Abstract
We treated 14 patients with GATA2 deficiency using a nonmyeloablative allogeneic hematopoietic stem cell transplantation (HSCT) regimen. Four patients received peripheral blood stem cells (PBSC) from matched-related donors (MRD), four patients received PBSC from matched-unrelated donors (URD), four patients received HSC from umbilical cord blood donors (UCB), and two patients received bone marrow cells from haploidentical related donors. MRD and URD recipients received conditioning with three days of fludarabine and 200cGy total body irradiation (TBI). Haploidentical related donor recipients and UCB recipients received cyclophosphamide and two additional days of fludarabine along with the 200 cGY TBI. MRD, URD, and UCB recipients received tacrolimus and sirolimus for post-transplant immunosuppression, whereas haploidentical recipients received high-dose cyclophosphamide followed by tacrolimus and mycophenolate mofetil. Eight patients are alive with reconstitution of the severely deficient monocyte, B-cell, and Natural Killer (NK) cell populations and reversal of the clinical phenotype at a median follow-up of 3.5 years. Two patients rejected the donor graft (one URD and one UCB), and one MRD recipient relapsed with myelodysplastic syndrome (MDS) post-transplant. We are currently using a high-dose conditioning regimen with busulfan and fludarabine in patients with GATA2 deficiency to achieve more consistent engraftment and eradication of the malignant myeloid clones.
Keywords: GATA2, hematopoietic stem cell transplant, myelodysplastic syndrome, familial acute myelogenous leukemia
INTRODUCTION
Expression of the GATA2 transcription factor is tightly regulated during hematopoiesis, and both over and under expression of GATA2 have been associated with myelodysplastic syndrome (MDS) and acute myelogenous leukemia (AML) [1, 2]. Recently, heterozygous sporadic or inherited mutations on one allele of the GATA2 gene have been shown to be responsible for a syndrome known variably as: “MonoMAC” (monocytopenia with nontuberculous mycobacterial infections (NTM) [3], DCML (dendritic, monocyte and lymphoid cell deficiency) [4], Emberger’s syndrome (congenital lymphedema with MDS and monosomy 7) [5], and familial MDS/AML [2].
Allogeneic hematopoietic stem cell transplantation represents a potentially curative therapy for patients with GATA2 deficiency, however these patients pose a particular therapeutic challenge due to the frequent co-morbidities associated with this disease. These co-morbidities include life-threatening infections secondary to deficiency of monocytes, NK cells, and B-cells, as well as pulmonary alveolar proteinosis (PAP) resulting from defective alveolar macrophages [6] [7]. In addition, the MDS in patients with GATA2 deficiency has the propensity to transform into AML or proliferative chronic myelomonocytic leukemia (CMML) [8].
We used a nonmyeloablative transplant regimen to treat patients with GATA2 deficiency because the majority of the patients in our initial cohort had severe underlying organ dysfunction and active infections at the time of transplant. In 2011 we published a pilot study describing encouraging results using a nonmyeloablative regimen in six patients with GATA2 deficiency [9]. Here, we describe the outcomes of the first 14 patients who received nonmyeloablative allogeneic HSCT for this newly described genetic syndrome, including the first two patients to receive a haploidentical transplant.
PATIENTS AND METHODS
Patients
This study was designed to determine the efficacy and safety of nonmyeloablative allogeneic HSCT for patients with GATA2 deficiency, or the MonoMAC syndrome, and was approved by the institutional review board of the National Cancer Institute. This study was independently monitored for safety and data accuracy (ClinicalTrials.gov number, NCT00923364). Patients between 12 to 60 years of age were eligible if they met the following criteria: 1) at least two episodes of life-threatening opportunistic infections; 2) mutation in the GATA2 gene, or a flow cytometry profile on peripheral blood demonstrating severe monocytopenia and CD19+ B-cell and CD3−CD56+ NK cell lymphopenia, consistent with the MonoMAC phenotype [10], 3) a 10/10 or 9/10 MRD or URD, a 4/6 or greater matched UCB, or a haploidentical related donor.
Patients were allowed to have MDS with one or more peripheral blood cytopenias and less than 5% blasts in the bone marrow in the absence of granulocyte colony stimulating factor (G-CSF).
