Abstract
We tested the hypothesis that exercise training would increase neuronal nitric oxide synthase (nNOS)-mediated inhibition of sympathetic vasoconstriction in resting and contracting skeletal muscle. Sprague–Dawley rats (n = 18) were randomized to sedentary or exercise-trained (40 m min−1, 5° grade; 5 days week−1 for 4 weeks) groups. Following completion of sedentary behaviour or exercise training, rats were anaesthetized and instrumented with a brachial artery catheter, femoral artery flow probe and stimulating electrodes on the lumbar sympathetic chain. The percentage change of femoral vascular conductance (%FVC) in response to sympathetic chain stimulations delivered at 2 and 5 Hz was determined at rest and during triceps surae muscle contraction before (control) and after selective nNOS blockade with S-methyl-l-thiocitrulline (SMTC, 0.6 mg kg−1, i.v.) and subsequent non-selective NOS blockade with l-NAME (5 mg kg−1, i.v.; SMTC + l-NAME). At rest, sympathetic vasoconstrictor responsiveness was greater (P < 0.05) in exercise-trained compared to sedentary rats in control, SMTC and SMTC + l-NAME conditions. During contraction, the constrictor response was not different (P > 0.05) between exercise trained (2 Hz: −11 ± 4%FVC; 5 Hz: −21 ± 5%FVC) and sedentary rats (2 Hz: −7 ± 6%FVC; 5 Hz: −18 ± 10%FVC) in control conditions. SMTC augmented (P < 0.05) sympathetic vasoconstriction in sedentary and exercise-trained rats; however, sympathetic vasoconstrictor responsiveness was greater (P < 0.05) in exercise-trained (2 Hz: −27 ± 5%FVC; 5 Hz: −39 ± 5%FVC) compared to sedentary (2 Hz: −17 ± 6%FVC; 5 Hz: −27 ± 8%FVC) rats during selective nNOS inhibition. SMTC + l-NAME further augmented (P < 0.05) sympathetic vasoconstrictor responsiveness by a similar magnitude (P > 0.05) in exercise-trained and sedentary rats. These data demonstrate that exercise training augmented nNOS-mediated inhibition of sympathetic vasoconstriction in contracting muscle.
Introduction
The sympathetic nervous system produces tonic vasoconstriction in the skeletal muscle vascular bed at rest and during exercise (Laughlin & Armstrong, 1987; Joyner et al. 1992; DiCarlo et al. 1996; O'Leary et al. 1997). Sympathetic vasoconstriction is opposed by local vasodilation and the blunting of vasoconstriction by substances released from the skeletal muscle and/or endothelium (Ohyanagi et al. 1992; Habler et al. 1997; Thomas & Victor, 1998; Chavoshan et al. 2002; Dinenno & Joyner, 2004; Jendzjowsky & DeLorey, 2013a). Several studies have shown that nitric oxide (NO) inhibits sympathetic vasoconstriction in resting and contracting skeletal muscle (Habler et al. 1997; Thomas & Victor, 1998; Chavoshan et al. 2002; Dinenno & Joyner, 2004; Jendzjowsky & DeLorey, 2013a,b2013b). The endothelial (eNOS) and neuronal (nNOS) isoforms of NO synthase (NOS) are constitutively expressed in skeletal muscle and the endothelium (Kobzik et al. 1994; Frandsen et al. 1996; Boulanger et al. 1998; Bachetti et al. 2004) and NO derived from both nNOS and eNOS has been shown to inhibit sympathetic vasoconstriction in resting and contracting muscle (Jendzjowsky & DeLorey, 2013a). However, NO derived from nNOS appears to make a larger contribution to the inhibition of sympathetic vasoconstriction during muscular contraction and may be particularly important for functional sympatholysis (Jendzjowsky & DeLorey, 2013a).
Our laboratory recently demonstrated that exercise training improved NO-mediated inhibition of sympathetic vasoconstriction in resting and contracting skeletal muscle (Jendzjowsky & DeLorey, 2013b). Exercise training has also been shown to increase the expression of both eNOS and nNOS enzymes and to increase NO bioavailability (Sun et al. 1994; Laughlin et al. 2001, 2003; Vassilakopoulos et al. 2003; McConell et al. 2007; Harris et al. 2008). Thus, NO derived from either or both eNOS and nNOS may be responsible for the greater NO-mediated inhibition of sympathetic vasoconstriction following exercise training. However, the effect of exercise training on NOS isoform-specific blunting of sympathetic vasoconstriction has not been investigated.
Therefore, the purpose of the present study was to utilize selective nNOS blockade and subsequent non-selective NOS blockade to investigate the effects of exercise training on: (1) nNOS- and eNOS-mediated inhibition of sympathetic vasoconstriction in resting and contracting skeletal muscle, and (2) the relative contribution of nNOS- and eNOS-derived NO to total NO-mediated inhibition of sympathetic vasoconstriction. It was hypothesized that exercise training would augment nNOS-mediated inhibition of sympathetic vasoconstriction in resting and contracting skeletal muscle.
Methods
Animals and animal care
Male Sprague–Dawley rats (2 months of age) were obtained from the institutional breeding colony and housed in pairs in a 12:12 h light–dark cycle, environmentally controlled (22–24°C, 40–70% humidity) room. Water and rat chow (Lab Diet 5001; PMI Nutrition, Brentwood, MO, USA) were freely available. All experiments were conducted in accordance with the Canadian Council on Animal Care Guidelines and Policies with approval from the Animal Care and Use Committee: Health Sciences for the University of Alberta.
Chronic endurance exercise training
All rats were habituated to the lab and exercise by walking on a treadmill (Panlab LE8710, Barcelona, Spain) 10 min day−1 for 5 days at 10 m min−1, 0° grade. Following familiarization, rats were randomly assigned to a sedentary time-control (n = 8) or heavy-intensity exercise training (n = 10; 40 m min−1, 5° grade) group. Exercise-trained rats trained 5 days week−1 for 4 weeks, while sedentary rats were handled and weighed daily. On the first training day, exercise-trained rats completed 15× 1 min intervals at 40 m min−1 5° grade interspersed with rest periods of equivalent duration. Each subsequent training day, run time was increased while rest time was maintained. This training progression allowed all rats in the exercise-trained group to run continuously for 600 m at the prescribed speed and grade within 11 ± 2 days. This training paradigm is regularly used in our laboratory and has been shown to increase heart mass, heart mass: body mass ratio, soleus citrate synthase activity and endothelium-dependent vasodilation (Jendzjowsky & DeLorey, 2011, 2012, 2013b).
Instrumentation
Approximately 24 h after the last training session anaesthesia was induced by inhalation of isoflurane (3–3.5%, balance O2). During isoflurane anaesthesia, the right jugular vein was cannulated and anaesthesia was subsequently maintained by i.v. infusion of α-chloralose (8–16 mg kg−1 h−1) and urethane (50–100 mg kg−1 h−1) with a syringe pump. Depth of anaesthesia was assessed by the stability of arterial blood pressure, heart rate (HR) and the absence of a withdrawal reflex in response to painful stimuli (i.e. paw-pinch). Core temperature was monitored by rectal probe and maintained at 36–37°C by an external heating pad (TCAT-2; Physitemp, Clifton, NJ, USA). A tracheotomy was performed to allow spontaneous breathing of room air. We have previously demonstrated the maintenance of arterial blood gases and acid–base status at rest and during contraction in this preparation (Jendzjowsky & DeLorey, 2013b). Thus, arterial blood gases and acid–base status were checked periodically to confirm the maintenance of normal values in these experiments (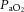 : 88–95 mmHg;
: 88–95 mmHg; 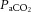 : 39–41 mmHg pH 7.39–7.42). The left brachial artery was cannulated and connected to a solid-state pressure transducer (Abbott, Chicago, IL, USA) for the continuous measurement of arterial blood pressure. Mean arterial pressure (MAP) and HR were derived from the arterial blood pressure waveform. The left femoral artery and vein were cannulated for the delivery of drugs. Blood flow was measured in the right femoral artery using a transit-time flow probe (0.7 V; Transonic Systems, Ithaca, NY, USA) and flow-meter (T106; Transonic Systems).
