Abstract
In healthy humans, ageing is typically associated with reduced skeletal muscle blood flow and vascular conductance during exercise. Further, there is a marked increase in resting sympathetic nervous system (SNS) activity with age, yet whether augmented SNS-mediated α-adrenergic vasoconstriction contributes to the age-associated impairment in exercising muscle blood flow and vascular tone in humans is unknown. We tested the hypothesis that SNS-mediated vasoconstriction is greater in older than young adults and limits muscle (forearm) blood flow (FBF) during graded handgrip exercise (5, 15, 25% maximal voluntary contraction (MVC)). FBF was measured (Doppler ultrasound) and forearm vascular conductance (FVC) was calculated in 11 young (21 ± 1 years) and 12 older (62 ± 2 years) adults in control conditions and during combined local α- and β-adrenoreceptor blockade via intra-arterial infusions of phentolamine and propranolol, respectively. Under control conditions, older adults exhibited significantly lower FBF and FVC at 15% MVC exercise (22.6 ± 1.3 vs. 29 ± 3.3 ml min−1 100 g forearm fat-free mass (FFM)−1 and 21.7 ± 1.2 vs. 33.6 ± 4.0 ml min−1 100 g FFM−1 100 mmHg−1; P < 0.05) and 25% MVC exercise (37.4 ± 1.4 vs. 46.0 ± 4.9 ml min−1 100 g FFM−1 and 33.7 ± 1.4 vs. 49.0 ± 5.7 ml min−1 100 g FFM−1 100 mmHg−1; P < 0.05), whereas there was no age group difference at 5% MVC exercise. Local adrenoreceptor blockade increased FBF and FVC at rest and during exercise in both groups, although the increase in FBF and FVC from rest to steady-state exercise was similar in young and older adults across exercise intensities, and thus the age-associated impairment in FBF and FVC persisted. Our data indicate that during graded intensity handgrip exercise, the reduced FVC and subsequently lower skeletal muscle blood flow in older healthy adults is not due to augmented sympathetic vasoconstriction, but rather due to impairments in local signalling or structural limitations in the peripheral vasculature with advancing age.
Introduction
The regulation of skeletal muscle blood flow and oxygen delivery to contracting skeletal muscle is complex and involves the mechanical effects of muscle contraction, local metabolic and endothelial-derived substances, and the sympathetic nervous system (SNS; Saltin et al. 1998). With advancing age in humans, skeletal muscle blood flow is reduced during dynamic exercise and this is due to a lower vascular conductance, which could ultimately contribute to age-associated reductions in aerobic exercise capacity (Poole et al. 2003; Proctor et al. 2004; Fleg et al. 2005; Burtscher, 2013), a primary predictor of mortality in both healthy and diseased ageing populations (Kokkinos et al. 2008). This lower muscle blood flow in older adults has been observed in studies involving both large (e.g. upright cycling) and small (e.g. handgrip and knee extensor) muscle mass exercise, the latter of which occurs independent of any limitations in central circulatory capacity (i.e. cardiac output) (Proctor et al. 1998; Poole et al. 2003; Lawrenson et al. 2004; Kirby et al. 2009, 2012; Barrett-O'Keefe et al. 2014). Although the majority of studies have demonstrated impaired blood flow and vascular conductance during exercise in older adults compared to their younger counterparts, the mechanisms underlying this impairment have yet to be fully elucidated.
With human ageing, several changes in both autonomic nervous system and peripheral vascular function occur that could conceivably lead to a net decline in skeletal muscle blood flow during exercise due to impaired local regulation of vascular tone. One such change is the marked ∼2- to 3-fold increase in basal SNS activity observed in older adults (Sundlof & Wallin, 1978; Davy et al. 1997), and one primary target of this sympathoexcitation with age is the skeletal muscle resistance vessels of both the upper (Dinenno et al. 2002a) and the lower limbs (Dinenno et al. 1999). How this elevated basal sympathoexcitation with age impacts muscle blood flow regulation during steady-state dynamic exercise is presently unknown.
Recently, Jackson et al. (2010) observed sympathetic α-adrenergic modulation of the rapid vasodilatation in response to single contractions in ageing mice. Under control conditions, these investigators found that contraction-induced rapid vasodilatation was significantly blunted in older male mice, similar to what we originally demonstrated in humans (Carlson et al. 2008; Kirby et al. 2009). Following α-blockade with phentolamine, the age group differences in male mice were abolished. An interesting observation in this study was that there were no age group differences in basal vasoconstrictor tone, and when low doses of noradrenaline were applied to vessels of young mice to stimulate α-adrenoreceptors in the absence of significant vasoconstriction, the rapid dilatory response was blunted similar to what was observed in the older mice. In a study designed to translate these findings to humans, Casey and Joyner (2012) also demonstrated that tonic sympathetic α-vasoconstriction limits the rapid dilatation to a single muscle contraction in the forearm of older men, explaining the majority of the impairment with age in humans. Thus, it appears that greater sympathetic outflow and tonic stimulation of post-junctional α-adrenoreceptors may attenuate the rapid vasodilatory and hyperaemic response to muscle contraction independent of greater vasoconstrictor tone, perhaps by interfering with signals associated with ascending vasodilatation from the microvascular network (Jackson et al. 2010). Whether age-related changes in sympathetic regulation of vascular tone limit muscle blood flow during steady-state exercise in older adults is currently unknown.
With this information as a background, the purpose of the present study was to test the hypothesis that compared to young adults, the lower forearm blood flow and vascular conductance during graded handgrip exercise in older adults is due to elevated α-adrenergic vasoconstrictor tone that restrains exercising muscle haemodynamics with age. To do so, we determined forearm blood flow and vascular conductance at rest and during graded exercise prior to and during local (intra-arterial) adrenoreceptor blockade to eliminate α-mediated vasoconstrictor influences on vascular control in young and older healthy adults.
Methods
Subjects
With Institutional Review Board approval and following written informed consent, 11 young (7 male, 4 female) and 12 older (8 male, 4 female) healthy subjects participated in the present study. All subjects were free from overt cardiovascular disease as assessed from a medical history, were sedentary to moderately active, non-smokers, non-obese, normotensive and not taking any medications including over-the-counter supplements (Table1). Older subjects were further evaluated for clinical evidence of cardiopulmonary disease with a physical examination and resting and maximal exercise electrocardiograms. Females were studied during the placebo phase of birth control or during the early follicular phase of their menstrual cycle to minimize any potential vascular effects of sex hormones and all older females were post-menopausal not taking hormone replacement. All studies were performed in the Human Cardiovascular Physiology Laboratory located at Colorado State University following a 12-h fast with the subjects in the supine position. The experimental arm of the subject was slightly elevated above heart level to minimize any potential influence of the muscle pump on forearm haemodynamics (Tschakovsky et al. 1996). All studies were performed according to the Declaration of Helsinki.
Table 1.
