Abstract
Transcription of inflammatory genes in innate immune cells is coordinately regulated by transcription factors, including NF-κB, and chromatin modifiers. However, it remains unclear how microbial sensing initiates chromatin remodeling. Here, we show that Akirin2, an evolutionarily conserved nuclear protein, bridges NF-κB and the chromatin remodeling SWI/SNF complex by interacting with BRG1-Associated Factor 60 (BAF60) proteins as well as IκB-ζ, which forms a complex with the NF-κB p50 subunit. These interactions are essential for Toll-like receptor-, RIG-I-, and Listeria-mediated expression of proinflammatory genes including Il6 and Il12b in macrophages. Consistently, effective clearance of Listeria infection required Akirin2. Furthermore, Akirin2 and IκB-ζ recruitment to the Il6 promoter depend upon the presence of IκB-ζ and Akirin2, respectively, for regulation of chromatin remodeling. BAF60 proteins were also essential for the induction of Il6 in response to LPS stimulation. Collectively, the IκB-ζ–Akirin2–BAF60 complex physically links the NF-κB and SWI/SNF complexes in innate immune cell activation. By recruiting SWI/SNF chromatin remodellers to IκB-ζ, transcriptional coactivator for NF-κB, the conserved nuclear protein Akirin2 stimulates pro-inflammatory gene promoters in mouse macrophages during innate immune responses to viral or bacterial infection.
Keywords: chromatin remodeling, cytokine, gene regulation, innate immunity, macrophages
See also: F Bonnay et al (October 2014)
Introduction
Innate immune cells such as macrophages sense molecular patterns from microorganisms and damaged cells (Beutler, 2009; Medzhitov, 2008). These molecular patterns are recognized by several classes of sensor proteins, such as Toll-like receptors (TLRs), RIG-I-like receptors (RLRs), Nod-like receptors (NLRs), and so on. TLRs and RLRs trigger signaling pathways leading to transcriptional expression of a set of genes involved in inflammation (Takeuchi & Akira, 2010). Systemic inflammation is mediated by the action of proinflammatory cytokines and chemokines such as tumor necrosis factor (TNF), interleukin (IL)-1β, IL-6, IL-12, and type I IFNs. It has been well documented that transcription factors such as NF-κB, AP-1, and IFN-regulatory factors (IRFs) are critical for the expression of these inflammatory genes (Honda & Taniguchi, 2006; Oeckinghaus et al, 2011). Moreover, inflammatory gene expression is controlled by various other mechanisms, such as modulation of the decay rate of induced mRNAs, even after nuclear translocation of transcription factors.
Epigenetic regulation of transcription constitutes another such mechanism. Mammalian DNA is tightly packed into chromatin which must be modified by the histone-modifying and ATP-dependent SWI/SNF family of remodeling complexes in order to facilitate gene activation (Medzhitov & Horng, 2009), thereby allowing transcription at specific loci (Cairns, 2009). Several transcription factors can interact with SWI/SNF complexes and recruit them to specific genes (Chi et al, 2004; Clapier & Cairns, 2009; Yudkovsky et al, 1999). For example, IFN-γ restores IL6 expression by facilitating TLR4-induced recruitment of chromatin remodeling machinery to the IL6 promoter and promoting IL6 locus accessibility in tolerized monocytes (Chen & Ivashkiv, 2010). Nucleosome remodeling also appears to contribute to the rapid induction of the p40 subunit of the proinflammatory cytokine interleukin-12 (IL-12) after LPS stimulation in murine macrophages (Weinmann et al, 1999). Nucleosome remodeling requires TLR4 signaling and new protein synthesis but is independent of the NF-κB subunit c-Rel, which is essential for transcription in macrophages (Weinmann et al, 2001).
The transcriptional induction of mammalian genes by TLRs and other stimuli can be classified in terms of their dependence on SWI/SNF. It was previously proposed that class of genes characterized by CpG island promoters facilitate promiscuous induction from constitutively active chromatin, although another major class consists of non-CpG island promoters that assemble into stable nucleosomes, resulting in SWI/SNF dependence and a requirement for transcription factors that promote selective nucleosome remodeling (Ramirez-Carrozzi et al, 2009). However, a more recent work suggests that some of CpG island containing genes also require new protein synthesis and are expressed with slow kinetics (Bhatt et al, 2012). The relationship between chromatin remodeling and transcription factor activation in cytokine gene expression is thus not well understood.
An interesting feature of the innate immune system is the high conservation among species from insects to mammals. Indeed, the role of the Toll protein in innate immunity was discovered through work on Drosophila. We previously identified Akirin, a nuclear factor regulating NF-κB-dependent transcription in a functional genome-wide RNA-mediated interference (RNAi) screening of Drosophila cell culture to isolate new components of the Imd pathway (Goto et al, 2008). Akirins are found in diverse animal species and possess two conserved regions at their N- and C-termini, the former containing a nuclear localization signal. Akirin has been reported to be crucial for regulating gene expression in a wide range of contexts (Goto et al, 2008; Macqueen & Johnston, 2009; Nowak et al, 2012; Salerno et al, 2009). It has also been established that Akirin has a promyogenic role during muscle regeneration in mice (Marshall et al, 2008). There are 2 Akirin family members in humans and mice. Akirin2, but not Akirin1, is critical for the production of IL-6 in response to TLR stimuli in mouse embryonic fibroblasts (MEFs) in addition to having a role in mouse development (Goto et al, 2008). As Akirin sequences show no obvious DNA- or RNA-binding motifs, they represent a potential link between NF-κB-induced transcription and upstream signaling.
In the present study, we examined the role of mouse Akirin2 in macrophages by generating a conditional allele. Akirin2 is essential for the expression of a set of inflammatory genes including Il6 downstream of TLRs and RLRs. In addition, mice lacking Akirin2 in macrophages show impaired cytokine production in response to Listeria infection and clearance of infecting bacteria in vivo. Akirin2-dependent genes tended to exhibit relatively few CpG islands in their promoters. These observations motivated us to examine how Akirin2 regulates inflammatory gene expression. Akirin2 was directly recruited to its target gene promoters and was found to control chromatin remodeling by recruiting BAF60 proteins, components of the SWI/SNF complex. Further, we identified IκB-ζ as an Akirin2-binding protein via the C-terminal region of Akirin2 and found that IκB-ζ and the NF-κB p50 subunit are required for the recruitment of Akirin2 to the Il6 promoter. Reciprocally, IκB-ζ was also recruited to the Il6 promoter in the presence of Akirin2. This study reveals that Akirin2 mediates the physical link between the NF-κB and SWI/SNF complexes and thereby represents a novel paradigm for providing tissue and target specificity for transcription factor interactions with chromatin remodeling machinery.
Results
Akirin2 deletion severely impairs proinflammatory cytokine production in macrophages
To investigate the role of Akirin2 in innate immune cells, we specifically ablated Akirin2 in myeloid cells by crossing Akirin2fl/fl mice with mice expressing the lysozyme promoter-driven Cre recombinase gene (LysM-Cre). Selective deletion of Akirin2 in myeloid cells of LysM-Cre+; Akirin2fl/fl mice was demonstrated by Northern blot and immunoblot analyses (Supplementary Fig S1A and B). Immunoblot analysis showed a modest decrease in Akirin2 protein expression in macrophages upon LPS stimulation (Supplementary Fig S1B). The frequency of T and B cells, macrophages and Neutrophils in the spleen, bone marrow (BM), and peritoneal exudate cells (PECs) was not altered between LysM-Cre+; Akirin2fl/+ or LysM-Cre+; Akirin2fl/fl mice (Supplementary Fig S2A–C).
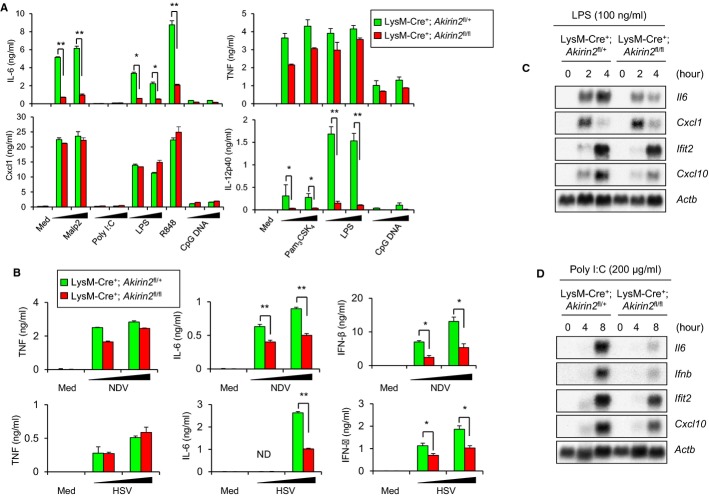
We first examined the responses of peritoneal macrophages to stimulation with TLR ligands and virus infection. Whereas the production of TNF and CXCL1 chemokine KC was comparable, macrophages from LysM-Cre+; Akirin2fl/fl mice produced significantly less IL-6 and IL-12p40 than those from LysM-Cre+; Akirin2fl/+ mice upon stimulation with various TLR ligands (Fig 1A). In contrast, macrophages lacking Akirin1 did not show any defects in the production of cytokines to TLR stimulation (data not shown), indicating that Akirin2, but not Akirin1, plays a critical role in the regulation of proinflammatory cytokine production. It has been shown that Newcastle disease virus (NDV) and Encephalomyocarditis virus (EMCV) infection is recognized by RIG-I and MDA-5, respectively, in macrophages, resulting in the production of type I IFNs. Akirin2-deficient macrophages produced significantly less IL-6 and IFN-β than did WT cells after NDV and EMCV infection (Fig 1B, Supplementary Fig S3). Furthermore, Herpes simplex virus (HSV)-mediated production of IL-6 and IFN-β was also severely impaired in the absence of Akirin2 in macrophages. Since recognition of HSV is mediated through STING by cytoplasmic DNA sensors including cyclic GMP-AMP synthase (Sun et al, 2013), these results indicate that Akirin2 plays a pivotal role in the production of IL-6, IL-12p40, and IFN-β in response to the recognition of pathogens via various classes of sensors in macrophages.
Figure 1. TLR-ligand- and virus infection-induced cytokine production and gene expression in Akirin2-deficient macrophages.
A, B Peritoneal macrophages from LysM-Cre+; Akirin2fl/+ and LysM-Cre+; Akirin2fl/fl mice were stimulated with various TLR ligands including Malp2 (1, 10 ng/ml), poly I:C (10, 100 μg/ml), LPS (10, 100 ng/ml), R848 (10 ng/ml) and CpG DNA (0.1, 1 μM) (A) or infected with HSV and NDV (MOI 1, 5) (B) for 24 h. Then, IL-6, TNF, CXCL-1, IL-12p40, and IFN-β concentrations in the culture supernatants were determined by ELISA. Error bars indicate mean ± SD. Results are representative of at least three independent experiments. Statistical significance was determined using the Student’s t-test. *P < 0.05; **P < 0.01.
C, D Peritoneal macrophages from LysM-Cre+; Akirin2fl/+ and LysM-Cre+; Akirin2fl/fl mice were stimulated with 100 ng/ml LPS (C) or poly I:C (200 μg/ml) (D) for indicated time period, and the total RNA extracted was subjected to Northern blot analysis for the expression of Il6, Ifnb, Cxcl1, Ifit2, Cxcl10, and Actb.
