Abstract
Pegylated interferon and ribavirin combination therapy is known to be effective in suppressing viral replication in 50–60% of hepatitis C virus (HCV) -infected patients. However, HCV-infected patients often exhibit varied responses to therapy. Therefore, the identification of immunological markers associated with the clinical outcomes of antiviral treatment is critical for improvement of therapeutic options. In this study, we aimed to investigate the ratio of CD4+ CD25+ FoxP3+ regulatory T (Treg) cells to interleukin-17A (IL-17A) -producing T helper type 17 (Th17) cells, and its association with clinical outcomes in response to anti-HCV treatment. In all, 114 patients with HCV infection received pegylated interferon-α2a and ribavirin therapy for 48 weeks, and the frequency of Treg cells and Th17 cells as well as the levels of secreted cytokines were longitudinally analysed by flow cytometry and ELISA. Treg cell proportions and IL-10 production were significantly elevated in HCV-infected patients, especially for HCV genotype 1b. However, the frequency of Th17 cells as well as the secretion of IL-17, IL-22 and IL-23 did not reveal notable difference between HCV infections and healthy individuals. Inhibition of HCV replication was accompanied by a reduction in Treg cells, but little influence on Th17 cells, which led to a significant decrease in Treg : Th17 ratios. Skewed Treg : Th17 ratios existed in chronic hepatitis C. HCV RNA load is closely associated with Treg : Th17 ratios during pegylated interferon-α2a and ribavirin treatment in HCV-infected patients. The imbalance of Treg cells to Th17 cells might play an important role in persistent HCV infection.
Keywords: antiviral therapy, hepatitis C virus, regulatory T cells, T helper type 17 cells
Introduction
The magnitude of the hepatitis C virus (HCV) infection problem is estimated to comprise 3% of the world population including past and current infections, with most cases being established chronic infections.1,2 Chronic HCV infection is a risk factor for the development of liver cirrhosis and hepatocellular carcinoma, resulting in the third-leading cause of all deaths from end-stage liver diseases.1 Although HCV has been described as a positive-stranded RNA virus and non-cytopathic pathogen, it can induce liver damage of variable severity by stimulating the immune response, which can simultaneously cause damage and protection. Therefore, the interplay between virus and host immune response may influence the outcome of HCV infection.3 The current standard therapy for HCV infection comprises the combination of pegylated interferon (peg-IFN) and ribavirin.4 The newly developed direct-acting antivirals were shown to improve the rate of sustained virological response, but they were not widely used for pharmaco-economic reasons.5 However, HCV-infected patients often exhibit varied responses to therapy. Therefore, the identification of immunological markers associated with the clinical outcomes in such cases is important to improve clinical management.
CD4+ T cells can differentiate into different lineages of T helper (Th) cells with distinct biological functions after activation. CD4+ CD25+ FoxP3+ regulatory T (Treg) cells and interleukin-17 (IL-17)-expressing T cells (Th17 cells) were proposed to be additional independent Th cell lineages.6,7 Previous studies have demonstrated that proportions of Treg cells were elevated in patients with chronic hepatitis C in peripheral blood and liver.8,9 The increase Treg cells showed HCV specificity through IL-10 production and suppressed HCV-specific CD8+ T cells during persistent infection.8,10,11 Standard anti-HCV treatment led to the decline of circulating and liver-infiltrating Treg cells and partial recovery of the impaired immune response.12,13 These findings revealed that Treg cells take part in regulation of the anti-HCV response. Moreover, Th17 cells were also enriched in intrahepatic and circulating conditions in HCV infection.14,15 Treg cells and Th17 cells may be generated from the same precursor T cells, and tumour growth factor-β1 is essential for both Treg cell and Th17 cell differentiation in a concentration-dependent manner.16 Recently, changes in the Treg cell and Th17 cell balance were reported to be involved in disease progression and persistent hepatitis B virus (HBV) infection.17,18 Hence, we hypothesized that an imbalance between Treg and Th17 cells participates in regulating the immune response during anti-HCV treatment. To test this possibility, we investigated the frequency of peripheral Treg cells and Th17 cells and related cytokine production, and thereby assessed the relationship between Treg : Th17 imbalance and effectiveness of IFN-α and ribavirin combination therapy.
