Abstract
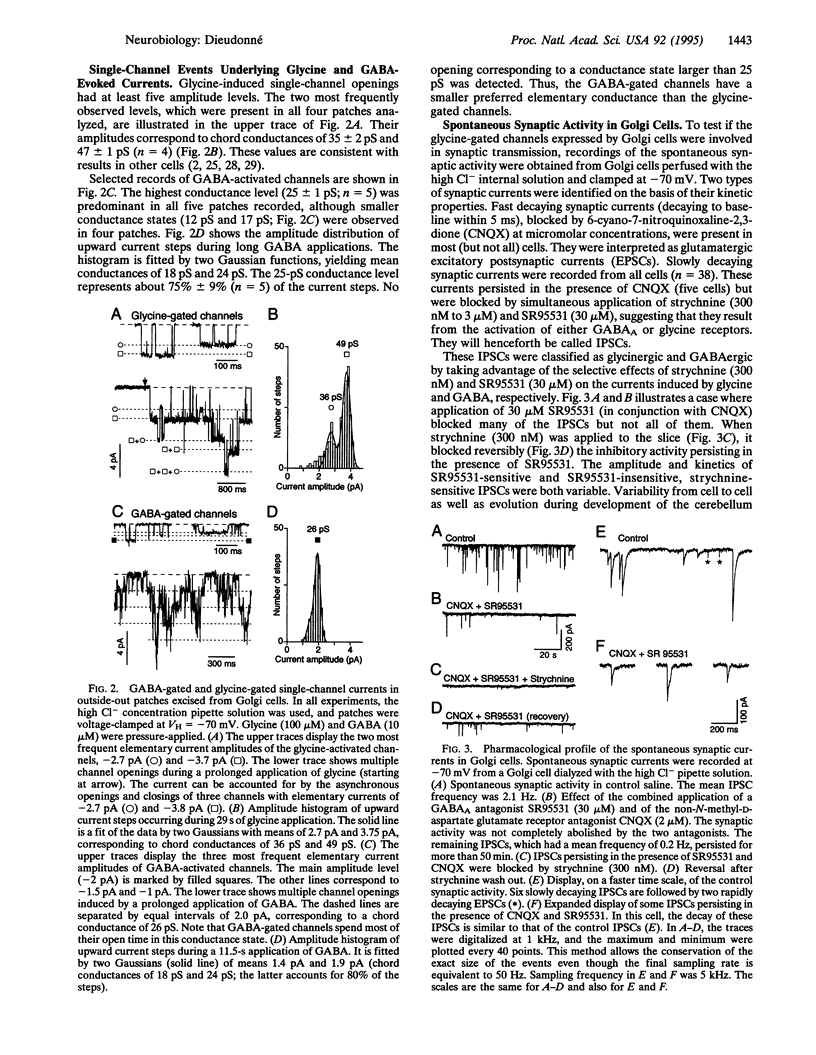
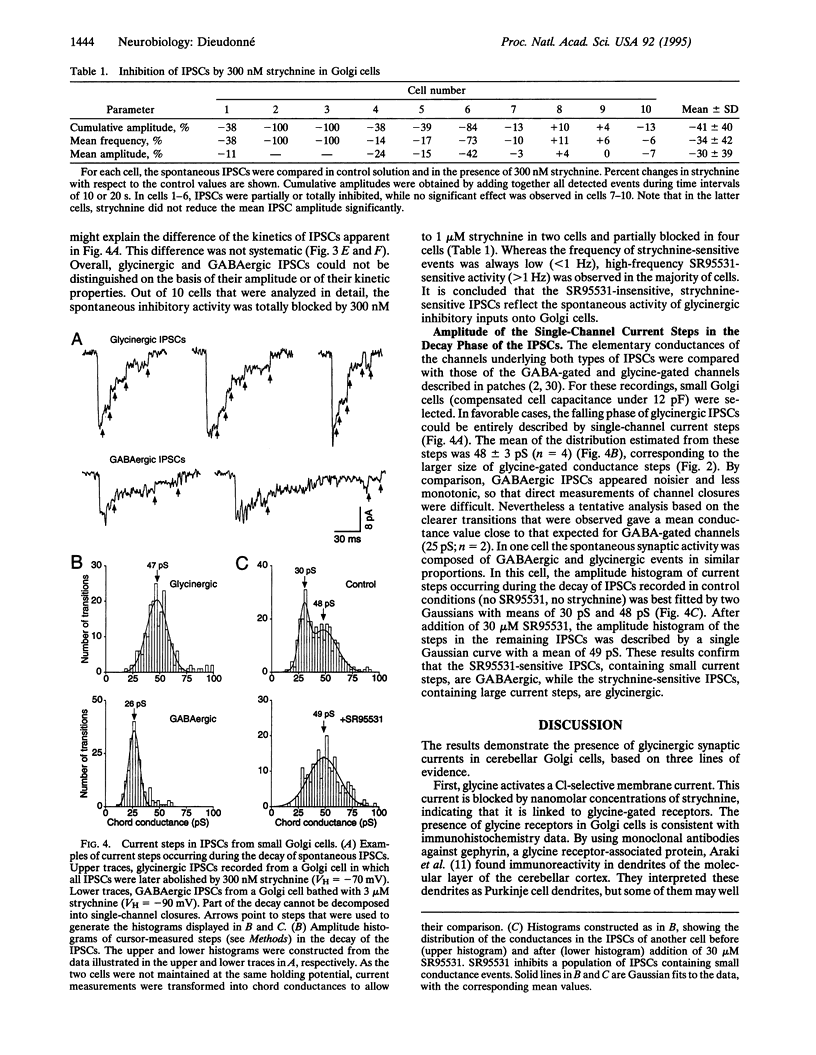
Recordings were made from Golgi cells in slices from rat cerebellar cortex using whole-cell and outside-out configurations of the patch-clamp technique. Exogenous glycine and gamma-aminobutyric acid (GABA) both activated chloride currents, which could be differentially blocked by strychnine and SR95531, respectively. Inhibitory synaptic currents occurred spontaneously in all Golgi cells. Some were blocked by strychnine while the others were blocked by SR95531. The single channel events occurring during the decay of these two types of inhibitory postsynaptic currents had different amplitudes, which matched the main conductance states of the channels gated by glycine and GABA in outside-out patches. It was concluded that Golgi cells receive both glycinergic and GABAergic synaptic inputs.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Kaneda M. Glycine-gated chloride current in acutely isolated rat hypothalamic neurons. J Neurophysiol. 1989 Dec;62(6):1400–1409. doi: 10.1152/jn.1989.62.6.1400. [DOI] [PubMed] [Google Scholar]
- Araki T., Yamano M., Murakami T., Wanaka A., Betz H., Tohyama M. Localization of glycine receptors in the rat central nervous system: an immunocytochemical analysis using monoclonal antibody. Neuroscience. 1988 May;25(2):613–624. doi: 10.1016/0306-4522(88)90263-1. [DOI] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Friz C. T. The nitrogen content of the water soluble proteins of three species of the free-living amoebae. Life Sci. 1968 Jun 15;7(12):583–585. doi: 10.1016/0024-3205(68)90078-7. [DOI] [PubMed] [Google Scholar]
- Gold M. R., Martin A. R. Characteristics of inhibitory post-synaptic currents in brain-stem neurones of the lamprey. J Physiol. 1983 Sep;342:85–98. doi: 10.1113/jphysiol.1983.sp014841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenningloh G., Pribilla I., Prior P., Multhaup G., Beyreuther K., Taleb O., Betz H. Cloning and expression of the 58 kd beta subunit of the inhibitory glycine receptor. Neuron. 1990 Jun;4(6):963–970. doi: 10.1016/0896-6273(90)90149-a. [DOI] [PubMed] [Google Scholar]