Next, we examined whether Akirin2 regulates IL-6 production at the level of gene expression. Northern blot analysis revealed that LPS-induced expression of genes encoding IL-6 and CXCL10 was severely impaired in LysM-Cre+; Akirin2fl/fl macrophages relative to control LysM-Cre+; Akirin2fl/+ cells, indicating that Akirin2 is critical for the expression of several LPS-inducible genes (Fig 1C). However, the induction of genes encoding IFIT2 and CXCL1 chemokine KC was similar in LysM-Cre+; Akirin2fl/+ and LysM-Cre+; Akirin2fl/fl macrophages. Gene induction in response to Poly I:C stimulation was similarly impaired in LysM-Cre+; Akirin2fl/fl macrophages (Fig 1D). Time course analysis revealed that LPS- and Poly I:C-induced Il6, Il12, and Ifnb, but not Cxcl1 expression was altered at all time points tested in the absence of Akirin2 in peritoneal (Supplementary Fig S4A and B) and BM-derived macrophages (BMDM) (Supplementary Fig S5). Thus, mouse Akirin2 regulates the expression of a set of LPS and Poly I:C inducible genes, including Il6, Il12b, and Ifnb.
Akirin2 is critical for the responses to Listeria monocytogenes infection in vivo
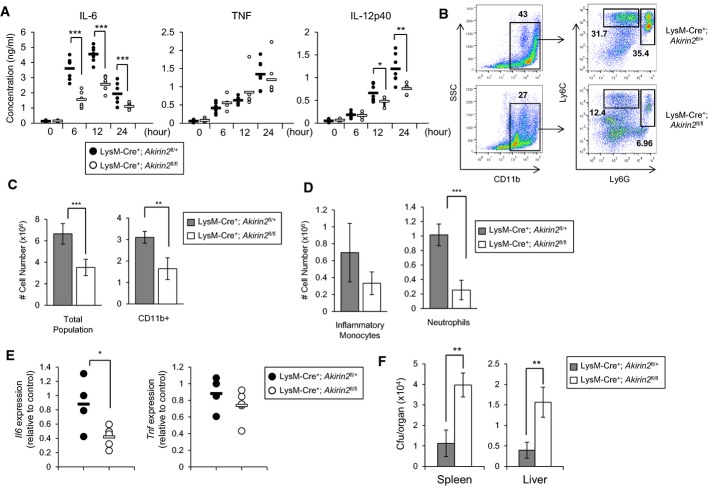
Next, we examined the innate immune responses to pathogen infection in vivo by using Listeria monocytogenes as a model. When LysM-Cre+; Akirin2fl/fl mice were intraperitoneally infected with Listeria monocytogenes, the production of IL-6 and IL-12p40, but not TNF, in the sera was severely impaired compared with LysM-Cre+; Akirin2fl/+ mice (Fig 2A). Consistently, Listeria-mediated infiltration of myeloid cells including CD11b+Ly6Ghi neutrophils to the peritoneal cavity was significantly reduced in LysM-Cre+; Akirin2fl/fl mice (Fig 2B and C). CD11b+Ly6ChiLy6Glo inflammatory monocytes also tended to require Akirin2 for their recruitment (Fig 2D). Furthermore, the expression of Il6, but not Tnf, in the PECs from Listeria-infected mice decreased in the absence of Akirin2 in macrophages (Fig 2E). Consistent with the impaired immune responses to infection, Akirin2 deficiency leads to impaired clearance of infected Listeria in the spleen and liver (Fig 2F). These results demonstrate that Akirin2 expressed in macrophages is critical for innate immune responses to Listeria infection and host defense in vivo.
Figure 2. Akirin2 is critical for innate immune responses to Listeria infection in vivo.
LysM-Cre+; Akirin2fl/+ and LysM-Cre+; Akirin2fl/fl mice (n = 7) were intraperitoneally infected with 5 × 105 Listeria monocytogenes/mice in a volume of 0.25 ml of PBS.
A Serum cytokine levels were determined by ELISA for IL-6, TNF, and IL-12p40 at indicated time periods following infection.
B PECs were prepared from LysM-Cre+; Akirin2fl/+ (n = 4) and LysM-Cre+; Akirin2fl/fl (n = 5) mice 24 h after Listeria infection, and the cell surface expression of myeloid cell markers was determined by flow cytometry. Frequencies of CD11b+Ly6Ghi neutrophils and CD11b+Ly6ChiLy6Glo inflammatory monocytes are indicated.
C, D The numbers of (C) total PECs, CD11b+ myeloid cells and (D) CD11b+Ly6ChiLy6Glo inflammatory monocytes and CD11b+Ly6Ghi neutrophils are shown.
E The levels of Il6 and Tnf mRNAs in the PECs were measured by qPCR.
F The bacterial load in the liver and spleen was measured by colony formation assay, and the number of bacterial colonies was then counted and expressed as CFU/organ (n = 4).
Data information: Error bars indicate mean ± s.d. Statistical significance was determined using the Student’s t-test. *P < 0.05; **P < 0.01; ***P < 0.001.
Akirin2 is required for LPS-induced nucleosome remodeling at the Il6 promoter region
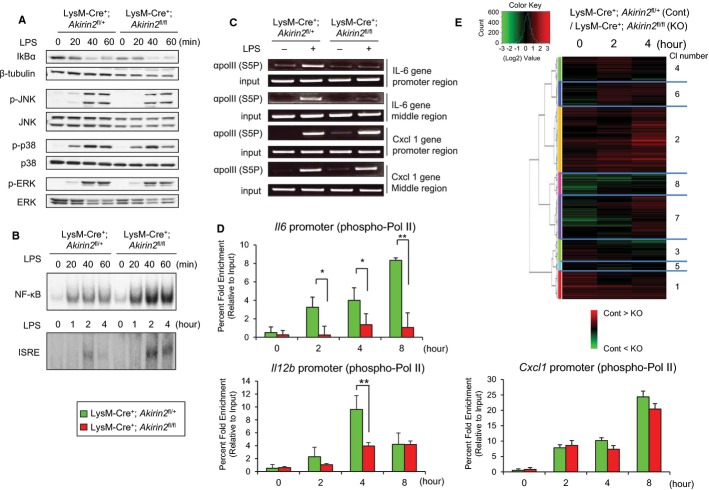
As Drosophila Akirin acts together with or downstream of Relish, we next examined the LPS-dependent activation of NF-κB in LysM-Cre+; Akirin2fl/fl macrophages. In response to these stimuli, neither degradation of IκBα (Fig 3A) nor induction of NF-κB DNA binding (Fig 3B) was impaired in LysM-Cre+; Akirin2fl/fl macrophages. In addition, LPS-induced phosphorylation of JNK and p38 was not altered between control and LysM-Cre+; Akirin2fl/fl macrophages (Fig 3A). These data indicate that mouse Akirin2 acts together with or downstream of NF-κB and MAP kinases in the control of TLR-inducible gene expression in macrophages.
Figure 3. Akirin2 is dispensable for NF-κB activation but decisive for the transcription progression.
A Whole-cell lysates were prepared from LysM-Cre+; Akirin2fl/+ and LysM-Cre+; Akirin2fl/fl PECs treated with LPS (100 ng/ml) for the indicated time period and IκBα, β-tubulin, p-JNK, JNK, p-p38, p38, p-ERK, and ERK expression were determined by Western blotting.
B Nuclear extracts were prepared from LysM-Cre+; Akirin2fl/+ and LysM-Cre+; Akirin2fl/fl PECs treated with LPS (100 ng/ml) for the indicated time period and NF-κB- and ISRE-DNA-binding activities were analyzed by electrophoretic mobility-shift assay (EMSA).
C, D ChIP experiments were performed with chromatin prepared from LysM-Cre+; Akirin2fl/+ and LysM-Cre+; Akirin2fl/fl PECs treated with or without LPS (1 μg/ml) for indicated time period. Antibody against phospho-RNA polymerase II (S5P) was used. Precipitated DNA was amplified by semi-quantitative PCR using primers specific for Il6 or Cxcl1 promoter or middle region (C), and precipitated DNA was quantified by real-time PCR using primers specific for Il6, Il12b, or Cxcl1 promoter region (D). ChIP values were normalized against the input and expressed as relative enrichment of the material precipitated by the indicated antibody on specific promoter (relative quantification using the comparative Ct method (2−ΔΔCt)). Error bars indicate mean ± SD. The results are representative of at least three independent experiments. Statistical significance was determined using the Student’s t-test. *P < 0.05; **P < 0.01.
E Microarray analysis was performed in PECs from LysM-Cre+; Akirin2fl/+ and LysM-Cre+; Akirin2fl/fl mice stimulated with LPS (100 ng/ml) for indicated time period using Affymetrix mouse genome 430 2.0 microarray chips. Robust multichip average (RMA) expression values were calculated using R package. For each probe, the changes in expression between LysM-Cre+; Akirin2fl/+ and LysM-Cre+; Akirin2fl/fl samples were defined as the difference between log2 values for LysM-Cre+; Akirin2fl/+ and LysM-Cre+; Akirin2fl/fl macrophages. The genes upregulated more than twofold in LysM-Cre+; Akirin2fl/+ macrophages after stimulation were selected. Hierarchical clustering classified the LPS-inducible genes to eight different clusters based on the expression levels in LysM-Cre+; Akirin2fl/+ and LysMCre/+; Akirin2fl/fl macrophages.
To examine if Akirin2 controls LPS-induced gene expression at the transcriptional level, we performed a chromatin-immunoprecipitation (ChIP) assay using anti-phospho-polymerase II (Pol II) Ab in LPS-stimulated macrophages. As shown in Fig 3C, phospho-Pol II was recruited to the Il6 and Cxcl1 promoters and coding regions in response to LPS stimulation in wild-type macrophages. However, its recruitment to the Il6 locus was severely impaired in the absence of Akirin2 (Fig 3C). Furthermore, impaired recruitment of phospho-Pol II to the Il6 and Il12b, but not Cxcl1, promoter was observed at various time points after LPS stimulation (Fig 3D). Likewise, the recruitment of phospho-Pol II to the Tnf promoter was normal in LysM-Cre+; Akirin2fl/fl macrophages (Supplementary Fig S6A). Collectively, these results clearly indicate that Akirin2 controls Il6 gene expression at the transcription level.
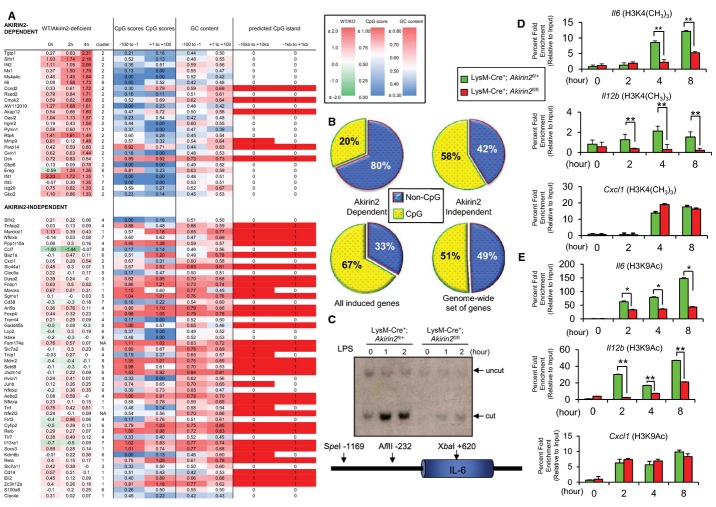
To comprehensively examine the effect of Akirin2 deficiency on LPS-induced gene expression in macrophages, we examined the genome-wide changes in gene expression in response to LPS by microarray analysis. We selected 1,054 genes whose expression levels were upregulated more than twofold in response to LPS stimulation in wild-type macrophages (Supplementary Table S1). Comparison of LPS-inducible genes between wild-type and LysM-Cre+; Akirin2fl/fl macrophages revealed that 187 genes were expressed less than twofold in the absence of Akirin2. Hierarchical clustering of the 1,054 genes divided the LPS-inducible genes into 8 different clusters based on their expression levels in LysM-Cre+; Akirin2fl/+ (Control) and LysM-Cre+; Akirin2fl/fl (KO) macrophages (Supplementary Table S1 and Fig 3E). Among these, gene clusters 1, 2, and 6 tended to be downregulated in LysM-Cre+; Akirin2fl/fl macrophages (Fig 3E). From these clusters, we next selected 25 genes whose expression levels were highly different in wild-type and LysM-Cre+; Akirin2fl/fl macrophages and designated these as Akirin2-dependent genes (Fig 4A). In addition, we selected 48 genes whose expression levels were comparable between wild-type and LysM-Cre+; Akirin2fl/fl macrophages as a reference.