Patients, materials and methods
Subjects
A total of 114 patients with HCV infection were enrolled in this study, including 44 with rapid virological response (RVR), 51 with early virological response (EVR), and 19 with non-response (NR). All patients were hospitalized or present for follow-up examinations in Tangdu Hospital from May 2009 to July 2012. The baseline characteristics of enrolled subjects are shown in Table 1. All patients received Pegasys [peg-IFN α2a (40KD); Roche, Shanghai, China] with ribavirin treatment for 48 weeks. Blood samples were taken on five occasions from all patients: baseline, 4, 12, 24 and 48 weeks. For normal controls (NCs), 24 healthy individuals matched for sex ratio and mean age with the patient groups were included. No enrolled participants were co-infected with HIV or other hepatitis viruses. Patients who received antiviral or immunomodulatory treatments within 1 year of baseline sampling were also excluded from the study. RVR was defined as undetectable HCV RNA at 4 weeks of therapy. EVR was defined as detectable HCV RNA at 4 weeks but undetectable at 12 weeks after initiation of treatment. NR was defined as less than 2 log10 copies/ml decrease in HCV RNA level at 12 weeks of therapy compared with baseline, and still having detectable HCV RNA at the end of standard therapy.19 The study protocol was approved by the ethics committee of the Fourth Military Medical University, and written informed consent was obtained from each subject.
Table 1.
Baseline clinical characteristics of enrolled subjects
| Group | NC | RVR | EVR | NR |
|---|---|---|---|---|
| Case | 24 | 44 | 51 | 19 |
| Sex (male/female) | 15/9 | 20/24 | 28/23 | 12/7 |
| Age (years) | 29 (23–36) | 31 (18–46) | 31 (19–65) | 28 (20–42) |
| ALT (U/l) | 25 (12–40) | 43 (9–142) | 56 (32–198) | 40 (21–173) |
| HCV RNA (log10 copies/ml) | N.D. | 4·32 (2·15–7·24) | 5·42 (2·72–6·21) | 4·82 (3·52–5·98) |
| HCV genotype (1b/2a) | N.D. | 30/14 | 33/18 | 16/3 |
Data are shown as median and range. NC, normal control; RVR, rapid virological response; EVR, early virological response; NR, Non-response; ND, not determined.
Virological and biochemical assessments
Serum HCV RNA was quantified with a commercial real-time PCR kit (PG Biotech, Shenzhen, China) with detection limit of 2 log10 copies/ml. Anti-HCV antibody was determined by commercial enzyme immunoassay kits (Jinhao Biotech, Beijing, China). Genotyping of HCV was performed using a second-generation line probe assay (Inno-Lipa II; Innogenetics, Zwijndre, Belgium). Serum biochemical assessments [including albumin, alanine aminotransferase (ALT), bilirubin, blood urea nitrogen, creatine and creatine kinase] were measured on an automatic analyser (Hitachi 7170A; Hitachi Ltd, Tokyo, Japan).
Peripheral blood mononuclear cell isolation and stimulation
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque (Sigma-Aldrich, St Louis, MO) density gradient centrifugation. The isolated PBMCs were then counted and checked for viability. Cells were cryopreserved at 5 × 106/ml in 10% DMSO and 90% fetal bovine serum (Invitrogen GIBCO, Grand Island, NY). On the day of the experiment, thawed cell populations were counted, and 106 PBMCs were stimulated with PMA (50 ng/ml) and ionomycin (1 μg/ml), with brefeldin A (10 μg/ml) for 5 hr for the measurement of Th17 cells.
Flow cytometry
The PBMCs were transferred into FACS tubes, and anti-CD3-peridinin chlorophyll protein (PerCP; BD Biosciences, San Jose, CA), anti-CD4-allophycocyanin (APC; eBioscience, San Diego, CA), and anti-CD25-phycoerythrin (PE; eBioscience) were added and incubated at 4° in the dark for 30 min. Cells were then fixed with 100 μl of Fixation & Permeabilization Medium A (Caltag Laboratories, Invitrogen, Carlsbad, CA) for 15 min of incubation, and then resuspended in 100 μl of Fixation & Permeabilization Medium B (Caltag Laboratories) containing anti-IL-17A-FITC (eBioscience) and anti-FoxP3-PE Cy7 for 20 min at room temperature. Samples were analysed with a FACSCalibur analyser (BD Biosciences Immunocytometry Systems, San Jose, CA). The isotype control antibodies were used to separate positive and negative cells in the PerCP, FITC, PE, PE Cy-7 and APC fluorescence channels. Acquisitions and analyses were performed with CellQuest Pro software (BD Biosciences Immunocytometry Systems), and data were analysed with flowjo version 5.7.2 for Windows (Tree Star Inc., Ashland, OR).