Characteristics of Allogeneic Hematopoietic Stem Cell Transplant
MRD and URD were required to be 18 years of age or older and matched at 10/10 or 9/10 HLA-A, B, C, DRB1, and DQB1 loci by high resolution typing [11]. In addition, MRD and haploidentical related donors were required to have either a normal GATA2 gene on DNA sequencing or normal blood monocyte, NK cell, and B cell counts, and no history of mycobacterial or any other opportunistic infections.
Haploidentical related donors were required to share one haplotype in common with the recipient, such that human leukocyte antigen (HLA) compatibility represented a minimum match of 5 out of 10 HLA loci. As with MRDs, exclusion criteria included mutation in GATA2, history of mycobacterial or other opportunistic infections, or abnormal monocyte, NK or B cell counts. If more than one haploidentical donor was available, each donor was evaluated individually in terms of overall health, ABO matching, CMV, etc. to select the best donor.
The conditioning regimen depended upon the type of donor graft. MRD or URD recipients received fludarabine 30 mg/m2/day on days −4, −3, and −2, and 200-cGy total body irradiation (TBI) on day −1. UCB recipients received cyclophosphamide 50 mg/kg IV on day −6, fludarabine 30 mg/m2/day IV for 5 days (days −6 to −2, and 200 cGy TBI on day −1. Haploidentical related donor recipients received fludarabine 30 mg/m2/day IV for 5 days (days −6 to −2), cyclophosphamide 14.5 mg/kg IV for 2 days (days −6 and −5), and 200 cGy TBI on day −1 [12].
The cell product also depended upon the type of transplant. MRD and URD received PBSC from their donors obtained using 5 days of granulocyte colony stimulating factor (G-CSF, filgrastim) (10 µg/kg/day) followed by apheresis with the goal of collecting at least 5 × 106 CD34+ cells/kg of the recipient's body weight. MRD and URD peripheral blood stem cells were infused fresh on day 0. UCB units were thawed and infused on day 0. Haploidentical related donor recipients received fresh or cryopreserved bone marrow cells on day 0 with a target dose of 2 × 108 total nucleated cells per body weight of the recipient. Graft-versus-host-disease (GVHD) prophylaxis was dependent upon the type of transplant. Tacrolimus and sirolimus were initiated on days −3 and −2, respectively in MRD, URD, and UCB recipients. The doses of both agents were titrated to achieve serum levels between 5 and 10 ng/mL. Immunosuppression was tapered at 6 months post-transplant if there was no evidence of GVHD. Post-transplant immunosuppression for haploidentical recipients consisted of cyclophosphamide 50mg/kg/day IV for 2 days on days +3 and +4 followed by tacrolimus and mycophenolate mofetil starting on day +5 [12]. Immunosuppression with mycophenolate was stopped at day +30, and immunosuppression with tacrolimus was tapered at 6 months post-transplant if there was no evidence of GVHD.
Supportive Care
Standard guidelines for supportive care established at the National Institutes of Health Clinical Center for patients undergoing allogeneic HSCT were used. These guidelines are in agreement with the international guidelines for preventing infectious complications among hematopoietic cell transplantation recipients [13].
For non-tuberculous mycobacterial (NTM) infections, infectious disease physicians with expertise in therapy for NTM infection were involved in the care of these patients. Treatment selection was based on the specific NTM species isolated [14]. When patients had had their NTM infection fully treated prior to the transplant, they were kept on prophylactic azithromycin until the time of transplant, and about a year after transplant. In cases in which the NTM infection was recent, all efforts were made to delay transplant until the NTM infection was under control, cultures became negative and lesions were stable or regressing on imaging studies. For patients whose infection episode was recent, or had bone involvement, and were still on a rifamycin as part of their treatment, this was changed to moxifloxacin before the start of the conditioning regimen, to avoid possible drug interactions. All patients had a macrolide as part of their treatment regimen or secondary prophylaxis; in all cases azithromycin was selected over clarithromycin to avoid possible drug interactions. Most patients that were still being treated for active infection at the time of transplant (double or triple therapy) were kept on all the anti-mycobacterial drugs at least 6–12 months after the transplant. Subsequently, these were discontinued, and the azithromycin was kept for at least 6 more months.
Immune reconstitution of T, B, and NK Cells and monocytes
CD14+ monocytes, CD3−/CD56+ NK cells, CD19+ B-lymphocytes, and CD3+ T- lymphocytes were quantified by flow cytometry using subset specific monoclonal antibodies pre-transplant and at designated intervals post-transplant.
