., Hayes J. A. Irreversible bronchial goblet cell metaplasia in hamsters with elastase-induced panacinar emphysema. J Clin Invest. 1977 Mar;59(3):397–404. doi: 10.1172/JCI108652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döring G., Obernesser H. J., Botzenhart K. Extrazelluläre Toxine von Pseudomonas aeruginosa. II. Einwirkung zweier gereinigter Proteasen auf die menschlichen Immunoglobuline IgG, IgA und sekretorisches IgA. Zentralbl Bakteriol A. 1981 Mar;249(1):89–98. [PubMed] [Google Scholar]
- FLOREY H. W. Electron microscopic observations on goblet cells of the rat's colon. Q J Exp Physiol Cogn Med Sci. 1960 Oct;45:329–336. doi: 10.1113/expphysiol.1960.sp001487. [DOI] [PubMed] [Google Scholar]
- FREEMAN J. A. Fine structure of the goblet cell mucous secretory process. Anat Rec. 1962 Dec;144:341–357. doi: 10.1002/ar.1091440406. [DOI] [PubMed] [Google Scholar]
- Franken C., Kramps J. A., Meyer C. J., Dijkman J. H. Localization of a low molecular weight protease inhibitor in the respiratory tract. Bull Eur Physiopathol Respir. 1980;16 (Suppl):231–236. doi: 10.1016/b978-0-08-027379-2.50024-7. [DOI] [PubMed] [Google Scholar]
- Freeman J. A. Goblet cell fine structure. Anat Rec. 1966 Jan;154(1):121–147. doi: 10.1002/ar.1091540111. [DOI] [PubMed] [Google Scholar]
- Gibson J. J., Jr, Brodsky R. E., Schultz M. G. Changing patterns of malaria in the United States. J Infect Dis. 1974 Nov;130(5):553–555. doi: 10.1093/infdis/130.5.553. [DOI] [PubMed] [Google Scholar]
- HOLLMANN K. H. The fine structure of the goblet cells in the rat intestine. Ann N Y Acad Sci. 1963 Mar 30;106:545–554. doi: 10.1111/j.1749-6632.1963.tb16664.x. [DOI] [PubMed] [Google Scholar]
- Homma J. Y., Abe C., Tanamoto K., Hirao Y., Morihara K., Tsuzuki H., Yanagawa R., Honda E., Aoi Y., Fujimoto Y. Effectiveness of immunization with single and multi-component vaccines prepared from a common antigen (OEP), protease and elastase toxoids of Pseudomonas aeruginosa on protection against hemorrhagic pneumonia in mink due to P. aeruginosa. Jpn J Exp Med. 1978 Apr;48(2):111–133. [PubMed] [Google Scholar]
- Iglewski B. H., Sadoff J. C. Toxin inhibitors of protein synthesis: production, purification, and assay of Pseudomonas aeruginosa toxin A. Methods Enzymol. 1979;60:780–793. doi: 10.1016/s0076-6879(79)60071-x. [DOI] [PubMed] [Google Scholar]
- Janoff A., Sloan B., Weinbaum G., Damiano V., Sandhaus R. A., Elias J., Kimbel P. Experimental emphysema induced with purified human neutrophil elastase: tissue localization of the instilled protease. Am Rev Respir Dis. 1977 Mar;115(3):461–478. doi: 10.1164/arrd.1977.115.3.461. [DOI] [PubMed] [Google Scholar]
- Johnson D. A., Carter-Hamm B., Dralle W. M. Inactivation of human bronchial mucosal proteinase inhibitor by Pseudomonas aeruginosa elastase. Am Rev Respir Dis. 1982 Dec;126(6):1070–1073. doi: 10.1164/arrd.1982.126.6.1070. [DOI] [PubMed] [Google Scholar]
- Johnson G. G., Morris J. M., Berk R. S. The extracellular protease from Pseudomonas aeruginosa exhibiting elastase activity. Can J Microbiol. 1967 Jun;13(6):711–719. doi: 10.1139/m67-093. [DOI] [PubMed] [Google Scholar]
- Konrádová V. The ultrastructure of the tracheal epithelium in rabbit. Folia Morphol (Praha) 1966;14(3):210–214. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liedtke C. M., Boat T. F., Rudolph S. A. Neurohormonal receptors and cyclic AMP-binding proteins in rabbit tracheal mucosa-submucosa. Biochim Biophys Acta. 1982 Nov 24;719(2):169–177. doi: 10.1016/0304-4165(82)90086-1. [DOI] [PubMed] [Google Scholar]
