Abstract
Zoledronic acid (ZA) is a potential immunotherapy for cancer because it can induce potent γδ T-cell-mediated anti-tumour responses. Clinical trials are testing the efficacy of intravenous ZA in cancer patients; however, the effects of systemic ZA on the activation and migration of peripheral γδ T cells remain poorly understood. We found that γδ T cells within ZA-treated peripheral blood mononuclear cells were degranulating, as shown by up-regulated expression of CD107a/b. Degranulation was monocyte dependent because CD107a/b expression was markedly reduced in the absence of CD14+ cells. Consistent with monocyte-induced degranulation, we observed γδ T-cell-dependent induction of monocyte apoptosis, as shown by phosphatidylserine expression on monocytes and decreased percentages of monocytes in culture. Despite the prevailing paradigm that ZA promotes tumour homing in γδ T cells, we observed down-modulation of their tumour homing capacity, as shown by decreased expression of the inflammatory chemokine receptors CCR5 and CXCR3, and reduced migration towards the inflammatory chemokine CCL5. Taken together our data suggest that ZA causes γδ T cells to target monocytes and down-modulate the migratory programme required for inflammatory homing. This study provides novel insight into how γδ T cells interact with monocytes and the possible implications of systemic use of ZA in cancer.
Keywords: cancer, monocytes, zoledronic acid, γδ T cells
Introduction
γδ T cells are a unique subset of T cells that express T-cell receptors (TCRs) composed of γ and δ chains. These cells contribute to immunosurveillance against pathogenic infections and malignant transformations,1–4 and are therefore potential targets for immunotherapy.5,6
Peripheral blood γδ T cells in humans typically constitute between 1% and 5% of circulating T cells, and predominantly express TCRs composed of Vγ9 and Vδ2 chains (these cells will be referred to hereafter as Vδ2 cells).7 The Vδ2 cell TCR detects phosphate-rich non-protein intermediates of the isoprenoid biosynthesis pathway.8 Phosphoantigens do not accumulate in healthy somatic cells to levels capable of stimulating Vδ2 cells, but in some tumours they are over-expressed, which subsequently renders the tumour susceptible to Vδ2 cell killing.9 Although the underlying mechanism of phosphoantigen recognition is poorly understood, evidence suggests that conventional antigen-presenting molecules are not involved, and recent studies have identified a critical role for butyrophilin-3(CD277).10 Vδ2 cell detection of phosphoantigens potentially contributes to tumour immunosurveillance; however, over-expression of phosphoantigens in tumour cells is not common to all types of cancer,11 and the activity of peripheral Vδ2 cells in certain cancer patients is suboptimal.12
Nitrogen-containing bisphosphonates (NBPs) are a group of synthetic compounds that inhibit the activity of farnesyl diphosphate synthase, a rate-limiting enzyme of isoprenoid biosynthesis responsible for converting dimethylallyl and geranyl diphosphate into downstream metabolites.13 By blocking the activity of this enzyme, NBPs can induce certain cell types to accumulate and over-express phosphoantigens to levels capable of stimulating Vδ2 cells.14 For example, in vitro studies have shown that certain malignant cells become more susceptible to Vδ2 cell killing when pre-treated with NBPs.15 Importantly, malignant cells are not the only cell type capable of taking up NBPs and over-expressing phosphoantigens; certain subsets of peripheral blood immune cells when treated with NBPs acquire the capacity to stimulate Vδ2 cells.16
Zoledronic acid (ZA) is currently the most potent NBP, which, although typically used to treat bone disorders, has potential as an immunotherapeutic drug for cancer.17,18 Early-phase clinical trials have begun to test the efficacy of intravenous ZA in cancer patients;19 however, the underlying mechanisms of action are poorly understood. Although in vitro studies have demonstrated that ZA-treated tumour cells up-regulate phosphoantigens and become more sensitive to Vδ2 cell cytotoxicity,20 it is unclear how much intravenous ZA reaches non-haematological tumours, and whether the resultant concentration in these tumours achieves a therapeutic dose.21 Recent evidence in humanized mice suggests that systemic ZA can increase phosphoantigen expression in subcutaneously implanted tumours and so facilitate Vδ2 cell-mediated killing;22 however, these observations have yet to be confirmed in humans. Indeed, ZA immunotherapy is more successful in patients with haematological malignancies; for example, in a clinical trial assessing the anti-cancer effects of intravenous ZA in renal cell carcinoma, malignant melanoma and acute myeloid leukaemia, objective clinical responses were only observed in patients with acute myeloid leukaemia.23 Intravenous ZA may also cause peripheral blood immune cells, such as monocytes, to accumulate phosphoantigens and activate Vδ2 cells. It is thought that peripheral activation of Vδ2 cells in this manner boosts tumour targeting, and in some clinical trials for intravenous ZA, Vδ2 cell activation has been shown to correlate with reduced tumour burden.24,25
Several studies have attempted peripheral activation of Vδ2 cells using intravenous ZA in combination with interleukin-2.23–26 It is generally assumed that activated Vδ2 cells have the capacity to leave the bloodstream and migrate to tumours; however, there is limited evidence for this process and further investigation is needed to determine the activity of ZA-stimulated Vδ2 cells in the periphery. Our data support the idea that ZA treatment of peripheral blood monocytes leads to Vδ2 cell activation, but further suggests that monocytes themselves become targets and apoptose. Moreover, an analysis of the migratory capacity of these Vδ2 cells indicates that they do not have the capacity to migrate to tumours. Our data provide novel insight into the γδ T-cell response during intravenous ZA immunotherapy, which will facilitate the further development and clinical application of this drug.
Materials and methods
Immune cell isolation and depletion
Anonymized human leucocyte cones were obtained from the UK Blood Transfusion Service, and peripheral blood mononuclear cells (PBMCs) were isolated by density-adjusted centrifugation using Histopaque-1077 (Sigma-Aldrich, Dorset, UK). Contaminating red blood cells and platelets were removed using ammonium chloride solution and slow speed centrifugation, respectively. PBMCs were washed three times in PBS (Sigma Aldrich) and resuspended in 45% RPMI-1640, 45% fetal bovine serum (FBS) and 10% DMSO (all from Sigma Aldrich) before being frozen and stored in liquid nitrogen. Monocytes and/or γδ T cells were isolated and/or depleted from PBMCs using magnetic bead separation. PBMCs were labelled with either CD14 or anti-TCR-γδ-conjugated microbeads (Miltenyi Biotec, Surrey, UK) according to the manufacturer’s instructions and passed through magnetic columns. Purity was then assessed by flow cytometric analysis of CD14 and TCR-γδ expression.