Figure 4. Classification of LPS-induced Akirin2-dependent and Akirin2-independent genes.
A 73 genes that are potently induced by LPS in mouse peritoneal macrophages are shown. Columns 2 to 4 show the difference (log2 ratios) in expression between LysM-Cre+; Akirin2fl/+ and LysM-Cre+; Akirin2fl/fl macrophages, for time points 0, 2, and 4 h. Column 5 shows the cluster index assigned by hierarchical clustering (see Fig 3E). Columns 6, 7 and 8, 9 show the CpG scores and GC content in the regions from −100 to −1 and from +1 to 100 relative to the transcription start sites. Column 10 and 11 show whether a CpG island is predicted (1) or not (0) within the regions −10 kb to +10 kb and −1 kb to +1 kb. Colors reflect the difference in gene expression, the CpG scores, and the GC content, as indicated in the respective color legends.
B These genes were further classified as Akirin2 dependent (lower frequency of CpG islands) and Akirin2 independent (higher frequency of CpG islands) genes. The pie charts show the fraction of genes associated with CpG islands on the region −1 kb to +1 kb for Akirin2-dependent and Akirin2-independent genes, as well as the entire LPS-inducible genes and genome-wide set of genes.
C Restriction enzyme accessibility assay was used to monitor LPS-induced nucleosome remodeling at the Il6 promoter. PECs from LysM-Cre+; Akirin2fl/+ and LysM-Cre+; Akirin2fl/fl were treated with LPS (100 ng/ml) for the indicated time period. Isolated nuclei were digested with AflII, and the cleaved DNA was analyzed by Southern blot using Il6 promoter-specific probe.
D, E ChIP experiments were performed with chromatin prepared from LysM-Cre+; Akirin2fl/+ and LysM-Cre+; Akirin2fl/fl PECs treated with or without LPS (1 μg/ml) for indicated time period. Antibody against trimethyl-histone (H3K4(CH3)3) (D) and acetylated histone (H3K9Ac) (E) was used. Precipitated DNA was quantified by real-time PCR using primers specific for Il6, Il12b, or Cxcl1 promoter region. ChIP values were normalized against the input and expressed as relative enrichment of the material precipitated by the indicated antibody on specific promoter (relative quantification using the comparative Ct method (2−ΔΔCt)). Error bars indicate mean ± s.d. The results are representative of at least three independent experiments. Statistical significance was determined using the Student’s t-test. *P < 0.05; **P < 0.01.
Using these two sets of genes, we first compared the promoter sequences of Akirin2-dependent and Akirin2-independent genes. However, we did not observe any significant differences in the frequency of NF-κB binding sites between Akirin2-dependent and Akirin2-independent gene promoters (Supplementary Fig S7). In contrast, the Akirin2-dependent genes tended to harbor significantly fewer CpG islands compared with Akirin2-independent genes (P = 0.0067), entire LPS-inducible genes (P = 1.4 × 10−6) or genes in the whole genome (P = 0.0016) (Fig 4A and B). These data demonstrate that among LPS-inducible genes, Akirin2 controls the expression of a set of genes whose promoter regions have relatively fewer CpG islands, suggesting a relationship between Akirin2 and chromatin remodeling.
Akirin2 is critical for chromatin remodeling of the Il6 promoter region
To investigate whether Akirin2 is involved in the regulation of chromatin remodeling, nuclei from unstimulated and LPS-stimulated peritoneal macrophages were treated with a restriction enzyme; after purification of genomic DNA and cleavage with a second enzyme, the efficiency of cleavage in the isolated nuclei was monitored by Southern blot (Zhou et al, 2004). Macrophages from LysM-Cre+; Akirin2fl/fl mice showed decreased accessibility to the Il6 promoter, indicating that Akirin2 is critical for opening the Il6 promoter and further nucleosome remodeling (Fig 4C).
We next investigated the involvement of Akirin2 in histone modification on the Il6 promoter region in response to LPS. We examined changes in the trimethylation of histone 3 lysine 4 (H3K4) as well as acetylation of H3K9 on the Il6, Il12b, Cxcl1, and Tnf promoters in response to LPS. Although trimethylation of H3K4 on the Cxcl1 and Tnf promoters was comparably induced in response to LPS stimulation in LysM-Cre+; Akirin2fl/+ and LysM-Cre+; Akirin2fl/fl macrophages, the Il6 and Il12b promoters required Akirin2 for inducing H3K4 trimethylation (Fig 4D, Supplementary Fig S6B). Likewise, the acetylation of H3K9 on the Il6 and Il12b, but not Cxcl1 and Tnf promoters, was also induced in response to LPS in an Akirin2-dependent manner (Fig 4E, Supplementary Fig 6C). These results indicate that Akirin2 is directly involved in the regulation of chromatin remodeling and histone modification at the Il6 promoter.
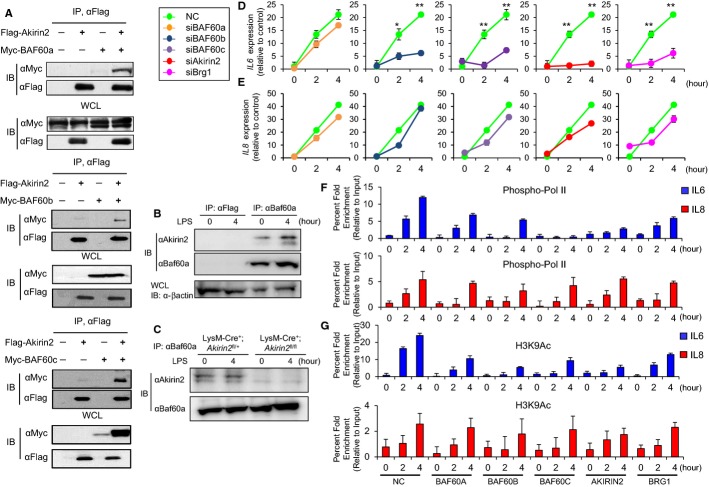
Akirin2 interacts with BAF60 family members, which are required for Il6 expression
One mechanism whereby Akirin might function as a general cofactor for gene expression would be through interactions with chromatin remodeling complexes. A Drosophila whole-genome yeast two-hybrid experiment (Giot et al, 2003) suggested that Akirin interacts with BAP60 (Brahma-associated protein), a core subunit of the Drosophila SWI/SNF class Brahma (BRM) chromatin remodeling complex (Moller et al, 2005). Mammals have a family of BAP60 homologues, comprised of BAF60a, BAF60b, and BAF60c, which are encoded by the SMARCD1, SMARCD2, and SMARCD3 genes, respectively (Puri & Mercola, 2012). Among these, BAF60c is preferentially expressed in the developing heart and somites during embryogenesis (Lickert et al, 2004). Furthermore, an equivalent activity of BAF60c in skeletal muscle differentiation and the molecular mechanism by which BAF60c promotes tissue-specific activation of gene expression has been described (Forcales et al, 2012). BAF60 proteins have been shown to interact with multiple transcription factors and are thought to bridge interactions between these transcription factors and SWI/SNF complexes, thereby allowing the recruitment of SWI/SNF to the target genes (Debril et al, 2004; Hsiao et al, 2003; Lickert et al, 2004).
To investigate if Akirin2 interacts with mammalian BAP60 homologues, we overexpressed Flag-tagged Akirin2 and Myc-tagged BAF60 family members (BAF60a, BAF60b and BAF60c proteins) in HEK293 cells. Immunoprecipitation of the cell lysates with anti-Flag antibody co-precipitated Myc-tagged BAF60a, BAF60b, and BAF60c (Fig 5A), indicating that Akirin2 can interact with BAF60 proteins in cells. Furthermore, endogenous Akirin2 co-precipitated with anti-BAF60a Ab in peritoneal macrophages irrespective of stimulation by LPS (Fig 5B), and the specificity of the Akirin2 precipitation was confirmed by the fact that the precipitated band was not observed in LysM-Cre+; Akirin2fl/fl macrophages (Fig 5C). These results suggest that endogenous Akirin2 associates with BAF60 proteins even in resting cells.
Figure 5. SWI/SNF components are required for the expression and histone modification of the IL6, but not IL8, promoter in response to IL-1β stimulation.
A Cell lysates prepared from HEK293 cells transfected with indicated expression plasmids were immunoprecipitated with anti-Flag antibody, followed by immunoblot analysis using anti-Myc and anti-Flag antibodies.
B PECs from LysM-Cre+; Akirin2fl/+ mice were treated with LPS (1 μg/ml) for 4 h. Cell lysates were collected and subjected to immunoprecipitation using anti-Flag and anti-BAF60a, followed by immunoblot with anti-Akirin2 and anti-BAF60a antibodies.
C PECs from LysM-Cre+; Akirin2fl/+ and LysM-Cre+; Akirin2fl/+ mice were treated with LPS (1 μg/ml) for 4 h. Cell lysates were collected and subjected to immunoprecipitation using anti-BAF60a, followed by immunoblot with anti-Akirin2 and anti-BAF60a antibodies.
D, E HeLa cells knocked down with indicated siRNAs as shown in Supplementary Fig S8A were stimulated with recombinant human IL-1β (10 ng/ml) for indicated time period. Cells were harvested for RNA isolation, and cDNA was subjected to quantitative PCR analysis for gene expression of IL6 (D) and IL8 (E). Samples were normalized using 18S rRNA.
F, G HeLa cells knocked down with siRNAs for indicated genes were stimulated with recombinant human IL-1β (10 ng/ml) for indicated time period. Cells were fixed, chromatin was prepared, and ChIP experiments were performed. Antibodies against phospho-RNA Pol II (S5P) (F) and Histone H3 (acetyl K9) (G) were used. Precipitated DNA was quantified by real-time PCR using primers specific for IL6 or IL8 promoter region.
Data information: ChIP values were normalized against the input and expressed as relative enrichment of the material precipitated by the indicated antibody on specific promoter (relative quantification using the comparative Ct method (2−ΔΔCt)). Error bars indicate mean ± s.d. The results are representative of at least three independent experiments. Statistical significance was determined using the Student’s t-test. *P < 0.05; **P < 0.01.
Source data are available online for this figure.
