Abstract
Cancer pain is a deleterious consequence of tumor growth and related inflammation. Opioids and antiinflammatory drugs provide first line treatment for cancer pain, but both are limited by side effects. Fufang Kushen injection (FKI) is GMP produced, traditional Chinese medicine used alone or with chemotherapy to reduce cancer-associated pain. FKI limited mouse sarcoma growth both in vivo and in vitro, in part, by reducing the phosphorylation of ERK and AKT kinases and BAD. FKI inhibited TRPV1 mediated capsaicin-induced ERK phosphorylation and reduced tumor-induced proinflammatory cytokine production. Thus, FKI limited cancer pain both directly by blocking TRPV1 signaling and indirectly by reducing tumor growth.
Keywords: Sarcoma, Hyperalgesia, TRPV1, ERK, Fufang Kushen injection
Introduction
Cancer pain is a frequent symptom in late stage cancer patients, but the molecular factors contributing to tumor-induced, increased sensitivity to nociceptive stimuli, known as tumor-associated hyperalgesia, remain unclear. Further, chemotherapy induces peripheral neuropathy giving rise to cancer related pain [1]. No effective way to deal with cancer related hyperalgesia has been developed [2–5]. Therefore, understanding the molecular basis for tumor-associated hyperalgesia and development of new therapies are urgently needed.
Cancer pain shares common features with neuropathic [6] and inflammatory pain [7]. Tumor invasion of tissue causes pressure or direct neuronal tissue damage resulting in neuropathic pain. We have therefore studied pain induced by injecting transplantable pleiotropic sarcoma (S-180) cells near the sciatic nerve as previously reported by Qi et al. and others [8–11]. Pain signals are transmitted by the transient receptor potential channel receptors; the vanilloid subfamily member 1 (TRPV1) is a member of this family of receptors [12]. Studies of TRPV1 deficient mice showed that this receptor plays a crucial role in responding to and conveying thermal, chemical and mechanical stimuli, which are associated with peripheral inflammation and neuropathies [12]. Deletion of this receptor results in inhibiting experimental inflammatory hyperalgesia and neurogenic inflammation pain [13]. The ERK signal pathway has recently been identified as central to pain sensitivity in inflammatory and neuropathic pain models [14–16]. A noxious stimulus or tissue injury rapidly leads to phosphorylation of ERK within a few minutes, which correlates well with the rate of development of pain hypersensitivity [17]. While pERK induction is well accepted as a marker of pain induced by thermal, chemical and mechanical stimulation, the role of TRPV1 induced pERK [18] has not been reported in a murine model of cancer-induced pain. Further, clinical pain studies [19] and rodent cancer-pain models [20,21] have shown increases in inflammatory cytokine expression (IL1β, CCL2, TNFα and IL6). In experimental pain models, inflammatory chemokines have been shown to increase sensitivity to pain and promote TRPV1 response to capsaicin [22]. The role of these cytokines in maintenance of TRPV1 and pERK expression and function needs to be evaluated.
Traditional Chinese medicines (TCM) are being employed globally to treat cancer. Fufang Kushen injection (FKI) is a TCM extracted from the roots of two herbs, Sophora flavescens and Heterosmilacis Japonica. This injectable formula is a patented (Chinese patent:ZL:97112532.5.) and standardized Good Manufacturing Process (GMP) entity. FKI is used alone or combined with conventional chemotherapy to treat lung cancer patients in China improving anticancer efficacy and reducing the side effects of chemotherapy [23,24]. Evaluation of anti-inflammatory compounds contained in Sophora flavescens and Heterosmilacis Japonica root extracts have identified several discrete alkaloids. For example, matrine and oxymatrine combined are the most abundant chemicals in FKI [23] that inhibit cytokine production, oxygen radical production and tumor cell growth in vitro [25–31]. However, they do not account for all the effects of FKI and a more complete mechanism(s) of FKI action and optimal dosing in combination with chemotherapy still needs to be determined.
Sarcomas are malignant tumors arising from connective tissue that have been shown to cause hyperalgesia due to pressure on nerves or destruction of nervous tissue by the tumor [32]. We have therefore evaluated the molecular effects of FKI in a murine model of sarcoma-induced hyperalgesia. Here, we report that FKI not only decreased the size of the tumor, but also decreased hyperalgesia induced by sarcoma as well as TRPV1 and ERK phosphorylation.
Materials and methods
Reagents
Anti-mouse antibodies to p-ERK (4370), T-ERK (9102), p-38 (4511p), p-NFkb (3033p), p-MEK (9154), T-AKT (9272), p-AKT (9275), p-BAD (4366) and GAPDH (2118) were purchased from Cell Signaling (Boston, MA). Anti-mouse antibody TRPV1 (NB100-1617) was purchased from Novus Biologicals (Littleton, CO) and anti-mouse P-TRPV1 from Abnova (PAB8499) (Walnut, CA). Cytokines were purchased from PeproTech (Rocky Hill, NJ). Chemicals, including capsaicin and matrine were obtained from Sigma/Aldrich (St. Louis, MO). FuFang Kushen injection was obtained from Shanxi Zhendong Pharmaceutical Co.Ltd (Shanxi, China).
Cell lines and proliferation assay
S-180 sarcoma cell line (ATCC, Manassas, VA) was maintained in MEM media containing 10% heat-inactivated fetal calf serum (Hyclone; Thermo) with nonessential amino acids (Gibco), 2 mM L-Glutamine and 100 U/ml penicillin and 100 µg/ ml streptomycin at 37 °C in 5% CO2 and saturated humidity. M3-9M sarcoma cells were a kind gift from Crystal Mackall, Bethesda, MD. 4T1 and Hepa1-6 were also obtained from ATCC. TRPV1-HEK cells were generated as previously described [22]. TRPV1-HEK cells were cultured in DMEM containing 10% heat-inactivated fetal calf serum with 2 mM L-Glutamine plus 100 U/ml penicillin and 100 µg/ml streptomycin at 37 °C in 5% CO2, with saturated humidity. Tumor cells were seeded at a density of 3 × 103 cells/well in a 96-well plate and subsequently incubated with FKI. After 24, 48 and 72 h of incubation, cells were pulsed with [3H]dT for 4 h (Amersham Pharmacia) at 1 µCi per well [33].
Mice
C57BL/6 mice, Swiss Webster and NIH Webster mice were provided by the Animal Production Area of the NCI (Frederick, MD). Frederick National Laboratory for Cancer Research is accredited by AAALAC International and follows the Public Health Service Policy for the Care and Use of Laboratory Animals. Animal care was provided in accordance with the procedures outlined in the “Guide for Care and Use of Laboratory Animals” (National Research Council; 1996; National Academy Press; Washington, D.C.). These studies were performed under approved protocol ASP 13–218.
Tumor-induced temperature sensitivity (hyperalgesia)
Our laboratory adapted a tumor-induced temperature sensitivity model from earlier studies [8–11]. Two million S-180 cancer cells cultured in vitro, washed and resuspended in buffered saline were inoculated into the muscular tissue of female Swiss Webster mice in the immediate vicinity of the sciatic nerve near the trochanter, immediately distal to where the posterior biceps semitendinosus branches off the common sciatic nerve. A negative control group was injected with PBS. Paw withdrawal latencies to radiant heat stimulation at 55 °C were measured before any procedure and on days 2, 4, 6, 8, 10, 12 after tumor inoculation. Treatment groups consisted of ≥10 animals. FKI was delivered i.p., in a dose range from 0 to 100 mg/ kg/mouse once or twice in 24 h; the tumor control groups received PBS i.p.
Embryonic liver cells harvest and proliferation assay
Embryonic liver cells were harvested from newborn Swiss Webster mice. Liver tissue was mechanically disrupted followed by filtration through 70 and 30 µm cell strainers. The resulting single cells were resuspended in DMEM (Life Technologies) supplemented with 10% heat-inactivated fetal calf serum, 50 uM 2-mercaptoethanol, 2 mM L-Glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin and plated at 0.5 × 105 cell/well into 96-well flat bottom plates. After 24 h of culture in 37 °C in 5% CO2 and saturated humidity increasing amounts of FKI were added to culture, after an additional 48 h the cells were subjected to 3H Thymidine uptake assay for 4 h.