Cell culture
The PBMCs were cultured in RPMI-1640 supplemented with 10% FBS, 2 mm l-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin (all from Sigma-Aldrich) at 37° with 5% CO2. PBMCs were seeded at a density of 5·0 × 106 cells/ml, and 200 μl of cell suspension was added per well of 96-well round-bottomed tissue culture plates (Corning, Corning, NY). Duration of cell culture for individual experiments is detailed in the figure legends. To measure interferon-γ accumulation, 1 μg/ml brefeldin A (Sigma-Aldrich) was added to the cells for the last 4 hr of culture. To measure degranulation, CD107a and CD107b antibodies along with 1 μg/ml monensin (Sigma-Aldrich) were added to the cells for the last 4 hr of culture. For monocyte apoptosis assays, γδ T-cell-depleted PBMCs were seeded at 1 × 106 to 1·25 × 106 cells/ml and 200 μl of cell suspension was added per well of 96-well round-bottomed tissue culture plates with or without 0·5 × 104 to 5·0 × 104 autologous γδ T cells. For some experiments, 1 μg/ml anti-TCR-γδ (clone IMMU510; Beckman Coulter, High Wycombe, UK) or matched isotype control antibody was added to the cultures. The lung carcinoma cell line A549 was cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS, 2 mm l-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin (all from Sigma-Aldrich) at 37° with 5% CO2. Cells were cultured with different concentrations of ZA (Sigma-Aldrich) for different time periods as indicated in the figure legends for individual experiments.
Transwell assays
Six hundred microlitres of migration buffer (RPMI-1640 containing 0·1% weight/volume fatty acid-free BSA, 2 mm l-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin; all from Sigma-Aldrich) with or without 200 ng/ml recombinant human CCL5 (R & D Systems, Abingdon, UK) was added to 24-well tissue culture plates (Corning). Polycarbonate transwell inserts with 5 μm pores (Corning) were added to the wells, and 100 μl of migration buffer containing 2·0 × 104 to 5·0 × 104 purified γδ T cells were added to the insert. The cells were incubated for 4 hr at 37° with 5% CO2. Cells in the bottom chamber were collected, stained for CD3 and Vδ2, and then counted using a flow cytometer to count the number of events acquired over a set time. Percentage of specific migration was calculated by expressing the number of cells in the bottom chamber as a percentage of the number of input cells, and then subtracting the amount of spontaneous migration.
Flow cytometry
Cells were washed in flow cytometry buffer (PBS supplemented with 1% weight/volume BSA and 0·1% weight/volume sodium azide; all from Sigma-Aldrich), and then stained with fluorochrome-conjugated antibodies according to the manufacturer’s instructions. For intracellular staining, cells were simultaneously fixed and permeabilized using 4% paraformaldehyde and 0·1% saponin solution (Cytofix/Cytoperm kit; Becton Dickinson, Oxford, UK) before staining with fluorochrome-conjugated antibodies according to the manufacturer’s instructions. The antibodies used throughout this study are as follows: CD3(SK7), CD14(HCD14), CD107a(H4A3), CD107b(H4B4), IFN-γ(B27) (Biolegend); Vδ2(B6), CD56(B159), CD8(SK1), CD14(MφP9), CD11c(B-ly6), perforin(δG9), CD195(2D7/CCR5), CD183(1C6/CXCR3) (Becton Dickinson); Vδ2(123R3) (Milenyi Biotec); CCR7(150503), CCR2(48607) (R & D Systems); Vδ1(TS8.2) (Beckman Coulter); granzyme B(GB12) (Caltag, Buckingham, UK). For some experiments, matched isotype controls were used to assess non-specific binding. For the monocyte apoptosis assay, cells were washed in flow cytometry buffer before resuspension in buffer supplemented with Ca2+ (annexin V binding buffer; Becton Dickinson) containing FITC- or phycoerythrin-conjugated annexin V (Becton Dickinson and Biolegend, respectively) at the manufacturer’s recommended dilutions. Cells were run on either a FACSCalibur or LSR II flow cytometer (both Becton Dickinson) and data were analysed using winmdi 2.9 or facsdiva 6 software (Becton Dickinson), respectively.
Statistical analyses
Gaussian distributions were assumed for all data sets. Student’s paired t-tests or analyses of variance followed by Bonferroni’s multiple comparison tests were used to compare data sets. All statistical analyses were carried out using graphpad prism 5 (Graphpad software, San Diego, CA). Statistical significance was reached when P < 0·05. *, ** and *** are used throughout the figures to indicate P values of < 0·05, < 0·01 and < 0·001, respectively.
Results
Vδ2 cells within ZA-treated PBMCs degranulate
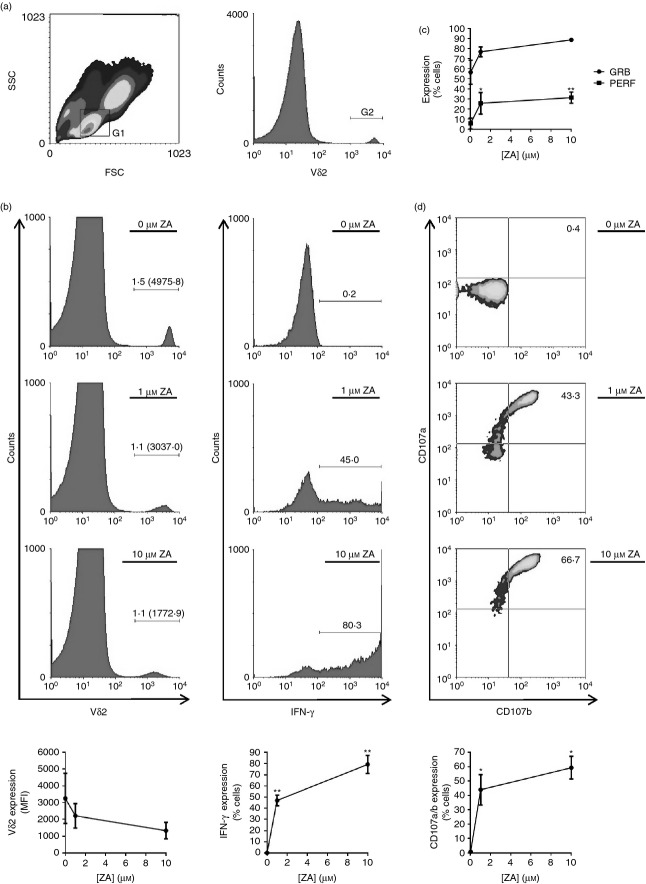
Previous studies have shown that ZA can indirectly activate γδ T cells by inducing the accumulation of phosphoantigens in immune cells,16 but it is currently unknown if ZA-treated immune cells subsequently become targets for γδ T cells. Therefore, we determined whether Vδ2 cells within ZA-treated PBMCs undergo degranulation; the process by which cytotoxic immune cells release their cytolytic granules during target cell recognition. PBMCs treated with ZA contained activated Vδ2 cells, as shown by down-regulated expression of the Vδ2 cell TCR and up-regulated expression of interferon-γ (Fig. 1a,b). Vδ2 cells in resting PBMCs expressed low levels of perforin and moderate levels of granzyme B; however, both molecules were markedly up-regulated by Vδ2 cells when PBMCs were treated with ZA (Fig. 1c). Interestingly, activated Vδ2 cells had undergone degranulation, as indicated by up-regulated expression of cell surface CD107a and CD107b (Fig. 1d). This effect was restricted to Vδ2 cells because CD107a expression was not up-regulated by Vδ1 cells, natural killer cells or CD8+ αβ T cells when PBMCs were treated with ZA (see Supporting information, Fig. S1).