: 39–41 mmHg pH 7.39–7.42). The left brachial artery was cannulated and connected to a solid-state pressure transducer (Abbott, Chicago, IL, USA) for the continuous measurement of arterial blood pressure. Mean arterial pressure (MAP) and HR were derived from the arterial blood pressure waveform. The left femoral artery and vein were cannulated for the delivery of drugs. Blood flow was measured in the right femoral artery using a transit-time flow probe (0.7 V; Transonic Systems, Ithaca, NY, USA) and flow-meter (T106; Transonic Systems).
Muscle contraction
The right sciatic nerve was exposed and instrumented with a cuff electrode. The triceps surae muscle group was then dissected free of all skin and connective tissue and attached to a force transducer (Model MLT1030/D; AD Instruments Colorado Springs, CO, USA) via the calcaneal tendon. Hind-limb contractions were produced by electrical stimulation of the sciatic nerve with Chart software (AD Instruments). Maximal contractile force (MCF) was determined by stimulation of the triceps surae muscle group with 25, 1 ms impulses delivered at 100 Hz, 10× motor threshold. The optimal muscle length for tension development was determined by progressively lengthening the muscle and repeating the nerve stimulation until a plateau in tension (peak – baseline) was observed. Rhythmic contractions of the triceps surae muscles at 60% MCF were produced by stimulation of the sciatic nerve with 0.1 ms pulses at 40 Hz in 250 ms trains at a rate of 60 trains min−1 at ∼6× motor threshold.
Lumbar sympathetic chain stimulation
A laparotomy was performed and the aorta and vena cava were temporarily retracted to attach a bipolar silver-wire stimulating electrode to the lumbar sympathetic chain at the L3/L4 Level. The electrodes were embedded and electrically isolated in a rapidly curing non-toxic silicone elastomer (Kwiksil; WPI, Sarasota, FL, USA). The electrodes were used to deliver constant current stimulations through an isolated stimulator (Digitimer DS3, Welwyn Garden City, Herts, UK).
Experimental procedures
Following the surgical procedure, a 30 min recovery period was used to establish baseline haemodynamic values. The cardiovascular response [change in MAP, HR, femoral artery blood flow (FBF) and femoral vascular conductance (FVC)] to sympathetic stimulation was then determined at rest and during contraction at 60% MCF. Sympathetic stimulations were delivered for 1 min at 2 and 5 Hz in random order in resting and contracting skeletal muscle. At rest, sympathetic stimulations were separated by ∼2 min of recovery to restore baseline haemodynamic values. Bouts of muscle contraction were 8 min in duration and sympathetic stimulations were delivered at 3 and 6 min after the onset of contraction.
Following data collection under control conditions, rats were allowed to recover for ∼20 min and then the selective nNOS antagonist S-methyl-l-thiocitrulline (SMTC, 0.6 mg kg−1, i.v.) was injected. Following stabilization of haemodynamic variables (∼20 min) lumbar sympathetic chain stimulations were repeated at rest and during skeletal muscle contraction. After another period of recovery (∼20 min) the non-selective NOS antagonist, Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME, 5 mg kg−1, i.v.) was injected. Following haemodynamic stabilization (∼20 min), lumbar sympathetic chain stimulations were repeated at rest and during skeletal muscle contraction (SMTC + l-NAME). We have previously demonstrated that the cardiovascular response to sympathetic stimulation and muscle force production are not altered over time when bouts of contraction are repeated in this manner (Jendzjowsky & DeLorey, 2013b). The doses of SMTC and l-NAME used in the present study were selected based on previous work in our laboratory (Jendzjowsky & DeLorey, 2013a) and other studies that have shown effective blockade of nNOS and all NOS isoforms at similar doses of SMTC (Ichihara & Navar, 1999; Wakefield et al. 2003; Seddon et al. 2008, 2009) and l-NAME (Thomas & Victor, 1998). We have also previously shown that selective nNOS blockade does not alter the subsequent determination of total NO-mediated inhibition of sympathetic vasoconstriction with l-NAME (Jendzjowsky & DeLorey, 2013a). Upon completion of all experiments, rats were killed with an overdose of anaesthetic (mixture of α-chloralose and urethane).
Effectiveness of nitric oxide synthase blockade
To assess the effectiveness of selective nNOS and non-selective NOS blockade, intra-arterial bolus injections of acetylcholine (ACh; 0.005 μg and 0.1 μg doses) were administered before and following SMTC and l-NAME. ACh was injected in small volumes (100 μl) over ∼5 s to avoid any flow-mediated vasodilation and each dose was separated by ∼5 min. Vehicle injections delivered in this manner did not alter FBF.
Pharmacology
All drugs were purchased from Sigma-Aldrich (Oakville, ON, Canada) and dissolved in 0.9% physiological saline.
Western blot analysis
Snap frozen (100–200 mg) soleus, medial gastrocnemius and lateral gastrocnemius muscles were homogenized in lysis buffer [20 mm Tris (pH 7.4), 5 mm EDTA, 10 mm sodium pyrophosphate tetrabasic, 100 mm sodium fluoride, 1% NP-40] containing 1× Halt Protease Inhibitor Cocktail (Pierce, Rockford, IL, USA). Protein concentrations were determined by bicinchoninic acid assay (Pierce). One hundred micrograms of total protein was separated on a 7.5% SDS-polyacrylamide gel and then transferred on to nitrocellulose membrane (0.2 μm; Biorad, Hercules, CA, USA). Membranes were incubated with antimouse inducible NOS, eNOS or nNOS (1:1000; BD Transduction Laboratories, Franklin Lakes, NJ, USA) and rabbit α-tubulin (1:1000; Abcam, Cambridge, MA, USA). Membranes were subsequently probed with IRDye® 800CW donkey antimouse IgG and IRDye® 680RD donkey antirabbit IgG secondary antibodies (1:10,000; Li-Cor Biosciences, Lincoln, NE, USA). The protein bands were detected and quantified by the Li-Cor Odyssey imager system v3.0. Results were normalized to α-tubulin.
Data analysis
Data were recorded using Chart data acquisition software (AD Instruments). Arterial blood pressure and FBF were sampled at 100 Hz and FVC was calculated. The change in HR, MAP, FBF and FVC in response to sympathetic stimulation was calculated as an absolute change and as a percentage change from the value preceding the sympathetic stimulation. The magnitude of sympathetic vasoconstrictor responses to lumbar chain stimulation was assessed by percentage changes in FVC because percentage changes in FVC accurately reflect percentage changes in resistance vessel radius (Buckwalter & Clifford, 2001; Thomas & Segal, 2004). The effect of SMTC and l-NAME on the magnitude of sympathetic vasoconstriction (decrease in %FVC) was determined by calculating the difference between sympathetic vasoconstriction achieved during control, SMTC and SMTC + l-NAME conditions. The increase in sympathetic vasoconstrictor responsiveness following treatment with SMTC is a measure of nNOS-mediated inhibition of sympathetic vasoconstriction and the difference between the constrictor response in the SMTC and SMTC + l-NAME conditions is a measure of eNOS-mediated inhibition of sympathetic vasoconstriction (Jendzjowsky & DeLorey, 2013a).