Spinal cord cell harvest and in vitro stimulation
Spinal cords were harvested from Swiss Webster mice and single cells prepared as described by Berghmans et al. [34]. Individual spinal cords were mechanically disrupted followed by filtering through 70 and 30 µm cell strainers. Single cells (1 × 106/well) were then plated into 24-well plates, in neurobasal medium (Life Technologies) supplemented with 10% heat-inactivated horse serum (Sigma-Aldrich). After 30 min of incubation at 37 °C in 5% CO2 and humidified air, to eliminate the influence of mechanical stimulation, specific stimuli were added for varying amounts of time. Following stimulation, cells were subjected to Western blotting.
Western blot analysis
Cells or tissues from spinal cord were lysed in ice-cold lysis buffer (62.5 mM Tris-HC1, pH 6.8, 2% w/v SDS, 10% glycerol, 50 mM DTT), briefly sonicated, and centrifuged at 14,000×g at 4 °C for 30 min. Total protein in the supernatant was determined using the BCA Protein assay (Pierce, Thermo Scientific). Equal amounts of protein were subjected to electrophoresis on NuPAGE Bis-Tris (Invitrogen, Carlsbad, CA) in 4–12% gradient gels in MES buffer and then transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA). The membranes were blocked in buffer (TBST: 0.25 M Tris base, 1.37 M NaCl, 0.03 M KCl, and 0.1%Tween 20, pH 7.4, with 5% nonfat milk) at room temperature for 1 h. The membranes were then incubated with rabbit polyclonal antibody against p-ERK, t-ERK, p-AKT, t-AKT, p-P38, p-NFkb (1:1000; Cell Signaling), rabbit anti-TRPV1 (1:1000), rabbit anti-phospho TRPV1 (1:500) and rabbit anti-GAPDH (1:1000) overnight at 4 °C. The blots were washed three times in TBST and incubated with HRP-conjugated anti-rabbit secondary Ab (1:2000) for 1 h at room temperature. Blots were then visualized with ECL reagent (GE Healthcare, Pittsburgh, PA). All Western blot band quantification steps were performed using custom ImageJ (National Institutes of Health; http://rsb.info.nih.gov/ij/) script.
Immunohistochemistry analysis
Mouse tissues were fixed in 10% neutral buffered formalin, routinely processed, and embedded in paraffin. Tissues were sectioned at 5 µm and stained with hematoxylin and eosin for histopathological examination. For immunohistochemistry, 5 µm sections were deparaffinized into ethanol and endogenous peroxidase activity was blocked using 0.6% H2O2 in methanol. Antigen retrieval was carried out by microwaving in citrate buffer and slides were stained with biotin-conjugated anti-CD45R/B220 (BD Biosciences #553086) 1:200 for B-cell lymphocytes. Detection of CD45R signal was performed using the avidin-biotinylated enzyme complex (Vector Laboratories) with 3,3’-diaminobenzidine (Sigma) as a chromagen. Slides were coun-terstained with hematoxylin. Slides were photographed using an Olympus BX40 microscope, Q-image RGB camera using Bioquant (Nashville, TN) software to capture and achieve the images.
Multiplex cytokine quantification
Following the last hypersensitivity study, mice were anesthetized and serum samples taken. The serum was analyzed by Leidos Clinical Services Program using a Meso Scale Discovery mouse proinflammatory 7-plex kit.
Statistical methods
Statistical analysis was performed using two-sided paired Student t test comparison with control. The p values <0.05 were considered statistically significant. Linear regression, least squares and Deming comparison were used to determine the slope and correlation within treatment groups and one-way ANOVA with post tests was used to determine the probability and dosing trend (GraphPad Prism, version 6.0c; GraphPad).
Results
FKI inhibited S-180 sarcoma cell proliferation: ERK and AKT phosphorylation
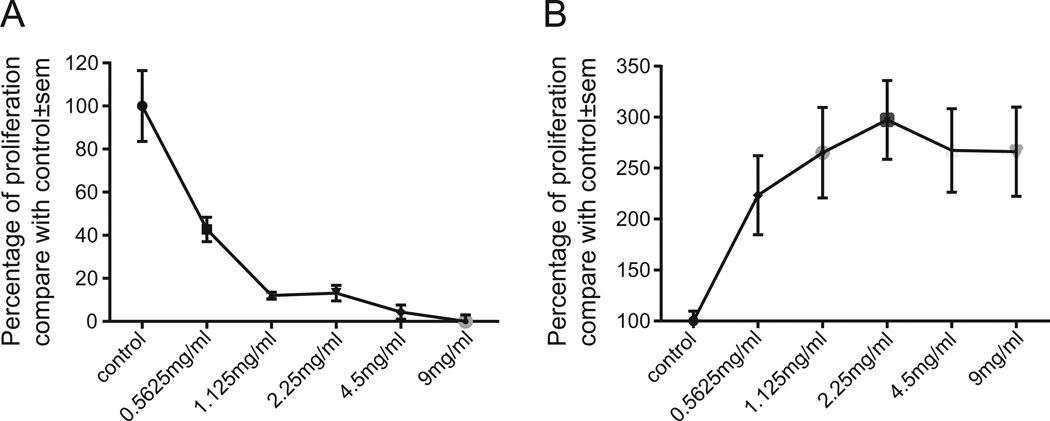
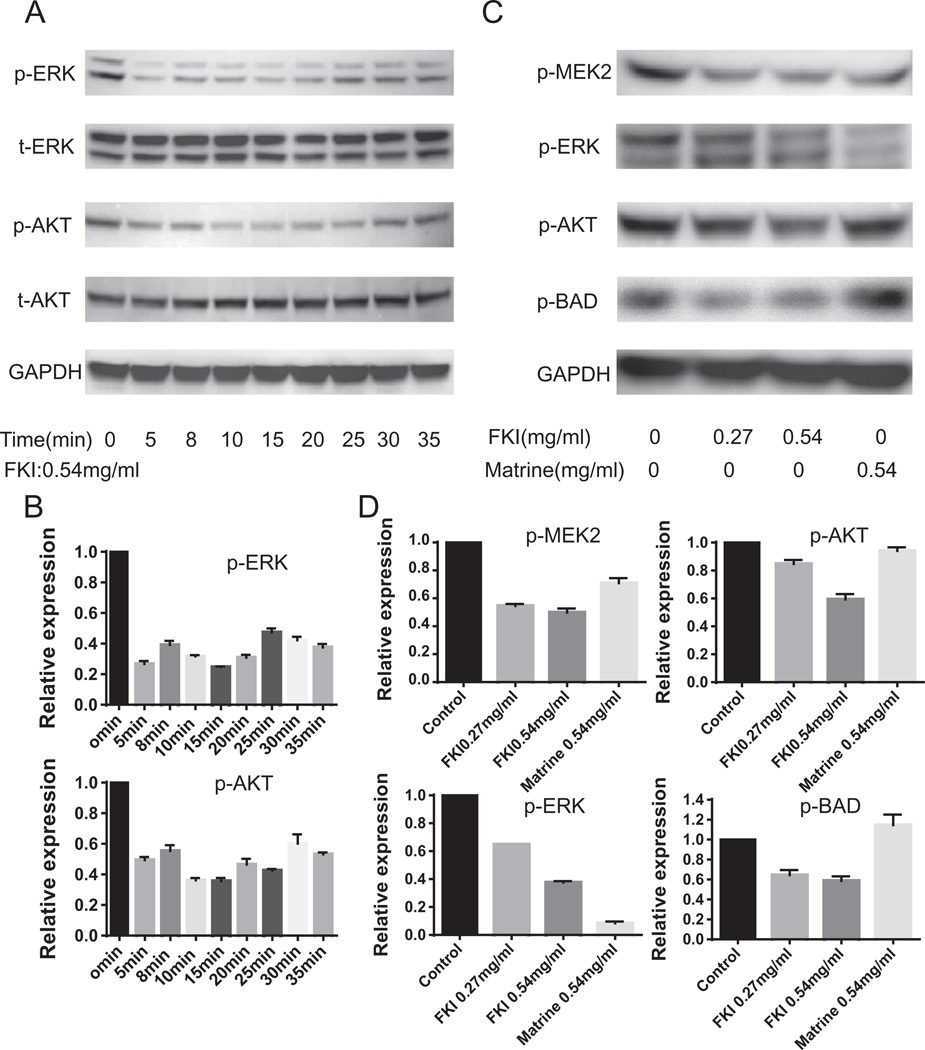
The proliferation of S-180 sarcoma cells was markedly inhibited by FKI as compared with the other cell lines (Fig. 1A and data not shown). The IC50 of FKI on S-180 cells at 48 h was 0.54 mg/ml. In contrast, the growth of embryonic liver cells was not inhibited by FKI (Fig. 1B). Further, we investigated short-term signal transduction pathways in S-180 cells activated by the IC50 dose of FKI. FKI in a time dependent manner inhibited ERK and AKT phosphorylation (Fig. 2A and 2C), but there was no effect on P38 phosphorylation (data not shown). The signal transduction pathway in tumor cells that includes ERK and AKT phosphorylation also includes BAD phosphorylation, a likely component of the pathway leading to S-180 death [35]. We therefore compared the ability of FKI and matrine, the chemically defined active metabolite of oxymatrine found in high abundance in FKI, to inhibit MEK, ERK, AKT and BAD phosphorylation. Although FKI more potently inhibited MEK2 and ERK phosphorylation, matrine also inhibited the phosphorylation of these two kinases. Unlike FKI, matrine did not inhibit AKT or BAD phosphorylation at tested concentrations. Thus FKI was more effective at inhibiting these signaling molecules than matrine (Fig. 2B and 2D).
