Abstract
The roles of Notch1 and Notch2 in T-cell function have been well studied, but the functional roles of Notch in B cells have not been extensively investigated, except for Notch2 involvement in peripheral marginal zone B-cell differentiation. This study examined the roles of Notch1 in murine primary B cells. During B-cell activation by B-cell receptor ligation, Notch1 was up-regulated while Notch2 was not. In addition, Notch1 up-regulation itself did not contribute to the further activation of B cells, but the Notch ligand was important for Notch1-mediated further B-cell activation. Moreover, Notch1 deficiency significantly decreased B-cell activation and antibody secretion under the presence of Notch ligand. These data suggest that Notch1 is an important mediator for enhancing B-cell activation and antibody secretion by Notch ligand.
Keywords: B cell, B-cell receptor, Notch ligand, Notch1
Introduction
Members of the mammalian Notch family of transmembrane receptors (Notch1–4) are constituents of a highly conserved pathway that participates in cell fate decisions.1–3 Signalling through the Notch receptor is initiated by interaction with one of its ligands (Delta or Serrate/Jagged) located on an adjacent cell, which results in a series of proteolytic cleavages by several proteases such as ADAM-family metalloproteases and γ-secretase.3–5 This leads to the release and nuclear translocation of the Notch intracellular domain (NICD),6 which converts the transcription factors CBF1, Su(H) and Lag-1 (CLS) from repressors to activators.7
Previous studies have shown that Notch1 signalling is important for T-cell lineage commitment from haematopoietic stem cell precursors. Cre-mediated deletion of the genes encoding Notch1 or CSL in adult mouse bone marrow results in impairment of early T-cell development and ectopic differentiation of immature B cells in the thymus.8,9 In contrast, retroviral expression of constitutively active Notch1 in bone marrow stem cells causes suppression of early B-cell development and the ectopic development of immature T cells in the bone marrow.10 Intrathymic T-cell development is also critically affected by signalling through Notch1, and members of two families of structurally distinct Notch ligands, Delta-like 1 and Jagged-1, are expressed by thymic epithelial cells and thymocytes.11,12 Many Notch receptors have specific roles in each step of T-cell differentiation in the thymus.13–16 Some reports suggest that peripheral T-cell activation can also be regulated by Notch signalling; for example, CSL-mediated Notch signalling enhances T-cell proliferation in the presence of antigen-presenting cells, and induces preferential polarization toward the T helper type 2 (Th2) lineage, while simultaneously inhibiting Th1 cell differentiation.17 In addition, recent findings show that Notch affects Th17 cell differentiation.18
With respect to Notch involvement in B-cell differentiation and function, it is well known that Notch2 plays a key role in the generation of marginal zone B cells in the mouse spleen. Inactivation of CSL in B lineage cells results in the complete loss of marginal zone B cells with a concomitant increase in follicular B cells, and conditional deletion of Notch2 in haematopoietic stem cells also leads to the loss of marginal zone B cells.19 Notch2 signalling is also important for regulating the balance between two peripheral B-cell populations (marginal zone and follicular B cells) in the spleen.20 However, despite these advances in our knowledge of Notch2 involvement in B-cell differentiation and function, there have been few reports on the functional relationship between Notch1 and B cells.
Notch1 is a member of the type 1 transmembrane protein family and contains an extracellular domain consisting of multiple epidermal growth factor-like repeats and an intracellular domain consisting of multiple different types of domains. This protein is involved in development, including T-cell lineage commitment.8,9 Some studies showed that Notch1 signalling can act as a potent inducer of T-cell and B-cell leukaemia by inhibiting apoptosis21,22 and others showed that Notch1 signalling can stimulate apoptosis and cell cycle arrest of a chicken B-cell line.23 However, the relationship between Notch1 signalling and peripheral mature B-cell activation is not yet fully understood.
In this study, we examined the role of Notch1 in peripheral B-cell activation. We found that Notch1 could increase marginal zone B-cell differentiation. We also found that Notch1 was important for peripheral B-cell activation and antibody secretion. Based on our data, we suggest that Notch1 contributes to differentiated peripheral B-cell activation through up-regulation of Notch1 expression by B-cell receptor (BCR) ligation.
Materials and methods
Mice and B-cell isolation
Mice with a conditional allele of Notch1 (B6.129X1-Notch1tm2Rko/GridJ, synonym: Notch1flox/flox),24 mice with an integration of the intracellular domain of the Notch1 receptor gene in the Rosa26 locus (Gt(ROSA)26Sortm1(Notch1)Dam, synonym: Rosa26NICD),25 and B6.129P2(C)-Cd19tm1(cre)Cgn/J (CD19-Cre) mice26,27 were obtained from The Jackson Laboratory (Bar Harbor, ME). All mice were kept in pathogen-free conditions in the animal-care facility at GIST (Gwangju, Korea). All experiments using mice were approved by the Institutional Animal Care and Use Committee of GIST. Primary mouse B cells were isolated by negative depletion of non-B cells to a purity of > 95% using an EasySep™ Mouse B-Cell Isolation Kit (Stemcell Technologies, Vancouver, BC, Canada). Mice aged 7–10 weeks were used for experiments.