Subject characteristics
| Variable | Young | Older |
|---|---|---|
| Male: female | 7:4 | 8:4 |
| Age | 21 ± 1 | 62 ± 2* |
| Height (cm) | 172.6 ± 3.5 | 173.0 ± 2.8 |
| Weight (kg) | 77.5 ± 4.4 | 77.6 ± 4.7 |
| BMI (kg m–2) | 25.8 ± 1.2 | 25.3 ± 0.8 |
| % Body fat | 25.7 ± 3.8 | 31.1 ± 2.4 |
| Whole body fat free mass (kg) | 54.1 ± 2.9 | 49.7 ± 2.7 |
| Forearm volume (ml) | 933.2 ± 67.4 | 908.4 ± 60.3 |
| Forearm fat free mass (g) | 764.7 ± 82.5 | 710.2 ± 64.1 |
| Maximal voluntary contraction (kg) | 39 ± 2 | 36 ± 4 |
| 5% Workload (kg) | 1.8 ± 0.2 | 1.8 ± 0.2 |
| 15% Workload (kg) | 5.5 ± 0.6 | 5.4 ± 0.5 |
| 25% Workload (kg) | 9.2 ± 0.9 | 9.0 ± 0.8 |
| Triglycerides (mmol l−1) | 2.2 ± 0.3 | 2.4 ± 0.3 |
| Cholesterol (mmol l−1) | 3.4 ± 0.1 | 4.2 ± 0.2* |
| HDL (mmol l−1) | 1.1 ± 0.1 | 1.2 ± 0.1 |
| LDL (mmol l−1) | 1.8 ± 0.1 | 2.4 ± 0.1* |
HDL, high-density lipoprotein; LDL, low-density lipoprotein. Values are mean ± SEM. *P < 0.05 vs. Young.
Arterial catheterization
The non-dominant arm was chosen to be the experimental arm and after local application of anaesthesia (2% lidocaine), a 20-guage, 7.6 cm catheter was inserted into the brachial artery via an aseptic technique. The catheter was connected to a pressure transducer for continuous monitoring of mean arterial pressure (MAP) as well as a three-port connector to allow for drug infusions and blood sampling (Dinenno et al. 2002b; Crecelius et al. 2011). Throughout the duration of the study, heparinized saline (2 U ml−1) was continuously infused at a rate of 3 ml min−1. Heart rate (HR) was monitored via three-lead ECG.
Body composition and forearm volume
Dual-energy X-ray absorptiometry (DEXA; Hologic, Bedford, MA, USA) was used to determine body composition. A regional analysis of the experimental forearm area (proximal to distal radio-ulnar joint) from the whole body DEXA scan was performed to determine both forearm volume (FAV) and forearm fat-free mass (FFM). Drug doses were normalized according to FAV (Dinenno et al. 2002a). Body mass index was calculated as body weight (kg) divided by height (metres) squared.
Graded handgrip exercise
Maximum voluntary contraction (MVC) was determined from the experimental arm for each subject as the average of at least three maximal squeezes of a handgrip dynamometer (Stoelting, Chicago, IL, USA) that were within 3% of each other. Using a pulley system with weights attached, graded handgrip exercise was performed whereby individuals contracted at 5, 15 and 25% of their MVC for 4 min at each workload. Handgrip exercise was performed for a total of 12 min with a duty cycle of 1 s contraction and 2 s relaxation using both audio and visual cues to ensure correct timing of contraction and relaxation (Dinenno & Joyner, 2003, 2004). These workloads represent approximately 15, 40 and 70% of maximum work rate (WRmax) as assessed by a graded maximal handgrip exercise test to fatigue.
Forearm blood flow (FBF) and vascular conductance (FVC)
Brachial artery mean blood velocity (MBV) was determined using a 4 MHz pulsed Doppler ultrasound probe (model 500M; Multigon Industries, Mt Vernon, NY, USA) which was securely fixed to the skin and the probe insonation angle was 45 deg relative to the skin (Dinenno & Joyner, 2003, 2004; Markwald et al. 2011). Brachial artery diameter was determined using a 7 MHz ultrasound probe (Sonos 4500; Hewlett Packard, Andover, MA, USA) and measurements were made in duplex mode at end-diastole in triplicate during steady-state conditions (Dinenno & Joyner, 2003, 2004; Markwald et al. 2011). FBF was calculated as FBF = MBV × π (brachial artery diameter/2)2 × 60, where FBF is in ml min−1, MBV is in cm s−1, the brachial diameter is in centimetres and 60 was used to convert from ml s−1 to ml min−1. As an index of vascular tone, FVC was calculated as (FBF/MAP)×100, and expressed as ml min−1 100 mmHg−1. All studies were performed in a cool (20°C) temperature-controlled environment with a fan directed toward the forearm to minimize the contribution of skin blood flow to forearm haemodynamics.
Regional adrenoreceptor blockade
To eliminate α-adrenergic-mediated vasoconstriction at rest and during graded handgrip exercise, we locally infused phentolamine mesylate (Bedford Laboratories, Bedford, OH, USA), a non-selective α-adrenoreceptor antagonist, for 10 min prior to exercise (12 μg dl−1 FAV min−1) and maintained the infusion throughout the exercise trial (5 μg dl−1 FAV min−1) (Dinenno et al. 2001, 2002aa). Administering a non-selective α-adrenergic antagonist can inhibit α2-adrenergic receptors on sympathetic nerve endings and facilitate noradrenaline release, which is able to bind β-adrenoreceptors located on the endothelium and vascular smooth muscle and elicit vasodilatation (Saeed et al. 1982). Therefore, to limit any contribution of β-adrenoreceptor-mediated vasodilatation, we locally infused propranolol hydrochloride (Baxter, Deerfield, IL, USA), a non-selective β-adrenoreceptor antagonist, for 10 min prior to exercise (10 μg dl FAV−1 min−1) and continued the infusion at a maintenance rate (5 μg dl FAV−1 min−1) throughout the exercise trial (Dinenno et al. 2001, 2002a,b). Doses of both α- and β-adrenoreceptor antagonists were previously demonstrated to be highly effective at blocking any vasoconstriction and vasodilatation to both α- and β-adrenoreceptor agonists, respectively (Johnsson, 1967; Dinenno et al. 2001, 2002a,b). Further, we recently demonstrated that a slightly lower (∼10%) loading and maintenance dose of phentolamine is effective at eliminating α-adrenoreceptor vasoconstriction over a 3-h time frame in the human forearm (Markwald et al. 2011).
Endothelium-dependent and endothelium-independent dilatation
To characterize resistance vessel function of the participants and to determine if α-adrenergic vasoconstrictor signalling limited endothelium-dependent or endothelium-independent vasodilatation, acetylcholine (Ach; Miochol-E; Novartis Inc., Basel, Switzerland) and sodium nitroprusside (SNP; Nitropress; Hospira Inc., Lake Forest, IL, USA) were locally administered at 16 and 4 μg 100 ml FAV−1 min−1 for 3 min each, respectively (Kirby et al. 2009). Drug order was randomized and a minimum of 10 min of rest occurred between drug administrations. Due to time constraints for a few participants, these infusions were performed in 8 young and 11 older subjects.
Experimental protocol
The overall study timeline is presented in Fig.1. All participants arrived in the morning after an overnight fast. All measurements were performed with the subjects in the supine position within a temperature-controlled room with a fan directed towards the arm to reduce skin blood flow. The order of all measures was randomized in both control and under adrenoreceptor blockade conditions. All study drugs were administered via brachial artery catheter and were dosed according to forearm volume and infused via a Harvard infusion syringe pump. Briefly, ∼30 min post-catheterization, endothelium-dependent and endothelium-independent vasodilatation was assessed, with a 10 min break between dilator infusions. Subsequent to vasodilator infusions and after a 20 min break, all subjects performed the graded exercise protocol under control (saline) conditions, consisting of 2 min of rest followed by 4 min of exercise at each workload (5, 15, and 25% MVC). During regional adrenoreceptor blockade, both the graded exercise protocol and assessment of endothelium-dependent and endothelium-independent vasodilatation were repeated in random order and in a similar time frame as described for the control (pre-blockade) trials.
Figure 1. Study timeline.

