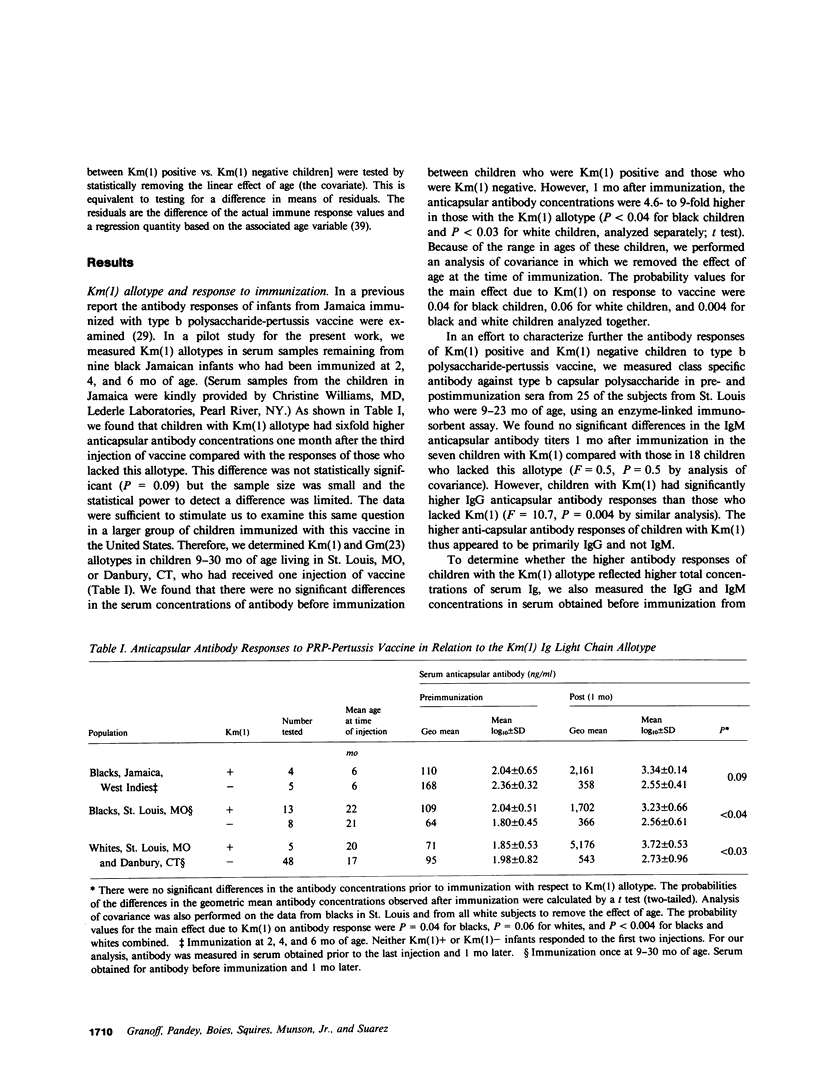
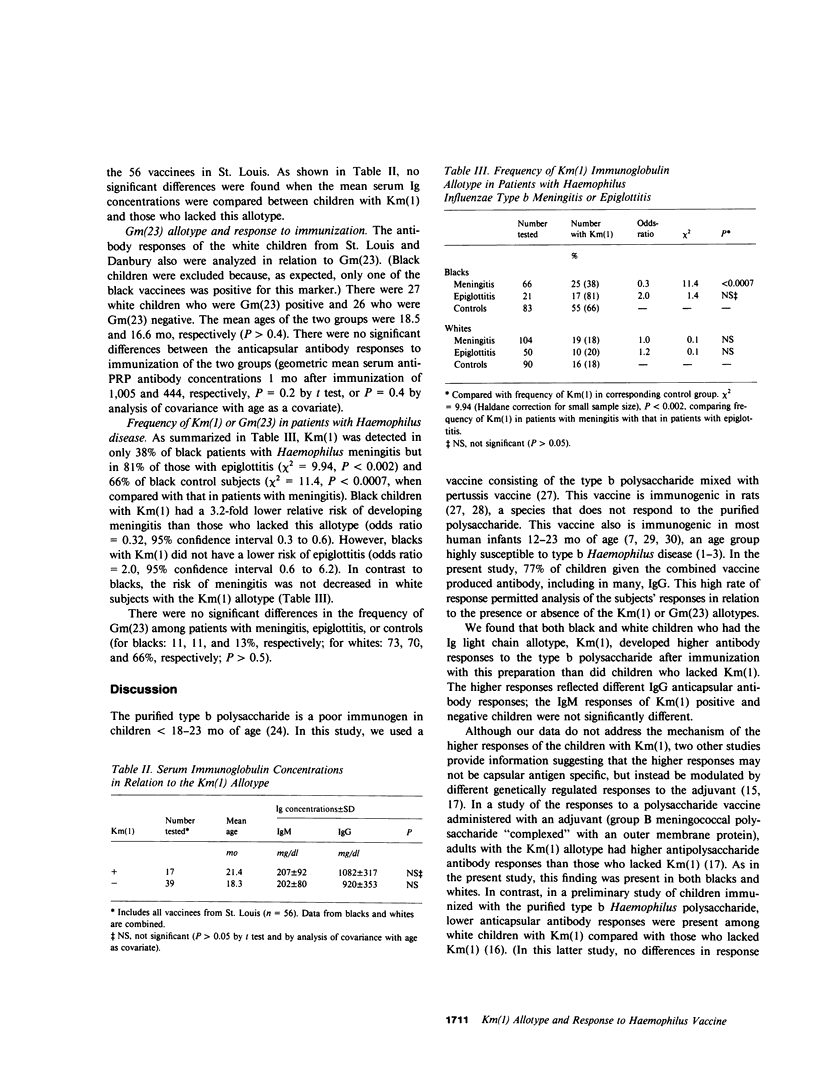
Abstract
In experimental animals, immune responses to certain antigens are regulated by immunoglobulin allotype-linked genes. In an effort to detect such genes in humans, we examined the antibody responses of 74 healthy children with different Km(1) or Gm(23) allotypes to a Haemophilus influenzae type b vaccine (type b polysaccharide capsule-pertussis vaccine). The anticapsular antibody responses of black or white children with the Km(1) allotype were 4.6- to 9.5-fold higher than those of children who lacked this determinant (P less than 0.004). No significant differences were found in antibody response with respect to the Gm(23) allotype. The frequencies of Km(1) and Gm(23) also were examined in 170 patients with Haemophilus meningitis, 71 patients with epiglottitis, and 173 control children. Km(1) was detected less frequently in black patients with meningitis (38%) than in those with epiglottitis (81%, P less than 0.002) or in controls (66%, P less than 0.0007). The relative risk of meningitis thus was 3.2-fold lower among black children with the Km(1) allotype than in those who lacked this allotype (odds ratio = 0.3, 95% confidence interval 0.2 to 0.6). However, the risk of meningitis was not decreased in white children with the Km(1) allotype (odds ratio = 1.0). There were no significant differences in the frequency of Gm(23) among the patient groups and controls. The Km(1) allotype but not the Gm(23) thus defines a subpopulation of children of both races who are high responders to this vaccine, and black children but not white children with the Km(1) allotype are at decreased risk of developing Haemophilus meningitis. These data indicate that in blacks, genes associated with Km(1) may affect immune response to a prototype type b Haemophilus vaccine, and perhaps interact with another factor related to race to affect susceptibility to Haemophilus meningitis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander H. E., Heidelberger M., Leidy G. The Protective or Curative Element in Type B H. influenzae Rabbit Serum. Yale J Biol Med. 1944 May;16(5):425–434. [PMC free article] [PubMed] [Google Scholar]
- Benacerraf B. Role of MHC gene products in immune regulation. Science. 1981 Jun 12;212(4500):1229–1238. doi: 10.1126/science.6165083. [DOI] [PubMed] [Google Scholar]
- Coulehan J. L., Hallowell C., Michaels R. H., Welty T. K., Lui N., Kuo J. S. Immunogenicity of a Haemophilus influenzae type b vaccine in combination with diphtheria-pertussis-tetanus vaccine in infants. J Infect Dis. 1983 Sep;148(3):530–534. doi: 10.1093/infdis/148.3.530. [DOI] [PubMed] [Google Scholar]