To gain functional insight into the specific role of BAF60 proteins and their relation to Akirin2 in TLR/IL-1R-induced gene expression, we knocked down BAF60 genes, the SWI/SNF catalytic subunit BRG1 and Akirin2 in HeLa cells (Supplementary Fig S8A) and in J774 macrophages (Supplementary Fig S8B and C). The expression of IL6 in response to IL-1β was severely impaired in Akirin2, BAF60b, BAF60c, and BRG1 depleted HeLa cells. In contrast, knockdown of BAF60a failed to affect IL6 expression in response to IL-1β stimulation (Fig 5D). However, the induction of genes encoding IL8, a counterpart of mouse KC, was comparable in control, Akirin2, and BAF60 depleted HeLa cells (Fig 5E). In addition, knockdown of Baf60 genes as well as Brg1 in the J774 mouse macrophage cell line led to the defects in the expression of Il6, but not Cxcl1, in response to LPS (Supplementary Fig S8D). In this cell line, Baf60a is also critical for the regulation of Il6 in contrast to HeLa cells. These results indicate that Akirin2 and BAF60 complex members are critical for regulating Il6 gene expression together with BRG1 in various cell types.
Although Akirin2 target genes have fewer CpG islands on their promoter regions, some of the target genes such as Cmpk2, Akap12, and Parp14 harbor a CpG island (Fig 4A). To investigate if these genes require chromatin remodeling for their expression, we examined their expression in Brg1 knockdown J774 macrophages in response to LPS. As shown in Supplementary Fig S9, LPS-induced expression of Cmpk2, Akap12, and Parp14 was impaired in Brg1 knockdown cells, indicating that Akirin2 target genes require SWI/SNF complex for their expression irrespective of the presence of CpG islands on their promoter regions.
Next, we examined the role of BAF60 proteins in the regulation of histone modification. Knockdown of BAF60b and BAF60c, but not BAF60a, in HeLa cells resulted in deficient recruitment of phospho-Pol II as well as H3K9 acetylation in response to IL-1β stimulation (Fig 5F and G). Similarly, downregulation of Akirin2 and Brg1 also impaired the recruitment of phospho-Pol II and acetylation of H3K9, further confirming that Akirin2, BAF60b, and BAF60c regulate Il6 gene expression through chromatin modification.
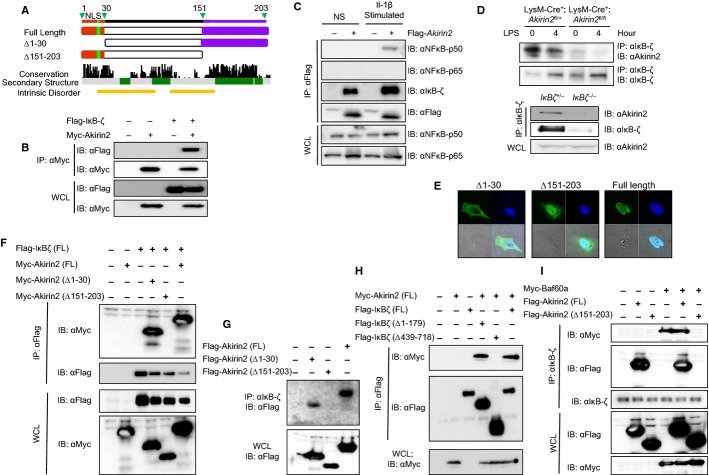
Finally, we investigated the interaction between Akirin2 and BAF60 family members at the residue level. The immunoprecipitation experiment further revealed that the Akirin2 mutants lacking N-terminal NLS (Δ1–30) or the C-terminal region (Δ151–203) were capable of co-precipitating with BAF60a or BAF60c (Supplementary Fig S10A and B), suggesting that Akirin2 interacts with BAF60 proteins via its central portion. Indeed, an alignment of 50 Akirin proteins among different species identified that the central portion is also highly conserved and is predicted to assume a helical secondary structure (Fig 6A).
Figure 6. Akirin2 interacts with IκB-ζ via the C-terminal conserved region.
A Schematic representation of Akirin2 conserved regions and mutant proteins used.
B Flag-IκB-ζ and/or Myc-Akirin2 were transfected in HeLa cells, and cell lysates were immunoprecipitated with anti-Myc Ab followed by immunoblot with anti-Flag and anti-Myc Ab.
C HeLa cells were transfected with Flag-Akirin2 plasmid. The cells were then stimulated with IL-1β for 12 h followed by the preparation of the cell lysates, and immunoprecipitation with anti-Flag Ab followed by immunoblot with anti-NF-κB p50, anti-NF-κB p65, anti-IκB-ζ, and anti-Flag Ab.
D PECs from LysM-Cre+; Akirin2fl/+ and LysM-Cre+; Akirin2fl/fl mice were treated with LPS (1 μg/ml) for 4 h. Cell lysates were collected and subjected to immunoprecipitation using anti-IκB-ζ Ab followed by immunoblot with anti-Akirin2 and anti-IκB-ζ Ab. PECs from IκBζ+/− and IκBζ−/− mice were harvested, and cell lysates were subjected to immunoprecipitation using anti-IκB-ζ Ab followed by immunoblot with anti-Akirin2 and anti-IκB-ζ Ab.
E Immunofluorescent staining of HeLa cells expressing Flag-Akirin2 mutants (green) together with DAPI (blue).
F HEK293 cells were transfected with indicated Myc-tagged Akirin2 mutant plasmids and Flag-tagged IκB-ζ. Cell lysates were collected and subjected to immunoprecipitation with anti-Flag Ab followed by immunoblot with anti-Myc and anti-Flag Ab.
G HeLa cells transfected with indicated Akirin2 mutant plasmids were stimulated with IL-1β for 12 h followed by the preparation of the cell lysates. Then, immunoprecipitation with anti-IκB-ζ Ab was performed followed by immunoblot with anti-Flag Ab.
H HEK293 cells were transfected with indicated Flag-tagged IκB-ζ mutant plasmids and Myc-tagged Akirin2. Cell lysates were collected and subjected to immunoprecipitation with anti-Flag Ab followed by immunoblot with anti-Myc and anti-Flag Ab.
I HEK293 cells were transfected with indicated Flag-tagged Akirin2 mutant plasmids and Myc-tagged BAF60a. Cell lysates were collected and subjected to immunoprecipitation with anti-IκB-ζ Ab followed by immunoblot with anti-Myc, anti-Flag, and anti-IκB-ζ Ab.
Data information: The results are representative of at least three independent experiments.
Source data are available online for this figure.
Akirin2 interacts with IκB-ζ via the C-terminal conserved region
We then further investigated the mechanism by which Akirin2 regulates gene expression in the course of TLR-induced macrophage activation. We hypothesized that Akirin2 acts in concert with NF-κB family members by physically associating with them, though we did not observe interaction with NF-κB family members (data not shown). On the other hand, it has been shown that Il6 and Il12b, but not Tnf, mRNA expression to TLR stimulation requires an IκB-like molecule called IκB-ζ (Yamamoto et al, 2004; Yamazaki et al, 2001). Another report suggested the involvement of IκB-ζ in the regulation of histone modifications, though the underlying mechanism is not well understood (Kayama et al, 2008). Interestingly, Flag-tagged IκB-ζ co-precipitated with Myc-tagged Akirin2, when overexpressed in HeLa cells (Fig 6B). Furthermore, immunoprecipitation of overexpressed Flag-tagged Akirin2 with anti-Flag Ab resulted in the co-precipitation of endogenous NF-κB p50, but not p65, subunit in HeLa cells upon IL-1β stimulation (Fig 6C). Interestingly, overexpressed Akirin2 could also precipitate endogenous IκB-ζ with and without stimulation (Fig 6C). When Flag-Akirin2 was abundantly expressed, the interaction between Flag-Akirin2 and IκB-ζ appears to be modestly increased probably due to the increase in the expression of IκB-ζ. Consistently, even endogenous Akirin2 co-precipitated with endogenous IκB-ζ in peritoneal macrophages (Fig 6D), and this interaction was not observed in IκB-ζ−/− or LysM-Cre+; Akirin2fl/fl macrophages (Fig 6D). Whereas IκB-ζ expression was induced in macrophages in response to LPS stimulation, the IκB-ζ–Akirin2 interaction was observed even in resting cells and modestly decreased in response to LPS stimulation (Fig 6D). This may be due to the modest decrease in the endogenous Akirin2 protein expression in response to LPS stimulation in macrophages (Supplementary Fig S1B). These results suggest that endogenous IκB-ζ expressed in resting cells can interact with endogenous Akirin2 irrespective of TLR stimulation. Then, we examined the region of Akirin2 responsible for interaction with IκB-ζ (Fig 6A). Antibody staining of the HeLa cells expressing Akirin2 mutants clearly showed a strict dependence on the NLS (Δ1–30) (Fig 6E). Whereas a Myc-tagged Akirin2 mutant lacking the N-terminal NLS co-precipitated with Flag-tagged IκB-ζ, deletion of the C-terminal conserved region of Akirin2 abrogated the interaction between IκB-ζ and Akirin2 upon overexpression (Fig 6F). Furthermore, endogenous IκB-ζ co-precipitated the Flag-tagged full-length and Δ1–30 Akirin2 mutant, but not the mutant lacking the C-terminal region (Fig 6G). Next, we examined regions of IκB-ζ responsible for interaction with Akirin2. IκB-ζ is comprised of an N-terminal NLS, a central NF-κB p50 subunit binding domain, and a C-terminal ankyrin-repeat domain (Motoyama et al, 2005). Although deletion of IκB-ζ N-terminal region (Δ1-179) did not affect the interaction with Akirin2, the mutant IκB-ζ lacking the C-terminal ankyrin-repeat region (Δ439–718) failed to co-precipitate with Akirin2 (Fig 6H). These data indicate that Akirin2 interacts with IκB-ζ, and the Akirin2 C-terminal conserved region is critical for the interaction with the ankyrin-repeat domain of IκB-ζ. Next, we investigated the relationship between IκB-ζ, Akirin2 and BAF60 family members. Interestingly, the immunoprecipitation experiment revealed that overexpressed BAF60a co-precipitated with endogenous IκB-ζ, and this interaction is probably mediated through the endogenous Akirin2, as endogenous IκB-ζ failed to co-precipitate BAF60a upon overexpression of the C-terminal deletion mutant of Akirin2 lacking the IκB-ζ binding domain (Fig 6I). Collectively, these results demonstrate that IκB-ζ can interact with BAF60 proteins via bridging by Akirin2.
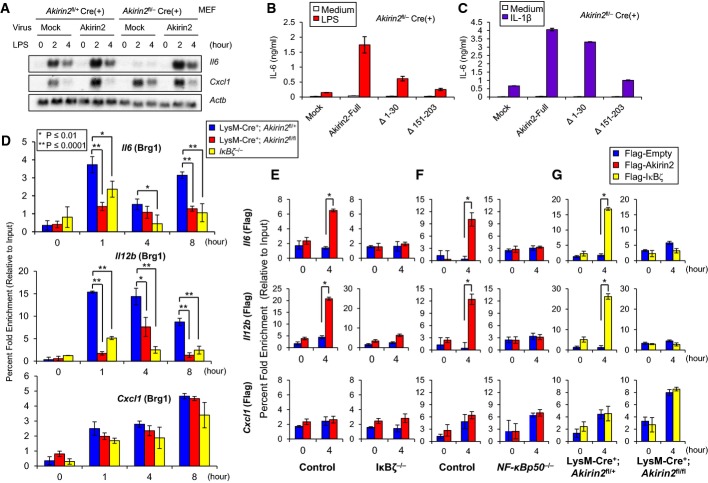
The IκB-ζ-Akirin2 cascade is critical for the recruitment of Brg1 to the Il6 promoter for transactivation
Next, we examined the role of the interaction between Akirin2 and IκB-ζ. The C-terminal part of Akirin2 is highly conserved among species, although the functional role of this domain is enigmatic. Thus, we investigated the role of the Akirin2 C-terminal region in response to TLR/IL-1R stimulation. Whereas deletion of Akirin2 in MEFs resulted in severe reduction in LPS-mediated expression of Il6, but not Cxcl1, consistent with a previous report (Goto et al, 2008), the expression of full-length Akirin2 rescued Il6 expression upon LPS stimulation at the mRNA and protein levels (Fig 7A). In contrast, the deletion of either the N-terminal or C-terminal resulted in defective rescue of IL-6 production in response to LPS or IL-1β stimulation (Fig 7B and C). Thus, these results suggest that the interaction between IκB-ζ and Akirin2 is critical for inducing a set of genes in response to TLR stimulation.