Figure 1.
Vδ2 cells within zoledronic acid (ZA) -treated peripheral blood mononuclear cells (PBMCs) degranulate. PBMCs were cultured overnight with 0, 1 or 10 μm ZA before flow cytometric analysis of Vδ2, interferon-γ (IFN-γ), granzyme B, perforin, CD107a and CD107b expression. (a) Representative flow cytometric plots depicting the gating strategy. Lymphocytes were gated according to size (forward scatter; FSC) and granularity (side scatter; SSC) using gate (G) 1, and Vδ2+ cells within G1 were gated using G2. (b) Representative flow cytometric plots and means ± SD for three different donors showing Vδ2 expression within G1 cells and IFN-γ expression within G1 + G2 cells. Numbers on flow cytometric plots are percentages of cells with mean fluorescence intensities (MFI) in parentheses. (c) Means ± SD for three different donors for granzyme B (GRB) and perforin (PERF) expression within G1 + G2 cells. (d) Representative flow cytometric plots and means ± SD for three different donors showing CD107a and CD107b expression within G1 + G2 cells. Numbers on flow cytometric plots are percentages of cells. For statistical analyses, analyses of variance were conducted followed by Bonferroni’s multiple comparison tests. * and ** indicate P values of < 0·05 and < 0·01, respectively, between treatment groups and controls.
Vδ2 cell degranulation within ZA-treated PBMCs is monocyte-dependent
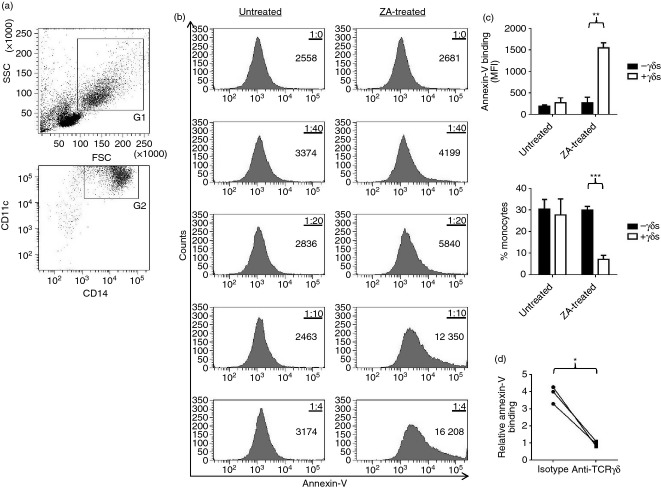
Recent evidence suggests that ZA-treated monocytes release soluble factors that can activate γδ T cells.27 To determine whether soluble factors are responsible for ZA-induced degranulation we set out to assess whether up-regulation of CD107a is contact dependent. We generated conditioned media by culturing ZA-pulsed γδ T-cell-depleted PBMCs, and then tested their capacity to induce degranulation in purified γδ T cells. We found that conditioned media from ZA-pulsed γδ T-cell-depleted PBMCs failed to induce CD107a expression on Vδ2 cells (Fig. 2a), suggesting that Vδ2 cell degranulation within ZA-treated PBMCs is dependent on contact between Vδ2 cells and a component of the remaining PBMCs. Roelofs et al.16 have previously shown that monocytes are the predominant PBMCs that over-express isopentenyl diphosphate following ZA treatment. Therefore, we tested whether Vδ2 cell degranulation within ZA-treated PBMCs is dependent on contact with monocytes by depleting CD14+ cells from PBMCs and subsequently assessing Vδ2 cell CD107a/b expression in response to ZA. We were able to completely and specifically deplete the CD14+ cell population from PBMCs (Fig. 2b). At 1 μm ZA, but not at 10 μm ZA, there was a marked reduction in Vδ2 cell degranulation in monocyte-depleted PBMCs compared with control PBMCs (Fig. 2c).
Figure 2.
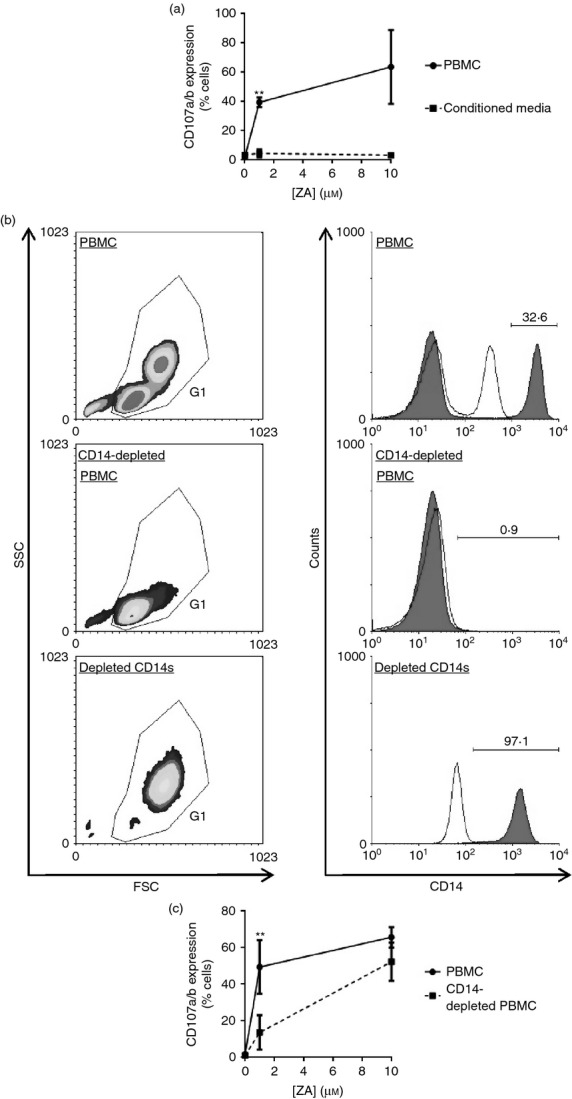
Vδ2 cell degranulation within zoledronic acid (ZA) -treated peripheral blood mononuclear cells (PBMCs) is monocyte-dependent. (a) γδ T-cell-depleted PBMCs were pulsed for 4 hr with 0, 1 or 10 μm ZA, then washed and cultured overnight in fresh media. Cell-free conditioned media were collected and cultured overnight with purified γδ T cells before measuring CD107a expression on Vδ2 cells. For matched donors, CD107a expression was also measured on Vδ2 cells in PBMCs that had been pulsed for 4 hr with 0, 1 or 10 μm ZA, then washed and cultured overnight in fresh media. Means ± SD for three different donors are shown. (b, c) PBMCs or monocyte-depleted PBMCs were cultured overnight with 0, 1 or 10 μm ZA before flow cytometric analysis of CD107a and CD107b expression on Vδ2 cells. (b) Flow cytometric plots representative of three different donors showing CD14 expression on PBMCs, monocyte-depleted PBMCs and the depleted monocytes. Debris was excluded according to forward scatter (FSC) and side scatter (SSC) using gate 1 (G1). CD14 expression on G1 cells is shown. The filled and open histogram plots are expression levels for CD14 and isotype control antibodies, respectively. Numbers are percentages of cells. (c) Means ± SD for three different donors for the percentage of CD107a+/CD107b+ cells within Vδ2 cells. For (a) and (c), ** indicates a P value < 0·01 for paired t-tests between the data sets at 1 μm ZA.