Mean HR, MAP, FBF and FVC were calculated during the minute immediately preceding the first sympathetic stimulation and the increase in each variable above the resting baseline was used to determine the cardiovascular response to contraction. Muscle force production was measured continuously and peak force development was determined for each muscle contraction. To compare force production between groups and experimental conditions a mean of peak contractile forces was calculated between minutes 3 and 7 (the period encompassing the sympathetic stimulations) for each contractile bout.
The magnitude of ACh-mediated vasodilation in control, SMTC and l-NAME conditions was calculated as the difference between the peak FVC response (∼3 s average) and the FVC baseline before ACh infusion (∼20 s average) and expressed as a percentage change from the FVC baseline. All data are expressed as mean ± s.d.
Statistics
The vasoconstrictor response to sympathetic stimulation was analysed by three-way repeated measures ANOVA (group × muscle contractile state × drug condition). The response to each frequency of sympathetic stimulation was analysed separately. The effect of muscle contractile state on the contribution of nNOS to the total NO-mediated inhibition of sympathetic vasoconstriction was determined by two-way repeated measures ANOVA. The effects of exercise training and drug treatment on baseline haemodynamics, the haemodynamic response to contraction, muscle contractile force and the vasodilator responses to ACh were determined by two-way repeated measures ANOVA. When significant F-ratios were detected, Student–Newman–Keuls post hoc tests were used to identify differences between specific groups and drug treatments. Differences in anthropometric measures were analysed by t test. P < 0.05 was considered statistically significant.
Results
Resting haemodynamics and indicators of training efficacy
All rats assigned to the exercise training group completed the prescribed training protocol. Body mass was lower (P < 0.05) and heart mass and the heart mass/body mass ratio were increased (P < 0.05) in exercise-trained compared to sedentary rats (Table1).
Table 1.
Indicators of training efficacy
| Group | Body mass (g) | Heart mass (g) | Heart mass/body mass ratio (mg g−1) |
|---|---|---|---|
| Sedentary | 450 ± 43 | 1.4 ± 0.2 | 3.11 ± 0.20 |
| Exercise trained | 394 ± 21* | 1.7 ± 0.2* | 4.31 ± 0.40* |
Values are means ± s.d. *Significant difference between groups. P < 0.05 was considered statistically significant.
Resting HR was not different (P > 0.05) between exercise-trained and sedentary rats. Selective nNOS inhibition with SMTC did not change (P > 0.05) HR in exercise-trained or sedentary rats; however, subsequent non-selective NOS inhibition with l-NAME decreased (P < 0.05) HR by a similar magnitude in exercise-trained and sedentary rats. Resting MAP was not different (P > 0.05) between exercise-trained and sedentary rats. SMTC caused a similar increase (P < 0.05) in MAP in exercise-trained and sedentary rats and l-NAME caused a further similar increase (P < 0.05) in MAP in exercise-trained and sedentary rats. Resting FBF was not different (P > 0.05) between exercise-trained and sedentary rats and was not altered by treatment with SMTC or l-NAME. Resting FVC was not different (P > 0.05) between exercise-trained and sedentary rats. SMTC reduced (P < 0.05) FVC by a similar magnitude in exercise-trained and sedentary rats (P > 0.05) and l-NAME further reduced (P < 0.05) FVC to a similar extent in exercise-trained and sedentary rats (Table2).
Table 2.
Basal haemodynamics
| Drug condition | Group | HR (beats min−1) | MAP (mmHg) | FBF (ml min−1) | FVC (ml min−1 mmHg−1) |
|---|---|---|---|---|---|
| Control | Sedentary | 410 ± 61 | 95 ± 16‡ | 3.1 ± 0.6 | 0.034 ± 0.010‡ |
| Exercise trained | 374 ± 40 | 104 ± 11‡ | 3.0 ± 0.4 | 0.030 ± 0.003‡ | |
| SMTC | Sedentary | 395 ± 43 | 104 ± 15‡ | 3.3 ± 1.1 | 0.032 ± 0.012‡ |
| Exercise trained | 374 ± 36 | 109 ± 15‡ | 2.7 ± 0.6 | 0.025 ± 0.005‡ | |
| SMTC + l-NAME | Sedentary | 353 ± 33*† | 124 ± 16‡ | 3.1 ± 1.5 | 0.025 ± 0.011‡ |
| Exercise trained | 352 ± 33*† | 133 ± 14‡ | 2.4 ± 0.7 | 0.018 ± 0.004‡ |
Values are means ± s.d. Abbreviations: FBF, femoral blood flow; FVC, femoral vascular conductance; HR, heart rate; MAP, mean arterial blood pressure; l-NAME, Nω-nitro-l-arginine methyl ester; SMTC, S-methyl-l-thiocitrulline. *Statistically significant difference from control condition. †Statistically significant difference from the SMTC condition. ‡Statistically significant difference between all drug conditions within each experimental group. P < 0.05 was considered statistically significant.
Endothelium-dependent vasodilation and effectiveness of nitric oxide synthase inhibition
ACh-mediated vasodilation was greater (P < 0.05) in exercise-trained compared to sedentary rats. SMTC did not alter (P > 0.05) ACh-mediated vasodilation in exercise-trained or sedentary rats (Fig.1). In contrast, subsequent treatment with l-NAME diminished (P > 0.05) ACh-induced vasodilation in both exercise-trained and sedentary rats (Fig.1).
Figure 1. Percentage change of femoral vascular conductance (FVC) in sedentary (white bars) and exercise trained rats (gray bars) in response to intra-arterial bolus injections of acetylcholine (ACh) at doses of 0.005 μg (open bars) and 0.1 μg (hatched bars).
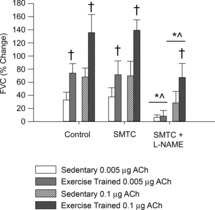
Values are mean ± SD. Statistically significant difference: ^from control condition; *from SMTC condition; and †between groups within a specified drug condition and dose. P < 0.05 was considered statistically significant. ACh, acetylcholine; FVC, femoral vascular conductance; SMTC, S-methyl-l-thiocitrulline.
Sympathetic vasoconstriction at rest and during muscular contraction
The response to sympathetic stimulation delivered at rest and during muscle contraction in a representative rat is shown in Fig.2. Absolute changes in HR, MAP, FBF and FVC in response to sympathetic stimulation at rest and during contraction are presented in Table3.
Figure 2. Original data from a representative rat illustrating the response of MAP, FBF, FVC and muscle contractile force at rest (A) and during muscular contraction at 60% of maximal contractile force (B).
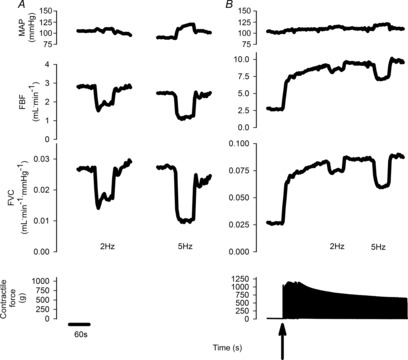
The arrow indicates the onset of contraction. Lumbar sympathetic nerve stimulations were delivered at 2 and 5 Hz in random order at rest and during contraction. FBF, femoral artery blood flow; FVC, femoral vascular conductance; MAP, mean arterial blood pressure.
Table 3.