Figure 7. The IκB-ζ-Akirin2 cascade is critical for the recruitment of Brg1 to the Il6 promoter for transactivation.
A MEFs from Akirin2fl/+ and Akirin2fl/− mice were infected with retrovirus expressing Cre and/or full-length Akirin2. Then, the cells were stimulated with LPS (1 μg/ml) followed by Northern blot analysis with indicated probes.
B, C MEFs from Akirin2fl/− mice were infected with retrovirus expressing Cre and indicated Akirin2 mutants. Then, the cells were stimulated with LPS (1 μg/ml) (B) and IL-1β (10 ng/ml) (C), and the production of IL-6 was determined by ELISA.
D ChIP experiments were performed with chromatin prepared from LysM-Cre+; Akirin2fl/+, LysM-Cre+; Akirin2fl/fl and IκB-ζ−/− PECs treated with or without LPS (1 μg/ml) for indicated time period. Antibody against Brg1 (J1) was used. Precipitated DNA was quantified by real-time PCR using primers specific for Il6, Il12b, or Cxcl1 promoter region.
E, F Retrovirally transduced IκB-ζ−/− (E) and NF-κBp50−/− (F) BMDM expressing Flag-tagged Akirin2 were then stimulated with LPS (1 μg/ml) for 4 h, and ChIP experiments were performed. Antibody against anti-Flag was used. Precipitated DNA was quantified by real-time PCR using primers specific for Il6, Il12b, or Cxcl1 promoter region.
G BM cells from LysM-Cre+; Akirin2fl/+ and LysM-Cre+; Akirin2fl/fl mice were infected with retrovirus expressing Flag-tagged empty or full-length IκB-ζ. BMDM were then stimulated with LPS (1 μg/ml) for 4 h, and ChIP experiments were performed. Antibody against anti-Flag was used. Precipitated DNA was quantified by real-time PCR using primers specific for Il6, Il12b, or Cxcl1 promoter region.
Data information: ChIP values were normalized against the input and expressed as relative enrichment of the material precipitated by the indicated antibody on specific promoter (relative quantification using the comparative Ct method (2−ΔΔCt)). Error bars indicate mean ± s.d. The results are representative of at least two independent experiments. Statistical significance was determined using the Student’s t-test. *P < 0.05; **P < 0.01.
Since IκB-ζ has been shown to be critical for the histone modification of its target genes in response to LPS, we next sought to determine if Akirin2 and IκB-ζ are required for the recruitment of the SWI/SNF remodeling complexes to the promoter regions of their target genes. ChIP experiments were performed using anti-BRG1 antibody, and sheared cross-linked chromatin was prepared from unstimulated or LPS-stimulated macrophage cells lacking Akirin2 or IκB-ζ. BRG1 recruitment to the Il6 and Il12b, but not Cxcl1 promoter, was observed 1, 4, and 8 h after LPS stimulation and was impaired in the macrophages from LysM-Cre+; Akirin2fl/fl mice as well as from IκB-ζ-deficient mice (Fig 7D). Thus, these results indicate that both Akirin2 and IκB-ζ are required for the recruitment of the SWI/SNF complex to the Il6 and Il12b promoter regions.
Next, we examined if Akirin2 is also recruited to the promoter regions of its target genes. We retrovirally expressed Flag-tagged Akirin2 in wild-type and NF-κBp50−/− BM macrophages and performed the ChIP assay using anti-Flag Ab upon LPS stimulation (Supplementary Fig S11A and B). The levels of total Akirin2 mRNA expression increased modestly in Flag-Akirin2 expressing cells (Supplementary Fig S11A). Interestingly, we observed the recruitment of Akirin2 to the Il6 and Il12b, but not Cxcl1, promoters in response to LPS stimulation (Fig 7E). In contrast, retroviral expression of Flag-tagged Akirin2 in IκB-ζ−/− and NF-κBp50−/− BM macrophages led to the abrogation of the recruitment of Akirin2 on the Il6 and Il12b promoters (Fig 7E and F), indicating that IκB-ζ is required for the recruitment of Akirin2 to its target gene promoters. We further examined the recruitment of IκB-ζ to the immune regulatory gene promoters by retrovirally expressing Flag-tagged IκB-ζ in LysM-Cre+; Akirin2fl/fl BM macrophages (Supplementary Fig S11C). The ChIP assay revealed that IκB-ζ is also recruited to the Il6 and Il12b, but not Cxcl1, promoters in response to LPS stimulation (Fig 7G). Interestingly, the recruitment of IκB-ζ to the promoters required the presence of Akirin2 in macrophages (Fig 7G), suggesting that IκB-ζ and Akirin2 function together in order to be recruited to the Il6 and Il12b promoters for inducing the gene expression.
Discussion
In Drosophila, Akirin is critical for gene expression downstream of the Imd pathway. We have previously shown that Akirin2, but not Akirin1, controls IL-6 production in mouse embryonic fibroblasts, probably downstream of NF-κB activation. However, the early embryonic lethality of Akirin2-deficient mice prevented examination of the role of this molecule in innate immune cells. Furthermore, the mechanisms of Akirin2-mediated control of IL-6 production have not been clarified. In the present study, we investigated the role of Akirin2 in macrophages by generating an Akirin2 conditional allele in mice, and we found that this molecule plays an essential role in the regulation of inflammatory gene expression in response to TLR stimulation as well as infection with viruses and a model bacteria, Listeria monocytogenes. Furthermore, Akirin2 in macrophages and neutrophils plays a role in the clearance of infecting bacteria. Thus, Akirin2 expressed in macrophages and neutrophils is critical for host defense against bacterial infection.
Akirin2 controls the TLR-induced transcription of a set of inflammation-related genes such as Il6, Il12b, and Ifnb. In contrast, Tnf and Cxcl1 are not regulated by Akirin2. It has been shown that Tnf gene expression is rapidly inducible compared with Il6 or Il12b. Cxcl1 was also reported to be a rapidly inducible gene in response to TNF stimulation. Therefore, it is suggested that Akirin2 is critical for the control of rather slowly inducible genes in the course of macrophage activation.
Promoters of Akirin2-dependent genes harbor significantly lower frequencies of CpG islands compared with Akirin2-independent genes, although there are some exceptions. Akirin2 is essential for the recruitment of the chromatin remodeling complex component Brg1 and histone modification in response to LPS stimulation. Nevertheless, CpG-containing Akirin2-dependent genes also require Brg1 for their expression, further supporting a role for Akirin2 in the control of genes that require chromatin remodeling for their expression. Mechanistically, we found that Akirin2 interacts with BAF60 proteins and IκB-ζ, and the complex is recruited to Il6 and Il12b promoters for inducing chromatin remodeling. Akirin2 and IκB-ζ are recruited to the Il6 and Il12b promoters and act together to recruit the SWI/SNF complex to the promoters of their target genes.
Dynamic changes in gene transcription in innate immune cells is pivotal for evoking inflammation in response to pathogen infection. Gene expression is, in turn, controlled by elaborate and complex mechanisms. Whereas the activation of transcription factors such as NF-κB, AP-1, and IRF-3/-7 is essential for the induction of gene expression, the kinetics and intensity of the change in gene transcription is also regulated by histone modification and changes in chromatin architecture. TLR-inducible genes have been roughly classified into rapidly induced genes and late induced genes. The rapidly induced genes are known to be induced by the action of NF-κB, AP-1, and IRF transcription factors and frequently have promoters containing CpG islands. TLR signaling leads to rapid recruitment of NF-κB as well as basic transcriptional machinery, p-TEFb phosphorylating Pol II to start generation of mature transcripts. On the other hand, the late induced genes are reported to be comprised of both CpG and low CpG island promoters (Bhatt et al, 2012). Low CpG island promoters are enriched in the highly induced genes and possibly require chromatin remodeling for transcription factor binding and general transcription initiation. Interestingly, genes regulated by the presence of Akirin2 show quite low numbers of CpG islands, and chromatin remodeling was impaired in the absence of Akirin2. We further found that Akirin2 is critical for recruiting the SWI/SNF complex to secondary gene promoters. These data demonstrate that Akirin2 functions as an adaptor molecule to tether the chromatin remodeling machinery to the inflammation-related gene promoters. Akirin is highly conserved among orthologs identified throughout metazoa including Drosophila, mice, teleosts, and humans (Macqueen & Johnston, 2009). The function of the Akirin protein has remained enigmatic; despite its high degree of conservation, it remains almost completely devoid of known functional domains, catalytic activity, or demonstrable DNA binding (Goto et al, 2008; Macqueen & Johnston, 2009; Nowak et al, 2012). Akirin2 contains two conserved helical regions, one located in the central region (residues 75-95) and one in the C-terminus (residues 143-193), separated from each other and from the N-terminus by regions predicted to be intrinsically disordered. Neither of the conserved helical regions show significant sequence similarity to proteins of known function, but conserved intrinsically disordered regions are often important for mediating protein–protein interactions (Dyson & Wright, 2005). Akirins function during immune and inflammatory responses in Drosophila as well as in mice. Importantly, Akirins appear to have conserved functions in both the Drosophila and mouse immunity pathways, reinforcing the notion that the role of the Akirin protein has been conserved throughout evolution. Our results give new insight into the role of Akirin2 in mediating NF-κB-dependent gene expression in a SWI-SNF dependent manner via IκB-ζ during inflammatory responses.
Akirin2 can interact with all 3 BAF60 proteins. Knockdown of all of the BAF60 proteins led to impaired Il6 gene expression in response to LPS in the J774 macrophage cell line, though BAF60b and BAF60c, but not BAF60a, were important for Il6 gene expression in response to IL-1β in HeLa cells. Although the function of BAF60a has not been well understood, downregulation of BAF60b and BAF60c have been reported to reduce the expression of certain muscle genes, such as muscle creatine kinase (MCK) (Forcales et al, 2012) and this peculiarity has been ascribed to the partial, functional redundancy between BAF60b and BAF60c (Takeuchi & Bruneau, 2009). Based on this evidence, as well as on our initial identification by co-immunoprecipitation experiments that Akirin2 interacts with both BAF60b and BAF60c, we restricted our analysis to HeLa cells in which Akirin2, Brg1, BAF60b or BAF60c were individually knocked down. BAF60b and BAF60c have been shown to be modified while regulating chromatin remodeling. Besides muscle cell development, BAF60c was reported to be involved in lipogenesis (Wang et al, 2013). Insulin signaling leads to the phosphorylation of BAF60c by atypical protein kinase C ζ/λ inducing its nuclear translocation and regulation of lipogenic genes. On the other hand, BAF60b was reported to interact with an E3 ubiquitin ligase Unkempt, and ubiquitination of BAF60b results in its degradation in a proteasome-dependent fashion (Lores et al, 2010). In this regard, it is also interesting to explore if the BAF60 proteins undergo modification in the course of inflammation via Akirin2. Further studies are required to address differential involvement of the BAF60 proteins in controlling inflammation in vivo.