ELISA
Concentrations of IFN-γ, IL-10, IL-17, IL-22 and IL-23 were measured using commercial ELISA kits (eBioscience) according to the manufacturer’s instructions.
Statistical analyses
Data were analysed using spss version 13.0 for Windows (SPSS, Chicago, IL). Mann–Whitney U-test was used for the comparison among groups. Pearson correlation tests were performed for correlation analysis. All tests were two-tailed and values of P < 0·05 was considered to indicate a significant difference.
Results
Increased ratio of Treg : Th17 in chronic hepatitis C patients compared with normal controls
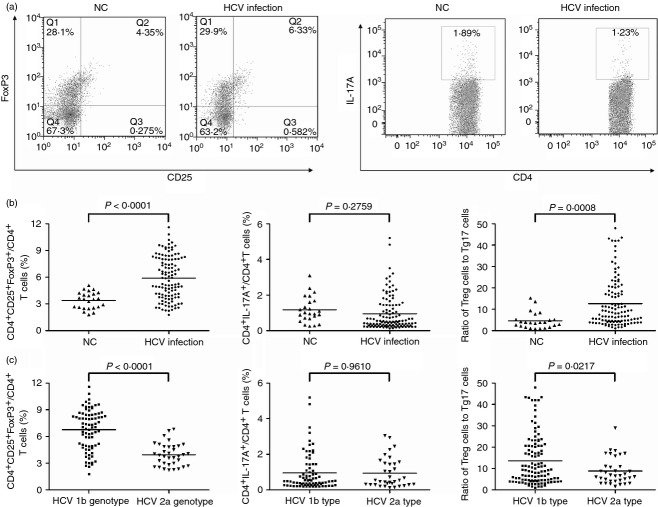
A total of 114 HCV-infected patients and 24 healthy individuals were enrolled in this study. We measured the frequency of CD3+ CD4+ CD25+ FoxP3+ T cells (Treg cells) and CD3+ CD4+ IL-17A+ T cells (Th17 cells) within a CD3+ CD4+ subset by flow cytometry. All subjects clearly displayed the two CD4+ T-cell subsets, and representative PBMC samples from both normal controls (NCs) and HCV-infected patients are shown in Fig. 1(a). There was a significantly higher frequency of CD4+ CD25+ FoxP3+ Treg cells in HCV-infected patients (5·91 ± 2·38%) when compared with NCs (3·40 ± 0·95%, P < 0·0001, Fig. 1b). Despite Th17 cells being generated from the same naive T cells that generate Treg cells, we did not find a remarkable difference in Th17 proportion when comparing NCs (1·18 ± 0·73%) and HCV-infected patients (0·95 ± 0·97%, P = 0·2759, Fig. 1b). To link Treg cells with Th17 cells, we use the ratio of Treg : Th17 cells as an index, and we observed a notably increased ratio in HCV-infected patients (Fig. 1b). Among the 114 enrolled HCV-infected individuals, 79 patients were HCV genotype 1b and the other 35 were genotype 2a. We further analysed the percentage of Treg cells and Th17 cells between the two different genotypes. As shown in Fig. 1(c), the percentage of Treg cells with CD4+ T cells were significantly higher in HCV genotype 1B groups (6·77 ± 2·26%) compared with HCV genotype 2a (3·96 ± 1·25%, P < 0·0001). However, there was no remarkable difference for Th17 cells between the two groups. Interestingly, the ratio of Treg cells to Th17 cells was also increased in HCV genotype 1b patients (Fig. 1c).
Figure 1.