Cell culture, antibodies, and plasmids
Dll1-expressing OP9 (OP9-Dll1) cells were used to provide B cells with the Notch ligand, and OP9 cells were used as the control. OP9, Dll1 expressing OP9 (OP9-Dll1) and HEK293 cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 5% fetal bovine serum. Isolated primary B cells or total splenocytes were cultured in RPMI-1640 media supplemented with 5% fetal bovine serum. Anti-GAPDH (H-12) antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Myc (9B11) antibody was purchased from Cell Signaling, Danvers, MA. Anti-Notch1 (EP1238Y) antibody was purchased from Epitomics (Burlingame, CA), which detect cytoplasmic region of Notch1. Anti-p65 and anti-TBP1 antibodies were purchased from Santa Cruz Biotechnology. Anti-haemagglutinin (HA-7) antibody was purchased from Sigma (St Louis, MO). Phycoerythrin (PE)-conjugated anti-mouse CD69 (H1.2F3), PE-conjugated anti-mouse CD86 (B7-2), PE-conjugated anti-mouse CD23 (B3B4), PE-conjugated anti-mouse CD43 (eBioR2/60), PE-conjugated anti-mouse IgM (II/41), peridinin chlorophyll protein (PerCP)-conjugated anti-mouse CD8 (53-6.7), PerCP-conjugated anti-mouse CD21 (8D9), PerCP-conjugated anti-mouse CD3ε (145-2C11), PerCP-conjugated anti-mouse IgD (11-26C), PerCP-conjugated anti-mouse CD25 (PC61.5), and allophycocyanin-conjugated anti-mouse B220 (RA3-6B2) antibodies were purchased from eBioscience (San Diego, CA). Live cells were stained for 1 hr at 4° and then fixed in 2·5% paraformaldehyde. Cell proliferation was assessed by staining with Cell Proliferation Dye eFluor® 670 according to the manufacturer’s protocols (eBioscience). This fluorescent dye binds to any cellular protein that contains primary amines. As cells divide, the dye is distributed equally between daughter cells, the number of which can be determined by measuring the successive halving of the fluorescence intensity of the dye. Hence, proliferation was measured by monitoring the decrease in the fluorescence intensity of this dye. Plasmids pMigR1-HA-mNICD1 and pMigR1-HA-mNICD2 were constructed via the insertion of the Notch1 intracellular domain (NICD1) or Notch2 intracellular domain (NICD2) sequences into pMigR1 plasmids, respectively. Cytoplasmic and nuclear extracts were prepared as described previously.28
Flow cytometry
Bone marrow cells, spleen cells and lymph node cells were stained with the indicated antibodies. For B-cell stimulation, purified primary B cells were activated by either 20 µg/ml of goat anti-µ F(ab)2 antibody (Jackson Laboratory) or 20 µg/ml of goat anti-µ F(ab)2 antibody plus 10 µg/ml of anti-mouse CD40 antibodies (eBioscience) for the indicated time and then stained with the indicated antibodies. The stained cells were analysed on a FACS Canto II (BD Bioscience, San Jose, CA), FACS Calibur (BD Bioscience) or Guava easyCyte HT (Millipore, Billerica, MA).
Enzyme-linked immunosorbent assay
Levels of secreted antibodies were analysed by isotype-specific ELISA (eBioscience). B cells were activated by 20 µg/ml of goat anti-µ F(ab)2 antibody and 10 µg/ml of anti-mouse CD40 antibodies with or without OP9-DLL1 cells. After 5 days, the culture medium was analysed according to the manufacturer’s protocols (eBioscience).
Retrovirus transduction
Recombinant retroviruses were produced in Phoenix-Eco cells by transfection of the cells with pMigR1 or pMigR1-HA-mNICD1. Recombinant retroviruses were harvested 48 hr after transfection. The harvested recombinant retroviruses were used for the infection of B cells stimulated with lipopolysaccharide. Then, the infected cells were rested for 5 days and re-stimulated with 20 µg/ml of goat anti-µ F(ab)2 antibody (Jackson Laboratory) or with both 20 µg/ml of goat anti-µ F(ab)2 antibody and 10 µg/ml of anti-mouse CD40 antibody. GFP+ cells were analysed because they represented cells that were infected with the recombinant retrovirus.
Quantitative RT-PCR
Total RNA was extracted from isolated B cells using Trizol reagent (Invitrogen, Carlsbad, CA) and complementary DNAs were generated by reverse transcription. Quantitative RT-PCR analyses were performed using SYBR mix (TaKaRa, Shiga, Japan) and Mx3005p (Stratagene, La Jolla, CA) with primers (5′–3′): Notch1 CAGCTTGCACAACCAGACAGAC (sense) and ACGGAGTACGGCCCATGTT (antisense); Notch2 ACAAATACTGTGCAGACCACTTCAA (sense) and AGCACCACGATGATCAGGGT (antisense); β-Actin GAAGTCCCTCACCCTCCCAA (sense) and GGCATGGACGCGACCA (antisense).
Luciferase reporter assay
HEK293 cells were seeded in 12-well plates at a density of 0·2 × 106 cells per well and transfected by using Lipofectamine 2000 (Invitrogen) with 100 ng of pHes1pro-luci or 100 ng of pNotch1pro, pReniall, and the appropriate DNAs. After 48 hr, the cell extracts were prepared and assayed for luciferase activity according to the instructions in the Luciferase Assay System (Promega, Madison, WI). Transfection efficiencies were normalized by renilla activity. All transfection experiments were performed in triplicate and repeated three times. Expression of mNICD1 or mNICD2 was confirmed by immunoblot analysis with anti-HA and anti-GAPDH antibodies.