γδ T cells induce monocyte apoptosis within ZA-treated PBMCs
We have shown that Vδ2 cell degranulation within ZA-treated PBMCs is dependent on contact with monocytes, suggesting that ZA may render monocytes as targets for Vδ2 cells. To further test this hypothesis we set out to assess the viability of ZA-treated monocytes after co-culture with Vδ2 cells. We observed high levels of spontaneous cell death during in vitro culture of purified monocytes, so rendering standard cytotoxicity assays using purified effector and target cells inappropriate (data not shown). We found that monocyte viability was consistently stable in PBMC cultures (data not shown), and opted to assess monocyte apoptosis within ZA-treated PBMCs in the absence or presence of γδ T cells using an annexin-V binding assay. γδ T cells were completely and specifically depleted from PBMCs, and isolated to high purity (mean ± SD for three different donors for the percentage of TCR-γδ+ cells within γδ T-cell-depleted PBMCs, depleted γδ T cells and isolated γδ T cells: 0·09 ± 0·03, 90·3 ± 3·2 and 96·5 ± 1·6, respectively). ZA did not affect annexin-V binding on monocytes in the absence of γδ T cells, suggesting that ZA does not directly induce apoptosis in monocytes nor does it render monocytes cytotoxic to non-γδ T cells (Fig. 3a–c). In the presence of γδ T cells, ZA caused a marked increase in the amount of annexin-V binding on monocytes (Fig. 3b,c). Consistent with this, we observed lower percentages of monocytes within ZA-treated PBMCs cultured in the presence of γδ T cells (Fig. 3c). To determine if these effects are TCR-mediated, we tested whether γδ T-cell-dependent ZA-induced apoptosis in monocytes could be blocked using TCR-γδ-specific antibodies. We observed complete blockade of annexin-V binding on monocytes when ZA-treated PBMCs were cultured in the presence of anti-TCR-γδ compared with isotype control antibodies (Fig. 3d).
Figure 3.
γδ T cells induce monocyte apoptosis within zoledronic acid (ZA) -treated peripheral blood mononuclear cells (PBMCs). γδ T-cell-depleted PBMCs were cultured for 48 hr with or without 1 μm ZA in the presence or absence of autologous γδ T cells before flow cytometric analysis of annexin-V binding on monocytes. (a) Flow cytometric plots representative of three different donors showing the monocyte gating strategy. Monocytes were gated according to forward scatter (FSC) and side scatter (SSC) using gate 1 (G1), and CD14 and CD11c expression using G2. (b) Flow cytometric plots for one donor showing annexin-V binding on monocytes when γδ T-cell-depleted PBMCs were reconstituted with different numbers of γδ T cells. The ratios of γδ T cell : γδ T-cell-depleted PBMCs are shown. Numbers on flow cytometric plots are mean fluorescence intensities (MFIs). (c) Top graph: Means ± SD for three different donors for annexin-V binding on monocytes within γδ T-cell-depleted PBMCs reconstituted with or without γδ T cells at a ratio of 5 : 1 treated with or without 1 μm ZA. Bottom graph: Means ± SD for three different donors for the percentage of monocytes within γδ T-cell-depleted PBMCs reconstituted with or without γδ T cells at a ratio of 5 : 1. (d) γδ T-cell-depleted PBMCs reconstituted with γδ T cells at a ratio of 10 : 1 were treated for 48 hr with or without 1 μm ZA in the presence of 1 μg/ml anti-T-cell receptor-(TCR)γδ or isotype control antibodies before flow cytometric analysis of annexin-V binding on monocytes. Data were standardized by expressing ZA scores relative to untreated scores. Data points for individual donors are shown. *, ** and *** indicate P values of < 0·05, < 0·01 and < 0·001, respectively, for paired t-tests between treatment groups.
ZA-treated PBMCs contain Vδ2 cells with reduced migratory capacity
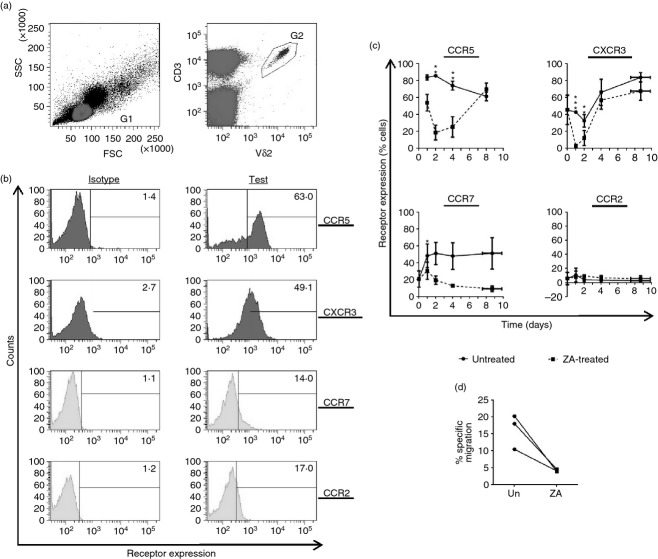
Current hypotheses state that intravenous ZA can activate peripheral γδ T cells and so augment their capacity to target tumour. Although γδ T cells activated by ZA-treated monocytes were able to further degranulate when co-cultured with ZA-treated A549 cells (see Supporting information, Fig. S2), these hypotheses depend on the assumption that γδ T cells can home to tumour following ZA treatment. When effector T cells encounter target cells they undergo marked changes in their migratory behaviour;28 therefore, we set out to investigate whether ZA-induced targeting of monocytes alters the chemokine profile of γδ T cells to assess their tumour homing potential. We measured the expression levels of four chemokine receptors previously implicated in γδ T-cell homing; these were CCR5, CXCR3, CCR7 and CCR2.28–30 We found that Vδ2 cells within resting PBMCs expressed high levels of CCR5, moderate levels of CXCR3 and low levels of CCR7 and CCR2 (Fig. 4a,b). CCR5, CXCR3 and CCR7 on Vδ2 cells were markedly reduced when PBMCs were treated with ZA; whereas, CCR2 levels remained unchanged (Fig. 4c). We then focused on the functionality of CCR5 because it has high expression on circulating Vδ2 cells and undergoes marked down-regulation following ZA treatment. We used transwells to test whether ZA-induced down-regulation of CCR5 on Vδ2 cells results in reduced migration towards the inflammatory chemokine and CCR5 ligand CCL5. Consistent with the down-regulation of CCR5 on the cell surface, we found that Vδ2 cells isolated from ZA-treated PBMCs displayed reduced migration towards CCL5 compared with Vδ2 cells isolated from control PBMCs (Fig. 4d).