The regulatory T (Treg) cell : T helper type 17 (Th17) cell ratio was increased in hepatitis C virus (HCV)-infected patients, especially in HCV genotype 1b patients. (a) Representative dot plots of CD25+ FoxP3+ and IL-17A+ expression in peripheral CD4+ T cells of normal controls (NC) and HCV-infected patients. The values in the quadrants indicate the percentage of each CD4+ T-cell subset. (b) The percentage of Treg cells, Th17 cells and the ratio of circulating Treg cells to Th17 cells in NC and HCV-infected patients. (c) The percentage of Treg cells, Th17 cells and the ratio of circulating Treg cells to Th17 cells in patients with HCV genotype 1b and 2a infection. Horizontal bars represent the mean values of indicated index.
Elevated IL-10 and decreased IFN-γ in the serum of HCV-infected patients
In order to learn about the cytokine production of Treg and Th17 cells in HCV-infected patients, we measured Treg- and Th17-related cytokines in the serum. Interleukin-10, which is important to mediate Treg suppression,20 was significantly increased in patients with HCV infection (31·29 ± 25·64 pg/ml) when compared with NCs (21·11 ± 13·98 pg/ml, P = 0·0375, Fig. 2a). However, cytokines related to Th17 cells, including IL-17, IL-22 and IL-23, did not reveal notable differences between NCs and HCV-infected patients (Fig. 2b–d). Moreover, we also investigated the serum concentration of IFN-γ, which plays an important role in controlling viral infection. Levels of IFN-γ showed a remarkable decrease in HCV-infected patients (13·30 ± 9·74 pg/ml) compared with NCs (23·63 ± 12·86 pg/ml, P = 0·0007, Fig. 2e).
Figure 2.
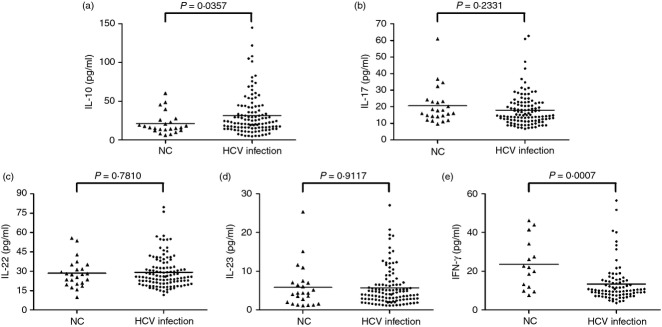
Serum levels of regulatory T (Treg) cells and T helper type 17 (Th17) -related cytokines in normal controls (NC) and hepatitis C virus (HCV) -infected patients. (a) Interleukin-10 (IL-10), (b) IL-17, (c) IL-22, (d) IL-23, and (e) interferon-γ (IFN-γ) were tested by ELISA. Horizontal bars represent the mean values of the indicated index.
Correlation between Treg : Th17 ratio and HCV RNA levels
Treg cells and Th17 cells are closely associated. Therefore, we investigated the relationship among Treg cells, Th17 cells and HCV RNA levels. As shown in Fig. 3, There was a positive correlation between frequency of Treg cells and HCV RNA (r = 0·232, P = 0·013, Fig. 3a). However, Th17 cell proportions did not reveal notable correlation with HCV RNA (r = 0·1136, P = 0·2288, Fig. 3b). We further investigated whether the circulating Treg : Th17 ratios were correlated with HCV replication. Bivariate correlation showed that Treg : Th17 ratios were directly and significantly associated with HCV RNA levels in patients with HCV infection (r = 0·447, P < 0·0001, Fig. 3c). Furthermore, neither IL-10 nor IFN-γ production was markedly associated with the viral titre or liver inflammation (P > 0·05, data not shown).
Figure 3.
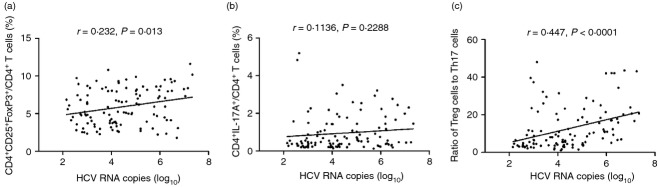
The relationship between regulatory T (Treg) cells, Y helper type 17 (Th17) cells, and Treg : Th17 ratio and viral loads in patients with hepatitis C virus (HCV) infection. (a) Frequency of Treg cells was positively correlated with HCV RNA. (b) Frequency of Th17 cells did not show significant correlation with HCV RNA. (c) Circulating Treg : Th17 ratio was positively correlated with HCV RNA.