Immunizations
NP-BSA, nitrophenol (NP)-chicken gamma globulin (CGG) NP-Ficoll, and NP-lipopolysaccharide (LPS) were purchased from Biosearch Technologies Inc. (Novato, CA) Briefly, Cd19Cre/+Notch1flox/flox and wild-type (Cd19Cre/+) mice (10–14 weeks old) were immunized intraperitoneally with NP-Ficoll (10 µg per mouse) to induce T-cell-independent type 2 immune responses, or with NP-LPS (5 µg per mouse) to induce T-cell-independent type 1 responses. The immunoglobulin concentration in the serum was measured 1 week later. To induce primary T-cell-dependent immune responses, mice were immunized intraperitoneally with alum-precipitated NP-CGG (200 µg per mouse) and then boosted with a second intraperitoneal injection of antigen (50 µg per mouse) 14 days later. Serum samples were collected 14 days after the antigen boost. NP-specific IgG and IgM in the sera were first captured with NP-BSA and then detected with isotype-specific horseradish peroxidase-conjugated anti-mouse immunoglobulin antibodies.
Results
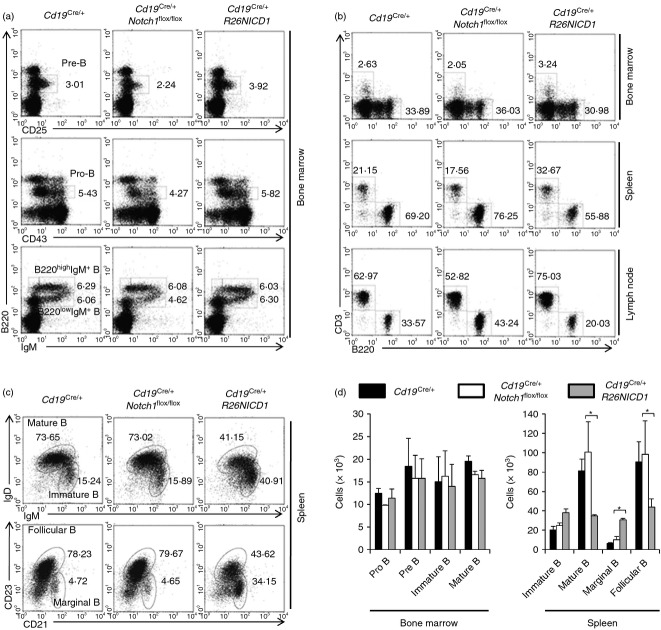
Notch1 gene deletion does not affect B-cell terminal differentiation, but activated Notch1 increased marginal zone B cells (B220+ CD21high CD23−)
The role of Notch1 in B-cell activation has not been clearly defined. In this study, B-cell development in the bone marrow of B-cell-specific NICD1-expressing mice (Cd19Cre/+ R26NICD1) and B-cell-specific Notch1 gene deleted mice (Cd19Cre/+ Notch1flox/flox) was similar to that in the bone marrow of wild-type mice (Cd19Cre/+) (Fig. 1a,b). In the periphery, the percentage of B cells in Cd19Cre/+ Notch1flox/flox mice slightly increased, but the percentage of B cells in Cd19Cre/+ R26NICD1 mice slightly decreased compared with wild-type mice (Cd19Cre/+) (Fig. 1b). Compared with wild-type mouse (Cd19Cre/+), the percentage and number of marginal zone B cells (B220+ CD21high CD23−) and immature B cells (B220+ IgMhigh IgDlow) in the spleens of Cd19Cre/+ R26NICD1 mice were increased while the populations of these cells in the spleens of Cd19Cre/+ Notch1flox/flox mice were not significantly different from those of the wild-type mouse (Cd19Cre/+) (Fig. 1c,d).
Figure 1.
Marginal zone B-cell population at the periphery was increased in B-cell-specific NICD1 expressing mice. (a) Flow cytometric analyses of surface markers of B-cell lineage development of bone marrow cells from Cd19Cre/+, Cd19Cre/+ Notch1flox/flox and Cd19Cre/+ R26NICD1 mice. (b) Flow cytometric analyses of surface markers of T-cell and B-cell populations of total cells from spleens and lymph node of Cd19Cre/+, Cd19Cre/+ Notch1flox/flox and Cd19Cre/+ R26NICD1 mice. (c) Flow cytometric analyses of surface markers of the B-cell subpopulation in spleen of Cd19Cre/+, Cd19Cre/+ Notch1flox/flox and Cd19Cre/+ R26NICD1 mice. (d) The number of each B-cell population in the bone marrows and spleens of Cd19Cre/+, Cd19Cre/+ Notch1flox/flox and Cd19Cre/+ R26NICD1 mice (n = 5 mice) is presented as mean ± SD. Mann–Whitney U-test: *P < 0·05.