Haemodynamic responses to sympathetic stimulation at rest and during muscle contraction.
| 2 Hz |
5 Hz |
|||||
|---|---|---|---|---|---|---|
| Muscle contractile state | Group | Drug condition | FBF (ml min−1) | FVC (ml min−1 mmHg−1) | FBF (ml min−1) | FVC (ml min−1 mmHg−1) |
| Rest | Sedentary | Control | −0.6 ± 0.2 | −0.008 ± 0.002 | −0.8 ± 0.3 | −0.011 ± 0.003 |
| SMTC | −0.8 ± 0.3 | −0.008 ± 0.002 | −1.1 ± 0.4 | −0.012 ± 0.003 | ||
| SMTC + l-NAME | −0.9 ± 0.3 | −0.009 ± 0.003 | −1.1 ± 0.4 | −0.011 ± 0.004 | ||
| Exercise trained | Control | −0.9 ± 1.0 | −0.010 ± 0.001 | −1.3 ± 0.2 | −0.015 ± 0.003 | |
| SMTC | −1.0 ± 0.3 | −0.010 ± 0.002 | −1.6 ± 0.4 | −0.015 ± 0.004 | ||
| SMTC + l-NAME | −1.2 ± 0.4 | −0.009 ± 0.002 | −1.5 ± 0.6 | −0.012 ± 0.004 | ||
| Contraction | Sedentary | Control | −0.5 ± 0.6 | −0.006 ± 0.005‡ | −1.1 ± 1.2 | −0.016 ± 0.008 |
| SMTC | −1.1 ± 0.8§ | −0.014 ± 0.004‡§ | −1.7 ± 1.3§ | −0.024 ± 0.007*§ | ||
| SMTC + l-NAME | −1.8 ± 1.0*†§ | −0.018 ± 0.005‡§ | −2.4 ± 1.3*§ | −0.026 ± 0.007*§ | ||
| Exercise trained | Control | −0.7 ± 0.4 | −0.010 ± 0.004 | −1.2 ± 0.6‡ | −0.018 ± 0.004 | |
| SMTC | −2.4 ± 0.6*§ | −0.025 ± 0.005*§ | −3.3 ± 0.6‡§ | −0.036 ± 0.006*§ | ||
| SMTC + l-NAME | −2.7 ± 0.8*§ | −0.024 ± 0.006*§ | −4.0 ± 1.0‡§ | −0.036 ± 0.006*§ | ||
Values are means ± s.d. Abbreviations: FBF, femoral blood flow; FVC, femoral vascular conductance; HR, heart rate; MAP, mean arterial blood pressure; l-NAME, Nω-nitro-l-arginine methyl ester; SMTC, S-methyl-l-thiocitrulline. *Statistically significant difference from control conditions within the specified contractile state. †Statistically significant difference from SMTC condition within the specified contractile state. ‡Statistically significant difference between all drug conditions within the specified contractile state. §Statistically significant difference between groups within specified drug condition and contractile state. P < 0.05 was considered statistically significant.
In resting skeletal muscle, sympathetic vasoconstrictor responsiveness was greater (P < 0.05) in exercise-trained compared to sedentary rats. Sympathetic vasoconstrictor responsiveness was increased by (P < 0.05) selective nNOS inhibition in exercise-trained and sedentary rats; however, the vasoconstrictor response was greater (P < 0.05) in exercise-trained compared to sedentary rats during treatment with SMTC. Subsequent treatment with l-NAME further increased (P < 0.05) sympathetic vasoconstrictor responsiveness in both exercise-trained and sedentary rats; however, the vasoconstrictor response remained greater (P < 0.05) in exercise-trained compared to sedentary rats (Fig.3).
Figure 3. The percentage change of femoral vascular conductance (FVC) in response to sympathetic stimulation at 2 Hz (top panels) and 5 Hz (bottom panels) in resting (left panels) and contracting muscle (right panels) during control conditions, following selective nNOS blockade with S-methyl-thio-citrulline (SMTC, 0.6 mg.kg−1 IV) and following subsequent non-selective NOS blockade with l-NAME (5 mg.kg−1 IV) in sedentary (white bars) and exercise trained (black bars) rats.
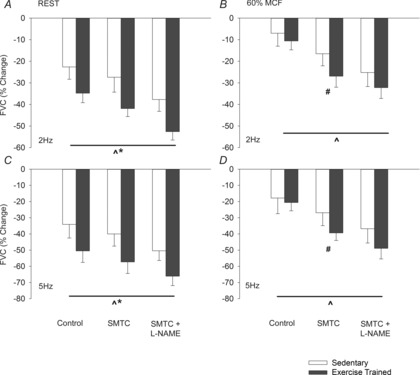
Values are means ± s.d. Significant difference between: ^all drug conditions; *groups during all drug conditions; and #training groups within specified drug condition. P < 0.05 was considered statistically significant. FVC, femoral vascular conductance; MCF, maximal contractile force; l-NAME, Nω-nitro-l-arginine methyl ester; SMTC, S-methyl-l-thiocitrulline.
Compared to rest, muscle contraction attenuated the vasoconstrictor response to sympathetic stimulation (P < 0.05) in control conditions and during selective nNOS and non-selective NOS inhibition (Fig.3 and Table2). During muscle contraction, sympathetic vasoconstrictor responsiveness was not different (P > 0.05) between exercise-trained and sedentary rats. Sympathetic vasoconstrictor responsiveness was increased by (P < 0.05) selective nNOS inhibition in both exercise-trained and sedentary rats; however, treatment with SMTC caused a larger increase (P < 0.05) in the vasoconstrictor response in exercise-trained compared to sedentary rats. l-NAME further increased (P < 0.05) sympathetic vasoconstrictor responsiveness in both exercise-trained and sedentary rats and the vasoconstrictor response was not different (P > 0.05) between exercise-trained and sedentary rats during non-selective NOS inhibition (Fig.3).
At rest, nNOS was responsible for a similar (P > 0.05) amount (∼35%) of total NO-mediated inhibition of sympathetic vasoconstriction in exercise-trained and sedentary rats. In contracting skeletal muscle, nNOS-mediated inhibition of sympathetic vasoconstriction was increased (P < 0.05) in both exercise-trained and sedentary rats. However, NO derived from nNOS was responsible for a greater (P < 0.05) proportion of total NO-mediated inhibition of sympathetic vasoconstriction in exercise-trained (∼75%) compared to sedentary (∼50%) rats (Fig.4).
Figure 4. Magnitude of increase in sympathetic vasoconstriction during selective nNOS inhibition (grey bars) and non-selective NOS inhibition (hatched grey bars) at rest (A) and during muscular contraction (C).
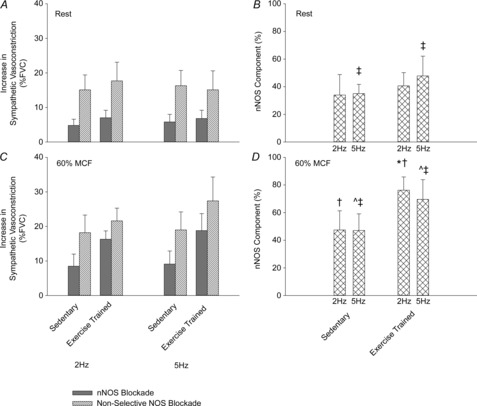
Contribution of NO derived from nNOS to total NO-mediated inhibition of sympathetic vasoconstriction at rest (B) and during muscular contraction (D). Values are means ± s.d. Main effect of ^muscle contraction and ‡exercise training. *Significant difference between exercise-trained and sedentary rats. P < 0.05 was considered statistically significant. FVC, femoral vascular conductance; MCF, maximal contractile force; nNOS, neuronal nitric oxide synthase.