- Crisel R. M., Baker R. S., Dorman D. E. Capsular polymer of Haemophilus influenzae, type b. I. Structural characterization of the capsular polymer of strain Eagan. J Biol Chem. 1975 Jul 10;250(13):4926–4930. [PubMed] [Google Scholar]
- Eardley D. D., Shen F. W., Cantor H., Gershon R. K. Genetic control of immunoregulatory circuits. Genes linked to the Ig locus govern communication between regulatory T-cell sets. J Exp Med. 1979 Jul 1;150(1):44–50. doi: 10.1084/jem.150.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granoff D. M., Basden M. Haemophilus influenzae infections in Fresno County, California: a prospective study of the effects of age, race, and contact with a case on incidence of disease. J Infect Dis. 1980 Jan;141(1):40–46. doi: 10.1093/infdis/141.1.40. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Boies E. G., Squires J. E., Pandey J. P., Suarez B. K., Oldfather J. W., Rodey G. E. Histocompatibility leukocyte antigen and erythrocyte MNSs specificities in patients with meningitis or epiglottitis due to Haemophilus influenzae type b. J Infect Dis. 1984 Mar;149(3):373–377. doi: 10.1093/infdis/149.3.373. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Daum R. S. Spread of Haemophilus influenzae type b: recent epidemiologic and therapeutic considerations. J Pediatr. 1980 Nov;97(5):854–860. doi: 10.1016/s0022-3476(80)80288-5. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Squires J. E., Munson R. S., Jr, Suarez B. Siblings of patients with Haemophilus meningitis have impaired anticapsular antibody responses to Haemophilus vaccine. J Pediatr. 1983 Aug;103(2):185–191. doi: 10.1016/s0022-3476(83)80342-4. [DOI] [PubMed] [Google Scholar]
- HALDANE J. B. The estimation and significance of the logarithm of a ratio of frequencies. Ann Hum Genet. 1956 May;20(4):309–311. doi: 10.1111/j.1469-1809.1955.tb01285.x. [DOI] [PubMed] [Google Scholar]
- Halsey N. A., Johansen T. L., Bowman L. C., Glode M. P. Evaluation of the protective efficacy of Haemophilus influenzae type b vaccines in an animal model. Infect Immun. 1983 Mar;39(3):1196–1200. doi: 10.1128/iai.39.3.1196-1200.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. K., Eardley D. D., Cantor H., Gershon R. K. Definition of two pathways for generation of suppressor T-cell activity. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3779–3781. doi: 10.1073/pnas.80.12.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Anderson P., Rosen F. S., Smith D. H. Characterization of human antibody to polyribophosphate, the capsular antigen of Hemophilus influenzae, type B. Clin Immunol Immunopathol. 1973 Jan;1(2):234–240. doi: 10.1016/0090-1229(73)90024-x. [DOI] [PubMed] [Google Scholar]