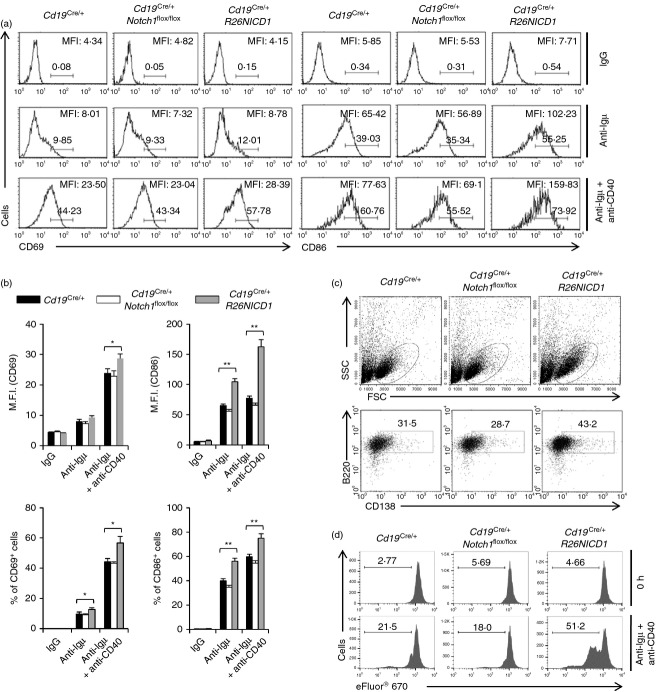
Expression of NICD1 in B-cell increases B-cell activation and proliferation
Usually, immature B cells (B220+ IgMhighIgDlow) and marginal zone B cell (B220+ CD21high CD23−) populations are less activated by BCR ligation than mature B-cell (B220+ IgMlow IgDhigh) and follicular B-cell (B220+ CD21+ CD23+) populations.29,30 To study B-cell activation, we examined the expression of activation markers, such as CD69 and CD86, because such markers are up-regulated on the surface of B cells upon BCR ligation. Upon stimulation with an anti-µ F(ab)2 antibody or with both anti-µ F(ab)2 and anti-CD40 antibodies, surface activation marker expression was significantly higher on B cells from Cd19Cre/+ R26NICD1 mice than on those from wild-type mice (Cd19Cre/+) (Fig. 2a,b). Notch1 gene deletion in B cells did not significantly affect B-cell activation under the same experimental conditions (Fig. 2a,b). In addition, following stimulation with both anti-µ F(ab)2 and anti-CD40 antibodies for 4 days, B cells from Cd19Cre/+ R26NICD1 mice induced the formation of more blasts and expressed a higher level of the plasma cell marker CD138 than B cells from wild-type mice (Cd19Cre/+) (Fig. 2c). Moreover, B cells from Cd19Cre/+ R26NICD1 mice induced more proliferation upon stimulation with anti-µ F(ab)2 plus anti-CD40 antibodies than B cells from wild-type mice (Cd19Cre/+), while the proliferation of specific Notch1 gene-deleted B cells was not appreciably different from that of wild-type B cells (Cd19Cre/+) (Fig. 2d).
Figure 2.
B cells from B-cell-specific NICD1-expressing mice were more activated by B-cell receptor (BCR) ligation than B cells from wild-type mouse. (a) B cells were isolated from the spleen of Cd19Cre/+, Cd19Cre/+ Notch1flox/flox and Cd19Cre/+ R26NICD1 mice. The isolated B cells were stimulated with anti-µ F(ab)2 or anti-µ F(ab)2 plus anti-CD40 antibodies for 16 hr. The activated cells were stained with fluorochrome-conjugated anti-CD69 and anti-CD86 antibodies and analysed by flow cytometry. (b) The summarized data from flow cytometry analyses are shown (± SD) for mean fluorescence intensity and percentage of gated populations. (c) The isolated B cells were stimulated with anti-µ F(ab)2 and anti-CD40 antibodies for 4 days. The cells were stained with a fluorochrome-conjugated anti-CD138 antibody and analysed by flow cytometry. (d) The isolated B cells were labelled with eFluor® 670 and stimulated with anti-µ F(ab)2 plus anti-CD40 antibodies for 4 days. Cells were analysed by flow cytometry. Data are representative of three independent experiments. Student’s t-test: *P < 0·05; **P < 0·01.
NICD1 expression in differentiated wild-type B cells also increased the B-cell activation level
To exclude the possibility of the above result being caused by developmental alterations, we introduced an NICD1 expression cassette into normal primary mouse B cells using a retroviral system (Fig. 3a). The empty retroviral vector was used as the control. After the introduction of the cassette, the cells were rested for 5 days and live B cells were isolated by Ficoll separation. Then, the cells were re-stimulated with either anti-µ F(ab)2 or anti-µ F(ab)2 plus anti-CD40, and activation levels were analysed by checking of CD69 and CD86 surface expression using flow cytometry (Fig. 3b). The results showed that NICD1 expression in primary B cells also increased B-cell activation upon stimulation (Fig. 3b,c).
Figure 3.
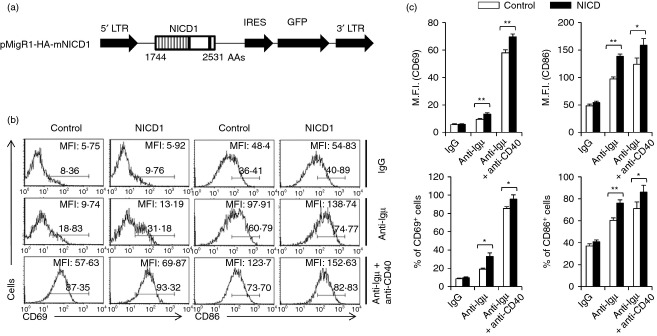
NICD1 expression in primary peripheral B cells increased B-cell activation. (a) Construction of mNICD1 expression retroviral vector. (b) B cells were isolated from C57BL/6 mice and the cells were infected with recombinant retroviruses for expression of mNICD1 and stimulated with either anti-µ F(ab)2 or anti-µ F(ab)2 plus anti-CD40 antibodies. (c) The summarized data from the flow cytometry analyses are shown (± SD) for mean fluorescence intensity and percentage of gated populations. Data are representative of three independent experiments. Data are representative of two independent experiments. Student’s t-test: *P < 0·05; **P < 0·01.