Hyperaemic response to contraction and muscle force production
The increase in HR, MAP, FBF and FVC in response to contraction was not different (P > 0.05) between groups or experimental conditions (Table4). Muscle force production was also not different (P > 0.05) between experimental conditions and between sedentary (control: 738 ± 112 g; SMTC: 778 ± 76 g; SMTC + l-NAME: 810 ± 40 g) and exercise-trained (control: 763 ± 66 g; SMTC: 827 ± 47 g; SMTC + l-NAME: 807 ± 42 g) rats.
Table 4.
Haemodynamic response to muscle contraction
| Drug condition | Group | HR (beats min−1) | MAP (mmHg) | FBF (ml min−1) | FVC (ml min−1 mmHg−1) |
|---|---|---|---|---|---|
| Control | Sedentary | 8 ± 8 | 7 ± 4 | 5.7 ± 1.2 | 0.052 ± 0.008 |
| Exercise trained | 12 ± 9 | 5 ± 3 | 6.6 ± 1.1 | 0.061 ± 0.008 | |
| SMTC | Sedentary | 7 ± 10 | 4 ± 2 | 5.5 ± 1.0 | 0.054 ± 0.007 |
| Exercise trained | 11 ± 10 | 4 ± 3 | 6.8 ± 1.1 | 0.064 ± 0.010 | |
| SMTC + l-NAME | Sedentary | 8 ± 8 | 4 ± 2 | 6.4 ± 1.7 | 0.051 ± 0.013 |
| Exercise trained | 11 ± 15 | 5 ± 4 | 7.3 ± 2.2 | 0.058 ± 0.016 |
Values are means ± s.d. Abbreviations: FBF, femoral blood flow; FVC, femoral vascular conductance; HR, heart rate; MAP, mean arterial blood pressure; l-NAME, Nω-nitro-l-arginine methyl ester; SMTC, S-methyl-l-thiocitrulline. Absolute increase of HR, MAP, FBF and FVC in response to muscular contraction.
Nitric oxide synthase expression
A main effect (P < 0.05) of exercise training on muscle nNOS expression was observed. The expression of nNOS was higher (P < 0.05; main effect) in medial and lateral gastrocnemius muscles compared to the soleus muscle. There was no interaction between experimental groups and individual muscle nNOS expression. eNOS expression was not different (P > 0.05) in exercise-trained and sedentary rats and was similar (P > 0.05) between muscles. Inducible NOS expression was not different (P > 0.05) in exercise-trained and sedentary rats, but was lower (P < 0.05) in the soleus compared to the medial and lateral gastrocnemius muscles (Fig.5).
Figure 5. eNOS (A), iNOS (B) and nNOS (C) expression from medial gastrocnemius (MG; white), lateral gastrocnemius (LG; light grey) and soleus (Sol; dark grey) muscles in sedentary (S) and exercise trained (Ex) groups. NOS expression was normalized to α-tubulin protein levels.
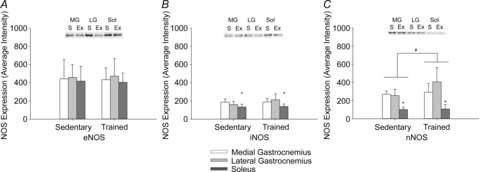
Values and means ± s.d. Significant difference between #groups (main effect) and *muscles (main effect). P < 0.05 was considered statistically significant. eNOS, endothelial nitric oxide synthase; Ex, exercise-trained; iNOS, inducible nitric oxide synthase; LG, lateral gastrocnemius; MG, medial gastrocnemius; nNOS, neuronal nitric oxide synthase; S, sedentary; Sol, soleus.
Discussion
The purpose of the present study was to investigate the effect of heavy-intensity exercise training on NOS isoform-specific inhibition of sympathetic vasoconstriction in resting and contracting skeletal muscle. The important novel finding from the present study was that heavy-intensity exercise training augmented nNOS-mediated inhibition of sympathetic vasoconstriction in contracting skeletal muscle. Consistent with improved nNOS-mediated vascular function, skeletal muscle nNOS expression was also greater in exercise-trained compared to sedentary rats. These data suggest that improvements in NO-dependent sympatholysis following exercise training are mediated by increased production of NO by nNOS.
Our laboratory and others have shown that NO inhibits sympathetic vasoconstriction in resting and contracting muscle (Habler et al. 1997; Thomas & Victor, 1998; Chavoshan et al. 2002; Donato et al. 2007; Jendzjowsky & DeLorey, 2013b). Consistent with previous findings from our laboratory (Jendzjowsky & DeLorey, 2013b), NO-mediated inhibition of sympathetic vasoconstriction in resting skeletal muscle was greater in exercise-trained compared to sedentary rats in the present study. At rest, the relative contribution of NO derived from nNOS to total NO-mediated inhibition of sympathetic vasoconstriction was similar between sedentary (∼35%) and exercise-trained (∼40%) rats suggesting that the increased NO-dependent inhibition of sympathetic vasoconstriction at rest in exercise-trained rats was a function of increases in both nNOS- and eNOS-mediated inhibition of vasoconstriction. We (Jendzjowsky & DeLorey, 2013a) and others (Habler et al. 1997) have previously shown that eNOS-derived NO is primarily responsible for inhibition of sympathetic vasoconstriction in resting skeletal muscle and the present data indicate that this is not altered by short-term heavy-intensity exercise training.
Previous investigations of the effects of exercise training on the inhibition of sympathetic vasoconstriction in contracting muscle (i.e. sympatholysis) have produced conflicting findings. Our laboratory has demonstrated that exercise training enhanced sympatholysis (i.e. a greater inhibition of sympathetic vasoconstriction during contraction) in a training intensity-dependent manner through a NO-dependent mechanism (Jendzjowsky & DeLorey, 2013b). Exercise training has also been shown to enhance sympatholysis through a NO-dependent mechanism in hypertensive rats (Mizuno et al. 2014). In contrast, short-term single limb exercise training did not alter sympatholysis in humans (Mortensen et al. 2012a; Wimer & Baldi, 2012). However, the maintenance of aerobic fitness throughout the lifespan maintained sympatholysis in older adults (Mortensen et al. 2012b) and prevented an age-associated decline in NO bioavailability (Nyberg et al. 2012). Consistent with our previous findings (Jendzjowsky & DeLorey, 2013b), exercise training enhanced NO-dependent inhibition of sympathetic vasoconstriction in the present study. The relative contribution of nNOS-derived NO to the inhibition of sympathetic vasoconstriction was markedly increased in exercise-trained rats in the present study. Our laboratory has previously shown that nNOS-derived NO makes a larger contribution to the inhibition of sympathetic vasoconstriction in contracting compared to resting skeletal muscle in untrained healthy rats (Jendzjowsky & DeLorey, 2013a). Other studies have also demonstrated that the nNOS-mediated NO production is increased during muscle contraction and NO derived from nNOS is particularly important for sympatholysis (Lau et al. 2000; Grange et al. 2001). Indeed, an impaired ability to inhibit sympathetic vasoconstriction has been reported in nNOS-null or nNOS-deficient populations (Thomas et al. 1998; Sander et al. 2000; Grange et al. 2001; Fadel et al. 2003). The present findings indicate that greater NO-dependent inhibition of vasoconstriction following exercise training is predominately due to increased nNOS-mediated inhibition of sympathetic vasoconstriction. These data suggest that exercise training may be a powerful therapy for vascular dysfunction in sedentary populations and clinical conditions where nNOS expression may be reduced. Further studies will be required to demonstrate this effect and to resolve the role of NO in the inhibition of sympathetic vasoconstriction in humans (Boushel et al. 2002; Chavoshan et al. 2002; Dinenno & Joyner, 2003; Rosenmeier et al. 2003).