In this study, we found that IκB-ζ was required for the recruitment of Akirin2 as well as Brg1 to the Il6 and Il12b promoters in macrophages in response to LPS. Reciprocally, Akirin2 is also required for the recruitment of IκB-ζ to the same promoters in response to LPS. The interaction between endogenous IκB-ζ and Akirin2 was also observed in resting macrophages. In addition, IκB-ζ was found to form a complex with BAF60 proteins via Akirin2. These data suggest that IκB-ζ is recruited to the LPS-inducible gene promoters together with Akirin2 and BAF60 proteins, where they induce chromatin remodeling. Since IκB-ζ has been shown to interact with NF-κB p50 subunit (Motoyama et al, 2005; Yamamoto et al, 2004), and NF-κB p50 translocate from the cytoplasm to the nucleus upon IL-1β/TLR stimulation, our results indicate that the complex of IκB-ζ-Akirin2-SWI/SNF interacts with the NF-κB p50 subunit via IκB-ζ upon IL-1β/TLR stimulation and the whole complex is then recruited to the Il6 and Il12b promoters. Therefore, it is possible that the specificity of Akirin2-dependent and Akirin2-independent genes is determined by the recruitment of the NF-κB p50 subunit to the promoters of its target genes. Future studies will identify the recruitment of NF-κB p50 subunit, IκB-ζ, and Akirin2 to each gene promoter in a comprehensive manner.
We have previously shown that IκB-ζ functions in the production of IFN-γ in natural killer (NK) cells in response to IL-12 and IL-18 stimulation. NK cells required IκB-ζ for inducing histone modifications in response to IL-12 and IL-18 stimulation (Miyake et al, 2010), suggesting that IκB-ζ also controls chromatin remodeling in NK cells. It has also been shown that IκB-ζ controls Th17 differentiation in T cells by regulating IL-17 gene expression via its promoter (Okamoto et al, 2010). In contrast, IκB-ζ expressed in epithelial cells of lacrimal glands is critical for the prevention of their apoptotic cell death, which leads to a pathology similar to Sjogren’s syndrome (Okuma et al, 2013), though its molecular mechanism is yet to be clarified. It is intriguing to speculate that IκB-ζ functions via Akirin2-mediated chromatin remodeling in various immune cell types. Further studies with conditional Akirin2-deficient mice will uncover the broad functional roles of Akirin2 in various cells types in vivo.
In addition to the regulation of Il6 and Il12b in response to stimulation with TLR ligands, Akirin2 was found to be important for the induction of type I IFNs and a set of IFN-inducible genes in response to RNA virus infection. It has been shown that a nucleosome masks the TATA box and the transcription start site of the human IFN-β promoter and that the nucleosome slides due to the action of SWI/SNF complex, enabling transcription (Agalioti et al, 2000). Further, a set of IFN-inducible genes require chromatin remodeling by SWI/SNF, a complex that includes a specific subunit, BAF200 (Yan et al, 2005). Thus, the involvement of Akirin2 in the regulation of IFN-inducible genes such as IL-6 in response to RLR stimulation can be explained by the regulation of chromatin remodeling of their promoter regions.
In addition to the control of innate immune responses, Drosophila Akirin has been shown to be required for Twist-mediated gene transcription by controlling chromatin remodeling during embryogenesis. Akirin mutant embryos had muscle defects in fly. Given that the lack of Akirin2 in mice results in early embryonic lethality, it will be interesting to investigate the role of Akirin2 in developmental tissues for controlling chromatin remodeling by coupling with various transcription factors.
In summary, this study clearly demonstrates that Akirin2 controls a set of inflammatory genes with non-CpG island promoters by recruiting the SWI/SNF complex and interacting with IκB-ζ. We believe that this is the first study unveiling the mechanism of initiation of chromatin remodeling after NF-κB activation in innate immune cells. Targeting Akirin2 in immune cells might be beneficial for ameliorating inflammatory diseases and may lead to new strategies for combating autoimmune diseases.
Materials and Methods
Mice, cells and reagents
Floxed Akirin2, IκBζ−/−, and NF-κBp50−/− mice were described previously (Goto et al, 2008; Sha et al, 1995; Yamamoto et al, 2004). Mice were housed in specific-pathogen-free conditions, and all animal experiments were done with the approval of the Animal Research Committee of the Research Institute for Microbial Diseases (Osaka University, Osaka, Japan), and of Kyoto University.
Peritoneal exudate cells were isolated from the peritoneal cavities of mice 3 days after injection with 2 ml of 4.0% Brewer’s thioglycollate medium (Sigma) by washing with ice-cold Hank’s buffered salt solution (Invitrogen). BMDMs were generated in RPMI-1640 medium containing 10% (vol/vol) FCS, 50 μM 2-mercaptoethanol, and 20 ng/ml M-CSF (Peprotech). J774 mouse macrophage cell line was cultured and maintained in DMEM containing 10% (vol/vol) FCS, 50 μM 2-mercaptoethanol, 100 U/ml penicillin, and 100 μg/ml streptomycin. TLR ligands, Malp2, were provided as described previously (Matsushita et al, 2009), LPS from Salmonella Minnesota Re595 (Sigma), Poly I:C from Amersham Biosciences; and R-848 and CpG oligonucleotide (ODN 1668) was used as described previously (Matsushita et al, 2009). Lipofectamine 2000, Lipofectamine RNAiMAX Reagent (Invitrogen) and FuGENE Transfection reagent (Promega) were used for transfection. Recombinant human IL-1β, mouse IL-6, IL-3, and stem cell factor were purchased from R&D Systems.
Measurement of cytokine production
Peritoneal macrophages (5 × 104) and BMDM (5 × 104) were stimulated for 24 h with Malp2, R848, LPS, Poly I:C, or with CpG DNA. Culture supernatants were collected and cytokine concentrations were measured with an ELISA kit (R&D Systems) for IL-6, TNF, IL-12p40, CXCL1, and (PBL interferon source) for IFN-β.
In vivo infection experiments with Listeria monocytogenes
Listeria monocytogenes bacteria were grown in tryptic soy broth (TSB) at 37°C for 6 h with shaking. Mice were intraperitoneally infected with 5 × 105 bacteria/mice in a volume of 0.25 ml of PBS. Serum was collected at the indicated time intervals and subsequently analyzed for cytokine concentrations with an ELISA kit (R&D Systems) for IL-6, TNF, and IL-12p40 production. Mice were sacrificed 24 h after infection, and PECs were harvested and subjected to quantitative PCR and flow cytometry analysis. For measuring the bacterial load, liver and spleen were aseptically removed from mice to produce a homogenized PBS suspension. The serially tenfold diluted homogenates by PBS of organs were placed on TSB agar plates and then were incubated at 37°C for 24 h. The number of bacterial colonies was then counted and expressed as CFU/organ.
Electrophoretic mobility shift assay (EMSA)
Macrophage cells from the peritoneal exudate (1 × 106) were treated with LPS for various time periods. Nuclear extracts were purified from cells, incubated with probes specific for the NF-κB and IRES DNA-binding sites, separated by electrophoresis, and visualized by autoradiography as described previously (Goto et al, 2008).
Co-immunoprecipitation
HEK293 or HeLa cells seeded on 60- or 100-mm dishes were transiently transfected with a total of 6–8 μg of the appropriate combination of plasmids. At 48 h after transfection, cells were lysed in lysis buffer (20 mM Tris–HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, and 1% (vol/vol) Nonidet P-40) containing complete mini protease inhibitor cocktail (Roche). Proteins were immunoprecipitated from lysates incubated overnight with an anti-flag-M2 monoclonal antibody (Sigma) and Dynabeads (Invitrogen) in lysis buffer. Immune complexes were washed three times with lysis buffer and suspended in SDS sample buffer (lysis buffer containing 3 mM Tris–HCl, pH 6.8, 2% (wt/vol) SDS, 5% (vol/vol) β-mercaptoethanol, 10% (vol/vol) glycerol and bromophenol blue). Samples were boiled for 5 min at 98°C and separated by SDS-PAGE and analyzed by immunoblot using anti-Flag, anti-Myc (Sigma), anti-NF-κB p50 (Santa Cruz H-119), anti-NF-κB p65 (Santa Cruz C-20), or anti-IκB-ζ antibodies.
Immunoblot analysis
Peritoneal exudate cells were cultured for 2 h in medium and were then stimulated with LPS for the indicated times and were lysed with lysis buffer (20 mM Tris–HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, and 1% (vol/vol) Nonidet P-40) containing complete mini protease inhibitor cocktail (Roche). Cell lysates were separated by standard SDS-PAGE and analyzed by immunoblot. Antibodies to the following proteins were used: phosphorylated Erk (Cell Signaling no. 9101), phosphorylated p38 (Cell Signaling 9211), p38 (Santa Cruz C-20), Erk (Santa Cruz K-23), IκBα (Santa Cruz C-21), phosphorylated JNK (Cell Signaling 9251), JNK (Santa Cruz C-17), and β-tubulin (Santa Cruz D-10).
Quantitative PCR analysis
Total RNA was isolated with TRIzol (Invitrogen), and reverse transcription was performed with ReverTra Ace (Toyobo) according to the manufacturer’s instructions. For quantitative PCR, cDNA fragments were amplified by real-time PCR Master Mix (Toyobo); fluorescence from the TaqMan probe for each cytokine was detected by a 7500 real-time PCR system (Applied Biosystems). To determine the relative induction of cytokine mRNA in response to various stimuli, the mRNA expression level of each gene was normalized to the expression level of 18S rRNA. All the experiments were performed in triplicate at least three times.
Flow cytometry
Antibodies for flow cytometry were purchased from BD Biosciences and Biolegend. Cells were washed in ice-cold flow cytometry buffer (0.5% (vol/vol) FCS and 2 mM EDTA in PBS, pH 7.5), then incubated with each antibody for 30 min, washed twice with flow cytometry buffer, and resuspended in an appropriate volume of flow cytometry buffer. Data were acquired on a FACS Canto II flow cytometer (BD Biosciences) and analyzed with FlowJo (Tree Star).
Restriction enzyme accessibility assay
Experiments were performed as described previously with little modifications (Ramirez-Carrozzi et al, 2006). Briefly, PECs (2 × 106 cells) were stimulated with LPS (100 ng/ml) for indicated time period, and the cell nuclei were isolated and digested with limiting amounts of restriction enzyme AflII (100 U) and were incubated for 15 min at 37°C, followed by genomic DNA isolation. Purified DNA (10–15 μg) was digested to completion to generate reference cleavage products using the following restriction enzymes: XbaI and SpeI for the Il6 promoter. Samples were analyzed by Southern blotting with 32P-labeled gene-specific probes designed at the Il6 promoter region (−1169 to +620).