Figure 4.
Zoledronic acid (ZA) -treated peripheral blood mononuclear cells (PBMCs) contain Vδ2 cells with reduced migratory capacity. (a–c) PBMCs were cultured with or without 1 μm ZA for 0, 1, 2, 4 and 8 days before flow cytometric analysis of the chemokine receptors CCR5, CXCR3, CCR7 and CCR2 on Vδ2 cells. (a) Flow cytometric plots representative of three different donors showing the gating strategy used. Lymphocytes were gated according to forward scatter (FSC) and side-scatter (SSC) using gate 1 (G1), and CD3+ Vδ2+ cells within G1 were gated using G2. (b) Flow cytometric plots representative of three different donors showing basal levels of the four chemokine receptors analysed. Numbers are percentages of cells. (c) Means ± SD for three different donors for the chemokine receptor expression on Vδ2 cells. Isotype scores were subtracted from test scores. (d) CCL5-induced migration across a 5-μm transwell was measured using Vδ2 cells isolated from PBMCs treated with or without 1 μm ZA for 48 hr (designated ‘ZA’ and ‘un’, respectively). *, ** and *** indicates P values of < 0·05, < 0·01 or < 0·001 for paired t-tests between untreated and ZA-treated data sets.
Discussion
The immunotherapeutic effects of intravenous ZA are currently being assessed in cancer. Current hypotheses for the mechanism of action state that ZA indirectly activates circulating γδ T cells by inducing phosphoantigen accumulation in peripheral blood monocytes.16 We argue that activation of circulating γδ T cells during intravenous ZA may induce targeting of monocytes and prevent extravasation of activated γδ T cells at the tumour. This concept may impact on the use of ZA in cancer immunotherapy, and highlights possible areas in which the clinical application of ZA could be improved.
We found that γδ T cells within ZA-treated PBMCs were exocytosing their cytolytic granules, suggesting that they may be targeting other immune cells. γδ T-cell degranulation was predominantly monocyte dependent at 1 μm ZA; however, at 10 μm ZA, γδ T-cell degranulation was monocyte independent. This result is consistent with monocytes being the most efficient PBMCs at endocytosing ZA,16 and further suggests that other PBMCs are targeted at higher concentrations of ZA. We and others have previously shown that subsets of circulating dendritic cells (DCs) mediate γδ T-cell activation.27,31 These cells constitute only a minor proportion of PBMCs, and therefore it seems plausible that higher concentrations of ZA are required to sensitize them to γδ T cells. However, whether circulating immune cells are subjected to concentrations of ZA as high as 10 μm during current treatment regimens remains unclear. On the surface of bone, where high concentrations of ZA are anticipated, ZA-mediated γδ T-cell activation is likely to be mediated by osteoclasts, which become sensitive to γδ T-cell killing following ZA treatment.32
Peripheral blood monocytes are the progenitor cells for inflammatory DCs and macrophages,33 both of which play demonstrable roles in generating protective adaptive immune responses against tumours.34,35 Our observation that ZA renders monocytes susceptible to γδ T-cell killing implies that intravenous ZA may hamper this important facet of the immune response, which may explain the, as yet, limited efficacy of ZA immunotherapy in cancer, as well as indicate limitations in the scope for potential combination therapy. However, the effects of intravenous NBPs on circulating monocytes in vivo remain poorly characterized. Recently, Welton et al.36 reported a transient increase in the percentage of circulating monocytes in a cohort of patients receiving intravenous ZA. Kalyan et al.37, on the other hand, have reported no change in monocyte percentages in a cohort of patients receiving intravenous ibandronate. Although these in vivo observations are inconsistent with the hypothesis that Vδ2 cells target ZA-treated monocytes, clinical trials have yet to document the effects of intravenous ZA on the absolute number of circulating monocytes; indeed, in the study by Welton et al.36 there was a concomitant decrease in the percentage of circulating T cells. Moreover, changes in either the absolute number or proportion of circulating monocytes do not take into account rates of monocyte turnover.
Previous studies suggest that there is a reciprocal stimulatory interaction between γδ T cells and antigen-presenting cells. Eberl et al.38 have shown in vitro that phosphoantigen-activated Vδ2 cells induce the differentiation of monocytes into inflammatory DCs, which is consistent with the observation by Welton et al.36 that CD40, CD80 and HLA-DR were up-regulated on peripheral blood monocytes from patients receiving intravenous ZA. In the context of our data, one could speculate that there is a balance between Vδ2 cell-dependent monocyte differentiation and targeting during intravenous ZA treatment, which may be dependent on the ratio of Vδ2 cells to monocytes. Indeed, we observed monocyte apoptosis at relatively high ratios of Vδ2 cells to monocytes, and the patient cohort evaluated by Welton et al.36 did not receive interleukin-2 and therefore did not have increased percentages of circulating Vδ2 cells. Although we modelled the effects of ZA on γδ T cells using donor PBMCs, which would contain higher percentages of γδ T cells compared with the immunocompromised PBMCs from patients,39 clinical trials have shown that patient γδ T cells undergo efficient expansion during ZA and interleukin-2 immunotherapy.23
Fiore et al. have shown that ZA-treated DCs can stimulate γδ T cells, which in turn enhance the capacity of these DCs to stimulate antigen-specific CD8+ αβ T cells.40,41 One possible explanation for why DCs in Fiore et al.’s system are protected from γδ T-cell attack, whereas monocytes in our system are not, is that DCs express serine protease inhibitors, which block the effects of granzyme B and protect against cytotoxic immune cells.42 Accordingly, Medema et al.42 have shown that serine protease inhibitors are detectable in mature but not in immature DCs, suggesting that monocytes may lack this protective mechanism. In contrast, Nussbaumer et al.27 have shown that CD56+ DCs are eliminated from ZA-treated PBMCs in a γδ T-cell-dependent manner. Recently, neutrophils have been shown to suppress phosphoantigen-induced activation of Vδ2 cells in a reactive oxygen species-dependent manner, which could further impact the cross-talk between Vδ2 cells and monocytes.43 However, it remains to be seen how this ties in with previous studies showing that ZA inhibits neutrophil function in terms of nicotinamide adenine dinucleotide phosphate oxidase activity.44
Circulating γδ T cells in humans are predominantly inflammatory-homing, and are therefore regarded as sentinels against infection and malignant transformation.29 Because target recognition can cause marked changes in the chemokine receptor profile of γδ T cells,28 we tested whether ZA-treated monocytes can induce similar effects. We observed marked down-regulation of CCR5 and CXCR3 expression on Vδ2 cells within ZA-treated PBMCs, which has important implications regarding subsequent migration to tumour. Within the tumour microenvironment there are tumour cells, stromal cells and resident/infiltrating immune cells, all of which have been shown to produce CCR5- and CXCR3-specific inflammatory chemokines that play a critical role in recruiting immune cells to the tumour.45–48 Although in vivo data for tumour infiltrating γδ T cells are limited, Cordova et al.49 have reported that γδ T cells represent the majority of infiltrating lymphocytes within melanoma lesions, and that percentages of infiltrating Vδ2 cells correlate with disease prognosis. Our data suggest that, although ZA can activate peripheral Vδ2 cells, it may prevent subsequent migration to and infiltration of tumour. Interestingly, we found that ZA-induced down-regulation of CCR5 and CXCR3 on Vδ2 cells was transient. This is particularly important considering that any increased sensitivity to γδ T-cell killing in the tumour mass following intravenous infusion of ZA may also be transient; indeed, Santolaria et al.50 have shown that when immunocompromised mice bearing subcutaneously implanted tumours were intravenously injected with pamidronate, the capacity for the tumour cells extracted from the tumour mass to induce γδ T-cell degranulation peaked at day one and then rapidly declined thereafter. In the context of our data this suggests that following intravenous ZA treatment the tumour may no longer be visible to circulating γδ T cells by the time the γδ T-cell cytotoxic and migratory potential has fully recovered. Our observations in vitro highlight the importance of a thorough investigation into the migration of γδ T cells and the concomitant susceptibility of the tumour mass during ZA immunotherapy, and investigations are currently underway.