Fig. 1.
FKI inhibited S-180 sarcoma cell proliferation. (A) FKI inhibited S-180 cells proliferation in a dose depended manner. (B) FKI promoted proliferation of newborn mouse liver cells. Relative viability of newborn mouse liver cells after incubation with increasing amounts of FKI for 48 h is shown. Cell proliferation activity was determined using tritiated thymidine uptake assay. Untreated control cells were assigned 100% and all increases or decreases are relative to the control culture. Mean and standard error of the mean are reported. N ≥ 6.
Fig. 2.
(A) Effect of FKI on ERK and AKT signaling pathway. FKI 0.54 mg/ml (48 h IC50) was used to stimulate S-180 cells for increasing amounts of time. Total (t) and phophospliorylated (p) ERK and AKT were determined by Western blotting. Both ERK and AKT phosphorylation was inhibited within 5 min of adding FKI. (B) Relative levels of ERK, and AKT signal for Fig. 2A. All Western blot bands were quantified using custom ImageJ (National Institutes of Health; http://rsb.info.nih.gov/ij/) script. The control or time zero time point was assigned a value of 1.0. (C) Comparing FKI and matrine effects on ERK/AKT and BAD signaling pathway. Western blotting was used to evaluate the amount of MEK2, ERK, AKT and BAD phosphorylation following stimulation of S-180 cells with equal concentrations of FKI or a chemical component, matrine. FKI inhibited phosphorylation of all four proteins; however, matrine only inhibited ERK phosphorylation. N = 3 representative Western result is shown. (D) Relative levels of ERK, AKT and BAD signal for Fig. 1C. All Western blot bands were quantified using custom ImageJ (National Institutes of Health; http://rsb.info.nih.gov/ij/) script. The control or time zero time point was assigned a value of 1.0.
Effect of FKI on capsaicin-induced ERK phosphorylation in TRPV1 transfected HEK cells and spinal cord cells
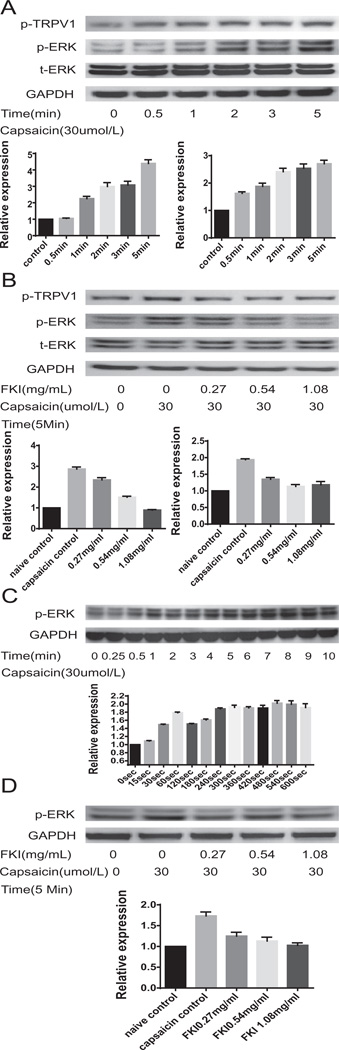
We utilized a TRPV1 transfected HEK cell line to show that capsaicin, which activates TRPV1 in a dose (0–120 umol/l) and time (0–5 min) dependent manner, induced TRPV1 and ERK phosphorylation (Fig. 3A). Capsaicin at 30 umol/1 (maximally effective dose in HEK cells see Supplementary Fig. S1) also induced ERK phosphorylation in spinal cord cells (Fig. 3C), indicating that TRPV1 is functional in such cells. We next investigated the effect of FKI on capsaicin-induced ERK phosphorylation. FKI (0.27–1.08 mg/ml) in a dose dependent manner inhibited capsaicin induced ERK phosphorylation in TRPV1-HEK cells (Fig. 3B) and also in spinal cord cells (Fig. 3D). This suggests that FKI directly inhibited TRPV1 -mediated signals associated with tumor-induced hyperalgesia.
Fig. 3.
Western blot analysis of capsaicin-induced ERK phosphorylation in TRPV1-HEK cells (A) or ex vivo spinal cord cells (C) shows FKI inhibits ERK phosphorylation. Capsaicin-induced ERK phosphorylation in TRPV1-HEK Cells in a dose dependent manner see Supplementary Fig. S1. Dose response was evaluated after 5-min stimulation. (A) Capsaicin-induced ERK phosphorylation in TRPV1-HEK cells is time dependent. Western blotting shows that ERK and TRPV1 phosphorylation increases from untreated control through 5 min. Relative expression of pTRPV1 is shown to the left below the Western blot, while relative expression of p-ERK is shown to the right. All Western blot bands were quantified using custom ImageJ (National Institutes of Health; http://rsb.info.nih.gov/ij/) script. The control or time zero time point was assigned a value of 1.0. (B) Capsaicin-induced TRPV1 and ERK phosphorylation in TRPV1-HEK cells is inhibited by FKI. Following 30 uM capsaicin stimulation for 5 min, Western blotting for phosphorylated ERK and TRPV1 shows a three-fold increase in ERK phosphorylation and a two-fold increase in TRPV1 phosphorylation. Adding FKI to stimulated cells reduces the phosphorylation to control levels. Relative expression of pTRPV1 is shown to the left below the Western blot, while relative expression of p-ERK is shown to the right. All Western blot bands were quantified using custom ImageJ (National Institutes of Health; http://rsb.info.nih.gov/ij/) script. The control or time zero time point was assigned a value of 1.0. (C) Capsaicin-induced ERK phosphorylation in ex vivo cultured spinal cord cells is time dependent. Western blotting shows that ERK phosphorylation increases from untreated control through 5 (300 s) min following 30 uM capsaicin stimulation of ex vivo cultured spinal cord cells. Relative expression levels are shown below the blot. (D) Capsaicin-induced ERK phosphorylation in cultured spinal cord cells is inhibited by FKI. Western blotting shows that ERK phosphorylation is increased following 30 uM capsaicin stimulation for 5 min of ex vivo cultured spinal cord cells. The capsaicin induced ERK phosphorylation is decreased when FKI is added to the culture. N = 3 representative Westerns shown. Relative expression levels are reported in graphs below blots.