Chromatin immunoprecipitation (ChIP)
ChIP was performed by using a ChIP assay kit (Millipore) as described elsewhere with little modification (Satoh et al, 2010). Briefly, PECs or BMDMs (2 × 106 cells) and HeLa cells (5 × 106) were stimulated with LPS (1 μg/ml) or recombinant hIL-1β (10 ng/ml) (R&D Systems), respectively, and were fixed with 1% formaldehyde (Nacalai Tesque, Japan) for 10 min at 37°C. Cells were then washed twice with ice-cold PBS and resuspended in SDS lysis buffer supplied with the kit. Lysates were sonicated using ultrasonicator (Covaris S2 and Bioruptor™) to obtain DNA fragments with a peak in size between 150 and 300 bp. Lysates pre-cleared with Dynabeads (Invitrogen) were incubated with antibodies against phospho-RNA Pol II (S5P), H3K9(Ac) or H3K4(Me)3 (Abcam), and Brg-1 (J1) and immunoprecipitated at 4°C overnight. The immune complexes were absorbed with beads. Beads were washed once with low salt buffer, high salt buffer, LiCl wash buffer, and twice with TE buffer. Immune complexes were extracted with elution buffer (50 mM Tris–HCl, pH-8.0; 10 mM EDTA; 1% SDS), and cross links were reversed by incubating overnight at 65°C. After RNAse treatment for 2 h at 37°C followed by proteinase K treatment for 2 h at 56°C, DNA was then purified via phenol-chloroform precipitation. The purified DNA was quantified and then used for qPCR analysis to assess the presence of target sequences. Quantitative RT-PCR was performed with SYBR green qPCR mix (Toyobo) in an Applied Biosystem 7500. Primers used for amplifying Il6, KC (Cxcl1), Tnf, Il12b, and IL8 loci were shown previously (Coles et al, 2010; Negishi et al, 2012; Ramirez-Carrozzi et al, 2006). ChIP values were normalized against the input and expressed as relative enrichment of the material precipitated by the indicated antibody on Il6 promoter (relative quantification using the comparative Ct method (2–ΔΔCt)). Error bars indicate mean ± s.d. The results are representative of at least three independent experiments.
Construction of expression plasmids
Akirin2, IκBζ, BAF60a, BAF60b, and BAF60c cDNA were obtained by PCR from a mouse cDNA library. Full-length or truncated Akirin2 cDNAs were cloned into the pcDNA-Flag or Myc tag vectors for overexpression or pMRX-Puro vector for retrovirus production (Matsushita et al, 2009). Full-length BAF60a, BAF60b, and BAF60c cDNAs were cloned into pcDNA3.1 (+) Myc tag vectors, and full-length or truncated IκBζ cDNAs were cloned into the Flag-tagged pEF-BOS vector for overexpression or pMRX-Puro vector for retrovirus production.
Retroviral transduction of Mouse Embryonic Fibroblasts (MEFs)
Akirin2−/− MEFs were established as described (Goto et al, 2008). Briefly, MEFs were isolated from Akirin2fl/fl mice at embryonic day 13.5. Cells were cultured in DMEM (supplemented with 10% (vol/vol) FCS, 10 mM sodium pyruvate, 2 μM L-glutamine, 50 μM 2-mercaptoethanol, 100 U/ml penicillin, 100 μg/ml streptomycin). Then, 48 h later, these cells were transduced with retroviral supernatant (supplemented with 10 ng/ml of polybrene) for 12 h. Virus was produced by PlatE packaging cells transfected with pMRX-Puro vector stably expressing Cre and/or full-length Akirin2 or deletion mutants. After incubation with retroviral supernatant (supplemented with polybrene), the cells were cultured in DMEM (supplemented with 10% (vol/vol) FCS, 10 mM sodium pyruvate, 2 μM L-glutamine, 50 μM 2-mercaptoethanol, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 μg/ml of puromycin). After 48 h in culture, cells were washed once with ice-cold PBS and the cells were harvested and used for further experiments.
Retroviral transduction of macrophages
Highly efficient retroviral transduction of macrophages was achieved by transduction of hematopoietic stem cells before differentiation into macrophages, as described previously (Satoh et al, 2010). BM was isolated from LysM-Cre+; Akirin2fl/+, LysM-Cre+; Akirin2fl/fl, NF-κBp50−/−, and IκBζ−/− deficient mice that had been injected intraperitoneally 4 days earlier with 5 mg of 5-fluorouracil (Nacalai tesque). Cells were cultured in stem cell media (DMEM supplemented with 15% FCS, 10 mM sodium pyruvate, 2 mM L-glutamine, 50 μM β-mercaptoethanol, 100 U/ml penicillin, 100 μg/ml streptomycin, 100 ng/ml stem cell factor, 10 ng/ml IL-6, and 10 ng/ml IL-3). Then, 48 h later, these cells were transduced with retroviral supernatant (supplemented with stem cell factor, IL-6, IL-3, and 10 ng/ml of polybrene) on two successive days. Virus was produced using PlatE. After the second transduction, cells were washed and resuspended in macrophage growth media (RPMI 1640 medium supplemented with 10% FCS, 10 mM HEPES, 10 mM sodium pyruvate, 2 mM L-glutamine, 50 μM β-mercaptoethanol, 100 U/ml penicillin, 100 μg/ml streptomycin, and 40 ng/ml macrophage colony-stimulating factor). The cells were cultivated for 7 days and were subjected to analysis.
Microarray analysis
Thioglycollate-elicited peritoneal macrophages were stimulated with LPS (100 ng/ml) for different time periods. Then, total RNA was extracted with TRIzol (Invitrogen Life Technologies) and further purified using an RNeasy kit (Qiagen). Biotin-labeled cDNA was synthesized from 100 ng of total RNA using the Ovation Biotin RNA amplification and labeling system (Nugen) according to the manufacturer’s protocol. Hybridization, staining, washing, and scanning of Affymetrix mouse Genome 430 2.0 microarray chips were conducted according to the manufacturer’s instructions. Data analysis was performed using R (http://www.r-project.org/). The data were normalized by robust multichip analysis (RMA) and log2-transformed expression values are shown. Genes with differential expression between control and Akirin2-deficient macrophages were clustered based on the similarity in the differences in microarray probe intensities between control and Akirin2-deficient macrophages for the time points 0, 2, and 4 h after stimulation. Clustering was done using hierarchical clustering using Ward’s method. After visual inspection, genes were divided into 8 clusters. MicroArray data are deposited in GEO (accession number GSE59319).
Analysis of CpG islands associated with Akirin2-dependent and Akirin2-independent genes
For Akirin2-dependent and Akirin2-independent genes, the GC content and CpG score of the genomic regions −100 to −1 and +1 to +100 relative to the transcription start sites were calculated. CpG island predictions for the mouse genome were obtained from the UCSC Genome Browser Database (Meyer et al, 2013). For the Akirin2-dependent and Akirin2-independent sets of genes, the number of genes with predicted CpG islands in the regions −1 kb to +1 kb and −10 kb to +10 kb relative to the transcription start site was counted. P-values for the difference in CpG island-associations between the Akirin2-dependent and Akirin2-independent sets were estimated using the Z-test for proportions, based on the predicted CpG islands in the region −1 kb to +1 kb. All analyses were based on the Refseq gene annotations for version mm10 (Dec. 2011, GRCm38) of the mouse genome.
RNA interference
siRNAs targeting human AKIRIN2, BAF60a, BAF60b, and BAF60c were designed and purchased from the Ambion (Supplementary Table S2). siRNAs targeting human BRG1 were designed and purchased from Dharmacon (Supplementary Table S2). HeLa cells were transfected with siRNA using Lipofectamine RNAiMax following the manufacturer’s instructions with little modifications. A retroviral vector containing a small hairpin RNA (shBAF60a, shBAF60b, shBAF60c, and shBrg1) was obtained by cloning the randomly designed annealed primers into BglII and XhoI sites of the pSUPER-retro-Puro (Oligoengine) following the manufacturer’s protocol. J774 cells were transfected with shRNA using FuGENE transfection reagent according to the manufacturer’s instructions with little modifications.
Statistical analysis
The statistical significance of differences was determined by the two-tailed Student’s t-test. Differences with a P-value of less than 0.05 were considered statistically significant.
Acknowledgments
We thank all the colleagues in our laboratory for helpful discussion and suggestions; E. Kamada, M. Kageyama, and Y. Asahira for secretarial assistance; and Y. Fujiwara and M. Kumagai for technical assistance. We thank Dr. Tatsushi Muta (Tohoku University) and Dr. Weidong Wang (NIH) for kindly providing anti-IκB-ζ and anti-Brg1 (J1) antibody, respectively. We also thank Dr. Masao Mitsuyama (Kyoto University) for kindly providing Listeria monocytogenes. NF-κBp50−/− mice were a kind gift from Dr. Eijiro Jimi (Kyushu Dental University). This work was supported in part by the Japan Society for the Promotion of Science (JSPS) through the Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program), and by JSPS Core-to-Core Program, A. Advanced Research Networks. This work was also supported in part by grants from Takeda Science Foundation, Naito Foundation, and Mochida Foundation. S.T. is supported by a research fellowship from the MEXT for the Promotion of Science for Young Scientists. J.-M.R. was supported by Centre National de la Recherche Scientifique, grants from Agence Nationale de la Recherche (LABEX: ANR-10-LABX-0036_NETRNA) and the European Research Council (Immunodroso2009-AdG-20090506).
Author contributions
ST performed most of the experiments. ST and OT designed experiments, analyzed the data, and wrote the manuscript. AV performed bioinformatics analysis. DMS performed structural modeling. KM helped with the experiments. DO, TI, and TM participated in discussions. JMR and JAH shared information on Drosophila and mammalian Akirin. SA and OT supervised the project.
Conflict of interest
The authors declare that they have no conflict of interest.
Note added in issue proof
Bonnay et al, published in this issue of The EMBO Journal, independently came to the conclusion that in the Drosophila innate immune response the conserved fly Akirin recruits the Osa-containing-SWI/SNF Brahma complex (BAP) to orchestrate NF-κB transcriptional selectivity.
Bonnay F, Nguyen XH, Cohen-Berros E, Troxler L, Batsche E, Camonis J, Takeuchi O, Reichhart JM, Matt N (2014) Akirin specifies NF-κB selectivity of Drosophila innate immune response via chromatin remodeling. EMBO J 33: 2349–2362.