Cytogenetics
Cytogenetic analysis was performed pre-transplant and at 28 days and 12 months post-transplant. When cytogenetic abnormalities were present, fluorescence in-situ hybridization was used to identify the specific chromosomal abnormalities.
Analysis of Chimerism
Engraftment of donor cells was assessed using polymorphisms in regions known to contain short tandem repeats. Peripheral blood CD14+, CD3−/CD56+, CD19+ and CD3+ subsets were isolated by flow cytometry at designated time points, and chimerism was assessed. In addition, CD14+/CD15+ myeloid cells and CD3+ T-lymphocytes were selected using immunobeads and chimerism was assessed on the selected cells.
Statistical Analysis
Descriptive statistics were used for chimerism, monocyte, NK and lymphocyte counts.
RESULTS
Study Population
Baseline characteristics of the 14 patients with mutations in GATA2, or the MonoMAC syndrome, who received allogeneic stem cell transplant, are shown in Table 1. The median age at the time of transplant was 33 years (range, 15 to 46 years). The majority of patients had suffered infections since childhood. The median duration of illness prior to transplant was 7.5 years (range, 1 to 28 years). All patients had characteristic flow cytometry findings in their peripheral blood with severely reduced CD14+ monocytes, CD19+ B-lymphocytes, and CD3−/CD56+ NK cells, along with normal to slightly reduced CD3+ T-lymphocytes.
Table 1.
Characteristics of Patients with GATA2 Deficiency Receiving HSCT
| Donor | Patient | Age at HSCT (years)/ sex |
Duration of Illness (years) |
Type of Infection | Pulmonary Manifestations |
Mutation | Family History* |
||
|---|---|---|---|---|---|---|---|---|---|
| NTM | Viral | Other | |||||||
| MRD | |||||||||
| 1 | 33/M | 24 | Disseminated MAI | Skin/ genital HPV | Invasive aspergillosis | PAP | delAinsGC G81fs |
− | |
| 2 | 46/F | 28 | Disseminated MAI, M. scrofulaceum, M fortuitum |
Skin HPV, VZV | Disseminated Aspergillosis |
PAP | R398W | + * | |
| 3 | 45/F | 2 | Disseminated MAI | VZV | - | Ground glass & reticular infiltrates |
Exon 6 skip | − | |
| 4 | 33/M | 5 | Disseminated MAI | Anal and genital HPV | - | PAP | T354M | + | |
| URD | |||||||||
| 5 | 33/M | 1 | Disseminated MAI | Skin HPV; molluscum contagiosum |
- | - | R398W | + * | |
| 6 | 23/F | 8 | Disseminated M abscessus |
Disseminated Nocardiosis | Reticular infiltrates/ bullae |
R398W | − | ||
| 7 | 38/F | 26 | MAI | HPV genital, disseminated VZV |
Culture (-) endocarditis, C difficile toxic megacolon |
Ground glass & reticular infiltrates |
N371K | Adopted | |
| 8 | 33/M | 13 | Granulomatous adenitis |
- | Necrotizing fasciitis | Ground glass & reticular infiltrates/ bullae |
T354M | + * | |
| UCB | |||||||||
| 9 | 41/F | 22 | Disseminated MAI and M. abscessus |
Skin and genital HPV | - | PAP | T354M | + * | |
| 10 | 15/M | 2 | Disseminated MAI | Skin HPV |
S. sanguinis osteo, C difficile toxic megacolon |
Reticular & nodular infiltrates/ bullae |
Ins 10bp D259fs |
− | |
| 11 | 29/M | 16 | M. kansasii | Skin and genital HPV | VRE empyema, Candida sinovitis, C. difficile |
Ground glass & reticular infiltrates |
R361del14 | − | |
| 12 | 27/F | 2 | Disseminated M. kansasii |
None | - | PAP | A318fs | − | |
| Haplo | |||||||||
| 13 | 22/F | 7 | None | Genital HPV, refractory genital HSV, disseminated CMV |
- | Ground glass infiltrates/ bullae |
G101fs | − | |
| 14 | 20/F | 1 | Disseminated MAI | Hydroa vaccineforme- like EBV+ T cell lymphoma |
Histoplasmosis | Reticular infiltrates | MonoMAC Syndrome** |
− | |
Abbreviations: NTM: Non-tuberculous mycobacteria; PAP: Pulmonary Alveolar Proteinosis; MAI: Mycobacterium Avium Intracellulare; VZV: Varicella zoster virus; HPV: Human Papilloma Virus; HSV: Human Herpes Simplex virus
Specifics of Family History: Patient 2: sister died at age 31 after HSCT, mother died at age 54 of CMML; Patient 4: cousin had leukemia and 2 sons with the same mutation but asymptomatic thus far age 3 and 10; Patient 5: mother died at age 32 of AML; Patient 8: father, aunt, and uncle had AML; Patient 9: son died age at 19 of AML.