- Hamann M., Desarmenien M., Desaulles E., Bader M. F., Feltz P. Quantitative evaluation of the properties of a pyridazinyl GABA derivative (SR 95531) as a GABAA competitive antagonist. An electrophysiological approach. Brain Res. 1988 Mar 1;442(2):287–296. doi: 10.1016/0006-8993(88)91514-4. [DOI] [PubMed] [Google Scholar]
- Ito S., Cherubini E. Strychnine-sensitive glycine responses of neonatal rat hippocampal neurones. J Physiol. 1991;440:67–83. doi: 10.1113/jphysiol.1991.sp018696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Osipchuk YuV, Vrublevsky S. V. Properties of glycine-activated conductances in rat brain neurones. Neurosci Lett. 1988 Feb 3;84(3):271–276. doi: 10.1016/0304-3940(88)90519-8. [DOI] [PubMed] [Google Scholar]
- Kuhse J., Schmieden V., Betz H. A single amino acid exchange alters the pharmacology of neonatal rat glycine receptor subunit. Neuron. 1990 Dec;5(6):867–873. doi: 10.1016/0896-6273(90)90346-h. [DOI] [PubMed] [Google Scholar]
- Lainé J., Axelrad H., Rahbi N. Intermediate cells of Lugaro are present in the immature rat cerebellar cortex at an earlier stage than previously thought. Neurosci Lett. 1992 Oct 12;145(2):225–228. doi: 10.1016/0304-3940(92)90028-6. [DOI] [PubMed] [Google Scholar]
- Llano I., Marty A., Armstrong C. M., Konnerth A. Synaptic- and agonist-induced excitatory currents of Purkinje cells in rat cerebellar slices. J Physiol. 1991 Mar;434:183–213. doi: 10.1113/jphysiol.1991.sp018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980 Aug;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malosio M. L., Marquèze-Pouey B., Kuhse J., Betz H. Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO J. 1991 Sep;10(9):2401–2409. doi: 10.1002/j.1460-2075.1991.tb07779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNiven A. I., Martin A. R. Effect of cytoplasmic chloride concentration on open-state preference of glycine-activated chloride channels in cultured spinal cord cells. J Neurophysiol. 1993 Mar;69(3):860–867. doi: 10.1152/jn.1993.69.3.860. [DOI] [PubMed] [Google Scholar]
- Midtgaard J. Membrane properties and synaptic responses of Golgi cells and stellate cells in the turtle cerebellum in vitro. J Physiol. 1992 Nov;457:329–354. doi: 10.1113/jphysiol.1992.sp019381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottersen O. P., Storm-Mathisen J., Somogyi P. Colocalization of glycine-like and GABA-like immunoreactivities in Golgi cell terminals in the rat cerebellum: a postembedding light and electron microscopic study. Brain Res. 1988 May 31;450(1-2):342–353. doi: 10.1016/0006-8993(88)91573-9. [DOI] [PubMed] [Google Scholar]