Following insertion of the brachial artery catheter, subjects rested quietly for 30–45 min and then either exercise or vasodilator infusion began (randomized). Endothelium-dependent and endothelium-independent vasodilatation was determined in random order utilizing local infusions of acetylcholine (Ach) and sodium nitroprusside (SNP), respectively. During the graded handgrip (HG) exercise trials, participants exercised for 4 min each at 5, 15 and 25% of their maximal voluntary contraction (MVC). The last 30 s of haemodynamics for baseline within a given trial, vasodilator infusion and each exercise workload was used to determine steady-state levels for each variable. Following the completion of control trials, phentolamine and propranolol were administered via the catheter and continuously infused to eliminate local sympathetic vasoconstriction, and the trials were repeated in random order.
Data acquisition/analysis
Data were collected and stored on a computer at 250 Hz and later analysed off-line with signal processing software (Windaq; DATAQ Instruments, Akron, OH, USA). MAP was determined from the brachial artery pressure waveform. FBF, FVC, HR and MAP represent an average of the last 30 s of each time period. For the exercise trials, FBF and FVC were normalized per 100 g FFM. A repeated measures ANOVA was used to determine differences within and between groups, with our primary interest in age group differences. When appropriate, post hoc comparisons were made using Tukey's HSD and significance was set at P < 0.05. All values are presented as means ± SEM.
Results
Subject characteristics
The mean age difference between young and older subjects was 41 years. There were no significant age group differences in any measure of whole body anthropometrics or regional tissue composition. MVC was not different between groups, and thus absolute exercise workloads were similar in young and older adults. Triglycerides and high-density lipoprotein-cholesterol were also not different between groups. Although within a normal range, older adults had significantly greater total and low-density lipoprotein-cholesterol (Table1).
FBF and FVC at rest and during graded handgrip exercise in control conditions
In the control trial, there were no differences in resting FBF between older and young adults (Older: 4.8 ± 0.3 vs. Young: 3.7 ± 0.3 ml min−1 100 g FFM−1). During exercise, FBF was significantly lower in older adults during both 15% MVC (22.6 ± 1.4 vs. 29.0 ± 3.3 ml min−1 100 g FFM−1; P < 0.05) and 25% MVC (37.4 ± 1.4 vs. 46.0 ± 4.9 ml min−1 100 g FFM−1; P < 0.05) handgrip exercise, whereas there was no age-associated impairment at 5% MVC (Older: 10.7 ± 0.8 vs. Young: 10.5 ± 1.3 ml min−1 100 g FFM−1; Fig.2A). While MAP was not significantly different between old and young at rest (Older: 94 ± 2 vs. Young: 88 ± 3 mmHg; P = 0.06), older adults had significantly elevated MAP across all exercise intensities (Table2).
Figure 2. Forearm blood flow.
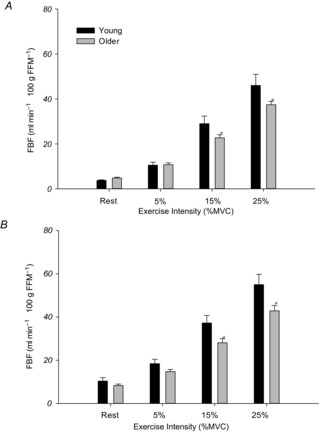
FBF per 100 g of forearm fat free mass (FFM) at rest and during exercise in control conditions (A) and during local adrenoreceptor blockade (B). In control conditions (A) older adults had significantly lower FBF at 15 and 25% MVC workloads. During local adrenoreceptor blockade (B) resting and exercising blood flows were greater in both groups compared to control conditions, but the age-associated impairment in exercising blood flow persisted at both 15 and 25% MVC. *P < 0.05 vs. Young.
Table 2.
Heart rate and blood pressure at rest and during graded handgrip exercise in control and local adrenoreceptor blockade conditions
| Baseline |
5% MVC |
15% MVC |
25% MVC |
|||||
|---|---|---|---|---|---|---|---|---|
| Young | Older | Young | Older | Young | Older | Young | Older | |
| Control | ||||||||
| HR (beats min−1) | 58 ± 3 | 57 ± 3 | 61 ± 3 | 64 ± 3 | 67 ± 4 | 65 ± 3 | 70 ± 5 | 71 ± 3 |
| MAP (mmHg) | 88.2 ± 2.9 | 94.0 ± 2.1 | 87.4 ± 2.6 | 100.1 ± 2.4* | 89.9 ± 2.9 | 103.9 ± 3.4* | 96.5 ± 3.1 | 110.4 ± 3.0* |
| α + β-Adrenoreceptor blockade | ||||||||
| HR (beats min−1) | 56 ± 4 | 56 ± 3 | 57 ± 3 | 58 ± 3 | 62 ± 4 | 62 ± 3 | 68 ± | 67 ± 4 |
| MAP (mmHg) | 89.3 ± 2.7 | 96.8 ± 4.0* | 89.9 ± 2.6 | 100.6 ± 3.5* | 93.1 ± 3.1 | 102.4 ± 3.0* | 96.5 ± 2.7 | 104.0 ± 3.6* |
Values are mean ± SEM. *P < 0.05 vs. Young.
Similar to FBF, there were no differences in resting FVC between older and young adults in control conditions (Older: 5.1 ± 0.4 vs. Young: 4.2 ± 0.4 ml min−1 100 g FFM−1 100 mmHg−1). Older adults exhibited significantly lower FVC during both 15% MVC (21.7 ± 1.2 vs. 33.6 ± 4.0 ml min−1 100 g FFM−1 100 mmHg−1; P < 0.05) and 25% MVC (33.7 ± 1.4 vs. 49.0 ± 5.7 ml min−1 100 g FFM−1 100 mmHg−1; P < 0.05) exercise, whereas there was no age-associated impairment at 5% MVC (Fig.3A).
Figure 3. Forearm vascular conductance.
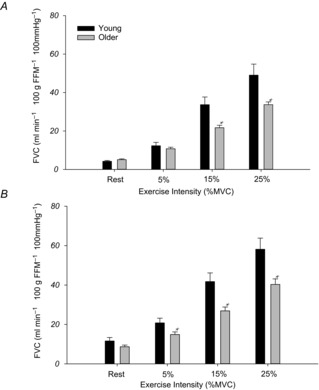
FVC per 100 g of forearm fat free mass (FFM) at rest and during exercise in control conditions (A) and during local adrenoreceptor blockade (B). In control conditions (A) older adults had significantly lower FVC at 15 and 25% MVC workloads. During local adrenoreceptor blockade (B) resting and exercising FVC were greater in both groups compared to control conditions, but the age-associated impairment in exercising FVC persisted at both 15 and 25% MVC, and was also evident at 5% MVC. *P < 0.05 vs. Young.
Effect of local adrenoreceptor blockade on FBF and FVC conductance at rest and during graded handgrip exercise
During local adrenoreceptor blockade, resting FBF increased significantly from control rest as expected in both groups (both P < 0.05), although there were no significant differences between older and young adults (Older: 8.3 ± 0.6 vs. Young: 10.3 ± 1.6 ml min−1 100 g FFM−1; P = 0.23; Fig.2B). During graded handgrip exercise in both groups, steady-state hyperaemia was augmented compared to control conditions (all P < 0.05), but the age-associated impairment in exercising FBF persisted at both 15% MVC (28.0 ± 1.8 vs. 37.2 ± 3.4 ml min−1 100 g FFM−1; P < 0.05) and 25% MVC (42.8 ± 2.5 vs. 54.9 ± 4.7 ml min−1 100 g FFM−1; P < 0.05, Fig.2B). There were no significant differences in FBF during 5% MVC exercise between older and young adults (Older: 14.7 ± 1.0 vs. Young: 18.4 ± 2.0 ml min−1 100 g FFM−1; P = 0.11). Additionally, MAP was significantly elevated in the older group at rest and across all exercise intensities (Table2).