We observed down-regulated chemokine receptor expression on γδ T cells following ZA-induced targeting of monocytes. This effect is likely to be mediated by the accumulation of phosphoantigens in monocytes because our data suggest that γδ T-cell degranulation and monocyte targeting is dependent on cell contact and TCR-γδ expression, respectively. In addition to causing the accumulation of upstream phosphoantigens, NBPs can also block the production of downstream farnesyl and geranylgeranyl diphosphate, both of which are required for protein prenylation.51 It is well established that dysfunctional prenylation of certain proteins disrupts a number of cell processes. In particular, cell migration has been shown to be dependent on the prenylation of small GTPases such as Ras and Rho.52 Accordingly, NBPs have been reported to hamper tumour cell migration, so limiting metastasis and invasion.53 This collateral effect of ZA, which has been well documented in tumour cells, could also contribute to the reduced migratory capacity of immune cells such as γδ T cells, and so hamper appropriate immune cell homing to and infiltration of tumours.
Our in vitro data suggest that ZA causes collateral targeting of monocytes by γδ T cells, an effect which might take place in patients undergoing intravenous infusion of ZA. We are currently investigating γδ T-cell activation in patients undergoing ZA immunotherapy, with particular focus on how it affects subsequent tumour targeting. Although our hypothesis is at odds with the aims of cancer immunotherapy, it is certainly in keeping with the limited responses observed for current treatment regimens that use intravenous infusion of ZA, and highlights areas in which the therapy may be improved. Indeed, it has been suggested that circulating γδ T cells become anergic during intravenous infusion of ZA, which is consistent with our hypothesis that ZA generates an abundance of inappropriate target cells in the periphery.19 We propose that instead of intravenous infusion, more targeted methods of administering ZA to the tumour mass should be explored. Intratumoural injection would be an interesting strategy to investigate in easily accessible lesions such as those associated with melanoma. Delivering ZA directly to the tumour mass would also circumvent the rapid binding of ZA to the hydroxyapatite crystals of the bone matrix that may limit its potential to reach tumours that are not closely associated with blood or bone.54 Such approaches could be combined with better ways of priming the anti-tumour programme in peripheral γδ T cells that do not elicit targeting of self; for instance, we have previously shown that bacillus Calmette–Guérin can indirectly activate γδ T cells without inducing cytotoxicity against immune cells.31,55
In conclusion, our data provide a novel insight into the complex immunological interaction between γδ T cells and monocytes, and suggest that ZA-induced activation of γδ T cells is a double-edged sword that can result in inappropriate targeting of self and reduced tumour-homing. These previously unexplored effects of ZA could prove to be useful in the design of future clinical trials using this drug.
Acknowledgments
DWF designed and performed the experiments, analysed the data, and wrote the manuscript. JC provided intellectual input, co-designed the experiments and edited the manuscript. AGD secured overall programme funding and provided additional intellectual input and translational relevance. MB-S developed the original project strategy and supervised previous reported work. In addition MB-S co-designed the experiments, secured project funding and edited the manuscript.
The authors thank the Cancer Vaccine Institute for funding this project.
Glossary
- DC
dendritic cell
- NBP
nitrogen-containing bisphosphonate
- PBMC
peripheral blood mononuclear cell
- TCR
T-cell receptor
- ZA
zoledronic acid
Disclosure
The authors declare no financial or commercial conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Vδ1 cells, natural killer cells and CD8+ αβ T cells within zoledronic acid (ZA)-treated peripheral blood mononuclear cells do not degranulate.
γδ T cells isolated from zoledronic acid (ZA) -treated peripheral blood mononuclear cells degranulate following subsequent co-culture with ZA-treated A549 cells.