Tumor induced inflammation and neuropathic pain
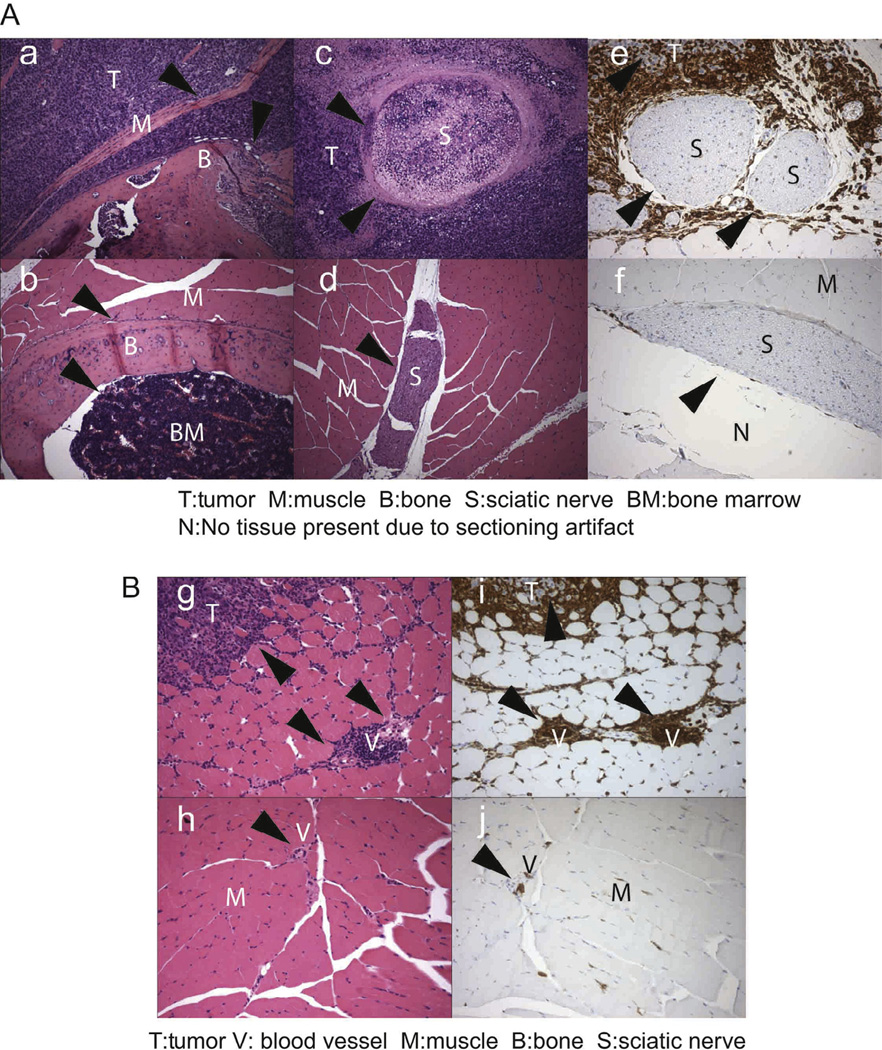
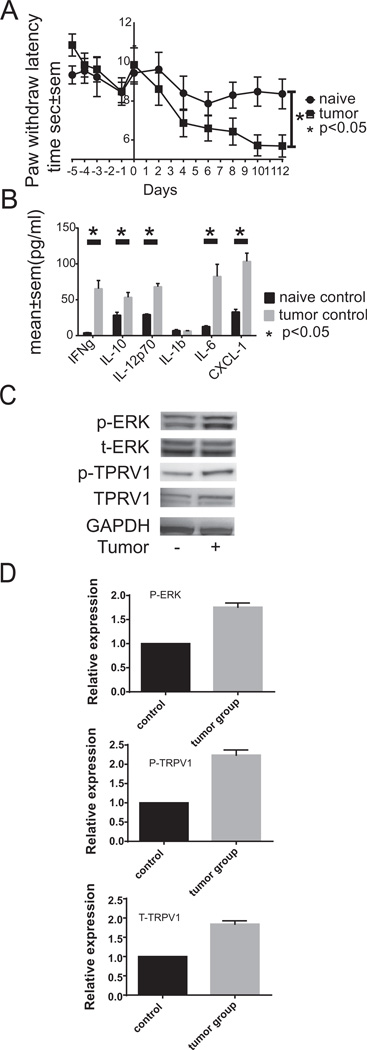
When mice were sacrificed following maximum growth of S-180 near the sciatic nerve, we observed that S-180 growth displaced muscle fibers (Fig. 4a, c, e, g and i) in tumor bearing mice as compared with naïve mice (Fig. 4b, d, f, h and j). Tumor (Fig. 4Ac versus d) and immune cells (Fig. 4Ae versus f) accumulated near the sciatic nerve of tumor bearing animals. We observed massive accumulation of CD45+ cells around the sciatic nerve as well as in the tumor (Fig. 3Ae). The nerve appears to be undergoing necrosis at the later stages of S-180 growth (Fig. 3Ac). However, we observed hyperalgesia as early as day 2 following S-180 sarcoma cell implantation near the sciatic nerve (Fig. 5A), which suggests that implanting the tumor induced the production of soluble inflammatory cytokines. We observed that IFNγ, IL-10, IL-6, IL-12p70, and CXCL1 (KC) were markedly increased in the serum of tumor bearing mice (Fig. 5B). Further, single spinal cord cells isolated from mice injected with S-180 cells showed increased pERK and TRPV1 expression (Fig. 5C). Taken together, these data suggest that the S-180 model of cancer pain maybe attributable to all three possible causes of pain; mechanical, neuropathic and inflammatory.
Fig. 4.
Histopathological analysis of S-180 tumor cells transplanted near the sciatic nerve of Swiss-Webster mice. (A) S-180 tumor cells grow around and into nervous tissue. (a) (200× HE staining) Cross section of S-180 sarcoma cells invading muscle and bone tissue. The arrows show the edge between sarcoma cells, muscle and bone. (b) (200× HE staining) A cross section of normal muscle and bone tissue. The arrows show the normal boundary of muscle, bone and bone marrow. (c) (200× HE staining) Cross section of sarcoma surrounding and invading nerve tissue. The arrows indicate the edge of tumor and nerve. (d)(200× HE staining) Section of normal muscle and nerve tissue. The arrow indicates sciatic nerve. (e) (200× IHC staining CD45) CD45+ immune cells (brown staining) surround sciatic nerve. The arrows indicate the edges of the nerve. (f) (200× IHC staining CD45) Section of control naïve sciatic nerve. The arrow indicates the sciatic nerve. N = 3 for each histopathology study. (B) S-180 tumor implantation increases CD45+ immune cell infiltration. (g)(200× HE staining): A cross section of thigh muscle and tumor cells surrounded by the immune cells in S-180 bearing mouse. The arrows indicate the location of mononuclear immune cells. (h) (200× HE staining) A cross section of naïve thigh muscle with normal vessel. The arrows indicate the location of a normal vessel, (i) (200× IHC staining) CD45+ immune cells (brown) surrounding tumor and blood vessels. The arrows mark the location of blood vessels and tumor cells (pale blue staining) surrounded by immune cells (brown), (j) (200× IHC staining) Normal vessel in naïve muscle. The arrow marks the location of a blood vessel. N = 3 for each histopathology study.
Fig. 5.
Tumor-induced hyperalgesia correlates with increased inflammatory cytokine and phosphorylated ERK and TRPV1 levels. (A) S-180 induces hypersensitivity to temperature (hyperalgesia). Paw withdrawal latency time measured in seconds was quantified over 17 days in response to 55 °C stimulation. The mean time and standard error of the mean for the tumor-free naïve mice (N = 10) is shown with circles, while that of the S-180 bearing mice is shown with squares. Deming Model II analysis of linear regression indicates that S-180 transplantation induces a significant change for the horizontal (p < 0.05). (B) S180 sarcoma induces inflammatory cytokine expression in vivo. Serum samples were subjected to Meso Scale Discovery analysis of murine inflammatory cytokines. Cytokine levels are reported as mean ± SEM in picograms/ml with the naïve control levels reported with black bars and the S-180 tumor samples being reported with gray bars. N = 5. (C) S-180 sarcoma induces ERK phosphorylation along with TRPV1 expression and phosphorylation in spinal cord cells. Spinal cords were isolated and quick frozen on day 12 following tumor implantation. Tissues were lysed and subjected to Western blotting for total ERK, phosphorylated ERK and TRPV1 protein levels. N = 3 representative Westerns shown. (D) Relative amounts of expression are reported. All Western blot bands were quantified using custom ImageJ (National Institutes of Health; http://rsb.info.nih.gov/ij/) script. The control or time zero time point was assigned a value of 1.0.