- Kagnoff M. F. Two genetic loci control the murine immune response to A-gliadin, a wheat protein that activates coeliac sprue. Nature. 1982 Mar 11;296(5853):158–160. doi: 10.1038/296158a0. [DOI] [PubMed] [Google Scholar]
- King S. D., Ramlal A., Wynter H., Moodie K., Castle D., Kuo J. S., Barnes L., Williams C. L. Safety and immunogenicity of a new Haemophilus influenzae type b vaccine in infants under one year of age. Lancet. 1981 Oct 3;2(8249):705–709. doi: 10.1016/s0140-6736(81)91045-x. [DOI] [PubMed] [Google Scholar]
- Käyhty H., Schneerson R., Sutton A. Class-specific antibody response to Haemophilus influenzae type b capsular polysaccharide vaccine. J Infect Dis. 1983 Oct;148(4):767–767. doi: 10.1093/infdis/148.4.767. [DOI] [PubMed] [Google Scholar]
- Morrow P. R., Rennick D. M., Benjamini E. The antibody response to a single antigenic determinant of the tobacco mosaic virus protein (TMVP): effects of allotype-linked genes and restricted heterogeneity of the response. J Immunol. 1983 Dec;131(6):2875–2881. [PubMed] [Google Scholar]
- Moxon E. R., Vaughn K. A. The type b capsular polysaccharide as a virulence determinant of Haemophilus influenzae: studies using clinical isolates and laboratory transformants. J Infect Dis. 1981 Apr;143(4):517–524. doi: 10.1093/infdis/143.4.517. [DOI] [PubMed] [Google Scholar]
- Pandey J. P., Fudenberg H. H., Virella G., Kyong C. U., Loadholt C. B., Galbraith R. M., Gotschlich E. C., Parke J. C., Jr Association between immunoglobulin allotypes and immune responses to Haemophilus influenzae and Meningococcus polysaccharides. Lancet. 1979 Jan 27;1(8109):190–192. doi: 10.1016/s0140-6736(79)90584-1. [DOI] [PubMed] [Google Scholar]
- Pandey J. P., Shannon B. T., Arala-Chaves M. P., Fudenberg H. H. Gm and Km frequencies in a Portuguese population. Hum Genet. 1982;61(2):154–156. doi: 10.1007/BF00274207. [DOI] [PubMed] [Google Scholar]
- Pandey J. P., Zollinger W. D., Fudenberg H. H., Loadholt C. B. Immunoglobulin allotypes and immune response to meningococcal group B polysaccharide. J Clin Invest. 1981 Nov;68(5):1378–1380. doi: 10.1172/JCI110387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parke J. C., Jr, Schneerson R., Robbins J. B. The attack rate, age incidence, racial distribution, and case fatality rate of Hemophilus influenzae type b meningitis in Mecklenbury County, North Carolina. J Pediatr. 1972 Oct;81(4):765–769. doi: 10.1016/s0022-3476(72)80099-4. [DOI] [PubMed] [Google Scholar]
- Peltola H., Käyhty H., Sivonen A., Mäkelä H. Haemophilus influenzae type b capsular polysaccharide vaccine in children: a double-blind field study of 100,000 vaccinees 3 months to 5 years of age in Finland. Pediatrics. 1977 Nov;60(5):730–737. [PubMed] [Google Scholar]