The improved nNOS-mediated inhibition of sympathetic vasoconstriction was not associated with an increased response of limb blood flow to contraction in exercise-trained rats in the present study. While the role of NO in the regulation of exercise hyperaemia remains controversial (Clifford & Hellsten, 2004), nNOS-derived NO appears to be important for the distribution of blood flow between muscles during exercise (Copp et al. 2013). Thus with respect to the control of limb blood flow in the present study, the greater nNOS-mediated inhibition of vasoconstriction in exercise-trained rats may have opposed training-induced alterations in sympathetic vasoconstrictor responsiveness and/or improved the distribution of blood flow between and within skeletal muscles during contraction.
In agreement with improved nNOS mediated vascular function following exercise training, skeletal muscle nNOS expression was greater in exercise trained compared to S rats (main effect of exercise training) in the present study. Increased nNOS expression has also been reported following 4 weeks of heavy-intensity swim training and 4 and 10 weeks of treadmill running in rats (Tatchum-Talom et al. 2000; Vassilakopoulos et al. 2003; Song et al. 2009) as well as 10 days of intense cycling exercise in humans (McConell et al. 2007). In contrast, 10 weeks of moderate intensity running in rats (Harris et al. 2008) and 6 weeks of endurance running or single leg knee extension exercise training in young, healthy males (Frandsen et al. 2000) did not increase nNOS protein expression. The reason for the conflicting findings between studies is not readily apparent. However, nNOS appears to be preferentially expressed in type II glycolytic muscle fibres (Kobzik et al. 1995) and therefore differences in training paradigms and muscle fibre types investigated may contribute to between study differences. Further investigation will be required to determine the sensitivity of nNOS expression to different patterns of muscle recruitment, intensities and volumes of training, as well as the time course of changes in nNOS expression during training.
Skeletal muscle eNOS expression was not different between exercise-trained and sedentary rats in the present study. Consistent with the present data, soleus muscle eNOS expression was not different between sedentary rats and rats that completed 10 weeks of treadmill exercise training (Harris et al. 2008). In contrast, greater eNOS expression has been reported in gastrocnemius and soleus muscles of treadmill-trained compared to sedentary rats (Balon & Nadler, 1997; Vassilakopoulos et al. 2003; Song et al. 2009). Increased eNOS expression has been reported in the endothelium from isolated aorta (McAllister & Price, 2010), coronary arterioles (Laughlin et al. 2001) and some, but not all, skeletal muscle arteries (Laughlin et al. 2004) following exercise training. eNOS expression was not measured in isolated vascular tissue in the present study; however, shear stress was probably elevated by exercise training and therefore an increase in vascular eNOS expression seems plausible. Nonetheless, if exercise training increased eNOS expression in the vascular endothelium in the present study, it did not appear to be responsible for the enhanced inhibition of sympathetic vasoconstriction following exercise training.
Experimental considerations and limitations
Consistent with a previous investigation in our laboratory (Jendzjowsky & DeLorey, 2013a), selective nNOS blockade with SMTC and subsequent infusion of l-NAME were used to determine nNOS-mediated inhibition of sympathetic vasoconstriction and the total magnitude of NO-mediated inhibition of sympathetic vasoconstriction, respectively. The difference between the constrictor response in the SMTC and SMTC + l-NAME conditions is an index of eNOS-mediated inhibition of sympathetic vasoconstriction (Jendzjowsky & DeLorey, 2013a). The vasodilator response to infusion of ACh was used to confirm the selectivity and effectiveness of NOS inhibition. Consistent with previous findings from our laboratory (Jendzjowsky & DeLorey, 2013a) and others (Copp et al. 2010, 2011, 2013), the vasodilator response to ACh was similar between control and SMTC conditions in the present study, indicating that SMTC selectively blocked nNOS without inhibition of eNOS. Subsequent non-selective NOS blockade with l-NAME significantly reduced ACh-mediated vasodilation indicating that subsequent treatment with l-NAME effectively inhibited eNOS-mediated vascular function in agreement with previous reports (Parkington et al. 2002).
Additional limitations inherent in the experimental preparation include the use of electrically evoked sympathetic activity and muscle contractions in anaesthetized rats that do not reflect naturally occurring patterns of nerve traffic in conscious animals/humans. Finally, muscle homogenates were utilized to investigate NOS expression and therefore tissue-specific (skeletal muscle, endothelium, nerve, smooth muscle) effects of exercise training on NOS expression could not be determined.
Conclusion
The present study demonstrated that exercise training increased skeletal muscle nNOS expression and markedly enhanced nNOS-mediated inhibition of sympathetic vasoconstriction. These data demonstrate that the greater NO-dependent sympatholysis in exercise-trained rats previously reported by our laboratory (Jendzjowsky & DeLorey, 2013b) is predominately attributable to greater nNOS-dependent blunting of sympathetic vasoconstriction. This study advances our mechanistic understanding of the vascular adaptations to exercise training. These data also suggest that the ability to regulate skeletal muscle vascular conductance and arterial blood pressure at rest and during exercise may be enhanced by exercise training. These exercise training induced adaptations may be particularly beneficial in sedentary individuals and pathophysiological conditions (e.g. heart failure, menopause, hypertension) where nNOS expression and nNOS-mediated vascular control may be reduced.
Acknowledgments
The authors would like to thank Dr Sandra Davidge and Ms Anita Quon for assistance with the measurement of NOS.
Glossary
- ACh
acetylcholine
- FBF
femoral artery blood flow
- FVC
femoral vascular conductance
- eNOS
endothelial nitric oxide synthase
- HR
heart rate
- MAP
mean arterial pressure
- MCF
maximal contractile force
- l-NAME
Nω-nitro-l-arginine methyl ester hydrochloride
- NO
nitric oxide
- NOS
nitric oxide synthase
- nNOS
neuronal nitric oxide synthase
- SMTC
S-methyl-l-thiocitrulline
Key points
Sympathetic nervous system activity causes tonic vasoconstriction in resting and contracting skeletal muscle. Nitric oxide (NO) has been shown to inhibit sympathetic vasoconstriction.
NO derived from both the neuronal and endothelial isoforms of NO synthase (NOS) has been shown to contribute to the inhibition of sympathetic vasoconstriction.
Our laboratory recently demonstrated that exercise training augmented NO-dependent inhibition of sympathetic vasoconstriction. However, the NOS isoform responsible for the increase in NO-mediated inhibition of sympathetic vasoconstriction following exercise training has not been established.
The present findings demonstrate that exercise training improves neuronal NOS-mediated inhibition of sympathetic vasoconstriction in contracting skeletal muscle.
Additional information
Competing interests
None declared.
Author contributions
N.G.J. contributed to the conception of the project; design of experiments; data collection, analysis and interpretation; and manuscript preparation and revision. T.P.J. contributed to data collection, analysis and interpretation; and manuscript preparation and revision. D.S.D. contributed to the conception of the project; design of experiments; data analysis and interpretation; and manuscript preparation and revision. All authors have read and approved the final submission.
Funding
This project was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC), Canadian Foundation for Innovation and Alberta Advanced Education and Technology. N.J. was supported by an NSERC Canada Graduate Doctoral Scholarship, University of Alberta Presidents’ Scholarship and Izaak Walton Killam Memorial Scholarship. T.J. was supported by an NSERC Canada Graduate Doctoral Scholarship and a Queen Elizabeth II Doctoral Scholarship from the University of Alberta.