Dynamic changes of Treg : Th17 ratio and related cytokines in response to anti-HCV therapy
All the 114 HCV-infected patients received peg-IFN-α2a with ribavirin therapy. We measured the HCV viral loads at baseline, 4, 12, 24 and 48 weeks post treatment, and found that 44 of the patients were RVR, 51 were EVR and 19 were NR. To assess the effect of anti-HCV therapy on Th cell subsets in patients with different responses to treatment, we longitudinally determined the frequency of Treg and Th17 cells in the peripheral blood of the patients. All patients showed distinct compositions of CD4+ T-cell subsets at different therapy time points. The frequency of Treg cells was reduced rapidly 4 weeks after initiation of antiviral therapy and continuously decreased during the observation period in patients with RVR (Fig. 4a). There was also a consistent trend of Treg cell reduction in EVR patients, but they still presented relatively high levels of Treg cells at 4 weeks (Fig. 4a). Moreover, there were no significant changes in response to antiviral therapy in patients with NC (Fig. 4a). In contrast, the frequencies of Th17 cells did not reveal remarkable changes in all patients in response to peg-IFN-α2a and ribavirin therapy (Fig. 4b). Interestingly, the Treg : Th17 ratios showed similar trends to Treg cells. NC patients revealed continuously high Treg : Th17 ratio (Fig. 4c). In contrast, Treg : Th17 ratios were sharply decreased after initiation of anti-HCV treatment in RVR patients, and a slow reduction of Treg : Th17 ratio was found in EVR patients. The Treg : Th17 ratios were maintained at low levels after 12 weeks of therapy during the observation period (Fig. 4c).
Figure 4.
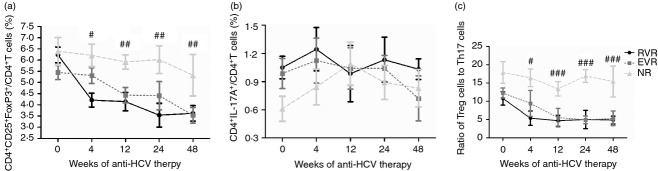
The frequency of regulatory T (Treg) cells and T helper type 17 (Th17) cells as well as Treg : Th17 ratio in patients treated with pegylated interferon-α2a (IFN-α2a) and ribavirin during the course of therapy. (a) The frequency of Treg cells was observed from baseline to the 48 weeks in rapid virological response (RVR), early virological response (EVR) and non-response (NR) patients. (b) The frequency of Th17 cells was observed from baseline to the 48 weeks in RVR, EVR and NR patients. (c) The ratio of Treg cells to Th17 cells was observed from baseline to the 48 weeks in RVR, EVR and NR patients. Error bars illustrate the standard errors. #P < 0·05, ##P < 0·01, ###P < 0·001 refers to the statistical differences among RVR, EVR and NR at each time-point.
Discussion
In the present study, we longitudinally monitored the impact of the peg-IFN-α2a and ribavirin induced suppression of HCV replication, and defined the relationship between HCV RNA and Treg : Th17 cell ratio. We found that the Treg : Th17 ratio was increased in HCV-infected patients, especially in HCV genotype 1b patients. In addition, inhibition of viral replication was associated with the decrease in Treg cells but had little influence in Th17 cells, which was manifested by a robust decrease of the Treg : Th17 ratio in patients with virological response (RVR and EVR) but less change in the NR patients. Hence, the imbalance of Treg : Th17 cells might play an important role in persistent HCV infection.