FKI inhibited tumor-induced hyperalgesia in S-180 tumor bearing mice
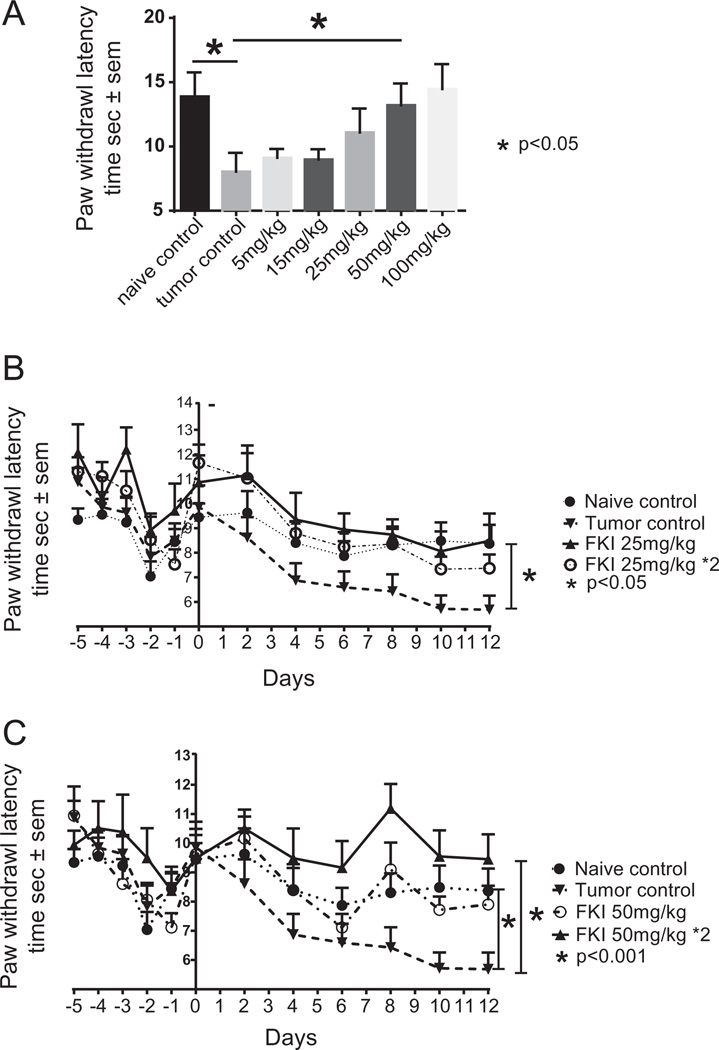
We investigated the dose and kinetics of FKI required to reduce S-180 tumor-induced pain. As shown in Fig. 6A, FKI treatment was maximally effective at or above 25 mg/kg over the 12 days of therapy in reducing tumor-induced hyperalgesia. Based on the pharmacokinetics of matrine, which showed a bioavailable half-life of 9 h [36–38], we attempted to maximize the therapeutic effect by using twice daily treatments. FKI injected twice daily appears to be more effective than once a day (Fig. 6B and C), at limiting both hyperalgesia as measured by mouse paw withdrawal and reducing tumor growth as measured by tumor weight. No significant side effects were observed by gross necropsy or behavioral changes in mice given 50 mg/kg twice daily. However, a once daily 100 mg/kg dose had a sedative effect.
Fig. 6.
FKI inhibited tumor-induced hyperalgesia in a dose dependent manner. (A) Dose response to FKI in the hyperalgesia model. Mean paw withdrawal latency time in seconds ± standard error of the mean is reported for naïve mice, untreated tumor control mice and mice receiving 25 mg/kg of FKI for 12 days. The day 12 times are reported. The 50 mg/kg dose resulted in significant increase in latency between tumor control and treatment p < 0.05. Howeverthe 100 mg/kg dose induced additional behavioral side effects. N = 10. (B) Comparison of once daily and twice daily 25 mg/ kg treatment on tumor-induced hyperalgesia. Mean paw withdrawal latency times in seconds ± standard error of the mean for naïve mice, untreated tumor control mice and mice receiving one or two 25 mg/kg treatments of FKI for 12 days are reported. N = 10. (C) Comparison of once daily and twice daily 50 mg/kg treatment on tumor-induced hyperalgesia. Mean paw withdrawal latency times in seconds ± standard error of the mean are reported for naïve mice, untreated tumor control mice and mice receiving one or two 50 mg/kg treatments of FKI for 12 days. N = 10. Deming analysis of linear regression was used to demonstrate difference from naïve control.
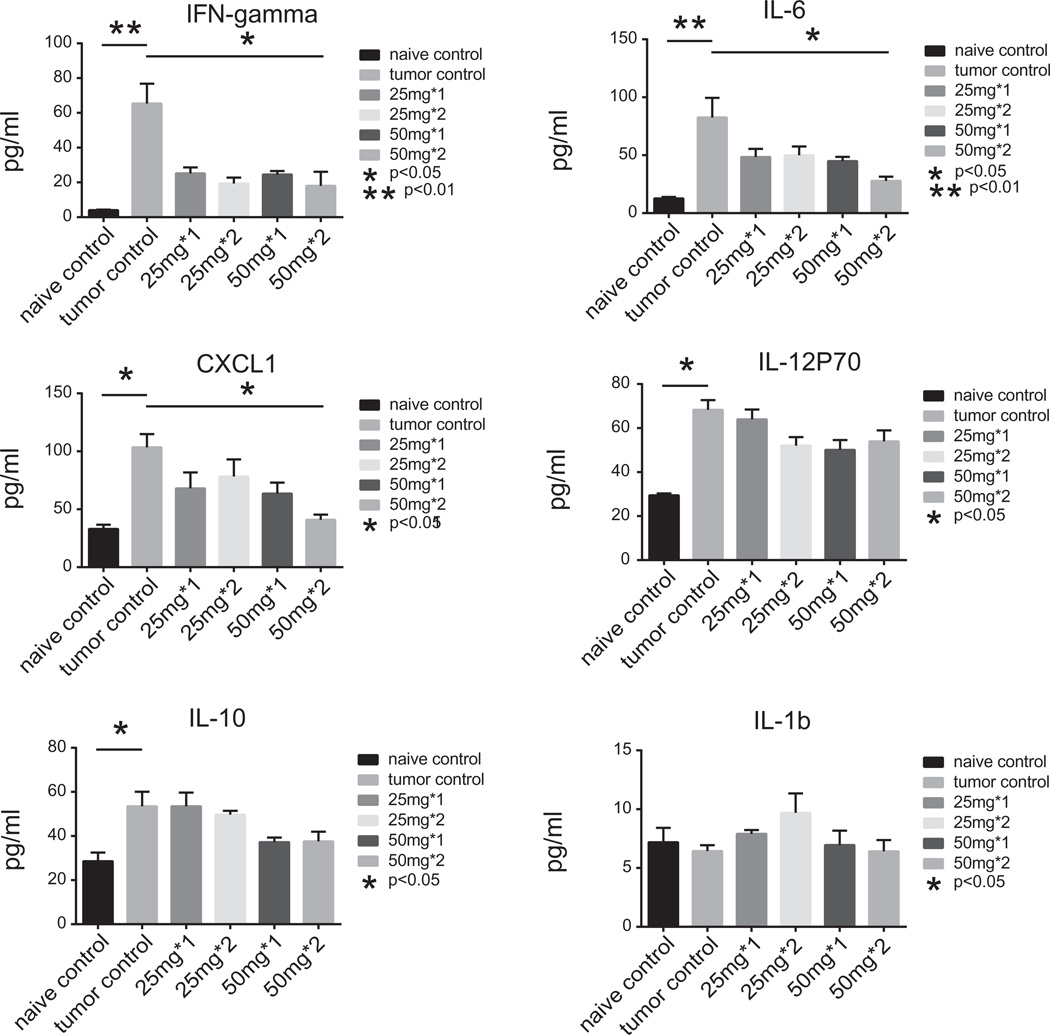
FKI inhibited inflammatory cytokine expression in vivo
In addition to the analgesic and anti-tumor effects of FKI, we evaluated the effects of FKI on tumor-induced cytokine production. The 50 mg/kg dose delivered once or twice daily markedly reduced IFN-γ, IL-6, and KC levels in the serum of tumor-bearing mice, but did not reduce IL-12p70 or IL10 levels (Fig. 7).
Fig. 7.