References
- Kabelitz D, Wesch D, Pitters E, Zoller M. Characterization of tumor reactivity of human Vγ9Vδ2 γδ T cells in vitro and in SCID mice in vivo. J Immunol. 2004;173:6767–76. doi: 10.4049/jimmunol.173.11.6767. [DOI] [PubMed] [Google Scholar]
- Sciammas R, Kodukula P, Tang Q, Hendricks RL, Bluestone JA. T cell receptor-γδ cells protect mice from herpes simplex virus type 1-induced lethal encephalitis. J Exp Med. 1997;185:1969–75. doi: 10.1084/jem.185.11.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Eltoum IE, Guo B, Beck BH, Cloud GA, Lopez RD. Protective immunosurveillance and therapeutic antitumor activity of γδ T cells demonstrated in a mouse model of prostate cancer. J Immunol. 2008;180:6044–53. doi: 10.4049/jimmunol.180.9.6044. [DOI] [PubMed] [Google Scholar]
- Ladel CH, Blum C, Dreher A, Reifenberg K, Kaufmann SH. Protective role of γ/δ T cells and α/β T cells in tuberculosis. Eur J Immunol. 1995;25:2877–81. doi: 10.1002/eji.1830251025. [DOI] [PubMed] [Google Scholar]
- Beetz S, Marischen L, Kabelitz D, Wesch D. Human γ/δ T cells: candidates for the development of immunotherapeutic strategies. Immunol Res. 2007;37:97–111. doi: 10.1007/BF02685893. [DOI] [PubMed] [Google Scholar]
- Bonneville M, Scotet E. Human Vγ9Vδ2 T cells: promising new leads for immunotherapy of infections and tumors. Curr Opin Immunol. 2006;18:539–46. doi: 10.1016/j.coi.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Kabelitz D, He W. The multifunctionality of human Vγ9Vδ2 γδ T cells: clonal plasticity or distinct subsets? Scand J Immunol. 2012;76:213–22. doi: 10.1111/j.1365-3083.2012.02727.x. [DOI] [PubMed] [Google Scholar]
- Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vγ2Vδ2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor γδ cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–8. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harly C, Guillaume Y, Nedellec S, et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood. 2012;120:2269–79. doi: 10.1182/blood-2012-05-430470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrobel P, Shojaei H, Schittek B, Gieseler F, Wollenberg B, Kalthoff H, Kabelitz D, Wesch D. Lysis of a broad range of epithelial tumour cells by human γδ T cells: involvement of NKG2D ligands and T-cell receptor- versus NKG2D-dependent recognition. Scand J Immunol. 2007;66:320–8. doi: 10.1111/j.1365-3083.2007.01963.x. [DOI] [PubMed] [Google Scholar]
- Argentati K, Re F, Serresi S, Tucci MG, Bartozzi B, Bernardini G, Provinciali M. Reduced number and impaired function of circulating γ δ T cells in patients with cutaneous primary melanoma. J Invest Dermatol. 2003;120:829–34. doi: 10.1046/j.1523-1747.2003.12141.x. [DOI] [PubMed] [Google Scholar]
- Keller RK, Fliesler SJ. Mechanism of aminobisphosphonate action: characterization of alendronate inhibition of the isoprenoid pathway. Biochem Biophys Res Commun. 1999;266:560–3. doi: 10.1006/bbrc.1999.1849. [DOI] [PubMed] [Google Scholar]
- Sun S, McKenna CE. Farnesyl pyrophosphate synthase modulators: a patent review (2006–2010) Expert Opin Ther Pat. 2011;21:1433–51. doi: 10.1517/13543776.2011.593511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Tanaka Y, Miyagawa F, Yamashita S, Minato N. Targeting of tumor cells for human γδ T cells by nonpeptide antigens. J Immunol. 2001;167:5092–8. doi: 10.4049/jimmunol.167.9.5092. [DOI] [PubMed] [Google Scholar]
- Roelofs AJ, Jauhiainen M, Monkkonen H, Rogers MJ, Monkkonen J, Thompson K. Peripheral blood monocytes are responsible for γδ T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br J Haematol. 2009;144:245–50. doi: 10.1111/j.1365-2141.2008.07435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo N, Meraviglia S, Scarpa F, et al. Aminobisphosphonate-activated γδ T cells in immunotherapy of cancer: doubts no more. Expert Opin Biol Ther. 2008;8:875–83. doi: 10.1517/14712598.8.7.875. [DOI] [PubMed] [Google Scholar]
- Gatti D, Adami S. New bisphosphonates in the treatment of bone diseases. Drugs Aging. 1999;15:285–96. doi: 10.2165/00002512-199915040-00004. [DOI] [PubMed] [Google Scholar]
- Fournie JJ, Sicard H, Poupot M, Bezombes C, Blanc A, Romagne F, Ysebaert L, Laurent G. What lessons can be learned from γδ T cell-based cancer immunotherapy trials? Cell Mol Immunol. 2013;10:35–41. doi: 10.1038/cmi.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattarollo SR, Kenna T, Nieda M, Nicol AJ. Chemotherapy and zoledronate sensitize solid tumour cells to Vγ9Vδ2 T cell cytotoxicity. Cancer Immunol Immunother. 2007;56:1285–97. doi: 10.1007/s00262-007-0279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss HM, Pfaar U, Schweitzer A, Wiegand H, Skerjanec A, Schran H. Biodistribution and plasma protein binding of zoledronic acid. Drug Metab Dispos. 2008;36:2043–9. doi: 10.1124/dmd.108.021071. [DOI] [PubMed] [Google Scholar]
- Benzaid I, Monkkonen H, Stresing V, Bonnelye E, Green J, Monkkonen J, Touraine JL, Clezardin P. High phosphoantigen levels in bisphosphonate-treated human breast tumors promote Vγ9Vδ2 T-cell chemotaxis and cytotoxicity in vivo. Cancer Res. 2011;71:4562–72. doi: 10.1158/0008-5472.CAN-10-3862. [DOI] [PubMed] [Google Scholar]
- Kunzmann V, Smetak M, Kimmel B, et al. Tumor-promoting versus tumor-antagonizing roles of γδ T cells in cancer immunotherapy: results from a prospective phase I/II trial. J Immunother. 2012;35:205–13. doi: 10.1097/CJI.0b013e318245bb1e. [DOI] [PubMed] [Google Scholar]
- Dieli F, Vermijlen D, Fulfaro F, et al. Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraviglia S, Eberl M, Vermijlen D, et al. In vivo manipulation of Vγ9Vδ2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin Exp Immunol. 2010;161:290–7. doi: 10.1111/j.1365-2249.2010.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang JM, Kaikobad MR, Wallace M, et al. Pilot trial of interleukin-2 and zoledronic acid to augment γδ T cells as treatment for patients with refractory renal cell carcinoma. Cancer Immunol Immunother. 2011;60:1447–60. doi: 10.1007/s00262-011-1049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaumer O, Gruenbacher G, Gander H, Thurnher M. DC-like cell-dependent activation of human natural killer cells by the bisphosphonate zoledronic acid is regulated by γδ T lymphocytes. Blood. 2011;118:2743–51. doi: 10.1182/blood-2011-01-328526. [DOI] [PubMed] [Google Scholar]
- Brandes M, Willimann K, Lang AB, Nam KH, Jin C, Brenner MB, Morita CT, Moser B. Flexible migration program regulates γδ T-cell involvement in humoral immunity. Blood. 2003;102:3693–701. doi: 10.1182/blood-2003-04-1016. [DOI] [PubMed] [Google Scholar]
- Glatzel A, Wesch D, Schiemann F, Brandt E, Janssen O, Kabelitz D. Patterns of chemokine receptor expression on peripheral blood γδ T lymphocytes: strong expression of CCR5 is a selective feature of Vδ2/Vγ9 γδ T cells. J Immunol. 2002;168:4920–9. doi: 10.4049/jimmunol.168.10.4920. [DOI] [PubMed] [Google Scholar]