- Liedtke C. M., Rudolph S. A., Boat T. F. Beta-adrenergic modulation of mucin secretion in cat trachea. Am J Physiol. 1983 May;244(5):C391–C398. doi: 10.1152/ajpcell.1983.244.5.C391. [DOI] [PubMed] [Google Scholar]
- Liu P. V. Exotoxins of Pseudomonas aeruginosa. I. Factors that influence the production of exotoxin A. J Infect Dis. 1973 Oct;128(4):506–513. doi: 10.1093/infdis/128.4.506. [DOI] [PubMed] [Google Scholar]
- Lyerly D. M., Kreger A. S. Importance of serratia protease in the pathogenesis of experimental Serratia marcescens pneumonia. Infect Immun. 1983 Apr;40(1):113–119. doi: 10.1128/iai.40.1.113-119.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLONIG G. A modified procedure for lead staining of thin sections. J Biophys Biochem Cytol. 1961 Dec;11:736–739. doi: 10.1083/jcb.11.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marom Z., Shelhamer J. H., Kaliner M. Effects of arachidonic acid, monohydroxyeicosatetraenoic acid and prostaglandins on the release of mucous glycoproteins from human airways in vitro. J Clin Invest. 1981 Jun;67(6):1695–1702. doi: 10.1172/JCI110207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Response of cultured mammalian cells to the exotoxins of Pseudomonas aeruginosa and Corynebacterium diphtheriae: differential cytotoxicity. Can J Microbiol. 1977 Feb;23(2):183–189. doi: 10.1139/m77-026. [DOI] [PubMed] [Google Scholar]
- Morihara K., Tsuzuki H., Oda K. Protease and elastase of Pseudomonas aeruginosa: inactivation of human plasma alpha 1-proteinase inhibitor. Infect Immun. 1979 Apr;24(1):188–193. doi: 10.1128/iai.24.1.188-193.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morihara K., Tsuzuki H., Oka T. Comparison of the specificities of various neutral proteinases from microorganisms. Arch Biochem Biophys. 1968 Mar 11;123(3):572–588. doi: 10.1016/0003-9861(68)90179-3. [DOI] [PubMed] [Google Scholar]
- Morihara K., Tsuzuki H. Phosphoramidon as an inhibitor of elastase from Pseudomonas aeruginosa. Jpn J Exp Med. 1978 Feb;48(1):81–84. [PubMed] [Google Scholar]
- Nadel J. A., Davis B. Parasympathetic and sympathetic regulation of secretion from submucosal glands in airways. Fed Proc. 1980 Nov;39(13):3075–3079. [PubMed] [Google Scholar]
- Neutra M. R., O'Malley L. J., Specian R. D. Regulation of intestinal goblet cell secretion. II. A survey of potential secretagogues. Am J Physiol. 1982 Apr;242(4):G380–G387. doi: 10.1152/ajpgi.1982.242.4.G380. [DOI] [PubMed] [Google Scholar]
- Ohlsson K., Tegner H., Akesson U. Isolation and partial characterization of a low molecular weight acid stable protease inhibitor from human bronchial secretion. Hoppe Seylers Z Physiol Chem. 1977 May;358(5):583–589. doi: 10.1515/bchm2.1977.358.1.583. [DOI] [PubMed] [Google Scholar]
- Peatfield A. C., Barnes P. J., Bratcher C., Nadel J. A., Davis B. Vasoactive intestinal peptide stimulates tracheal submucosal gland secretion in ferret. Am Rev Respir Dis. 1983 Jul;128(1):89–93. doi: 10.1164/arrd.1983.128.1.89. [DOI] [PubMed] [Google Scholar]
- Pinkett M. O., Strewler G. J., Anderson W. B. Stimulation of adenylate cyclase activity by a protease which activates cyclic nucleotide phosphodiesterase. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1159–1165. doi: 10.1016/0006-291x(79)91158-6. [DOI] [PubMed] [Google Scholar]