FKI inhibited tumor-induced inflammatory cytokine expression in vivo. Serum samples were subjected to Meso Scale Discovery analysis for murine inflammatory cytokines. Cytokine levels are reported as mean ± SEM in pg/ml. The individual cytokines or chemokines (KC also known as CXCL1) are indicated at the top of each graph. Compared on each graph are the serum cytokines levels detected from naïve, untreated tumor and those from 25 or 50 mg/kg FKI given once (l×) or twice (2×) daily for 12 days. Statistical differences are indicated between tumor control and naïve mice and tumor control and 50 mg/kg × 2 treatments. N = 5.
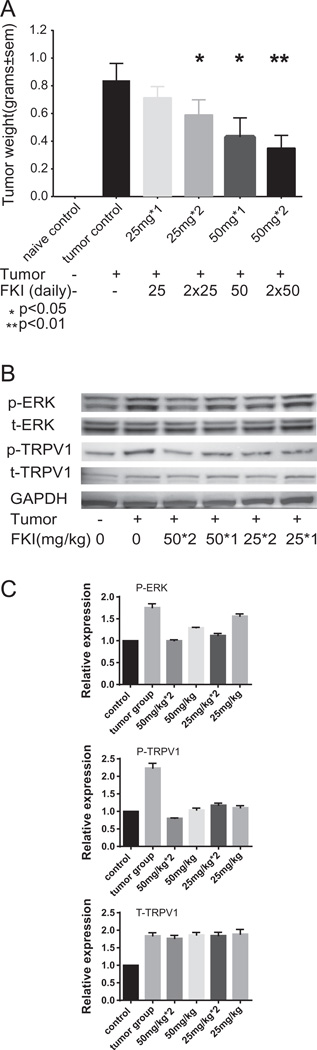
FKI inhibited tumor growth and TRPV1-ERK phosphorylation in vivo
FKI delivered intraperitoneally to S-180 bearing mice reduced the growth of tumor implanted in the thigh muscle near the sciatic nerve in a dose dependent manner (Fig. 8A). Increasing the dose to twice daily further decreased the tumor growth by at least 50% compared with PBS treated tumor bearing mice.
Fig. 8.
FKI treatment inhibited S180 tumor growth, which correlates with inhibited phosphorylation of ERK and TRPV1 in vivo. (A) FKI, in dose dependent manner, inhibited S180 tumor growth in vivo. After 12 days of in vivo treatment, mice were sacrificed. The weight of the non-tumor bearing left leg was subtracted from the weight of the tumor bearing right leg. Mean tumor weights in grams ± SEM are reported for untreated tumor control vs. once (1×) or twice (2×) daily treated at 25 mg/kg or 50 mg/kg tumors. Statistical difference is observed at 50 mg/kg treatment. N = 10 per group. (B) FKI inhibited ERK and TRPV1 Phosphorylation in vivo. Spinal cords were isolated and quick frozen at day 12 following tumor implantation. Tissues were lysed and subjected to Western blotting for total ERK, phosphorylated ERK and TRPV1 protein levels. N = 3 representative Westerns shown. (C) Relative amounts of expression are reported FKI inhibited ERK and TRPV1 Phosphorylation in vivo. All Western blot bands were quantified using custom ImageJ (National Institutes of Health; http://rsb.info.nih.gov/ij/) script. The control or time zero time point was assigned a value of 1.0. Mice treated twice daily (2×) with 50 mg/kg FKI had phosphorylated ERK and TRPV1 levels similar to tumor naïve (0) mice while untreated tumor bearing mice had ≥2-foId higher phosphorylation levels.
Western blotting demonstrated that FKI inhibited both tumor-induced TRPV1 and ERK phosphorylation in spinal cord cells. Cells were isolated following in vivo FKI treatment. In contrast to the increase in phosphorylation, total TRPV1 levels were not changed by FKI (Fig. 8B). Therefore, in the S-180 model of cancer-induced pain, FKI limited tumor growth and tumor-induced hyperalgesia based on several possible mechanisms including; limiting inflammatory cytokine production; directly reducing tumor growth and inhibiting TRPV1 mediated ERK activation.
Discussion
Late stage tumor patients can experience increased hyperalgesia which can be further enhanced by both chemotherapy and opioid treatment [5]. Therefore, we have investigated several mechanisms of action for a TCM therapy, FKI, based on its known clinical efficacy in reducing tumor growth and tumor-associated hyperalgesia. We utilized the pleotropic mouse sarcoma S-180 model, which is characterized by both neuropathic and inflammation-induced hyperalgesia (Figs. 4 and 5B), for our studies. The S-180 tumor, like many tumors, exhibits elevated levels of phosphorylated ERK, AKT and BAD (Fig. 2), which are often induced by growth factor-dependent signals [39]. The activation of the ERK signaling pathway has recently also been associated with neuropathic pain through activation of TRPV-1 and other ion channel receptor signaling cascades [40,41]. Here we show that ERK is phosphorylated in spinal cord cells following tumor implantation, and FKI reduces both this phosphorylation and tumor-induced hyperalgesia (Figs. 6 and 8B).
Further, FKI inhibits inflammatory cytokine production in mice. To further evaluate the mechanisms of action for FKI, we demonstrated a direct effect of FKI on tumor cell growth and TRPV1-mediated signal transduction. FKI also inhibited capsaicin-TRPV1-induced ERK phosphorylation in both TRPV1 transfected HEK cells and cultured spinal cord cells. Thus, FKI inhibited both tumor growth and tumor-induced hyperalgesia in part by inhibiting tumor-induced inflammation and the TRPV1-ERK pathway.
Anti-inflammatory and cytotoxic components of Sophora flavescens and Heterosmilacis Japonica have previously been identified [25,26,28,30,42–44]. The inhibitory concentrations for these individual compounds depends on the cell type being targeted, but tend to require greater than 1.2 mg/ml, which is at least twice the concentration of FKI required to limit S-180 cell growth in vitro. This observation suggests that chemical mixtures from plant extracts have an advantage over single agent in therapies.
Quinolizidine alkaloids, like matrine, appear to inhibit tumor cell growth by driving apoptosis through mitochondrial dependent signal cascades [45]. However, in our studies matrine at equal dosing was not as effective as FKI at inhibiting S-180 growth or signal cascades including pBAD, suggesting that the formulation of FKI results in an enhanced response over the effect of individual chemicals. Recently, Guenther et al. reported that combining inhibitors of PI3K/ Akt/mTor and Ras/MEK/ERK pathways synergistically induced apoptosis in sarcoma cell lines [46]. They observed reduced phosphorylation of both Akt and ERK when cell lines were given U0126, a MEK inhibitor, and PI103 a PI3K/mTor inhibitor. This observation is similar to our observation that FKI inhibits ERK and Akt phosphorylation (Fig. 2) and we have extended this in vitro observation with the in vivo observation that FKI treatment reduced tumor growth (Fig. 8).
While the potent effects of FKI in clinical settings are beginning to undergo quantitative statistical analysis [47], the quality and reproducibility of FKI preparations remains in question. These are common concerns with many traditional Chinese medicinals. China’s State Food and Drug Administration (SFDA) is currently revising the quality control standards for FKI. The new standards include testing the quality of the plant roots grown under good agricultural practice for the presence and concentration of reference chemicals. Furthermore the final good manufacturing practice product is tested for pyrogenic activity, quantification of reference components by MS-HPLC that are further compared with a reference standard [48], These added quality control standards should reduce concerns related to safety and reproducibility for any pharmaceutical and thereby broaden the clinical use of FKI as a cancer therapy.
Chemotherapies targeting mTOR, e.g., rapamycin, sirolimus, ridaforolimus, are used to treat sarcomas and have been reported to enhance neuronal excitability resulting in hyperalgesia when delivered systematically [49]. However, when delivered by intrathecal injection to the spine these same chemotherapies inhibit neuropathic pain [50]. Melemedjian et al. [50] report that mTOR inhibitors activate ERK pathways leading to spontaneous pain, however, combining rapamycin treatment with metformin, which blocks metabolic disease in part by blocking the S6 kinase cascade, blocked ERK phosphorylation and development of spontaneous pain. Sorafenib is a chemotherapy broadly used to treat sarcomas [49] that appears to act in part by inhibiting ERK phosphorylation [51]. Taken together, these studies suggest that our future studies should evaluate the effect of FKI on rapamycin and sorafenib mediated anti-cancer effects considering the chemotherapy delivery site, possible synergistic effects and overall effects on cancer-associated pain.