References
- Bachetti T, Comini L, Curello S, Bastianon D, Palmieri M, Bresciani G, Callea F. Ferrari R. Co-expression and modulation of neuronal and endothelial nitric oxide synthase in human endothelial cells. J Mol Cell Cardiol. 2004;37:939–945. doi: 10.1016/j.yjmcc.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Balon TW. Nadler JL. Evidence that nitric oxide increases glucose transport in skeletal muscle. J Appl Physiol. 1997;82:359–363. doi: 10.1152/jappl.1997.82.1.359. [DOI] [PubMed] [Google Scholar]
- Boulanger CM, Heymes C, Benessiano J, Geske RS, Levy BI. Vanhoutte PM. Neuronal nitric oxide synthase is expressed in rat vascular smooth muscle cells: activation by angiotensin II in hypertension. Circ Res. 1998;83:1271–1278. doi: 10.1161/01.res.83.12.1271. [DOI] [PubMed] [Google Scholar]
- Boushel R, Langberg H, Gemmer C, Olesen J, Crameri R, Scheede C, Sander M. Kjaer M. Combined inhibition of nitric oxide and prostaglandins reduces human skeletal muscle blood flow during exercise. J Physiol. 2002;543:691–698. doi: 10.1113/jphysiol.2002.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter JB. Clifford PS. The paradox of sympathetic vasoconstriction in exercising skeletal muscle. Exerc Sport Sci Rev. 2001;29:159–163. doi: 10.1097/00003677-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Chavoshan B, Sander M, Sybert TE, Hansen J, Victor RG. Thomas GD. Nitric oxide-dependent modulation of sympathetic neural control of oxygenation in exercising human skeletal muscle. J Physiol. 2002;540:377–386. doi: 10.1113/jphysiol.2001.013153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford PS. Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol. 2004;97:393–403. doi: 10.1152/japplphysiol.00179.2004. [DOI] [PubMed] [Google Scholar]
- Copp SW, Hirai DM, Schwagerl PJ, Musch TI. Poole DC. Effects of neuronal nitric oxide synthase inhibition on resting and exercising hindlimb muscle blood flow in the rat. J Physiol. 2010;588:1321–1331. doi: 10.1113/jphysiol.2009.183723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp SW, Hirai DM, Ferguson SK, Musch TI. Poole DC. Role of neuronal nitric oxide synthase in modulating microvascular and contractile function in rat skeletal muscle. Microcirculation. 2011;18:501–511. doi: 10.1111/j.1549-8719.2011.00111.x. [DOI] [PubMed] [Google Scholar]
- Copp SW, Holdsworth CT, Ferguson SK, Hirai DM, Poole DC. Musch TI. Muscle fibre-type dependence of neuronal nitric oxide synthase-mediated vascular control in the rat during high speed treadmill running. J Physiol. 2013;591:2885–2896. doi: 10.1113/jphysiol.2013.251082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCarlo SE, Chen CY. Collins HL. Onset of exercise increases lumbar sympathetic nerve activity in rats. Med Sci Sports Exerc. 1996;28:677–684. doi: 10.1097/00005768-199606000-00006. [DOI] [PubMed] [Google Scholar]
- Dinenno FA. Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory? J Physiol. 2003;553:281–292. doi: 10.1113/jphysiol.2003.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA. Joyner MJ. Combined NO and PG inhibition augments a-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol. 2004;287:H2576–H2584. doi: 10.1152/ajpheart.00621.2004. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Lesniewski LA. Delp MD. Ageing and exercise training alter adrenergic vasomotor responses of rat skeletal muscle arterioles. J Physiol. 2007;579:115–125. doi: 10.1113/jphysiol.2006.120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel PJ, Zhao W. Thomas GD. Impaired vasomodulation is associated with reduced neuronal nitric oxide synthase in skeletal muscle of ovariectomized rats. J Physiol. 2003;549:243–253. doi: 10.1113/jphysiol.2003.038828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen U, Lopez-Figueroa M. Hellsten Y. Localization of nitric oxide synthase in human skeletal muscle. Biochem Biophys Res Commun. 1996;227:88–93. doi: 10.1006/bbrc.1996.1472. [DOI] [PubMed] [Google Scholar]
- Frandsen U, Hoffner L, Betak A, Saltin B, Bangsbo J. Hellsten Y. Endurance training does not alter the level of neuronal nitric oxide synthase in human skeletal muscle. J Appl Physiol. 2000;89:1033–1038. doi: 10.1152/jappl.2000.89.3.1033. [DOI] [PubMed] [Google Scholar]
- Grange RW, Isotani E, Lau KS, Kamm KE, Huang PL. Stull JT. Nitric oxide contributes to vascular smooth muscle relaxation in contracting fast-twitch muscles. Physiol Genomics. 2001;5:35–44. doi: 10.1152/physiolgenomics.2001.5.1.35. [DOI] [PubMed] [Google Scholar]
- Habler HJ, Wasner G. Janig W. Attenuation of neurogenic vasoconstriction by nitric oxide in hindlimb microvascular beds of the rat in vivo. Hypertension. 1997;30:957–961. doi: 10.1161/01.hyp.30.4.957. [DOI] [PubMed] [Google Scholar]
- Harris MB, Mitchell BM, Sood SG, Webb RC. Venema RC. Increased nitric oxide synthase activity and Hsp90 association in skeletal muscle following chronic exercise. Eur J Appl Physiol. 2008;104:795–802. doi: 10.1007/s00421-008-0833-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara A. Navar LG. Neuronal NOS contributes to biphasic autoregulatory response during enhanced TGF activity. Am J Physiol Renal Physiol. 1999;277:F113–F120. doi: 10.1152/ajprenal.1999.277.1.F113. [DOI] [PubMed] [Google Scholar]
- Jendzjowsky NG. DeLorey DS. A prospective evaluation of non-interval- and interval-based exercise training progressions in rodents. Appl Physiol Nutr Metab. 2011;36:723–729. doi: 10.1139/h11-092. [DOI] [PubMed] [Google Scholar]
- Jendzjowsky NG. DeLorey DS. Short-term exercise training augments sympathetic vasoconstrictor responsiveness and endothelium-dependent vasodilation in resting skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2012;303:R332–R339. doi: 10.1152/ajpregu.00053.2012. [DOI] [PubMed] [Google Scholar]
- Jendzjowsky NG. DeLorey DS. Role of neuronal nitric oxide in the inhibition of sympathetic vasoconstriction in resting and contracting skeletal muscle of healthy rats. J Appl Physiol (1985) 2013a;115:97–106. doi: 10.1152/japplphysiol.00250.2013. [DOI] [PubMed] [Google Scholar]
- Jendzjowsky NG. DeLorey DS. Short-term exercise training enhances functional sympatholysis through a nitric oxide-dependent mechanism. J Physiol. 2013b;591:1535–1549. doi: 10.1113/jphysiol.2012.238998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner MJ, Nauss LA, Warner MA. Warner DO. Sympathetic modulation of blood flow and O2 uptake in rhythmically contracting human forearm muscles. Am J Physiol. 1992;263:H1078–1083. doi: 10.1152/ajpheart.1992.263.4.H1078. [DOI] [PubMed] [Google Scholar]
- Kobzik L, Reid MB, Bredt DS. Stamler JS. Nitric oxide in skeletal muscle. Nature. 1994;372:546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- Kobzik L, Stringer B, Balligand JL, Reid MB. Stamler JS. Endothelial type nitric oxide synthase in skeletal muscle fibers: mitochondrial relationships. Biochem Biophys Res Commun. 1995;211:375–381. doi: 10.1006/bbrc.1995.1824. [DOI] [PubMed] [Google Scholar]