Immune-mediated liver damage is a vital pathogenesis of HCV infection. Previous studies demonstrated that HCV viral clearance was associated with vigorous and sustained virus-specific CD4+ and CD8+ T-cell responses.21 Although increased Treg cells were found in peripheral blood and liver of HCV-infected patients, controversy remains as to the dynamics immune response to HCV and effects of therapy on Treg cells. Wang et al.13 found that combination therapy of peg-IFN-α2a and ribavirin resulted in a decline in HCV RNA with a simultaneous decrease in Treg cells. In contrast, Akiyama et al.12 revealed an increase Treg cells in PBMC but a significant reduction of Treg cells in liver-infiltrating lymphocytes in sustained virological responders, which indicated that liver-resident Treg cells may predict the results of treatment. However, a more recent study demonstrated that successful anti-HCV therapy does not lead to normalization of the local immune response to a resting state with continuous presence of high levels of Treg cells in the liver, suggesting ongoing residual Treg cell regulation of immunopathology post treatment.22 In our study, we found that peripheral Treg cells are closely associated with HCV viral loads. The frequency of Treg cells as well as the Treg-related cytokine IL-10 were elevated and slightly correlated with HCV RNA before treatment. Treg cell proportions did not change significantly in NR patients, whose HCV RNA levels were consistently detectable during anti-HCV therapy. In contrast, the percentage of Treg cells notably reduced in response to combination therapy in both RVR and EVR patients. Furthermore, the level of Treg cells was also remarkably lower in RVR than in EVR patients at 4 weeks after initiation of therapy, since RVR patients reached a complete virological response while EVR patients still had detectable HCV RNA at 4 weeks. Moreover, Treg cells showed similarly low levels in RVR and EVR patients at 12 weeks when both groups reached undetectable HCV RNA in the serum.
Both Th17 cells and Th17-secreting cytokines contributed to inflammation and the anti-microbial immune response.23,24 Our previous study demonstrated that anti-HBV could induce the reduction in the percentages of HBV-specific Th17 cells and IL-22 production.25 Therefore, we hypothesized that Th17 cells and secreting cytokines may decrease during anti-HCV treatment. Unexpectedly, we found that the percentage of Th17 cells and IL-17, IL-22 and IL-23 concentrations neither revealed differences compared with NC nor correlated with viral load before therapy. Also, there was no significant change in Th17 cell proportions during treatment. This is not consistent with other studies.26,27 More recently, Wang et al.28 reported that the up-regulation of IL-17 production in CD4+ T cells in chronic hepatitis C patients, and elevated Tim-3 signalling on CD14+ monocytes impaired the balance of IL-12/IL-23 through the intracellular signal transducer and activator of transcription 3 signalling, which led to the IL-17 secretion and Th17 cell development. Afford et al.29 also confirmed that vascular cell adhesion molecule 1 (VCAM-1) promotes liver inflammation by inducing lymphocyte recruitment and Th17 cell survival. Those studies revealed the association between Th17 cells and liver inflammation. However, the ALT levels of enrolled patients in our study were relatively low with the median level of 40–60 U/l. This may be one of the reasons why we did not find the association between Th17 cells and ALT levels in our study. Altogether, based on the results from the current experiments, Th17 signalling may not be involved in the pathogenesis of HCV infection.
The developmental pathway for Treg and Th17 cells are reciprocally interconnected and there is an important plasticity between Treg and Th17 cells.30 Hence, Treg : Th17 balance could impact the process and outcome of autoimmune and inflammatory diseases. Studies on HBV have demonstrated that imbalance of Treg : Th17 ratios was found in chronic hepatitis B, and inhibition of viral replication led to a decrease in Treg cells and a concomitant increase in Th17 cells.17,18,31–33 However, no study has described the importance of the skewed Treg : Th17 ratios during anti-HCV therapy in hepatitis C patients. In this study, we found that the imbalance of Treg : Th17 ratios occurred in HCV infection. As there was a decline of Treg cells but stable Th17 cells during anti-HCV therapy, we assumed that Treg cells contributed significantly to the skewed Treg : Th17 ratios. Importantly, Treg : Th17 ratios were similar in patients with RVR and EVR at 4 weeks after treatment. Treg : Th17 ratios may be more sensitive than Treg cells alone to predict the virological response during peg-IFN-α2a and ribavirin combination therapy. However, the limited sampling of patient blood precluded us from analysing the very early response to anti-HCV therapy. We will further investigate the Treg : Th17 ratios at 1 and 2 weeks post initiation.
In conclusion, the current study highlights that the skewed Treg : Th17 ratios existed in patients with chronic hepatitis C. HCV RNA load is closely associated with Treg : Th17 cell ratios during peg-IFN-α2a and ribavirin treatment in HCV-infected patients. The Treg : Th17 ratio in HCV-infected patients might be used as a biomarker for the likelihood of treatment failure. These data implied that the imbalance of Treg to Th17 cells might play an important role in persistent HCV infection, and the Treg : Th17 differentiation pathway could be deliberately manipulated to treat HCV infection.