Supporting Information
Supplementary information for this article is available online: http://emboj.embopress.org
References
- Agalioti T, Lomvardas S, Parekh B, Yie J, Maniatis T, Thanos D. Ordered recruitment of chromatin modifying and general transcription factors to the IFN-beta promoter. Cell. 2000;103:667–678. doi: 10.1016/s0092-8674(00)00169-0. [DOI] [PubMed] [Google Scholar]
- Beutler B. Microbe sensing, positive feedback loops, and the pathogenesis of inflammatory diseases. Immunol Rev. 2009;227:248–263. doi: 10.1111/j.1600-065X.2008.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt DM, Pandya-Jones A, Tong AJ, Barozzi I, Lissner MM, Natoli G, Black DL, Smale ST. Transcript dynamics of proinflammatory genes revealed by sequence analysis of subcellular RNA fractions. Cell. 2012;150:279–290. doi: 10.1016/j.cell.2012.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns BR. The logic of chromatin architecture and remodelling at promoters. Nature. 2009;461:193–198. doi: 10.1038/nature08450. [DOI] [PubMed] [Google Scholar]
- Chen J, Ivashkiv LB. IFN-gamma abrogates endotoxin tolerance by facilitating Toll-like receptor-induced chromatin remodeling. Proc Natl Acad Sci USA. 2010;107:19438–19443. doi: 10.1073/pnas.1007816107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi T, Yan Z, Xue Y, Wang W. Purification and functional analysis of the mammalian SWI/SNF-family of chromatin-remodeling complexes. Methods Enzymol. 2004;377:299–316. doi: 10.1016/S0076-6879(03)77018-9. [DOI] [PubMed] [Google Scholar]
- Clapier CR, Cairns BR. The biology of chromatin remodeling complexes. Annu Rev Biochem. 2009;78:273–304. doi: 10.1146/annurev.biochem.77.062706.153223. [DOI] [PubMed] [Google Scholar]
- Coles AH, Gannon H, Cerny A, Kurt-Jones E, Jones SN. Inhibitor of growth-4 promotes IkappaB promoter activation to suppress NF-kappaB signaling and innate immunity. Proc Natl Acad Sci USA. 2010;107:11423–11428. doi: 10.1073/pnas.0912116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debril MB, Gelman L, Fayard E, Annicotte JS, Rocchi S, Auwerx J. Transcription factors and nuclear receptors interact with the SWI/SNF complex through the BAF60c subunit. J Biol Chem. 2004;279:16677–16686. doi: 10.1074/jbc.M312288200. [DOI] [PubMed] [Google Scholar]
- Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- Forcales SV, Albini S, Giordani L, Malecova B, Cignolo L, Chernov A, Coutinho P, Saccone V, Consalvi S, Williams R, Wang K, Wu Z, Baranovskaya S, Miller A, Dilworth FJ, Puri PL. Signal-dependent incorporation of MyoD-BAF60c into Brg1-based SWI/SNF chromatin-remodelling complex. EMBO J. 2012;31:301–316. doi: 10.1038/emboj.2011.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giot L, Bader JS, Brouwer C, Chaudhuri A, Kuang B, Li Y, Hao YL, Ooi CE, Godwin B, Vitols E, Vijayadamodar G, Pochart P, Machineni H, Welsh M, Kong Y, Zerhusen B, Malcolm R, Varrone Z, Collis A, Minto M, et al. A protein interaction map of Drosophila melanogaster. Science. 2003;302:1727–1736. doi: 10.1126/science.1090289. [DOI] [PubMed] [Google Scholar]
- Goto A, Matsushita K, Gesellchen V, El Chamy L, Kuttenkeuler D, Takeuchi O, Hoffmann JA, Akira S, Boutros M, Reichhart JM. Akirins are highly conserved nuclear proteins required for NF-kappaB-dependent gene expression in drosophila and mice. Nat Immunol. 2008;9:97–104. doi: 10.1038/ni1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6:644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- Hsiao PW, Fryer CJ, Trotter KW, Wang W, Archer TK. BAF60a mediates critical interactions between nuclear receptors and the BRG1 chromatin-remodeling complex for transactivation. Mol Cell Biol. 2003;23:6210–6220. doi: 10.1128/MCB.23.17.6210-6220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayama H, Ramirez-Carrozzi VR, Yamamoto M, Mizutani T, Kuwata H, Iba H, Matsumoto M, Honda K, Smale ST, Takeda K. Class-specific regulation of pro-inflammatory genes by MyD88 pathways and IkappaBzeta. J Biol Chem. 2008;283:12468–12477. doi: 10.1074/jbc.M709965200. [DOI] [PubMed] [Google Scholar]
- Lickert H, Takeuchi JK, Von Both I, Walls JR, McAuliffe F, Adamson SL, Henkelman RM, Wrana JL, Rossant J, Bruneau BG. Baf60c is essential for function of BAF chromatin remodelling complexes in heart development. Nature. 2004;432:107–112. doi: 10.1038/nature03071. [DOI] [PubMed] [Google Scholar]
- Lores P, Visvikis O, Luna R, Lemichez E, Gacon G. The SWI/SNF protein BAF60b is ubiquitinated through a signalling process involving Rac GTPase and the RING finger protein Unkempt. FEBS J. 2010;277:1453–1464. doi: 10.1111/j.1742-4658.2010.07575.x. [DOI] [PubMed] [Google Scholar]
- Macqueen DJ, Johnston IA. Evolution of the multifaceted eukaryotic akirin gene family. BMC Evol Biol. 2009;9:34. doi: 10.1186/1471-2148-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall A, Salerno MS, Thomas M, Davies T, Berry C, Dyer K, Bracegirdle J, Watson T, Dziadek M, Kambadur R, Bower R, Sharma M. Mighty is a novel promyogenic factor in skeletal myogenesis. Exp Cell Res. 2008;314:1013–1029. doi: 10.1016/j.yexcr.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Matsushita K, Takeuchi O, Standley DM, Kumagai Y, Kawagoe T, Miyake T, Satoh T, Kato H, Tsujimura T, Nakamura H, Akira S. Zc3h12a is an RNase essential for controlling immune responses by regulating mRNA decay. Nature. 2009;458:1185–1190. doi: 10.1038/nature07924. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol. 2009;9:692–703. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- Meyer LR, Zweig AS, Hinrichs AS, Karolchik D, Kuhn RM, Wong M, Sloan CA, Rosenbloom KR, Roe G, Rhead B, Raney BJ, Pohl A, Malladi VS, Li CH, Lee BT, Learned K, Kirkup V, Hsu F, Heitner S, Harte RA, et al. The UCSC Genome Browser database: extensions and updates 2013. Nucleic Acids Res. 2013;41:D64–D69. doi: 10.1093/nar/gks1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake T, Satoh T, Kato H, Matsushita K, Kumagai Y, Vandenbon A, Tani T, Muta T, Akira S, Takeuchi O. IkappaBzeta is essential for natural killer cell activation in response to IL-12 and IL-18. Proc Natl Acad Sci USA. 2010;107:17680–17685. doi: 10.1073/pnas.1012977107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller A, Avila FW, Erickson JW, Jackle H. Drosophila BAP60 is an essential component of the Brahma complex, required for gene activation and repression. J Mol Biol. 2005;352:329–337. doi: 10.1016/j.jmb.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Motoyama M, Yamazaki S, Eto-Kimura A, Takeshige K, Muta T. Positive and negative regulation of nuclear factor-kappaB-mediated transcription by IkappaB-zeta, an inducible nuclear protein. J Biol Chem. 2005;280:7444–7451. doi: 10.1074/jbc.M412738200. [DOI] [PubMed] [Google Scholar]
- Negishi H, Yanai H, Nakajima A, Koshiba R, Atarashi K, Matsuda A, Matsuki K, Miki S, Doi T, Aderem A, Nishio J, Smale ST, Honda K, Taniguchi T. Cross-interference of RLR and TLR signaling pathways modulates antibacterial T cell responses. Nat Immunol. 2012;13:659–666. doi: 10.1038/ni.2307. [DOI] [PubMed] [Google Scholar]
- Nowak SJ, Aihara H, Gonzalez K, Nibu Y, Baylies MK. Akirin links twist-regulated transcription with the Brahma chromatin remodeling complex during embryogenesis. PLoS Genet. 2012;8:e1002547. doi: 10.1371/journal.pgen.1002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Iwai Y, Oh-Hora M, Yamamoto M, Morio T, Aoki K, Ohya K, Jetten AM, Akira S, Muta T, Takayanagi H. IkappaBzeta regulates T(H)17 development by cooperating with ROR nuclear receptors. Nature. 2010;464:1381–1385. doi: 10.1038/nature08922. [DOI] [PubMed] [Google Scholar]
- Okuma A, Hoshino K, Ohba T, Fukushi S, Aiba S, Akira S, Ono M, Kaisho T, Muta T. Enhanced Apoptosis by Disruption of the STAT3-IkappaB-zeta Signaling Pathway in Epithelial Cells Induces Sjogren’s Syndrome-like Autoimmune Disease. Immunity. 2013;38:450–460. doi: 10.1016/j.immuni.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Puri PL, Mercola M. BAF60 A, B, and Cs of muscle determination and renewal. Genes Dev. 2012;26:2673–2683. doi: 10.1101/gad.207415.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Carrozzi VR, Nazarian AA, Li CC, Gore SL, Sridharan R, Imbalzano AN, Smale ST. Selective and antagonistic functions of SWI/SNF and Mi-2beta nucleosome remodeling complexes during an inflammatory response. Genes Dev. 2006;20:282–296. doi: 10.1101/gad.1383206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno MS, Dyer K, Bracegirdle J, Platt L, Thomas M, Siriett V, Kambadur R, Sharma M. Akirin1 (Mighty), a novel promyogenic factor regulates muscle regeneration and cell chemotaxis. Exp Cell Res. 2009;315:2012–2021. doi: 10.1016/j.yexcr.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Satoh T, Takeuchi O, Vandenbon A, Yasuda K, Tanaka Y, Kumagai Y, Miyake T, Matsushita K, Okazaki T, Saitoh T, Honma K, Matsuyama T, Yui K, Tsujimura T, Standley DM, Nakanishi K, Nakai K, Akira S. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol. 2010;11:936–944. doi: 10.1038/ni.1920. [DOI] [PubMed] [Google Scholar]
- Sha WC, Liou HC, Tuomanen EI, Baltimore D. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi JK, Bruneau BG. Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature. 2009;459:708–711. doi: 10.1038/nature08039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wong RH, Tang T, Hudak CS, Yang D, Duncan RE, Sul HS. Phosphorylation and recruitment of BAF60c in chromatin remodeling for lipogenesis in response to insulin. Mol Cell. 2013;49:283–297. doi: 10.1016/j.molcel.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann AS, Mitchell DM, Sanjabi S, Bradley MN, Hoffmann A, Liou HC, Smale ST. Nucleosome remodeling at the IL-12 p40 promoter is a TLR-dependent, Rel-independent event. Nat Immunol. 2001;2:51–57. doi: 10.1038/83168. [DOI] [PubMed] [Google Scholar]
- Weinmann AS, Plevy SE, Smale ST. Rapid and selective remodeling of a positioned nucleosome during the induction of IL-12 p40 transcription. Immunity. 1999;11:665–675. doi: 10.1016/s1074-7613(00)80141-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Yamazaki S, Uematsu S, Sato S, Hemmi H, Hoshino K, Kaisho T, Kuwata H, Takeuchi O, Takeshige K, Saitoh T, Yamaoka S, Yamamoto N, Yamamoto S, Muta T, Takeda K, Akira S. Regulation of Toll/IL-1-receptor-mediated gene expression by the inducible nuclear protein IkappaBzeta. Nature. 2004;430:218–222. doi: 10.1038/nature02738. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Muta T, Takeshige K. A novel IkappaB protein, IkappaB-zeta, induced by proinflammatory stimuli, negatively regulates nuclear factor-kappaB in the nuclei. J Biol Chem. 2001;276:27657–27662. doi: 10.1074/jbc.M103426200. [DOI] [PubMed] [Google Scholar]
- Yan Z, Cui K, Murray DM, Ling C, Xue Y, Gerstein A, Parsons R, Zhao K, Wang W. PBAF chromatin-remodeling complex requires a novel specificity subunit, BAF200, to regulate expression of selective interferon-responsive genes. Genes Dev. 2005;19:1662–1667. doi: 10.1101/gad.1323805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkovsky N, Logie C, Hahn S, Peterson CL. Recruitment of the SWI/SNF chromatin remodeling complex by transcriptional activators. Genes Dev. 1999;13:2369–2374. doi: 10.1101/gad.13.18.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Nazarian AA, Smale ST. Interleukin-10 inhibits interleukin-12 p40 gene transcription by targeting a late event in the activation pathway. Mol Cell Biol. 2004;24:2385–2396. doi: 10.1128/MCB.24.6.2385-2396.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.