During adrenoreceptor blockade, resting FVC increased significantly from control rest as expected in both groups (both P < 0.05), but there were no significant differences between older and young adults (Older: 8.6 ± 0.8 vs. Young: 11.5 ± 1.7 ml min−1 100 g FFM−1 100 mmHg−1; P = 0.07). During handgrip exercise, FVC was significantly lower at all workloads in older adults: 5% (14.9 ± 1.2 vs. 20.7 ± 2.3 ml min−1 100 g FFM−1 100 mmHg−1), 15% (26.8 ± 1.9 vs. 41.7 ± 4.3 ml min−1 100 g FFM−1 100 mmHg−1) and 25% MVC (40.3 ± 2.8 vs. 58.1 ± 5.6 ml min−1 100 g FFM−1 100 mmHg−1; all P < 0.05) (Fig.3B).
Sympathetic restraint of FBF and FVC
In an effort to quantify the degree of sympathetic α-adrenergic restraint of exercising muscle blood flow and vascular tone, we calculated the increase in absolute FBF and FVC from rest to steady-state exercise at each exercise intensity in both control and α-blockade conditions. In control conditions, older adults had a significantly smaller increase in FBF from rest to both 15% (ΔFBF: 144 ± 12 vs. 182 ± 10 ml min−1; P < 0.05) and 25% MVC handgrip exercise (ΔFBF: 246 ± 16 vs. 298 ± 17 ml min−1; P < 0.05) (Fig.4A). During local adrenoreceptor inhibition, older adults still exhibited smaller increases in blood flow at both 15% MVC (ΔFBF: 159 ± 16 vs. 194 ± 15 ml min−1; P < 0.05) and 25% MVC (ΔFBF: 264 ± 27 vs. 313 ± 21 ml min−1; P < 0.05).
Figure 4. Changes in forearm haemodynamics.
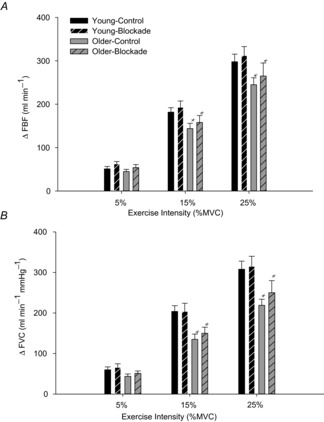
Increase in forearm haemodynamics from rest to steady-state exercise in control conditions (A) and during local adrenoreceptor blockade (B). In control conditions, older subjects had a significantly smaller increase in FBF (A) and FVC (B) from rest compared to young adults at both 15 and 25% MVC workloads. During adrenoreceptor blockade, older adults still had a significantly smaller increase in FBF (A) and FVC (B) from rest at both 15 and 25% MVC workloads. *P < 0.05 vs. Young.
Additionally, the increase in FVC from rest to exercise in control conditions was significantly lower in older adults at both 15% MVC (ΔFVC: 135 ± 13 vs. 205 ± 14 ml min−1 100 mmHg−1; P < 0.05) and 25% MVC (ΔFVC: 216 ± 14 vs. 308 ± 20 ml min−1 100 mmHg−1; P < 0.05, Fig.4B). During local adrenoreceptor inhibition, this age-associated impairment persisted at both 15% MVC (ΔFVC: 150 ± 14 vs. 204 ± 20 ml min−1 100 mmHg−1; P < 0.05) and 25% MVC (ΔFVC: 250 ± 27 vs. 315 ± 23 ml min−1 100 mmHg−1; P < 0.05). The increase in FVC from rest to exercise was not different from control conditions in either group, indicating that α-adrenergic vasoconstrictor tone did not limit skeletal muscle vasodilatation during exercise (Fig.4B).
Endothelium-dependent and endothelium-independent vasodilatation
Absolute FBF and FVC at rest and in response to Ach and SNP prior to and during sympathetic blockade are shown in Table3. Compared to young adults, older adults demonstrated a smaller increase in FBF and FVC to Ach (ΔFBF: 80 ± 18 vs. 228 ± 69 ml min−1; ΔFVC: 85 ± 19 vs. 274 ± 84 ml min−1 100 mmHg−1; both P < 0.05), whereas the responses to SNP were similar in older and young adults (ΔFBF: Older: 133 ± 20 vs. Young: 170 ± 26 ml min−1; ΔFVC: Older: 169 ± 25 vs. Young: 228 ± 34 ml min−1 100 mmHg−1). During local adrenoreceptor inhibition, despite having a significantly greater increase in FBF and FVC to each vasodilator, the age-associated impairment to Ach persisted (ΔFBF: 178 ± 27 vs. 312 ± 54 ml min−1 mmHg−1; ΔFVC: 184 ± 32 vs. 356 ± 63 ml min−1 100 mmHg−1; both P < 0.05) whereas the increase to SNP remained similar between older and young adults (ΔFBF: Older: 206 ± 26 vs. Young: 263 ± 37 ml min−1, P = 0.20; ΔFVC: Older: 250 ± 31 vs. Young: 350 ± 40 ml min−1 100 mmHg−1, P = 0.08).
Table 3.
Heart rate, blood pressure and forearm haemodynamics during infusion of acetylcholine and sodium nitroprusside
| Baseline |
Ach |
Baseline |
SNP |
|||||
|---|---|---|---|---|---|---|---|---|
| Young | Older | Young | Older | Young | Older | Young | Older | |
| Control | ||||||||
| HR (beats min−1) | 52 ± 3 | 59 ± 3 | 52 ± 3 | 60 ± 3 | 52 ± 3 | 61 ± 3 | 64 ± 4 | 66 ± 4 |
| MAP (mmHg) | 86.4 ± 3.0 | 94.0 ± 2.3* | 84.7 ± 3.2 | 93.6 ± 2.6* | 86.9 ± 3.4 | 94.2 ± 4.2* | 75.6 ± 2.3 | 81.8 ± 3.2 |
| FBF (ml min−1) | 30.9 ± 4.4 | 39.1 ± 4.2 | 258.8 ± 71.0 | 119.0 ± 20.1* | 30.1 ± 4.2 | 33.9 ± 3.6 | 200.1 ± 28.6 | 167.0 ± 21.6 |
| FVC (ml min−1 100 mmHg−1) | 35.8 ± 5.0 | 41.2 ± 4.2 | 309.9 ± 86.4 | 125.9 ± 20.4* | 34.7 ± 4.7 | 36.4 ± 3.4 | 263.2 ± 36.5 | 205.8 ± 27.5 |
| α + β-Adrenoreceptor blockade | ||||||||
| HR (beats min−1) | 52 ± 3 | 60 ± 3 | 55 ± 3 | 64 ± 3 | 52 ± 2 | 60 ± 3 | 61 ± 3 | 68 ± 3 |
| MAP (mmHg) | 89.5 ± 3.8 | 98.9 ± 2.7* | 87.6 ± 2.8 | 99.7 ± 3.1* | 92.1 ± 3.1 | 99.1 ± 3.4* | 78.1 ± 2.1 | 86.2 ± 2.8* |
| FBF (ml min−1) | 93.1 ± 17.5 | 75.2 ± 7.5 | 405.5 ± 60.5 | 252.7 ± 29.4* | 82.6 ± 7.9 | 74.0 ± 7.1 | 346.0 ± 41.3 | 279.8 ± 30.2 |
| FVC (ml min−1 100 mmHg−1) | 102.0 ± 16.5 | 75.7 ± 7.5 | 457.7 ± 68.3 | 259.7 ± 34.4* | 89.3 ± 7.8 | 74.4 ± 6.4 | 439.6 ± 50.2 | 324.0 ± 34.5* |
Values are mean ± SEM. *P < 0.05 vs. Young. Young: n = 8, Older: n = 11.