- Perlmutter R. M., Hansburg D., Briles D. E., Nicolotti R. A., Davie J. M. Subclass restriction of murine anti-carbohydrate antibodies. J Immunol. 1978 Aug;121(2):566–572. [PubMed] [Google Scholar]
- Pincus D. J., Morrison D., Andrews C., Lawrence E., Sell S. H., Wright P. F. Age-related response to two Haemophilus influenzae type b vaccines. J Pediatr. 1982 Feb;100(2):197–201. doi: 10.1016/s0022-3476(82)80634-3. [DOI] [PubMed] [Google Scholar]
- Reed T. E. Critical tests of hypotheses for race mixture using Gm data on American Caucasians and Negroes. Am J Hum Genet. 1969 Jan;21(1):71–83. [PMC free article] [PubMed] [Google Scholar]
- Robbins J. B., Parke J. C., Jr, Schneerson R., Whisnant J. K. Quantitative measurement of "natural" and immunization-induced Haemophilus influenzae type b capsular polysaccharide antibodies. Pediatr Res. 1973 Mar;7(3):103–110. doi: 10.1203/00006450-197303000-00001. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Barrera O., Sutton A., Robbins J. B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980 Aug 1;152(2):361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneerson R., Rodrigues L. P., Parke J. C., Jr, Robbins J. B. Immunity to disease caused by Hemophilus influenzae type b. II. Specificity and some biologic characteristics of "natural," infection-acquired, and immunization-induced antibodies to the capsular polysaccharide of Hemophilus influenzae type b. J Immunol. 1971 Oct;107(4):1081–1089. [PubMed] [Google Scholar]
- Siber G. R., Schur P. H., Aisenberg A. C., Weitzman S. A., Schiffman G. Correlation between serum IgG-2 concentrations and the antibody response to bacterial polysaccharide antigens. N Engl J Med. 1980 Jul 24;303(4):178–182. doi: 10.1056/NEJM198007243030402. [DOI] [PubMed] [Google Scholar]
- Vyas G. N., Fudenberg H. H., Pretty H. M., Gold E. R. A new rapid method for genetic typing of human immunoglobulins. J Immunol. 1968 Feb;100(2):274–279. [PubMed] [Google Scholar]
- Weller P. F., Smith A. L., Anderson P., Smith D. H. The role of encapsulation and host age in the clearance of Haemophilus influenzae bacteremia. J Infect Dis. 1977 Jan;135(1):34–41. doi: 10.1093/infdis/135.1.34. [DOI] [PubMed] [Google Scholar]
- Whisnant J. K., Rogentine G. N., Gralnick M. A., Schlesselman J. J., Robbins J. B. Host factors and antibody response Haemophilus influenza type b meningitis and epiglottitis. J Infect Dis. 1976 Apr;133(4):448–455. [PubMed] [Google Scholar]
- Whittingham S., Mathews J. D., Schanfield M. S., Matthews J. V., Tait B. D., Morris P. J., Mackay I. R. Interactive effect of Gm allotypes and HLA-B locus antigens on the human antibody response to a bacterial antigen. Clin Exp Immunol. 1980 Apr;40(1):8–15. [PMC free article] [PubMed] [Google Scholar]