- Reimer A., Klementsson K., Ursing J., Wretlind B. The mucociliary activity of the respiratory tract. I. Inhibitory effects of products of Pseudomonas aeruginosa on rabbit trachea in vitro. Acta Otolaryngol. 1980 Nov-Dec;90(5-6):462–469. doi: 10.3109/00016488009131749. [DOI] [PubMed] [Google Scholar]
- Rhodin J. A. The ciliated cell. Ultrastructure and function of the human tracheal mucosa. Am Rev Respir Dis. 1966 Mar;93(3 Suppl):1–15. doi: 10.1164/arrd.1966.93.3P2.1. [DOI] [PubMed] [Google Scholar]
- Rinderknecht H., Geokas M. C., Silverman P., Haverback B. J. A new ultrasensitive method for the determination of proteolytic activity. Clin Chim Acta. 1968 Aug;21(2):197–203. doi: 10.1016/0009-8981(68)90127-7. [DOI] [PubMed] [Google Scholar]
- STEMPAK J. G., WARD R. T. AN IMPROVED STAINING METHOD FOR ELECTRON MICROSCOPY. J Cell Biol. 1964 Sep;22:697–701. doi: 10.1083/jcb.22.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz D. R., Miller K. D. Elastase of Pseudomonas aeruginosa: inactivation of complement components and complement-derived chemotactic and phagocytic factors. Infect Immun. 1974 Jul;10(1):128–135. doi: 10.1128/iai.10.1.128-135.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelhamer J. H., Marom Z., Kaliner M. Immunologic and neuropharmacologic stimulation of mucous glycoprotein release from human airways in vitro. J Clin Invest. 1980 Dec;66(6):1400–1408. doi: 10.1172/JCI109993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman J. M., Cheng P. W., Tandler B., Boat T. F. Mucous glycoproteins from cat tracheal goblet cells and mucous glands separated with EDTA. Am Rev Respir Dis. 1981 Oct;124(4):476–479. doi: 10.1164/arrd.1981.124.4.476. [DOI] [PubMed] [Google Scholar]
- Specian R. D., Neutra M. R. Mechanism of rapid mucus secretion in goblet cells stimulated by acetylcholine. J Cell Biol. 1980 Jun;85(3):626–640. doi: 10.1083/jcb.85.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton J. Manipulating cardiac murmurs. Chest. 1982 Feb;81(2):135–136. doi: 10.1378/chest.81.2.135. [DOI] [PubMed] [Google Scholar]
- Tandler B., Walter R. J. Epon-maraglas embedment for electron microscopy. Stain Technol. 1977 Jul;52(4):238–239. doi: 10.3109/10520297709116783. [DOI] [PubMed] [Google Scholar]
- Taylor N. S., Pollack M. Purification of Pseudomonas aeruginosa exotoxin by affinity chromatography. Infect Immun. 1978 Jan;19(1):66–70. doi: 10.1128/iai.19.1.66-70.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trier J. S., Rubin C. E. Electron microscopy of the small intestine: a review. Gastroenterology. 1965 Nov;49(5):574–603. [PubMed] [Google Scholar]
- Wiggins J., Elliott J. A., Stevenson R. D., Stockley R. A. Effect of corticosteroids on sputum sol-phase protease inhibitors in chronic obstructive pulmonary disease. Thorax. 1982 Sep;37(9):652–656. doi: 10.1136/thx.37.9.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R. E., Boat T. F., Doershuk C. F. Cystic fibrosis. Am Rev Respir Dis. 1976 Jun;113(6):833–878. doi: 10.1164/arrd.1976.113.6.833. [DOI] [PubMed] [Google Scholar]
- Wretlind B., Kronevi T. Experimental infections with protease-deficient mutants of Pseudomonas aeruginosa in mice. J Med Microbiol. 1978 May;11(2):145–154. doi: 10.1099/00222615-11-2-145. [DOI] [PubMed] [Google Scholar]