Discussion
The primary findings of the present study are as follows. First, compared to young adults, older adults demonstrate impaired skeletal muscle blood flow during graded handgrip exercise, and this is due to reductions in vascular conductance. Second, the increase in muscle blood flow and vascular conductance during exercise after removal of sympathetic α-adrenergic vasoconstrictor tone in older adults was lower compared to young adults, similar to what was observed under control conditions. As such, the age-associated impairment in exercising muscle blood flow and vascular conductance persisted during local blockade of sympathetically mediated vasoconstriction. Third, older subjects exhibited significantly lower endothelium-dependent vasodilatation that persisted during local blockade of α-adrenoreceptors. The collective data indicate that the age-related impairments in skeletal muscle blood flow and vascular conductance are not due to greater sympathetic vasoconstriction during graded forearm exercise in older adults.
Age and basal forearm sympathetic α-adrenergic vasoconstrictor tone
Previous studies in the forearm circulation have demonstrated either reduced or similar basal levels of sympathetic vasoconstriction in older compared to young healthy humans, despite greater SNS activity (Hogikyan & Supiano, 1994; Dinenno et al. 2002a; Casey & Joyner, 2012). In the present study, local α-adrenoreceptor blockade via intra-arterial phentolamine increased resting FBF and FVC in both young and older subjects, and the older subjects tended to have lower FBF (P = 0.2) and FVC (P = 0.07) upon removal of sympathetic vasoconstrictor tone. Although not the primary purpose of this experiment, these data are in general agreement with previous studies on this topic, and are consistent with experimental data indicating reduced post-junctional α-adrenoreceptor responsiveness to endogenous noradrenaline release in the face of elevated skeletal muscle sympathetic outflow with advancing age in humans (Hogikyan & Supiano, 1994; Dinenno et al. 2002a; Smith et al. 2007).
Sympathetic α-adrenergic vasoconstriction during graded handgrip exercise
To our knowledge, this is the first study to determine the role of sympathetic α-adrenergic vasoconstriction in modulating blood flow to active skeletal muscle during graded, steady-state handgrip exercise in both young and older adults. Previously, Joyner et al. (1992) employed stellate ganglionic blockade to remove efferent sympathetic outflow in young adults and measured FBF (venous occlusion plethysmography) in response to incremental, rhythmic isometric handgrip exercise to exhaustion (1 min stages; 4 min total). The investigators found that the change in FBF above resting values was similar at mild to moderate workloads, but was significantly increased at the highest workloads, indicating that sympathetic vasoconstriction restrained blood flow. One consideration, beyond use of a different handgrip exercise modality and protocol (e.g. non-steady-state conditions), is the use of venous occlusion plethysmography to measure blood flow during exercise, a technique that requires the subjects to stop exercising during the measurement period and thus can overestimate blood flow to the muscle (Lutjemeier et al. 2005). In contrast, use of Doppler ultrasound permits the continuous measure of active muscle blood flow, including both anterograde and retrograde flows, while subjects continue to contract their muscles. In the present study, during local adrenoreceptor blockade, blood flow and vascular conductance were elevated at rest and during exercise in a parallel fashion (Figs 2 and 3), indicating that there was not an exercise intensity-dependent degree of sympathetic restraint in young adults during handgrip exercise. In fact, the increase in FBF and FVC from rest to steady-state exercise did not significantly differ between control and blockade conditions (Fig.4). These data are consistent with observations by Wilkins et al. (2008) who demonstrated similar changes in FBF and FVC after local α-blockade during two separate trials of dynamic handgrip exercise (10 and 20% MVC). Note that the workloads utilized in our study (5, 15 and 25% MVC) represent a range of ∼15–70% of maximum work rate as determined by a maximal graded handgrip exercise to fatigue (Table4). Thus, in this model of small muscle mass steady-state forearm exercise, there does not appear to be any sympathetic restraint of muscle blood flow and vascular conductance across a wide range of workloads beyond what is present under resting conditions.
Table 4.
Maximal work rate (WRmax) during handgrip exercise
| Sex | MVC (kg) | MVC (lb) | WRmax (lb) | %WRmax (5% MVC) | %WRmax (15% MVC) | %WRmax (25% MVC) |
|---|---|---|---|---|---|---|
| F | 30 | 66 | 23.4 | 14 | 42 | 70.5 |
| F | 32 | 70.4 | 23.4 | 14.9 | 45.1 | 75.2 |
| M | 43 | 94.6 | 40 | 12.5 | 37.8 | 63.06 |
| F | 30 | 66 | 28.4 | 11.6 | 34.9 | 58.1 |
| M | 40 | 88 | 28.4 | 15.4 | 46.4 | 77.4 |
| M | 42 | 92.4 | 33.4 | 13.7 | 41.4 | 69.1 |
| Mean | 36.17 | 79.57 | 29.50 | 13.68 | 41.27 | 68.89 |
| SD | 6.15 | 13.52 | 6.36 | 1.43 | 4.34 | 7.28 |
| SE | 2.51 | 5.52 | 2.60 | 0.59 | 1.77 | 2.97 |
In 6 young adults (3 female, 3 male), using the same pulley system utilized in the study, graded handgrip exercise to fatigue was performed whereby individuals contracted to a duty cycle of 1 s contraction and 2 s relaxation using both audio and visual cues to ensure correct timing of contraction and relaxation. The starting weight was 5 lbs and increased by 2.5 lbs each minute until the subject was no longer able to perform a full contraction. The average test duration was 12 min, similar to that obtained during cycle ergometer or treadmill-based graded exercise tests. MVC, maximum voluntary contraction.
Age and sympathetic α-adrenergic vasoconstriction during graded handgrip exercise
The primary purpose of the present investigation was to determine whether elevated sympathetic α-adrenergic vasoconstriction in older adults explains the impaired muscle blood flow and vascular conductance during graded, steady-state handgrip exercise with age. Consistent with prior observations from our laboratory (Kirby et al. 2009, 2012), FBF and FVC were similar at rest and during mild exercise (5% MVC), but were significantly reduced in older adults during higher intensity exercise (15 and 25% MVC; Fig.2A). Upon removal of sympathetic vasoconstriction, blood flow was similar in young and older adults during mild exercise, but remained significantly lower at the two higher exercise intensities in older adults (Fig.2B). In slight contrast, FVC was lower in older adults during all exercise intensities after local α-adrenoreceptor blockade (Fig.3B). Further, the change in FBF and FVC above resting values in response to exercise were similar across all workloads in both groups of subjects prior to and during adrenoreceptor inhibition (Fig.4), and this increase above rest was impaired in the older adults at the two higher exercise intensities. Taken together, the collective data clearly indicate that augmented sympathetic vasoconstriction or ‘restraint’ does not explain the age-related impairment in muscle blood flow and vascular conductance during graded handgrip exercise in humans.