Acknowledgments
We thank the volunteers who generously participated in this study. Chunqiu Hao, Yun Zhou, Yu He and Chao Fan performed the study. Li Sun, Xin Wei, Linxu Wang, Meijuan Peng and Pingzhong Wang enrolled the patients. Chunqiu Hao, Jianqi Lian and Zhansheng Jia interpreted and analysed the data, and prepared the manuscript. Jianqi Lian and Zhansheng Jia designed and supervised the study.
Glossary
- APC
allophycocyanin
- EVR
early virological response
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- IFN
interferon
- IL
interleukin
- NC
normal control
- NR
non-response
- PBMC
peripheral blood mononuclear cells
- PE
phycoerythrin
- peg-IFN
pegylated interferon
- PerCP
peridinin chlorophyll protein
- RVR
rapid virological response
- Th cells
T helper cells
- Treg
regulatory T
- TGF
tumour growth factor
Disclosures
None.
References
- Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107–15. doi: 10.1111/j.1469-0691.2010.03432.x. [DOI] [PubMed] [Google Scholar]
- Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20:17–35. doi: 10.1055/s-2000-9505. [DOI] [PubMed] [Google Scholar]
- Fierro NA, Gonzalez-Aldaco K, Torres-Valadez R, Martinez-Lopez E, Roman S, Panduro A. Immunologic, metabolic and genetic factors in hepatitis C virus infection. World J Gastroenterol. 2014;20:3443–56. doi: 10.3748/wjg.v20.i13.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHutchison JG, Fried MW. Current therapy for hepatitis C: pegylated interferon and ribavirin. Clin Liver Dis. 2003;7:149–61. doi: 10.1016/s1089-3261(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Delemos AS, Chung RT. Hepatitis C treatment: an incipient therapeutic revolution. Trends Mol Med. 2014;20:315–21. doi: 10.1016/j.molmed.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of TH17 cells. Nature. 2008;453:1051–7. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Liu C, Nelson DR. An immunomodulatory role for CD4+ CD25+ regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004;40:1062–71. doi: 10.1002/hep.20454. [DOI] [PubMed] [Google Scholar]
- Claassen MA, de Knegt RJ, Tilanus HW, Janssen HL, Boonstra A. Abundant numbers of regulatory T cells localize to the liver of chronic hepatitis C infected patients and limit the extent of fibrosis. J Hepatol. 2010;52:315–21. doi: 10.1016/j.jhep.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Boettler T, Spangenberg HC, Neumann-Haefelin C, et al. T cells with a CD4+CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection. J Virol. 2005;79:7860–7. doi: 10.1128/JVI.79.12.7860-7867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushbrook SM, Ward SM, Unitt E, Vowler SL, Lucas M, Klenerman P, Alexander GJ. Regulatory T cells suppress in vitro proliferation of virus-specific CD8+ T cells during persistent hepatitis C virus infection. J Virol. 2005;79:7852–9. doi: 10.1128/JVI.79.12.7852-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama M, Ichikawa T, Miyaaki H, et al. Relationship between regulatory T cells and the combination of pegylated interferon and ribavirin for the treatment of chronic hepatitis type C. Intervirology. 2010;53:154–60. doi: 10.1159/000274976. [DOI] [PubMed] [Google Scholar]
- Wang JP, Zhang Y, Wei X, et al. Circulating Toll-like receptor (TLR) 2, TLR4, and regulatory T cells in patients with chronic hepatitis C. APMIS. 2010;118:261–70. doi: 10.1111/j.1600-0463.2010.02586.x. [DOI] [PubMed] [Google Scholar]
- Chang Q, Wang YK, Zhao Q, Wang CZ, Hu YZ, Wu BY. Th17 cells are increased with severity of liver inflammation in patients with chronic hepatitis C. J Gastroenterol Hepatol. 2012;27:273–8. doi: 10.1111/j.1440-1746.2011.06782.x. [DOI] [PubMed] [Google Scholar]
- Foster RG, Golden-Mason L, Rutebemberwa A, Rosen HR. Interleukin (IL)-17/IL-22-producing T cells enriched within the liver of patients with chronic hepatitis C viral (HCV) infection. Dig Dis Sci. 2012;57:381–9. doi: 10.1007/s10620-011-1997-z. [DOI] [PubMed] [Google Scholar]