- Pourcho R. G., Goebel D. J., Jojich L., Hazlett J. C. Immunocytochemical evidence for the involvement of glycine in sensory centers of the rat brain. Neuroscience. 1992;46(3):643–656. doi: 10.1016/0306-4522(92)90151-q. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Momiyama A., Hirai K., Hishinuma F., Akagi H. Functional correlation of fetal and adult forms of glycine receptors with developmental changes in inhibitory synaptic receptor channels. Neuron. 1992 Dec;9(6):1155–1161. doi: 10.1016/0896-6273(92)90073-m. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Momiyama A. Single-channel currents underlying glycinergic inhibitory postsynaptic responses in spinal neurons. Neuron. 1991 Dec;7(6):965–969. doi: 10.1016/0896-6273(91)90341-v. [DOI] [PubMed] [Google Scholar]
- Triller A., Cluzeaud F., Korn H. gamma-Aminobutyric acid-containing terminals can be apposed to glycine receptors at central synapses. J Cell Biol. 1987 Apr;104(4):947–956. doi: 10.1083/jcb.104.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombley P. Q., Shepherd G. M. Glycine exerts potent inhibitory actions on mammalian olfactory bulb neurons. J Neurophysiol. 1994 Feb;71(2):761–767. doi: 10.1152/jn.1994.71.2.761. [DOI] [PubMed] [Google Scholar]
- Twyman R. E., Macdonald R. L. Kinetic properties of the glycine receptor main- and sub-conductance states of mouse spinal cord neurones in culture. J Physiol. 1991 Apr;435:303–331. doi: 10.1113/jphysiol.1991.sp018512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkin G. P., Csillag A., Balázs R., Kingsbury A. E., Wilson J. E., Johnson A. L. Localization of high affinity [3H]glycine transport sites in the cerebellar cortex. Brain Res. 1981 Jul 6;216(1):11–33. doi: 10.1016/0006-8993(81)91275-0. [DOI] [PubMed] [Google Scholar]
- van den Pol A. N., Gorcs T. Glycine and glycine receptor immunoreactivity in brain and spinal cord. J Neurosci. 1988 Feb;8(2):472–492. doi: 10.1523/JNEUROSCI.08-02-00472.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