Our findings during steady-state handgrip exercise contrast recent observations in response to single muscle contractions in both mice and humans. Specifically, Jackson et al. (2010) reported that the rapid onset vasodilatation of second-order (2A) arterioles in the mouse gluteus maximus muscle in response to a single tetanic muscle contraction was significantly blunted with age in males, corroborating original findings from our laboratory in humans (Carlson et al. 2008; Kirby et al. 2009). Interestingly, α-adrenergic blockade via phentolamine normalized the responses in older male mice. Casey and Joyner (2012) followed up these observations in the human forearm and also found that local α-adrenoreceptor blockade appeared to normalize single contraction-induced vasodilatation and hyperaemia in older adults. Note here that both of these observations regarding sympathetic modulation of contraction-induced dilatation occurred in the absence of greater basal vasoconstrictor tone. It is also of interest that topical noradrenaline in the young mice and acute sympathetic activation in young adults blunted rapid vasodilatation similar to older male mice and older humans, and this occurred in the absence of resting arteriolar diameter changes or changes in forearm haemodynamics, respectively (i.e. vasoconstriction), leading to the conclusion that subtle tonic activation of α-adrenoreceptors with age impairs rapid vasodilatation. Neither of these studies determined the role of sympathetic vasoconstriction during graded steady-state exercise, and thus our study is the first to directly assess this in ageing humans. This differing ability of tonic sympathetic α-adrenoreceptor activation to modulate vasodilatory signalling probably reflects differential control mechanisms involved in response to a brief, single contraction versus steady-state exercise (i.e. repeated contractions). For example, our laboratory has previously demonstrated that the age-associated impairment in rapid vasodilatation that occurs following a single muscle contraction is not impacted by intra-arterial infusion of ascorbic acid (vitamin C), whereas the blood flow impairment during steady-state exercise was reversed with infusion of ascorbic acid (Kirby et al. 2009) due to improvements in nitric oxide bioavailability (Crecelius et al. 2010). Thus, although there is clear interest in understanding multiple components of the hyperaemic response to exercise from both a basic physiology and ageing and disease standpoint, the collective data indicate that the ability of tonic sympathetic α-adrenoreceptor activation to modulate vasodilatory signalling in older humans differs for a single contraction and continuous, steady-state exercise.
Endothelium-dependent and endothelium-independent vasodilatation
Older subjects exhibited attenuated vasodilatation to the endothelium-dependent agonist Ach. Endothelial function declines with age and numerous studies note a significant decline in vasodilatation to Ach with ageing (Taddei et al. 2001; Kirby et al. 2009). Following local adrenoreceptor blockade we repeated the infusions of Ach and SNP to determine whether sympathetically mediated vasoconstrictor signalling limited the degree of endothelium-dependent or -independent vasodilatation. Both young and older adults demonstrated greater forearm vasodilatation to each agonist following local adrenoreceptor blockade, although the age impairment to Ach persisted. Thus, our data indicate that impaired endothelial vasodilator function with age is independent of α-adrenergic vasoconstriction in humans. Although not statistically significant, older adults also exhibited attenuated vasodilatation to the endothelium-independent vasodilator SNP, and this appeared more pronounced after α-blockade. Our laboratory has previously observed an age-associated impairment in the responsiveness to SNP (Kirby et al. 2009), and thus it is possible that both impairments in endothelial-dependent signalling and altered smooth muscle responsiveness to local vasodilating substances could be involved in the impaired vascular control during exercise with age.
Ageing and impaired control of skeletal muscle vascular tone during exercise
Potential mechanisms
In the present study, older adults exhibited lower FBF and elevated MAP during handgrip exercise (15 and 25% MVC), a model of exercise that clearly does not press the limits of central circulatory capacity. Despite this elevated perfusion pressure in older adults, net skeletal muscle blood flow remained lower and therefore the impairment is probably due to altered local regulation of skeletal muscle vascular tone. Indeed, FVC was significantly lower in older adults during handgrip exercise in both the control and the α-adrenergic blockade conditions. Thus, it appears that impairments in the bioavailability of, or responsiveness to, local vasodilating substances are probably contributing to the reductions in vascular conductance during exercise with age. In this context, studies have demonstrated that the contribution of endothelial-derived vasodilator substances such as nitric oxide (NO) and prostaglandins are reduced during exercise with advancing age (Schrage et al. 2007), and acute increases in NO bioavailability can improve exercise hyperaemia via local vasodilatation (Kirby et al. 2009; Crecelius et al. 2010). Another local vasodilator that could be involved in the age-related decline in exercise hyperaemia is intravascular ATP. ATP can be released from erythrocytes and endothelial cells in response to stimuli associated with active muscle, including mechanical forces (e.g. resistance vessel compression and shear stress) and haemoglobin deoxygenation (Ellsworth & Sprague, 2012; Crecelius et al. 2013; Kirby et al. 2013), and we have recently demonstrated that in contrast to young adults, plasma ATP draining contracting muscle does not increase in older humans and this could be due to impaired ATP release from erythroctyes (Kirby et al. 2012).
In addition to attenuated bioavailability of local vasodilatory signalling substances, elevated production of local vasoconstrictors may occur with age. In this context, there is an increase in the production of vasoconstricting prostaglandins (Taddei et al. 1995) and endothelin (Donato et al. 2009; Barrett-O'Keefe et al. 2014), the latter which has recently been shown to limit skeletal muscle blood flow during dynamic knee extensor exercise in older adults (Barrett-O'Keefe et al. 2014). Thus, this potential shift in the balance between vasodilatory and vasoconstrictor substances at the local level may limit the ability of older adults to adequately vasodilate and increase blood flow and oxygen delivery during exercise. Finally, it is possible that structural changes in the peripheral vasculature may limit the local vasodilatation during exercise with age (Bearden et al. 2004).
Traditionally, muscle blood flow and oxygen delivery during dynamic exercise are determined by tissue metabolic demand and in the present study both relative and absolute workloads were similar between young and older adults. During dynamic exercise, it has been demonstrated that the lower skeletal muscle perfusion and oxygen delivery during submaximal exercise is compensated for by greater oxygen extraction in older adults, thus maintaining ‘normal’ oxygen consumption for the work performed (Poole et al. 2003). Interestingly, some reports have shown that the metabolic cost of walking is ∼30% greater in older adults across a variety of walking speeds (Mian et al. 2006), and recent work utilizing magnetic resonance spectroscopy reports that the ATP cost of dynamic plantar flexion is significantly elevated in older adults compared to young adults (Layec et al. 2014). Thus, during dynamic exercise, it appears that the impaired local vasodilatation and subsequent blood flow and oxygen delivery with age is not due to a reduction in metabolic demand, and if anything may occur in the face of greater metabolic cost of contractions for a given workload.
Experimental considerations and limitations
In the present study, we utilized the forearm model to investigate the role of the SNS in modulating active muscle blood flow and vascular tone in young and older adults. Use of the forearm model allows for the study of muscle blood flow regulation during graded exercise that is not limited by central circulatory factors (e.g. cardiac output). Therefore, the attenuated blood flow observed in older adults is attributed to an impaired local regulation of vascular tone with age. Additionally, examining the steady-state blood flow response during graded exercise with this model provides insight into whether impairments in local vascular control and subsequent attenuated blood flow and oxygen delivery may contribute to the age-related decline in aerobic capacity, a predictor of mortality among healthy and diseased older adults. From a practical standpoint, our findings could have implications for blood flow and oxygen and nutrient delivery to active muscle of older adults during structured exercise that most individuals perform at moderate intensities for prolonged periods of time (e.g. walking/jogging or cycling at a given pace or intensity).