No identifiable mutation in GATA2, but expressed only the paternal allele of GATA2 at the mRNA level.
Susceptibility to opportunistic infections, especially with NTM mycobacteria species, is a hallmark of the MonoMAC syndrome, and all but two patients had a history of an NTM infection (Table 1). Severe DNA viral infections, most frequently human papilloma virus (HPV), were common, presumably due to the lack of NK cells. One patient had a severe refractory human herpes simplex virus infection. Over one-half of the patients in our cohort had serious fungal and/or bacterial infections pre-transplant.
Pulmonary disease was also common in this cohort with five patients having PAP (Table 1). Two patients were on supplemental oxygen prior to transplant due to this condition. The severity of the disease is evidenced by the fact that one patient (patient 1) was considered to be a lung transplant candidate prior to HSCT and ultimately received a double lung transplant 4 years after HSCT. In addition, patient 2 was transplanted while intubated following pre-transplant whole lung lavage for severe PAP.
The patients in this study had a variety of mutations in GATA2, the two most common being T354M and R398W (Table 1). Patient 14 did not have an identifiable mutation in an exon of GATA2, but expressed only the paternal allele of GATA2 at the mRNA level. Five patients had a parent or sibling with a GATA2 mutation, and the remaining nine patients did not have a family history of the syndrome in a first-degree relative.
All patients had bone marrow involvement with 12 patients (86%) meeting WHO criteria for MDS. Most patients had refractory cytopenia with multilineage dysplasia (RCMD) at the time of diagnosis. Two patients had progressed to refractory anemia with excess blasts (RAEB) prior to transplant (Table 2). In contrast to typical MDS, the majority of patients had bone marrow biopsies that were hypocellular with reticulin fibrosis. On cytogenetic analysis, four patients had monosomy 7, an abnormality known to be associated with a poor prognosis in MDS and AML (Table 2).
Table 2.
Reversal of MDS and Cytogenetic Abnormalities after HSCT
| Donor | Patient | Pre Transplant Bone Marrow | Pre Transplant Treatment |
2 years Post Transplant Bone Marrow | ||||
|---|---|---|---|---|---|---|---|---|
| Diagnosis | Cytogenetics | Cellularity** | Diagnosis | Cytogenetics | Cellularity** | |||
| MRD | ||||||||
| 1 | RCMD | Normal | Hypocellular | None | Trilineage hematopoiesis |
46, XY | Normocellular | |
| 2 | RCMD | N/A | Hypocellular | None | ND | ND | ND | |
| 3 | RCMD | Trisomy 1q | Hypocellular Gr 1–2 Fibrosis |
None | Trilineage hematopoiesis |
46, XY | Hypocellular Gr 1 Fibrosis |
|
| 4 | RCMD | Trisomy 8 | Hypocellular Gr 1–2 Fibrosis |
None | Trilineage hematopoiesis |
46, XX | Hypocellular Gr 1–2 Fibrosis |
|
| URD | ||||||||
| 5 | RCMD | -Y | Hypocellular Gr 2 Fibrosis |
None |
*Trilineage hematopoiesis |
46, XX | Normocellular Gr 2 Fibrosis |
|
| 6 | RCMD | Trisomy 8 | Hypocellular Gr 1–2 Fibrosis |
None | Trilineage hematopoiesis |
46. XX | Hypocellular Gr 1–2 Fibrosis |
|
| 7 | RAEB1 | Monosomy 7 | Hypercellular | 3+7 | Trilineage hematopoiesis |
46, XX | Normocellular Gr 1 Fibrosis |
|
| RCMD | Trisomy 8 | Hypocellular | None | ND | ND | ND | ||
| UC | ||||||||
| 9 | RAEB2 | Monosomy 6, +r | Normocellular Gr 3 Fibrosis |
3+7 | Trilineage hematopoiesis |
46, XY | Hypocellular Gr 3–4 Fibrosis |
|
| 10 | RCMD | Monosomy 7 | Hypocellular | None | Trilineage hematopoiesis |
46, XY | Hypocellular | |
| 11 | RCMD | Monosomy 7, Trisomy 21 |
Hypocellular Gr 2 Fibrosis |
None | ND | ND | ND | |
| 12 | RCMD | Monosomy 6 | Hypocellular Gr 2 Fibrosis |
None | ND | ND | ND | |
| Haplo | ||||||||
| 13 | CMML | Monosomy −7q Trisomy 8 |
Hypercellular Gr 2 Fibrosis |
HIDAC, Melphalan |
ND | ND | ND | |
| 14 | HLH, EBV (+) T cell LPD |
Normal | Hypocellular | Alemtuzumab,et oposide (HLH) |
*Trilineage hematopoiesis, no EBV detected |
N/A | Hypocellular | |
Abbreviations: RAEB: Refractory Anemia with Excess Blasts; LPD: lymphoproliferative disorder; N/A: not available; ND: Not done as patient died prior to 2 years.