- Lanca T, Costa MF, Goncalves-Sousa N, Rei M, Grosso AR, Penido C, Silva-Santos B. Protective role of the inflammatory CCR2/CCL2 chemokine pathway through recruitment of type 1 cytotoxic γδ T lymphocytes to tumor beds. J Immunol. 2013;190:6673–80. doi: 10.4049/jimmunol.1300434. [DOI] [PubMed] [Google Scholar]
- Fowler DW, Copier J, Wilson N, Dalgleish AG, Bodman-Smith MD. Mycobacteria activate gammadelta T-cell anti-tumour responses via cytokines from type 1 myeloid dendritic cells: a mechanism of action for cancer immunotherapy. Cancer Immunol Immunother. 2012;61:535–47. doi: 10.1007/s00262-011-1121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Q, Shibata H, Oda A, et al. Targeting myeloma-osteoclast interaction with Vγ9Vδ2 T cells. Int J Hematol. 2011;94:63–70. doi: 10.1007/s12185-011-0885-9. [DOI] [PubMed] [Google Scholar]
- Yona S, Jung S. Monocytes: subsets, origins, fates and functions. Curr Opin Hematol. 2010;17:53–9. doi: 10.1097/MOH.0b013e3283324f80. [DOI] [PubMed] [Google Scholar]
- Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–47. doi: 10.1016/s0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- Allavena P, Sica A, Garlanda C, Mantovani A. The yin-yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155–61. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- Welton JL, Morgan MP, Marti S, Stone MD, Moser B, Sewell AK, Turton J, Eberl M. Monocytes and γδ T cells control the acute-phase response to intravenous zoledronate: insights from a phase IV safety trial. J Bone Miner Res. 2013;28:464–71. doi: 10.1002/jbmr.1797. [DOI] [PubMed] [Google Scholar]
- Kalyan S, Quabius ES, Wiltfang J, Monig H, Kabelitz D. Can peripheral blood γδ T cells predict osteonecrosis of the jaw? an immunological perspective on the adverse drug effects of aminobisphosphonate therapy. J Bone Miner Res. 2013;28:728–35. doi: 10.1002/jbmr.1769. [DOI] [PubMed] [Google Scholar]
- Eberl M, Roberts GW, Meuter S, Williams JD, Topley N, Moser B. A rapid crosstalk of human γδ T cells and monocytes drives the acute inflammation in bacterial infections. PLoS Pathog. 2009;5:e1000308. doi: 10.1371/journal.ppat.1000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini I, Pacini S, Galimberti S, Taddei MR, Romanini A, Petrini M. Impaired function of γδ lymphocytes in melanoma patients. Eur J Clin Invest. 2011;41:1186–94. doi: 10.1111/j.1365-2362.2011.02524.x. [DOI] [PubMed] [Google Scholar]
- Fiore F, Castella B, Nuschak B, et al. Enhanced ability of dendritic cells to stimulate innate and adaptive immunity on short-term incubation with zoledronic acid. Blood. 2007;110:921–7. doi: 10.1182/blood-2006-09-044321. [DOI] [PubMed] [Google Scholar]
- Conti L, Casetti R, Cardone M, Varano B, Martino A, Belardelli F, Poccia F, Gessani S. Reciprocal activating interaction between dendritic cells and pamidronate-stimulated γδ T cells: role of CD86 and inflammatory cytokines. J Immunol. 2005;174:252–60. doi: 10.4049/jimmunol.174.1.252. [DOI] [PubMed] [Google Scholar]
- Medema JP, Schuurhuis DH, Rea D, et al. Expression of the serpin serine protease inhibitor 6 protects dendritic cells from cytotoxic T lymphocyte-induced apoptosis: differential modulation by T helper type 1 and type 2 cells. J Exp Med. 2001;194:657–67. doi: 10.1084/jem.194.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbione F, Gabelloni ML, Ernst G, et al. Neutrophils suppress γδ T-cell function. Eur J Immunol. 2014;44:819–30. doi: 10.1002/eji.201343664. [DOI] [PubMed] [Google Scholar]
- Kuiper JW, Forster C, Sun C, Peel S, Glogauer M. Zoledronate and pamidronate depress neutrophil functions and survival in mice. Br J Pharmacol. 2012;165:532–9. doi: 10.1111/j.1476-5381.2011.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham KA, Parsonage G, Bhatt RI, Wallace DM, Deshmukh N, Chaudhri S, Adams DH, Lee SP. T lymphocyte recruitment into renal cell carcinoma tissue: a role for chemokine receptors CXCR3, CXCR6, CCR5, and CCR6. Eur Urol. 2012;61:385–94. doi: 10.1016/j.eururo.2011.10.035. [DOI] [PubMed] [Google Scholar]
- Berghuis D, Santos SJ, Baelde HJ, Taminiau AH, Egeler RM, Schilham MW, Hogendoorn PC, Lankester AC. Pro-inflammatory chemokine–chemokine receptor interactions within the Ewing sarcoma microenvironment determine CD8+ T-lymphocyte infiltration and affect tumour progression. J Pathol. 2011;223:347–57. doi: 10.1002/path.2819. [DOI] [PubMed] [Google Scholar]
- Hong M, Puaux AL, Huang C, et al. Chemotherapy induces intratumoral expression of chemokines in cutaneous melanoma, favoring T-cell infiltration and tumor control. Cancer Res. 2011;71:6997–7009. doi: 10.1158/0008-5472.CAN-11-1466. [DOI] [PubMed] [Google Scholar]
- Chew V, Chen J, Lee D, et al. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut. 2012;61:427–38. doi: 10.1136/gutjnl-2011-300509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordova A, Toia F, La Mendola C, et al. Characterization of human γδ T lymphocytes infiltrating primary malignant melanomas. PLoS ONE. 2012;7:e49878. doi: 10.1371/journal.pone.0049878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santolaria T, Robard M, Leger A, Catros V, Bonneville M, Scotet E. Repeated systemic administrations of both aminobisphosphonates and human Vγ9Vδ2 T cells efficiently control tumor development in vivo. J Immunol. 2013;191:1993–2000. doi: 10.4049/jimmunol.1300255. [DOI] [PubMed] [Google Scholar]
- Gruenbacher G, Thurnher M. Mevalonate metabolism in cancer. Cancer Lett. 2014 doi: 10.1016/j.canlet.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Thurnher M, Gruenbacher G, Nussbaumer O. Regulation of mevalonate metabolism in cancer and immune cells. Biochim Biophys Acta. 2013;1831:1009–15. doi: 10.1016/j.bbalip.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Sawada K, Morishige K, Tahara M, Kawagishi R, Ikebuchi Y, Tasaka K, Murata Y. Alendronate inhibits lysophosphatidic acid-induced migration of human ovarian cancer cells by attenuating the activation of rho. Cancer Res. 2002;62:6015–20. [PubMed] [Google Scholar]
- Lambrinoudaki I, Christodoulakos G, Botsis D. Bisphosphonates. Ann N Y Acad Sci. 1092;2006:397–402. doi: 10.1196/annals.1365.036. [DOI] [PubMed] [Google Scholar]
- Fowler DW, Copier J, Dalgleish AG, Bodman-Smith MD. Tripartite immune cell co-operation in the bacillus Calmette–Guérin-induced activation of γδ T cells. Immunol Cell Biol. 2013;91:461–8. doi: 10.1038/icb.2013.30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Vδ1 cells, natural killer cells and CD8+ αβ T cells within zoledronic acid (ZA)-treated peripheral blood mononuclear cells do not degranulate.
γδ T cells isolated from zoledronic acid (ZA) -treated peripheral blood mononuclear cells degranulate following subsequent co-culture with ZA-treated A549 cells.