- Lau KS, Grange RW, Isotani E, Sarelius IH, Kamm KE, Huang PL. Stull JT. nNOS and eNOS modulate cGMP formation and vascular response in contracting fast-twitch skeletal muscle. Physiol Genomics. 2000;2:21–27. doi: 10.1152/physiolgenomics.2000.2.1.21. [DOI] [PubMed] [Google Scholar]
- Laughlin MH. Armstrong RB. Adrenoreceptor effects on rat muscle blood flow during treadmill exercise. J Appl Physiol. 1987;62:1465–1472. doi: 10.1152/jappl.1987.62.4.1465. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Pollock JS, Amann JF, Hollis ML, Woodman CR. Price EM. Training induces nonuniform increases in eNOS content along the coronary arterial tree. J Appl Physiol. 2001;90:501–510. doi: 10.1152/jappl.2001.90.2.501. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Welshons WV, Sturek M, Rush JW, Turk JR, Taylor JA, Judy BM, Henderson KK. Ganjam VK. Gender, exercise training, and eNOS expression in porcine skeletal muscle arteries. J Appl Physiol. 2003;95:250–264. doi: 10.1152/japplphysiol.00061.2003. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Woodman CR, Schrage WG, Gute D. Price EM. Interval sprint training enhances endothelial function and eNOS content in some arteries that perfuse white gastrocnemius muscle. J Appl Physiol. 2004;96:233–244. doi: 10.1152/japplphysiol.00105.2003. [DOI] [PubMed] [Google Scholar]
- McAllister RM. Price EM. Effects of exercise training on vasodilatory protein expression and activity in rats. Eur J Appl Physiol. 2010;110:1019–1027. doi: 10.1007/s00421-010-1584-6. [DOI] [PubMed] [Google Scholar]
- McConell GK, Bradley SJ, Stephens TJ, Canny BJ, Kingwell BA. Lee-Young RS. Skeletal muscle nNOSm protein content is increased by exercise training in humans. Am J Physiol Regul Integr Comp Physiol. 2007;293:R821–R828. doi: 10.1152/ajpregu.00796.2006. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Iwamoto GA, Vongpatanasin W, Mitchell JH. Smith SA. Exercise training improves functional sympatholysis in spontaneously hypertensive rats through a nitric oxide-dependent mechanism. Am J Physiol Heart Circ Physiol. 2014;307:H242–H251. doi: 10.1152/ajpheart.00103.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Morkeberg J, Thaning P, Hellsten Y. Saltin B. Two weeks of muscle immobilization impairs functional sympatholysis but increases exercise hyperemia and the vasodilatory responsiveness to infused ATP. Am J Physiol Heart Circ Physiol. 2012a;302:H2074–H2082. doi: 10.1152/ajpheart.01204.2011. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Nyberg M, Winding K. Saltin B. Lifelong physical activity preserves functional sympatholysis and purinergic signalling in the ageing human leg. J Physiol. 2012b;590:6227–6236. doi: 10.1113/jphysiol.2012.240093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg M, Blackwell JR, Damsgaard R, Jones AM, Hellsten Y. Mortensen SP. Lifelong physical activity prevents an age-related reduction in arterial and skeletal muscle nitric oxide bioavailability in humans. J Physiol. 2012;590:5361–5370. doi: 10.1113/jphysiol.2012.239053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary DS, Robinson ED. Butler JL. Is active skeletal muscle functionally vasoconstricted during dynamic exercise in conscious dogs? Am J Physiol Regul Integr Comp Physiol. 1997;272:R386–R391. doi: 10.1152/ajpregu.1997.272.1.R386. [DOI] [PubMed] [Google Scholar]
- Ohyanagi M, Nishigaki K. Faber JE. Interaction between microvascular alpha1- and alpha2-adrenoceptors and endothelium-derived relaxing factor. Circ Res. 1992;71:188–200. doi: 10.1161/01.res.71.1.188. [DOI] [PubMed] [Google Scholar]
- Parkington HC, Chow JA, Evans RG, Coleman HA. Tare M. Role for endothelium-derived hyperpolarizing factor in vascular tone in rat mesenteric and hindlimb circulations in vivo. J Physiol. 2002;542:929–937. doi: 10.1113/jphysiol.2002.021030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmeier JB, Fritzlar SJ, Dinenno FA. Joyner MJ. Exogenous NO administration and a-adrenergic vasoconstriction in human limbs. J Appl Physiol. 2003;95:2370–2374. doi: 10.1152/japplphysiol.00634.2003. [DOI] [PubMed] [Google Scholar]
- Sander M, Chavoshan B, Harris SA, Iannaccone ST, Stull JT, Thomas GD. Victor RG. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2000;97:13818–13823. doi: 10.1073/pnas.250379497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon MD, Chowienczyk PJ, Brett SE, Casadei B. Shah AM. Neuronal nitric oxide synthase regulates basal microvascular tone in humans in vivo. Circulation. 2008;117:1991–1996. doi: 10.1161/CIRCULATIONAHA.107.744540. [DOI] [PubMed] [Google Scholar]
- Seddon M, Melikian N, Dworakowski R, Shabeeh H, Jiang B, Byrne J, Casadei B, Chowienczyk P. Shah AM. Effects of neuronal nitric oxide synthase on human coronary artery diameter and blood flow in vivo. Circulation. 2009;119:2656–2662. doi: 10.1161/CIRCULATIONAHA.108.822205. [DOI] [PubMed] [Google Scholar]
- Song W, Kwak HB, Kim JH. Lawler JM. Exercise training modulates the nitric oxide synthase profile in skeletal muscle from old rats. J Gerontol Series A Biol Sci Med Sci. 2009;64:540–549. doi: 10.1093/gerona/glp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Huang A, Koller A. Kaley G. Short-term daily exercise activity enhances endothelial NO synthesis in skeletal muscle arterioles of rats. J Appl Physiol. 1994;76:2241–2247. doi: 10.1152/jappl.1994.76.5.2241. [DOI] [PubMed] [Google Scholar]
- Tatchum-Talom R, Schulz R, McNeill JR. Khadour FH. Upregulation of neuronal nitric oxide synthase in skeletal muscle by swim training. Am J Physiol Heart Circ Physiol. 2000;279:H1757–H1766. doi: 10.1152/ajpheart.2000.279.4.H1757. [DOI] [PubMed] [Google Scholar]
- Thomas GD. Segal SS. Neural control of muscle blood flow during exercise. J Appl Physiol. 2004;97:731–738. doi: 10.1152/japplphysiol.00076.2004. [DOI] [PubMed] [Google Scholar]
- Thomas GD. Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol. 1998;506(Pt 3):817–826. doi: 10.1111/j.1469-7793.1998.817bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GD, Sander M, Lau KS, Huang PL, Stull JT. Victor RG. Impaired metabolic modulation of a-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci U S A. 1998;95:15090–15095. doi: 10.1073/pnas.95.25.15090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilakopoulos T, Deckman G, Kebbewar M, Rallis G, Harfouche R. Hussain SN. Regulation of nitric oxide production in limb and ventilatory muscles during chronic exercise training. Am J Physiol Lung Cell Mol Physiol. 2003;284:L452–L457. doi: 10.1152/ajplung.00270.2002. [DOI] [PubMed] [Google Scholar]
- Wakefield ID, March JE, Kemp PA, Valentin JP, Bennett T. Gardiner SM. Comparative regional haemodynamic effects of the nitric oxide synthase inhibitors, S-methyl-l-thiocitrulline and l-NAME, in conscious rats. Br J Pharmacol. 2003;139:1235–1243. doi: 10.1038/sj.bjp.0705351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimer GS. Baldi JC. Limb-specific training affects exercise hyperemia but not sympathetic vasoconstriction. Eur J Appl Physiol. 2012;112:3819–3828. doi: 10.1007/s00421-012-2359-z. [DOI] [PubMed] [Google Scholar]