Given recent work indicating that tonic sympathetic activation with age limits the vasodilator and hyperaemic responses to a single forearm contraction in older adults (Casey & Joyner, 2012), we believed it was important to extend these observations to graded, steady-state exercise in humans. It is important to note that, similar to prior studies from our laboratory and others (Kirby et al. 2009, 2012; Casey & Joyner, 2012), findings from the present study of impaired blood flow and vascular conductance during handgrip exercise with age cannot be explained by differences in absolute work performed by young and older adults (Table1), nor can they be explained by age-related changes in forearm muscle mass. Nonetheless, our conclusion that the age-associated impairment in the regulation of skeletal muscle blood flow is not due to elevated sympathetic vasoconstrictor tone may not be applicable to the lower limb. Thus, future studies are needed to directly determine the role of sympathetic vasoconstriction in regulating vascular tone and thus blood flow delivery to skeletal muscle during lower limb exercise (e.g. knee extensor or cycling) in young and older adults.
One limitation of our study is the inability to determine how local adrenoreceptor inhibition affects the distribution of blood flow to the forearm during handgrip exercise. Recently, Heinonen et al. (2013) assessed the distribution of blood flow via positron emission tomography during knee extensor exercise in young adults both in control conditions and following local α-adrenergic blockade. The investigators observed that although total thigh blood flow was increased during α-blockade, blood flow to contracting muscle was unchanged whereas there was an increase in blood flow to inactive regions of the exercising limb (Heinonen et al. 2013). In the present study, despite increases in resting bulk FBF during α-blockade, the increase in blood flow (and vascular conductance) from baseline to steady-state exercise was not different between the two conditions, suggesting that the SNS did not limit the increase in blood flow during exercise handgrip in either young or older adults. Despite our inability to assess the regional distribution of blood flow to the forearm during α-adrenergic blockade, our findings are in general agreement with Heinonen et al. (2013) such that the SNS does not appear to limit the blood flow response during exercise with a relatively small muscle mass (forearm and quadriceps, respectively).
Conclusion
Skeletal muscle blood flow during exercise is typically reduced with age in humans and this is associated with lower vascular conductance. The collective findings from the present study indicate that elevated sympathetic vasoconstriction is not mechanistically linked to impaired muscle vasodilatation and perfusion during graded, steady-state handgrip exercise in older healthy humans. As such, following local α- and β-adrenoreceptor inhibition, muscle blood flow and vascular conductance remained ∼20–30% lower in older compared to young adults. This is of interest given recent data in humans indicating that greater tonic stimulation of α-adrenoreceptors can limit the dilatory and hyperaemic responses to single contractions in older adults, and indicates different and/or possibly more complex regulatory mechanisms during steady-state exercise. Furthermore, our data suggest that in this model of exercise, the impairment in vascular control appears to be due to alterations in local vasodilatory, local vasoconstricting or perhaps structural changes in the peripheral vasculature that occur with age.
Acknowledgments
We thank Anne R. Crecelius, Christopher M. Hearon Jr, Matthew L. Racine and Hannah K. Scott for their assistance with the project and the subjects who volunteered to participate.
Glossary
- Ach
acetylcholine
- DEXA
dual-energy X-ray absorptiometry
- FAV
forearm volume
- FBF
forearm blood flow
- FFM
forearm fat-free mass
- FVC
forearm vascular conductance
- HR
heart rate
- MAP
mean arterial pressure
- MBV
mean blood velocity
- MVC
maximal voluntary contraction
- SNP
sodium nitroprusside
- SNS
sympathetic nervous system
Key points
Human ageing is associated with attenuated skeletal muscle blood flow during exercise. It is unknown whether elevated sympathetic nervous system activity with ageing limits skeletal muscle blood flow during exercise. Determining the role of the sympathetic nervous system in regulating blood flow in healthy ageing is clinically relevant as age-related changes in the sympathetic nervous system are exacerbated with many diseases associated with impaired tissue perfusion.
In the present study, compared to young adults, older adults demonstrate impaired skeletal muscle blood flow during graded handgrip exercise, and this is due to reductions in vascular conductance.
Second, the increase in muscle blood flow and vascular conductance during exercise after removal of sympathetic α-adrenergic vasoconstrictor tone in older adults was lower compared to young adults, similar to what was observed under control conditions.
As such, the age-associated impairment in exercising muscle blood flow and vascular conductance persisted during local adrenoreceptor blockade.
Additional information
Competing interests
The authors have no competing interests.
Author contributions
F.D., J.R., G.L. and D.L.: conception and design of experiments. F.D., J.R., G.L. and D.L.: collection, analysis and interpretation of data. F.D. and J.R.: drafting the article or revising it critically for important intellectual content.
Funding
This research was supported by National Institutes of Health award HL095573 (F.A.D.).
References
- Barrett-O'Keefe Z, Ives SJ, Trinity JD, Morgan G, Rossman MJ, Donato AJ, Runnels S, Morgan DE, Gmelch BS, Bledsoe AD, Richardson RS. Wray DW. Endothelin-A-mediated vasoconstriction during exercise with advancing age. J Gerontol A Biol Sci Med Sci. 2014 doi: 10.1093/gerona/glu065. DOI: 10.1093/gerona/glu065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden SE, Payne GW, Chisty A. Segal SS. Arteriolar network architecture and vasomotor function with ageing in mouse gluteus maximus muscle. J Physiol. 2004;561:535–545. doi: 10.1113/jphysiol.2004.068262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtscher M. Exercise limitations by the oxygen delivery and utilization systems in aging and disease: coordinated adaptation and deadaptation of the lung–heart muscle axis – a mini-review. Gerontology. 2013;59:289–296. doi: 10.1159/000343990. [DOI] [PubMed] [Google Scholar]
- Carlson RE, Kirby BS, Voyles WF. Dinenno FA. Evidence for impaired skeletal muscle contraction-induced rapid vasodilation in aging humans. Am J Physiol Heart Circ Physiol. 2008;294:H1963–H1970. doi: 10.1152/ajpheart.01084.2007. [DOI] [PubMed] [Google Scholar]
- Casey DP. Joyner MJ. Influence of α-adrenergic vasoconstriction on the blunted skeletal muscle contraction-induced rapid vasodilation with aging. J Appl Physiol. 2012;113:1201–1212. doi: 10.1152/japplphysiol.00734.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Richards JC. Dinenno FA. Mechanical effects of muscle contraction increase intravascular ATP draining quiescent and active skeletal muscle in humans. J Appl Physiol. 2013;114:1085–1093. doi: 10.1152/japplphysiol.01465.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Voyles WF. Dinenno FA. Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol. 2010;299:H1633–H1641. doi: 10.1152/ajpheart.00614.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Voyles WF. Dinenno FA. Augmented skeletal muscle hyperaemia during hypoxic exercise in humans is blunted by combined inhibition of nitric oxide and vasodilating prostaglandins. J Physiol. 2011;589:3671–3683. doi: 10.1113/jphysiol.2011.209486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy KP, Jones PP. Seals DR. Influence of age on the sympathetic neural adjustments to alterations in systemic oxygen levels in humans. Am J Physiol. 1997;273:R690–R695. doi: 10.1152/ajpregu.1997.273.2.R690. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Dietz NM. Joyner MJ. Aging and forearm postjunctional α-adrenergic vasoconstriction in healthy men. Circulation. 2002a;106:1349–1354. doi: 10.1161/01.cir.0000028819.64790.be. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Eisenach JH, Dietz NM. Joyner MJ. Post-junctional α-adrenoceptors and basal limb vascular tone in healthy men. J Physiol. 2002b;540:1103–1110. doi: 10.1113/jphysiol.2001.015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Jones PP, Seals DR. Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation. 1999;100:164–170. doi: 10.1161/01.cir.100.2.164. [DOI] [PubMed] [Google Scholar]