Extensive investigation into the cause and elevation of cancer pain has identified several partially effective therapies, each with their own limitations. Pharmaceutical inhibitors of TRPV1 resulted in hyperthermia [52–54]. Opioids by cross talking with both the TRPV1 signal cascade and cytokine or chemokine receptors signal cascades reduce pain sensation at the cost of immune competence [55]. Thus FKI represents a novel, complementary therapy for the control of tumor growth and tumor associated pain.
Supplementary Material
Acknowledgments
Funding
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and in part supported by grants No. 2006DFA31700 from the Ministry of Science and Technology of China and No. 30600839, No. 30672764 from National Natural Science Foundation of China.
Appendix: Supplementary material
Supplementary data to this article can be found online at doi:10.1016/j.canlet.2014.08.037.
Footnotes
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government.
Conflict of interest
None.
References
- 1.Argyriou AA, Kyritsis AP, Makatsoris T, Kalofonos HP. Chemotherapy-induced peripheral neuropathy in adults: a comprehensive update of the literature. Cancer Manag. Res. 2014;6:135–147. doi: 10.2147/CMAR.S44261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Modesto-Lowe V, Girard L, Chaplin M. Cancer pain in the opioid-addicted patient: can we treat it right? J. Opioid Manag. 2012;8:167–175. doi: 10.5055/jom.2012.0113. [DOI] [PubMed] [Google Scholar]
- 3.Ahlbeck K. Opioids: a two-faced Janus. Curr. Med. Res. Opin. 2011;27:439–448. doi: 10.1185/03007995.2010.545379. [DOI] [PubMed] [Google Scholar]
- 4.Brant JM. The global experience of cancer pain. Asian Pac. J. Cancer Prev. 2010;11(Suppl. 1):7–12. [PubMed] [Google Scholar]
- 5.Thapa D, Rastogi V, Ahuja V. Cancer pain management-current status. J. Anaesthesiol. Clin. Pharmacol. 2011;27:162–168. doi: 10.4103/0970-9185.81820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gradl G, Gaida S, Gierer P, Mittlmeier T, Vollmar B. In vivo evidence for apoptosis, but not inflammation in the hindlimb muscle of neuropathic rats. Pain. 2004;112:121–130. doi: 10.1016/j.pain.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Liou JT, Liu FC, Mao CC, Lai YS, Day YJ. Inflammation confers dual effects on nociceptive processing in chronic neuropathic pain model. Anesthesiology. 2011;114:660–672. doi: 10.1097/ALN.0b013e31820b8b1e. [DOI] [PubMed] [Google Scholar]
- 8.Qi J, Buzas K, Fan H, Cohen JI, Wang K, Mont E, et al. Painful pathways induced by TLR stimulation of dorsal root ganglion neurons. J. Immunol. 2011;186:6417–6426. doi: 10.4049/jimmunol.1001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee HJ, Lee JH, Lee EO, Lee HJ, Kim KH, Lee KS, et al. Substance P and beta endorphin mediate electroacupuncture induced analgesic activity in mouse cancer pain model. Acupunct. Electrother Res. 2009;34:27–40. doi: 10.3727/036012909803861095. [DOI] [PubMed] [Google Scholar]
- 10.Shimoyama M, Tanaka K, Hasue F, Shimoyama N. A mouse model of neuropathic cancer pain. Pain. 2002;99:167–174. doi: 10.1016/s0304-3959(02)00073-8. [DOI] [PubMed] [Google Scholar]
- 11.Asai H, Ozaki N, Shinoda M, Nagamine K, Tohnai I, Ueda M, et al. Heat and mechanical hyperalgesia in mice model of cancer pain. Pain. 2005;117:19–29. doi: 10.1016/j.pain.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 13.Karai L, Brown DC, Mannes AJ, Connelly ST, Brown J, Gandal M, et al. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J. Clin. Invest. 2004;113:1344–1352. doi: 10.1172/JCI20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Q, Garraway SM, Weyerbacher AR, Shin SJ, Inturrisi CE. Activation of the neuronal extracellular signal-regulated kinase 2 in the spinal cord dorsal horn is required for complete Freund’s adjuvant-induced pain hypersensitivity. J. Neurosci. 2008;28:14087–14096. doi: 10.1523/JNEUROSCI.2406-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo SW, Liu MG, Long YL, Ren LY, Lu ZM, Yu HY, et al. Region- or state-related differences in expression and activation of extracellular signal-regulated kinases (ERKs) in naive and pain-experiencing rats. BMC Neurosci. 2007;8:53. doi: 10.1186/1471-2202-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat. Neurosci. 1999;2:1114–1119. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- 17.Gao YJ, R Ji R. c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J. 2009;2:11–17. doi: 10.2174/1876386300902010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han Y, Li Y, Xiao X, Liu J, Meng XL, Liu FY, et al. Formaldehyde up-regulates TRPV1 through MAPK and PI3K signaling pathways in a rat model of bone cancer pain. Neurosci. Bull. 2012;28:165–172. doi: 10.1007/s12264-012-1211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Homma Y, Nomiya A, Tagaya M, Oyama T, Takagaki K, Nishimatsu H, et al. Increased mRNA expression of genes involved in pronociceptive inflammatory reactions in bladdertissue of interstitial cystitis. J. Urol. 2013;190:1925–1931. doi: 10.1016/j.juro.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 20.Gui Q, Xu C, Zhuang L, Xia S, Chen Y, Peng P, et al. A new rat model of bone cancer pain produced by rat breast cancer cells implantation of the shaft of femur at the third trochanter level. Cancer Biol. Ther. 2013;14:193–199. doi: 10.4161/cbt.23291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lautner MA, Ruparel SB, Patil MJ, Hargreaves KM. In vitro sarcoma cells release a lipophilic substance that activates the pain transduction system via TRPV1. Ann. Surg. Oncol. 2011;18:866–871. doi: 10.1245/s10434-010-1328-1. [DOI] [PubMed] [Google Scholar]
- 22.Zhang N, Inan S, Cowan A, Sun R, Wang JM, Rogers TJ, et al. A proinflammatory chemokine, CCL3, sensitizes the heat- and capsaicin-gated ion channel TRPV1. Proc. Natl. Acad. Sci. U.S.A. 2005;102:4536–4541. doi: 10.1073/pnas.0406030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu W, Lin H, Zhang Y, Chen X, Hua B, Hou W, et al. Compound Kushen Injection suppresses human breast cancer stem-like cells by down-regulating the canonical Wnt/beta-catenin pathway. J. Exp. Clin. Cancer Res. 2011;30:103. doi: 10.1186/1756-9966-30-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan C-X, Lin C-L, Liang L, Zhao Y-Y, Liu J, Cui J, et at. Enhancing effect of compound Kusheng injection in combination with chemotherapy for patients with advanced non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi. 2010;32:294–297. [PubMed] [Google Scholar]
- 25.Berghe WV, De Naeyer A, Dijsselbloem N, David J-P, De Keukeleire D, Haegeman G. Attenuation of ERK/RSK2-driven NFkappaB gene expression and cancer cell proliferation by kurarinone, a lavandulyl flavanone isolated from Sophora flavescens ait. roots. Endocr. Metab. Immune Disord. Drug Targets. 2011;11:247–261. doi: 10.2174/187153011796429790. [DOI] [PubMed] [Google Scholar]
- 26.Dong X-Q, Du Q, Yu W-H, Zhang Z-Y, Zhu Q, Che Z-H, et al. Antiinflammatory effects of oxymatrine through inhibition of nuclear factor-kappa B and mitogen-activated protein kinase activation in lipopolysaccharide-induced BV2 microglia cells. Iran. J. Pharm. Res. 2013;12:165–174. [PMC free article] [PubMed] [Google Scholar]
- 27.Han JM, Jin Y-Y, Kim HY, Park KH, Lee WS, Jeong T-S. Lavandulyl flavonoids from sophora flaveseens suppress lipopolysaccharide-induced activation of nuclear factor-kappa B and mitogen-activated protein kinases in RAW264.7 cells. Biol. Pharm. Bull. 2010;33:1019–1023. doi: 10.1248/bpb.33.1019. [DOI] [PubMed] [Google Scholar]