Results of last available marrow. Patient 5 refused bone marrow biopsies as of 6 months post transplant. Patient 9 is only 9 months post transplant.
Cellularity for age (100% - age +/− 10%)
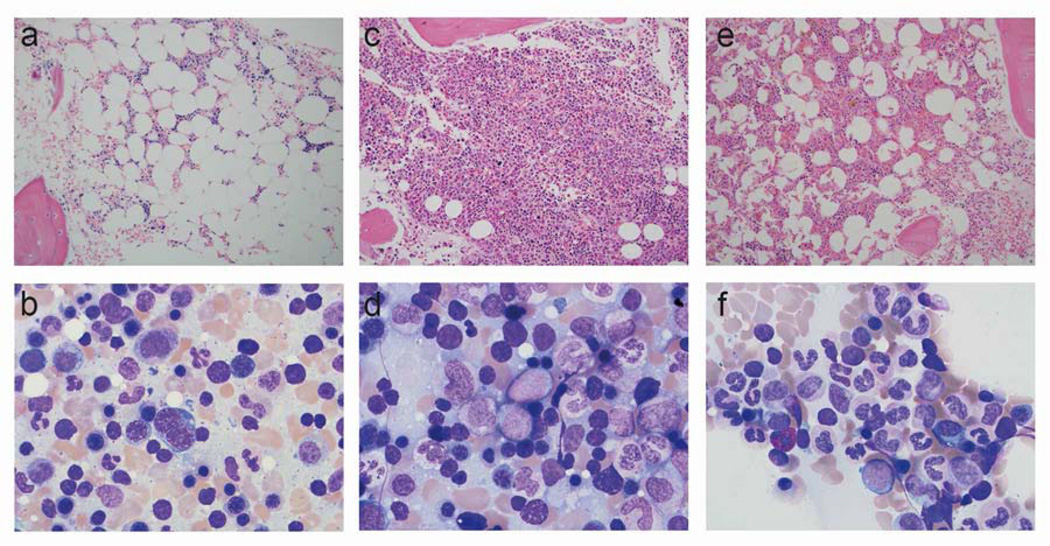
The typical progression of the bone marrow histology in GATA2 deficiency is shown (patient 7) (Figure 1). Approximately two years prior to transplant, her marrow was hypocellular with reduced granulocyte precursors and monocytes and normal cytogenetics (Figure 1, panels A and B). Over a two-year period, her marrow became hypercellular with 5 to 7% blasts, consistent with a diagnosis of RAEB-1, and cytogenetic analysis revealed a new monosomy 7 (Figure 1, panels C and D). Six months after induction chemotherapy and an unrelated donor transplant, the bone marrow histology and cytogenetic analysis were normal (Figure 1, panels E and F).
Figure 1.
Typical evolution of pathologic changes in the bone marrow in GATA2 deficiency. Bone marrow findings for patient 7. (A), (C) and (E) show bone marrow biopsy results 2 years prior to transplant, 2 months prior to transplant and 6 months post transplant respectively. (B), (D) and (F) show bone marrow aspirates from the same time points. This demonstrates the progression of the bone marrow from hypocellular to hypercellular and finally to normal trilineage hematopoiesis.