Notch1 expression level was increased by BCR stimulation
It is well known that Notch1 is predominantly expressed in peripheral CD4+ T cells17 and Notch2 is more highly expressed in marginal zone B cells than in other B-cell populations.19 However, the levels of Notch1 and Notch2 have yet to be determined in the B-cell activation state. Quantitative PCR methods were used to analyse the changes in expression of Notch1 and Notch2 in activated B cells after stimulation with anti-µ F(ab)2 antibodies. The mRNA level of Notch1, but not of Notch2, was markedly higher in activated B cells than in unactivated B cells, while the level of Notch2 mRNA was higher than the level of Notch1 mRNA in resting B cells (Fig. 4a). B-cell activating factor also increased the level of Notch1, but not of Notch2 in B cells (see Supporting information, Fig. S1). In addition, we found that Notch1 protein levels were also higher in activated B cells. However, the level of nuclear localized NICD1 in B cells was not changed by stimulation with anti-µ F(ab)2 (Fig. 4b). These data confirm previous data showing that the Notch ligand is essential for Notch1-mediated enhancement of B-cell activation. In addition, surface expression of Notch1 on B cells was increased by stimulation with anti-µ F(ab)2 (Fig. 4c).
Figure 4.
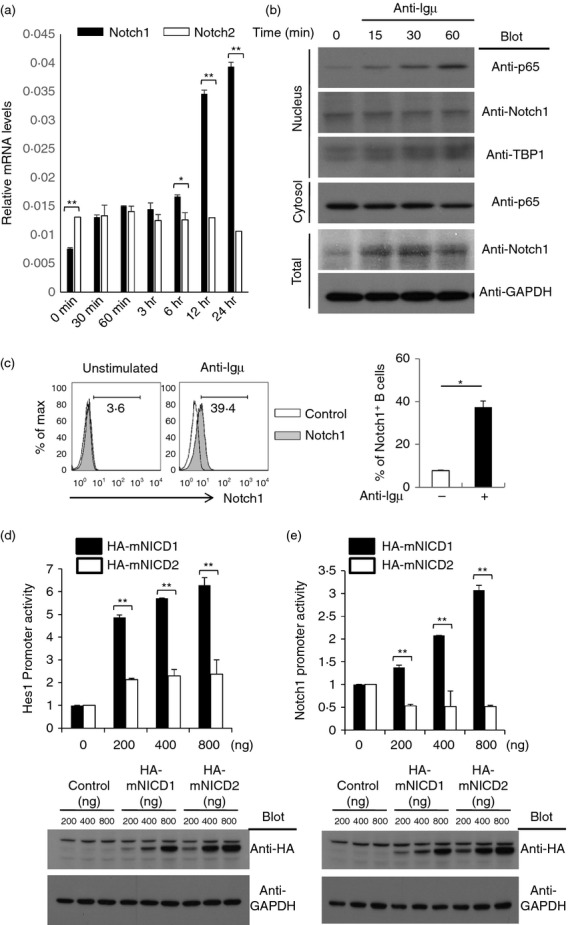
Notch1 level was greatly increased by B-cell receptor ligation while the Notch2 level was slightly increased. (a) B cells were isolated from C57BL/6 mice and stimulated with an anti-µ F(ab)2 antibody for the indicated amount of time. RNA was isolated from the stimulated cells. The mRNA levels of Notch1 and Notch2 were analysed by quantitative RT-PCR analysis and are shown relative to the level to GAPDH mRNA. (b) From the B cells stimulated with anti-µ F(ab)2, total proteins, nuclear extracts and cytosolic proteins were prepared and the proteins were analysed by immunoblot analysis with the indicated antibodies. (c) The isolated B cells were stimulated with anti-µ F(ab)2 or anti-µ F(ab)2 antibodies for 24 hr. The activated cells were stained with fluorochrome-conjugated anti-Notch1 antibodies and analysed by flow cytometry. (d, e) Hes-1 promoter activities (d) and Notch1 promoter activities (e) were analysed by luciferase reporter assays as described in the Materials and methods. Reporter plasmids were co-transfected with the indicated amounts of empty control vector or HA-mNICD1 or HA-mNICD2 expression vectors into HEK293 cells. Expression of mNICD1 and mNICD2 was analysed by immunoblot analysis with anti-HA antibodies. Data are representative of three (a, c, d and e) or two (b) independent experiments. Student’s t-test: *P < 0·05; **P < 0·01.
It has been reported that NICD1 shares target genes with NICD2, and that NICD1 more strongly activates shared target gene transcription than NICD2.31 The results showed that the NICD1 and NICD2 expression vectors were effective in increasing the promoter activity of Hes1, a target gene of Notch, when they were transfected at the same amount into HEK293 cells. However, NICD1 more strongly activated Hes1 promoter activity than NICD2 (Fig. 4c). In addition, Notch1 promoter activity was increased by NICD1 over-expression, while NICD2 did not affect Notch1 promoter activity (Fig. 4d).