- 28.Kim B-H, Won C, Lee Y-H, Choi JS, Noh KH, Han S, et at. Sophoraflavanone G induces apoptosis of human cancer cells by targeting upstream signals of STATs. Biochem. Pharmacol. 2013;86:950–959. doi: 10.1016/j.bcp.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Li T, Wong VKW, Yi XQ, Wong YE, Zhou H, Liu L. Matrine induces cell anergy in human Jurkat T cells through modulation of mitogen-activated protein kinases and nuclear factor of activated T-cells signaling with concomitant up-regulation of anergy-associated genes expression. Biol. Pharm. Bull. 2010;33:40–46. doi: 10.1248/bpb.33.40. [DOI] [PubMed] [Google Scholar]
- 30.Liu XS, Jiang J. Enhancement of imatinib-induced apoptosis by matrine in bcr/abl-positive leukemia K562 cells. Pharm. Biol. 2006;44:287–291. [Google Scholar]
- 31.Liu XS, Jiang JK, Jiao XY, Wu YE, Lin JH. Matrine-induced apoptosis in leukemia U937 cells: involvement of caspases activation and MAPK-independent pathways. Planta Med. 2006;72:501–506. doi: 10.1055/s-2006-931534. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Willcockson HH, Valtschanoff JG. Vanilloid receptor TRPV1-mediated phosphorylation of ERK in murine adjuvant arthritis. Osteoarthritis Cartilage. 2009;17:244–251. doi: 10.1016/j.joca.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shevach EM. Labeling cells in microtiter plates for determination of [3H]thymidine uptake. Curr. Protoc. Immunol. 2001;21 doi: 10.1002/0471142735.ima03ds21. Appendix 3-Appendix 3D. [DOI] [PubMed] [Google Scholar]
- 34.Berghmans N, Nuyts A, Uyttenhove C, Van Snick J, Opdenakker G, Heremans H. Interferon-gamma orchestrates the number and function of Thl7 cells in experimental autoimmune encephalomyelitis. J. Interferon Cytokine Res. 2011;31:575–587. doi: 10.1089/jir.2010.0137. [DOI] [PubMed] [Google Scholar]
- 35.Hayakawa J, Ohmichi M, Kurachi H, Kanda Y, Hisamoto K, Nishio Y, et al. Inhibition of BAD phosphorylation either at serine 112 via extracellular signal-regulated protein kinase cascade or at serine 136 via Akt cascade sensitizes human ovarian cancer cells to cisplatin. Cancer Res. 2000;60:5988–5994. [PubMed] [Google Scholar]
- 36.Liu J, Xue M, Huang X, Wang S, Jiang Z, Zhang L. [Pharmacokinetic of four alkaloids of Yanshu injection in Beagel dogs] Zhongguo Zhong Yao Za Zhi. 2012;37:1845–1849. [PubMed] [Google Scholar]
- 37.Li ZW, Li GF, Zhang JH. [Pharmacokinetics and bioavailability of sustained-release tablets of matrine in dogs] Zhong Yao Cai. 2010;33:1293–1296. [PubMed] [Google Scholar]
- 38.Wang Y, Ma Y, Li X, Qin F, Lu X, Li F. Simultaneous determination and pharmacokinetic study of oxymatrine and matrine in beagle dog plasma after oral administration of Kushen formula granule, oxymatrine and matrine by LC-MS/MS. Biomed. Chromatogr. 2007;21:876–882. doi: 10.1002/bmc.834. [DOI] [PubMed] [Google Scholar]
- 39.Danial NN. BAD: undertaker by night, candyman by day. Oncogene. 2008;27(Suppl. 1):S53–S70. doi: 10.1038/onc.2009.44. [DOI] [PubMed] [Google Scholar]
- 40.Wang LN, Yao M, Yang JP, Peng J, Peng Y, Li CF, et al. Cancer-induced bone pain sequentially activates the ERK/MAPK pathway in different cell types in the rat spinal cord. Mol. Pain. 2011;7:48. doi: 10.1186/1744-8069-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gimenez-Cassina A, Martinez-Francois JR, Fisher JK, Szlyk B, Polak K, Wiwczar J, et al. BAD-dependent regulation of fuel metabolism and K(ATP) channel activity confers resistance to epileptic seizures. Neuron. 2012;74:719–730. doi: 10.1016/j.neuron.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai Y, Chen T, Xu Q. Astilbin suppresses collagen-induced arthritis via the dysfunction of lymphocytes. Inflamm. Res. 2003;52:334–340. doi: 10.1007/s00011-003-1179-3. [DOI] [PubMed] [Google Scholar]
- 43.Xu Q, Yuan KW, Lu JF, Wang R, Wu FH. A new strategy for regulating the immunological liver injury - effectiveness of DTH-inhibiting agents on dth-induced liver injury to picryl chloride. Pharmacol. Res. 1997;36:401–409. doi: 10.1006/phrs.1997.0249. [DOI] [PubMed] [Google Scholar]
- 44.Liu G, Dong J, Wang H, Hashi Y, Chen S. Characterization of alkaloids in Sophora flavescens Ait. by high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2011;54:1065–1072. doi: 10.1016/j.jpba.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 45.Lin Z, Huang CF, Liu XS, Jiang J. In vitro anti-tumour activities of quinolizidine alkaloids derived from Sophora flavescens Ait. Basic Clin. Pharmacol. Toxicol. 2011;108:304–309. doi: 10.1111/j.1742-7843.2010.00653.x. [DOI] [PubMed] [Google Scholar]
- 46.Guenther MK, Graab U, Fulda S. Synthetic lethal interaction between PI3K/ Akt/mTOR and Ras/MEK/ERK pathway inhibition in rhabdomyosarcoma. Cancer Lett. 2013;337:200–209. doi: 10.1016/j.canlet.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Sun Q, Ma W, Gao Y, Zheng W, Zhang B, Peng Y. Meta-analysis: therapeutic effect of transcatheter arterial chemoembolization combined with compound kushen injection in hepatocellular carcinoma. Afr. J. Tradit. Complement. Altern. Med. 2012;9:178–188. doi: 10.4314/ajtcam.v9i2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.China Food and Drug Adminstration. Notice on GMP certification (for injections, radioactive drugs and biologicals defined by drug regulatory department under the State Council) GMP Certification. 2013 [Google Scholar]
- 49.Forscher C, Mita M, Figlin R. Targeted therapy for sarcomas. Biologics. 2014;8:91–105. doi: 10.2147/BTT.S26555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Melemedjian OK, Khoutorsky A, Sorge RE, Yan J, Asiedu MN, Valdez A, et al. mTORC1 inhibition induces pain via IRS-1-dependent feedback activation of ERK. Pain. 2013;154:1080–1091. doi: 10.1016/j.pain.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pignochino Y, Grignani G, Cavalloni G, Motta M, Tapparo M, Bruno S, et al. Sorafenib blocks tumour growth, angiogenesis and metastatic potential in preclinical models of osteosarcoma through a mechanism potentially involving the inhibition of ERK1/2, MCL-1 and ezrin pathways. Mol. Cancer. 2009;8:118. doi: 10.1186/1476-4598-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weil A, Moore SE, Waite NJ, Randall A, Gunthorpe MJ. Conservation of functional and pharmacological properties in the distantly related temperature sensors TRVP1 and TRPM8. Mol. Pharmacol. 2005;68:518–527. doi: 10.1124/mol.105.012146. [DOI] [PubMed] [Google Scholar]
- 53.Ferrer-Montiel A, Fernandez-Carvajal A, Planells-Cases R, Fernandez-Ballester G, Gonzalez-Ros JM, Messeguer A, et al. Advances in modulating thermosensory TRP channels. Expert Opin. Ther. Pat. 2012;22:999–1017. doi: 10.1517/13543776.2012.711320. [DOI] [PubMed] [Google Scholar]
- 54.Honore P, Chandran P, Hernandez G, Gauvin DM, Mikusa JP, Zhong C, et al. Repeated dosing of ABT-102, a potent and selective TRPV1 antagonist, enhances TRPV1-mediated analgesic activity in rodents, but attenuates antagonist-induced hyperthermia. Pain. 2009;142:27–35. doi: 10.1016/j.pain.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Finley MJ, Happel CM, Kaminsky DE, Rogers TJ. Opioid and nociceptin receptors regulate cytokine and cytokine receptor expression. Cell. Immunol. 2008;252:146–154. doi: 10.1016/j.cellimm.2007.09.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.