- Zhou L, Lopes JE, Chong MM, et al. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature. 2008;453:236–40. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JY, Song CH, Shi F, Zhang Z, Fu JL, Wang FS. Decreased ratio of Treg cells to Th17 cells correlates with HBV DNA suppression in chronic hepatitis B patients undergoing entecavir treatment. PLoS ONE. 2010;5:e13869. doi: 10.1371/journal.pone.0013869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue-Song L, Cheng-Zhong L, Ying Z, Mo-Bin W. Changes of Treg and Th17 cells balance in the development of acute and chronic hepatitis B virus infection. BMC Gastroenterol. 2012;12:43. doi: 10.1186/1471-230X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liver E. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392–420. doi: 10.1016/j.jhep.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–16. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin C, Thimme R. Adaptive immune responses in hepatitis C virus infection. Curr Top Microbiol Immunol. 2013;369:243–62. doi: 10.1007/978-3-642-27340-7_10. [DOI] [PubMed] [Google Scholar]
- Claassen MA, de Knegt RJ, Janssen HL, Boonstra A. Retention of CD4+ CD25+ FoxP3+ regulatory T cells in the liver after therapy-induced hepatitis C virus eradication in humans. J Virol. 2011;85:5323–30. doi: 10.1128/JVI.02551-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–6. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Bi Y, Liu G, Yang R. Th17 cell induction and immune regulatory effects. J Cell Physiol. 2007;211:273–8. doi: 10.1002/jcp.20973. [DOI] [PubMed] [Google Scholar]
- Hao C, Wang J, Kang W, et al. Kinetics of Th17 cytokines during telbivudine therapy in patients with chronic hepatitis B. Viral Immunol. 2013;26:336–42. doi: 10.1089/vim.2013.0032. [DOI] [PubMed] [Google Scholar]
- Balanescu P, Ladaru A, Voiosu T, Nicolau A, Ene M, Balanescu E. Th17 and IL-17 immunity in chronic hepatitis C infection. Rom J Intern Med. 2012;50:13–8. [PubMed] [Google Scholar]
- Sousa GM, Oliveira IS, Andrade LJ, Sousa-Atta ML, Parana R, Atta AM. Serum levels of Th17 associated cytokines in chronic hepatitis C virus infection. Cytokine. 2012;60:138–42. doi: 10.1016/j.cyto.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Wang JM, Shi L, Ma CJ, et al. Differential regulation of interleukin-12 (IL-12)/IL-23 by Tim-3 drives TH17 cell development during hepatitis C virus infection. J Virol. 2013;87:4372–83. doi: 10.1128/JVI.03376-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afford SC, Humphreys EH, Reid DT, et al. Vascular cell adhesion molecule 1 expression by biliary epithelium promotes persistence of inflammation by inhibiting effector T-cell apoptosis. Hepatology. 2014;59:1932–43. doi: 10.1002/hep.26965. [DOI] [PubMed] [Google Scholar]
- Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev. 2014;13:668–77. doi: 10.1016/j.autrev.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Su ZJ, Yu XP, Guo RY, Ming DS, Huang LY, Su ML, Deng Y, Lin ZZ. Changes in the balance between Treg and Th17 cells in patients with chronic hepatitis B. Diagn Microbiol Infect Dis. 2013;76:437–44. doi: 10.1016/j.diagmicrobio.2013.04.026. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zheng Y, Huang Z, Tian Y, Zhou J, Mao Q, Wu Y, Ni B. Activated IL-23/IL-17 pathway closely correlates with increased Foxp3 expression in livers of chronic hepatitis B patients. BMC Immunol. 2011;12:25. doi: 10.1186/1471-2172-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai S, Zhang L, Dang S, Yu Y, Zhao Z, Zhao W, Liu L. The ratio of Th-17 to Treg cells is associated with survival of patients with acute-on-chronic hepatitis B liver failure. Viral Immunol. 2011;24:303–10. doi: 10.1089/vim.2010.0135. [DOI] [PubMed] [Google Scholar]