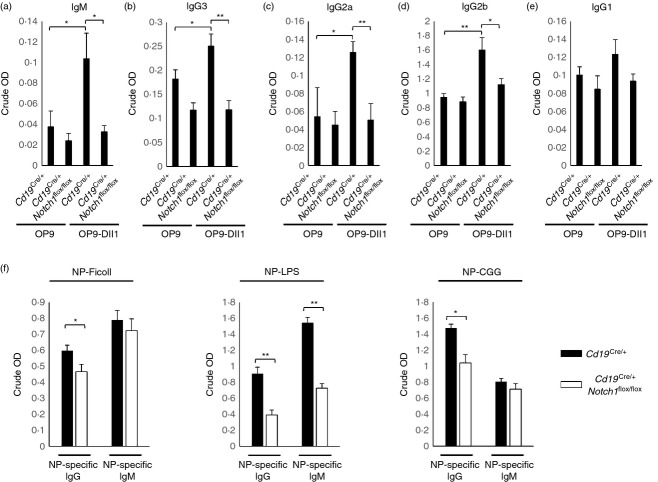
Notch1 deficiency abrogated the Notch ligand-mediated increase in B-cell activation
The above data showed that NICD1 expression affected B-cell activation and proliferation, but Notch1 gene deletion did not significantly affect B-cell activation (Fig. 2a,b). A previous study showed that the Notch ligand enhances B-cell activation,32 and our data also showed that the Notch ligand increased B-cell activation. Isolated primary B-cell activation levels were increased when isolated primary B cells were co-cultured with OP9-Dll1 cells, which express Notch ligand Dll1 on the cell surface during B-cell activation by BCR ligation (Fig. 5a,b). The increase in the level of B-cell activation was reduced by treatment with γ-secretase inhibitors, such as N-[N-(3,5-Difluorophenacetyl-L-alanyl)]-S-phenylglycine tert.butyl ester (DAPT) which suggests that the increased activation of B cells by OP9-Dll9 cells is dependent on Notch activation (Fig. 5a,b). We examined the role of Notch1 in the Notch ligand-mediated increase of B-cell activation by co-culturing Notch1 gene-deleted B cells with OP9-Dll1 cells. The activation levels of Notch1 gene-deleted B cells from Cd19Cre/+ Notch1flox/flox mice were significantly lower than those of B cells from wild-type mice (Cd19Cre/+) (Fig. 5c,d).
Figure 5.
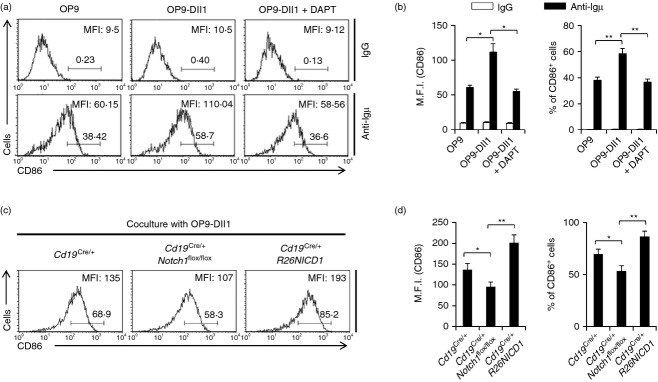
Notch1 deficiency abrogated Notch ligand-mediated increase in B-cell activation. (a) B cells were isolated from C57BL/6 mice and the cells were stimulated by anti-µ F(ab)2 antibodies with either OP9, OP9-Dll1 or OP9-Dll1 plus 50 µm of N-[N-(3,5-Difluorophenacetyl-L-alanyl)]-S-phenylglycine tert.butyl ester (DAPT) for 16 hr. The cells were stained with fluorochrome-conjugated anti-CD86 antibodies and analysed by flow cytometry. (b) The summarized data from the flow cytometry analyses are shown (± SD) for mean fluorescence intensity and percentage of gated populations. (c) B cells were isolated from the spleens of Cd19Cre/+, Cd19Cre/+ Notch1flox/flox and Cd19Cre/+ R26NICD1 mice and stimulated by anti-µ F(ab)2 antibodies with OP9-Dll1 for 16 hr. The cells were stained with fluorochrome-conjugated anti-CD86 antibodies and analysed by flow cytometry. (d) The summarized data from the flow cytometry analyses are shown (± SD) for mean fluorescence intensity and percentage of gated populations. Data are representative of two (a, b) or three (c, d) independent experiments. Student’s t-test: *P < 0·05; **P < 0·01.
Notch1 deficiency abrogated Notch ligand-mediated increase in B-cell antibody secretion
We isolated primary B cells from wild-type (Cd19Cre/+) and Cd19Cre/+ Notch1flox/flox mice and the isolated cells were activated by anti-µ F(ab)2 and anti-CD40 with or without OP9-Dll1 cells. Notch1-deficient B cells showed a significant decrease in the secretion of antibodies, such as IgM (Fig. 6a), IgG2a (Fig. 6b), IgG2b (Fig. 6c), and IgG3 (Fig. 6d) compared with wild-type cells except IgG1 (Fig. 6e). In addition to measuring the levels of secreted antibodies in vitro, we examined the effect of Notch1 on antibody secretion in vivo (Fig. 6f). Immunization with NP-Ficoll (T-cell independent type 2) and NP-LPS induced NP-specific antibody production in both wild-type (Cd19Cre/+) and Cd19Cre/+ Notch1flox/flox mice. The data showed that NP-specific IgG antibody production by NP-Ficoll-immunized Cd19Cre/+ Notch1flox/flox mice, and NP-specific IgG and IgM production by NP-LPS immunized Cd19Cre/+ Notch1flox/flox mice, was significantly lower than that in wild-type (Cd19Cre/+) mice. In addition, NP-specific IgG production in NP-CGG-immunized Cd19Cre/+ Notch1flox/flox mice was significantly lower than that in wild-type (Cd19Cre/+) mice.
Figure 6.
Notch1 deficiency decreases in B-cell antibody secretion. (a–e) B cells were isolated from the spleens of Cd19Cre/+ and Cd19Cre/+Notch1flox/flox mice and stimulated by anti-µ F(ab)2 plus anti-CD40 antibodies for 5 days with or without OP9-Dll1 cells and secreted IgM (a), IgG2a(b), IgG2b(c) or IgG3(d), or IgG1(e) levels were analysed by ELISA. (f) Analysis of antigen-specific antibody production in response to the T-cell independent antigens NP-Ficoll (TI type 2) or with NP-LPS (TI type 1) and TD antigen (NP-CGG). Data are representative of two independent experiments. Student’s t-test: * P < 0·05; **P < 0·01.