- Dinenno FA. Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory? J Physiol. 2003;553:281–292. doi: 10.1113/jphysiol.2003.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA. Joyner MJ. Combined NO and PG inhibition augments α-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol. 2004;287:H2576–H2584. doi: 10.1152/ajpheart.00621.2004. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Tanaka H, Stauffer BL. Seals DR. Reductions in basal limb blood flow and vascular conductance with human ageing: role for augmented α-adrenergic vasoconstriction. J Physiol. 2001;536:977–983. doi: 10.1111/j.1469-7793.2001.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K. Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297:H425–H432. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth ML. Sprague RS. Regulation of blood flow distribution in skeletal muscle: role of erythrocyte-released ATP. J Physiol. 2012;590:4985–4991. doi: 10.1113/jphysiol.2012.233106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG. Lakatta EG. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- Heinonen I, Wendelin-Saarenhovi M, Kaskinoro K, Knuuti J, Scheinin M. Kalliokoski KK. Inhibition of α-adrenergic tone disturbs the distribution of blood flow in the exercising human limb. Am J Physiol Heart Circ Physiol. 2013;305:H163–H172. doi: 10.1152/ajpheart.00925.2012. [DOI] [PubMed] [Google Scholar]
- Hogikyan RV. Supiano MA. Arterial α-adrenergic responsiveness is decreased and SNS activity is increased in older humans. Am J Physiol. 1994;266:E717–E724. doi: 10.1152/ajpendo.1994.266.5.E717. [DOI] [PubMed] [Google Scholar]
- Jackson DN, Moore AW. Segal SS. Blunting of rapid onset vasodilatation and blood flow restriction in arterioles of exercising skeletal muscle with ageing in male mice. J Physiol. 2010;588:2269–2282. doi: 10.1113/jphysiol.2010.189811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson G. The effects of intra-arterially administered propranolol and H 56–28 on blood flow in the forearm–a comparative study of two beta-adrenergic receptor antagonists. Acta Pharmacol Toxicol. 1967;25:63–74. doi: 10.1111/j.1600-0773.1967.tb02997.x. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Nauss LA, Warner MA. Warner DO. Sympathetic modulation of blood flow and O2 uptake in rhythmically contracting human forearm muscles. Am J Physiol. 1992;263:H1078–H1083. doi: 10.1152/ajpheart.1992.263.4.H1078. [DOI] [PubMed] [Google Scholar]
- Kirby BS, Crecelius AR, Richards JC. Dinenno FA. Sources of intravascular ATP during exercise in humans: critical role for skeletal muscle perfusion. Exp Physiol. 2013;98:988–998. doi: 10.1113/expphysiol.2012.071555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Crecelius AR, Voyles WF. Dinenno FA. Impaired skeletal muscle blood flow control with advancing age in humans: attenuated ATP release and local vasodilation during erythrocyte deoxygenation. Circ Res. 2012;111:220–230. doi: 10.1161/CIRCRESAHA.112.269571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG. Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol. 2009;587:1989–2003. doi: 10.1113/jphysiol.2008.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkinos P, Myers J, Kokkinos JP, Pittaras A, Narayan P, Manolis A, Karasik P, Greenberg M, Papademetriou V. Singh S. Exercise capacity and mortality in black and white men. Circulation. 2008;117:614–622. doi: 10.1161/CIRCULATIONAHA.107.734764. [DOI] [PubMed] [Google Scholar]
- Lawrenson L, Hoff J. Richardson RS. Aging attenuates vascular and metabolic plasticity but does not limit improvement in muscle VO2 max. Am J Physiol Heart Circ Physiol. 2004;286:H1565–H1572. doi: 10.1152/ajpheart.01070.2003. [DOI] [PubMed] [Google Scholar]
- Layec G, Trinity JD, Hart CR, Kim SE, Groot HJ, Le Fur Y, Sorensen JR, Jeong EK. Richardson RS. In vivo evidence of an age-related increase in ATP cost of contraction in the plantar flexor muscles. Clin Sci (Lond) 2014;126:581–592. doi: 10.1042/CS20130442. [DOI] [PubMed] [Google Scholar]
- Lutjemeier BJ, Miura A, Scheuermann BW, Koga S, Townsend DK. Barstow TJ. Muscle contraction–blood flow interactions during upright knee extension exercise in humans. J Appl Physiol. 2005;98:1575–1583. doi: 10.1152/japplphysiol.00219.2004. [DOI] [PubMed] [Google Scholar]
- Markwald RR, Kirby BS, Crecelius AR, Carlson RE, Voyles WF. Dinenno FA. Combined inhibition of nitric oxide and vasodilating prostaglandins abolishes forearm vasodilatation to systemic hypoxia in healthy humans. J Physiol. 2011;589:1979–1990. doi: 10.1113/jphysiol.2011.205013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian OS, Thom JM, Ardigo LP, Narici MV. Minetti AE. Metabolic cost, mechanical work, and efficiency during walking in young and older men. Acta Physiol (Oxf) 2006;186:127–139. doi: 10.1111/j.1748-1716.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- Poole JG, Lawrenson L, Kim J, Brown C. Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol. 2003;284:H1251–H1259. doi: 10.1152/ajpheart.00790.2002. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Koch DW, Newcomer SC, Le KU, Smithmyer SL. Leuenberger UA. Leg blood flow and VO2 during peak cycle exercise in younger and older women. Med Sci Sports Exerc. 2004;36:623–631. doi: 10.1249/01.mss.0000121951.10417.b5. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL. Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol. 1998;85:68–75. doi: 10.1152/jappl.1998.85.1.68. [DOI] [PubMed] [Google Scholar]
- Saeed M, Sommer O, Holtz J. Bassenge E. Alpha-adrenoceptor blockade by phentolamine causes beta-adrenergic vasodilation by increased catecholamine release due to presynaptic alpha-blockade. J Cardiovasc Pharmacol. 1982;4:44–52. doi: 10.1097/00005344-198201000-00008. [DOI] [PubMed] [Google Scholar]
- Saltin B, Radegran G, Koskolou MD. Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand. 1998;162:421–436. doi: 10.1046/j.1365-201X.1998.0293e.x. [DOI] [PubMed] [Google Scholar]
- Schrage WG, Eisenach JH. Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol. 2007;579:227–236. doi: 10.1113/jphysiol.2006.124313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EG, Voyles WF, Kirby BS, Markwald RR. Dinenno FA. Ageing and leg postjunctional α-adrenergic vasoconstrictor responsiveness in healthy men. J Physiol. 2007;582:63–71. doi: 10.1113/jphysiol.2007.130591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundlof G. Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol. 1978;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A. Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I. Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Shoemaker JK. Hughson RL. Vasodilation and muscle pump contribution to immediate exercise hyperemia. Am J Physiol. 1996;271:H1697–H1701. doi: 10.1152/ajpheart.1996.271.4.H1697. [DOI] [PubMed] [Google Scholar]
- Wilkins BW, Pike TL, Martin EA, Curry TB, Ceridon ML. Joyner MJ. Exercise intensity-dependent contribution of β-adrenergic receptor-mediated vasodilatation in hypoxic humans. J Physiol. 2008;586:1195–1205. doi: 10.1113/jphysiol.2007.144113. [DOI] [PMC free article] [PubMed] [Google Scholar]