Discussion
Notch1 is important for fate determination of haematopoietic stem cells to the T-cell lineage.3 In addition to T-cell development, Notch1 is also involved in peripheral T-cell activation. The role of Notch2 in B cells has been extensively investigated because Notch2 gene disruption blocks B-cell differentiation in the peripheral marginal zone. By contrast, the role of Notch1 has not been extensively investigated because Notch1 gene disruption does not affect B-cell terminal differentiation. In this work, we identified the roles of Notch1 in B-cell activation. We found that Notch1 expression was rapidly induced following BCR ligation, while Notch2 expression was not altered. This result implies that the expressed Notch1 may have a role in B-cell activation. In our data, the B-cell activation level was not increased without the Notch ligand when B cells were stimulated with either anti-µ F(ab)2 or anti-µ F(ab)2 plus anti-CD40. These data indicated that antigen recognition by BCR may up-regulate the Notch1 level and Notch ligand expression accessory cells may increase the activation of up-regulated Notch1. This idea was supported by the data showing that NICD1 expression in B cells enhances BCR-mediated B-cell activation, and that Notch1 gene deletion in B cells abolished Notch ligand-mediated enhancement of B-cell activation upon BCR ligation. Hence, our data clearly show that Notch1 is involved in B-cell activation. This agrees with recent studies showing that follicular dendritic cells express Notch ligand, and that this contributes to increased B-cell activation including antibody production.33
In addition to B-cell activation, NICD1 expression in B cells increased marginal zone B cells while Notch1 gene deletion in B cells did not affect the marginal zone B-cell population. Hence, these data suggest that marginal zone B-cell differentiation can be induced if the level of activated Notch1 is sufficient. However, in actuality, only Notch2 is involved in marginal zone B-cell differentiation in in vivo,19 whereas Notch1 and Notch2 are activated by common Notch ligands.2,3 In addition, a recently published paper showed that Notch2 expression control by interferon regulatory factor 4 is important for positioning of mature B cells.34 Our data showed that BCR-mediated B-cell activation significantly increased the Notch1 expression, but not Notch2 expression. Previous studies and the data presented in this study show that Notch1 more dramatically increased target gene expression than Notch2.31 Notch2 is therefore possibly important for B-cell differentiation and mature B-cell positioning while Notch1 is important for B-cell activation in the periphery through up-regulation of Notch1.
In this study, we showed that Notch1 is important for the further activation of activated peripheral B cells based on data we obtained using Notch1 gene deleted and NICD1 expression mouse systems. Hence, the Notch ligand may be helpful for enhancing B-cell responses. In future studies, the distinct roles of Notch1 and Notch2 as well as their shared roles in B-cell function and differentiation and in B-cell-mediated inflammatory diseases will be further investigated.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0029179), by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A111838) and by the “GIST-Caltech research Collaboration” and Bioimaging research center project through a grant provided by GIST in 2014.
Glossary
- APC
allophycocyanin
- BCR
B-cell receptor
- CSL
CBF1, Su(H), and Lag-1
- FBS
fetal bovine serum
- HA
haemagglutinin
- NICD
notch intracellular domain
- PE
phycoerythrin
- PerCP
peridinin chlorophyll protein
- Th2
T helper type 2
- TI
T-cell-independent
- TD
T-cell-dependent
Author contributions
SP conceived and designed the experiments; JK WK SP, performed the experiments, analysed data and contributed reagents, materials and analysis tools. JK and SP wrote the paper.
Disclosures
The authors declare no financial or commercial conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Notch1 mRNA level is increased by B-cell activating factor stimulation.
References
- Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995;268:225–32. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- Radtke F, MacDonald HR, Tacchini-Cottier F. Regulation of innate and adaptive immunity by Notch. Nat Rev Immunol. 2013;13:427–37. doi: 10.1038/nri3445. [DOI] [PubMed] [Google Scholar]
- Guruharsha KG, Kankel MW, Artavanis-Tsakonas S. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat Rev Genet. 2012;13:654–66. doi: 10.1038/nrg3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Pompa JL, Wakeham A, Correia KM, et al. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development. 1997;124:1139–48. doi: 10.1242/dev.124.6.1139. [DOI] [PubMed] [Google Scholar]
- Kuroda K, Tani S, Tamura K, Minoguchi S, Kurooka H, Honjo T. Delta-induced Notch signaling mediated by RBP-J inhibits MyoD expression and myogenesis. J Biol Chem. 1999;274:7238–44. doi: 10.1074/jbc.274.11.7238. [DOI] [PubMed] [Google Scholar]
- Maillard I, Fang T, Pear WS. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Annu Rev Immunol. 2005;23:945–74. doi: 10.1146/annurev.immunol.23.021704.115747. [DOI] [PubMed] [Google Scholar]
- Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–6. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, Aguet M. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–58. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- Wilson A, MacDonald HR, Radtke F. Notch 1-deficient common lymphoid precursors adopt a B cell fate in the thymus. J Exp Med. 2001;194:1003–12. doi: 10.1084/jem.194.7.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pui JC, Allman D, Xu L, et al. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11:299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- Kaneta M, Osawa M, Sudo K, Nakauchi H, Farr AG, Takahama Y. A role for pref-1 and HES-1 in thymocyte development. J Immunol. 2000;164:256–64. doi: 10.4049/jimmunol.164.1.256. [DOI] [PubMed] [Google Scholar]
- Anderson G, Pongracz J, Parnell S, Jenkinson EJ. Notch ligand-bearing thymic epithelial cells initiate and sustain Notch signaling in thymocytes independently of T cell receptor signaling. Eur J Immunol. 2001;31:3349–54. doi: 10.1002/1521-4141(200111)31:11<3349::aid-immu3349>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Sambandam A, Maillard I, Zediak VP, Xu L, Gerstein RM, Aster JC, Pear WS, Bhandoola A. Notch signaling controls the generation and differentiation of early T lineage progenitors. Nat Immunol. 2005;6:663–70. doi: 10.1038/ni1216. [DOI] [PubMed] [Google Scholar]
- Bain G, Engel I, Robanus ME, et al. E2A deficiency leads to abnormalities in αβ T-cell development and to rapid development of T-cell lymphomas. Mol Cell Biol. 1997;17:4782–91. doi: 10.1128/mcb.17.8.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofani M, Zuniga-Pflucker JC. Notch promotes survival of pre-T cells at the β-selection checkpoint by regulating cellular metabolism. Nat Immunol. 2005;6:881–8. doi: 10.1038/ni1234. [DOI] [PubMed] [Google Scholar]
- Shah DK, Zuniga-Pflucker JC. Notch receptor-ligand interactions during T cell development, a ligand endocytosis-driven mechanism. Curr Top Microbiol Immunol. 2012;360:19–46. doi: 10.1007/82_2012_225. [DOI] [PubMed] [Google Scholar]
- Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–26. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- Bassil R, Orent W, Elyaman W. Notch signaling and T-helper cells in EAE/MS. Clin Dev Immunol. 2013;2013:570731. doi: 10.1155/2013/570731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Chiba S, Ichikawa M, et al. Notch2 is preferentially expressed in mature B cells and indispensable for marginal zone B lineage development. Immunity. 2003;18:675–85. doi: 10.1016/s1074-7613(03)00111-0. [DOI] [PubMed] [Google Scholar]
- Hozumi K, Negishi N, Suzuki D, et al. δ-like 1 is necessary for the generation of marginal zone B cells but not T cells in vivo. Nat Immunol. 2004;5:638–44. doi: 10.1038/ni1075. [DOI] [PubMed] [Google Scholar]
- Jundt F, Acikgoz O, Kwon SH, et al. Aberrant expression of Notch1 interferes with the B-lymphoid phenotype of neoplastic B cells in classical Hodgkin lymphoma. Leukemia. 2008;22:1587–94. doi: 10.1038/leu.2008.101. [DOI] [PubMed] [Google Scholar]
- Rosati E, Sabatini R, Rampino G, et al. Constitutively activated Notch signaling is involved in survival and apoptosis resistance of B-CLL cells. Blood. 2009;113:856–65. doi: 10.1182/blood-2008-02-139725. [DOI] [PubMed] [Google Scholar]
- Morimura T, Goitsuka R, Zhang Y, Saito I, Reth M, Kitamura D. Cell cycle arrest and apoptosis induced by Notch1 in B cells. J Biol Chem. 2000;275:36523–31. doi: 10.1074/jbc.M006415200. [DOI] [PubMed] [Google Scholar]
- Yang X, Klein R, Tian X, Cheng HT, Kopan R, Shen J. Notch activation induces apoptosis in neural progenitor cells through a p53-dependent pathway. Dev Biol. 2004;269:81–94. doi: 10.1016/j.ydbio.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A. 2003;100:14920–5. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 1997;25:1317–8. doi: 10.1093/nar/25.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SG, Long M, Kang JA, et al. The kinase PDK1 is essential for B-cell receptor mediated survival signaling. PLoS ONE. 2013;8:e55378. doi: 10.1371/journal.pone.0055378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SG, Ryu HM, Lim SO, Kim YI, Hwang SB, Jung G. Interferon-γ inhibits hepatitis B virus-induced NF-κB activation through nuclear localization of NF-κB-inducing kinase. Gastroenterology. 2005;128:2042–53. doi: 10.1053/j.gastro.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Chung JB, Sater RA, Fields ML, Erikson J, Monroe JG. CD23 defines two distinct subsets of immature B cells which differ in their responses to T cell help signals. Int Immunol. 2002;14:157–66. doi: 10.1093/intimm/14.2.157. [DOI] [PubMed] [Google Scholar]
- Benschop RJ, Melamed D, Nemazee D, Cambier JC. Distinct signal thresholds for the unique antigen receptor-linked gene expression programs in mature and immature B cells. J Exp Med. 1999;190:749–56. doi: 10.1084/jem.190.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z, Friedmann DR, VanderWielen BD, Collins KJ, Kovall RA. Characterization of CSL (CBF-1, Su(H), Lag-1) mutants reveals differences in signaling mediated by Notch1 and Notch2. J Biol Chem. 2012;287:34904–16. doi: 10.1074/jbc.M112.403287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Calamito M, Srivastava B, Maillard I, Pear WS, Allman D. Notch activity synergizes with B-cell-receptor and CD40 signaling to enhance B-cell activation. Blood. 2007;109:3342–50. doi: 10.1182/blood-2006-09-046698. [DOI] [PubMed] [Google Scholar]
- Yoon SO, Zhang X, Berner P, Blom B, Choi YS. Notch ligands expressed by follicular dendritic cells protect germinal center B cells from apoptosis. J Immunol. 2009;183:352–8. doi: 10.4049/jimmunol.0803183. [DOI] [PubMed] [Google Scholar]
- Simonetti G, Carette A, Silva K, et al. IRF4 controls the positioning of mature B cells in the lymphoid microenvironments by regulating NOTCH2 expression and activity. J Exp Med. 2013;210:2887–902. doi: 10.1084/jem.20131026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Notch1 mRNA level is increased by B-cell activating factor stimulation.