Pre-transplant chemotherapy was required in three patients. Two patients (patients 7 and 9) required one cycle of chemotherapy with idarubicin and cytosine arabinoside (7 + 3) prior to transplant since the bone marrow blast count was greater than 5% (Table 2). One patient (patient 13) presented with an MDS/myeloproliferative syndrome (CMML) and received three cycles of chemotherapy (7 + 3, then high dose cytosine arabinoside and melphalan) prior to transplant. This patient had received chemotherapy for AML seven years earlier and had achieved a complete remission.
Transplant characteristics
This cohort of patients received a cross-section of graft sources with four MRD, four URD, four UCB, and two haploidentical related donors. Peripheral blood stem cells were used in all MRD and URD donors, and bone marrow was used for haploidentical related donor recipients. Cell doses were comparable in the MRD, URD, and haploidentical related donor groups (Table 3).
Table 3.
Characteristics of Hematopoietic Stem Cell Grafts and Outcome of HSCT
| Donor | Patient | Cell Source |
HLA Match |
Composition of Donor Graft | Infections Post HSCT |
GVHD | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|
| CD34+ × 106/kg | CD3+ × 106/kg | Acute | Chronic * | ||||||
| MRD | |||||||||
| 1 | PBSC | 10/10 | 7.0 | 247 | CRBSI | Gr. II skin, liver | Severe | Alive 4.7y | |
| 2 | PBSC | 10/10 | 4.1 | 380 | IFI, CMV viremia, BSI |
Gr IV skin, liver | N/A | Died day 90 - sepsis, GVHD | |
| 3 | PBSC | 10/10 | 6.0 | 174 | - | Gr II skin, gut | - | Alive 3.2y Relapsed 1.4y, now 1.8y post HSCT #2 |
|
| 4 | PBSC | 10/10 | 6.4 | 330 | - | - | Mild | Alive at 2.3y | |
| URD | |||||||||
| 5 | PBSC | 10/10 | 6.87 | 354 | - | - | - | Alive 4.5y | |
| 6 | PBSC | 10/10 | 8.25 | 207 | - | Gr III skin, gut, liver |
Mild | Alive 3.5y | |
| 7 | PBSC | 9/10 | 7.25 | 447 | - | - | - | Alive 2.3y | |
| 8 | PBSC | 10/10 | 6.3 | 188 | CMV, IFI | Gr IV skin, gut, liver |
- | Rejected 10m, retransplanted, died at 1.5y |
|
| UCB | |||||||||
| 9 | Single UC |
4/6 | 0.78 | 3.48 | CRBSI, IFI, CMV-viremia |
- | - | Developed donor cell AML 2.5y post HSCT and died at 3y |
|
| 10 | Double UC1 |
4/6 | 0.18 | 5.04 | FN, endocarditis |
Gr.I-II skin, gut | - | Alive 3.4y | |
| UC2 | 4/6 | 0.65 | 10.5 | ||||||
| 11 | Double UC1 |
4/6 | 0.23 | 6.8 | VRE sepsis, candida sepsis |
N/A | N/A | Died day 7 - sepsis | |
| UC2 | 4/6 | ||||||||
| 12 | Double UC1 |
4/6 | 0.16 | ND | VRE sepsis | Gr I-II gut | - | Rejected, retransplanted, died day 295. | |
| UC2 | 4/6 | 0.57 | ND | ||||||
| Haplo | |||||||||
| 13 | BM | 5/10 | 4.2 | 43.2 | ARDS | N/A | N/A | Died day 1 - ARDS | |
| 14 | BM | 7/10 | 6 | 55 |
Aeromonas sp. gastroenteritis |
Gr I Skin | Alive 8m | ||
Abbreviations: PBSC: Peripheral Blood Stem Cells; BM: bone marrow; CRBI: catheter related blood stream infection; IFI: invasive fungal infection; BSI: bloodstream infection; VRE: Vancomycin resistant enterococcus; FN: Febrile Neutropenia; N/A: not applicable as patient died early post transplant; ND: not done; ARDS: acute respiratory distress syndrome; UC: Umbilical Cord.
According to NIH Criteria for Severity
Reconstitution of Hematopoietic Compartments Following Transplant
Reconstitution of the cellular compartments that were severely deficient pre-transplant represented a primary objective of this study. The median time to neutrophil engraftment (defined as a neutrophil count of >0.5 × 109 cells/L for three consecutive days) for recipients of MRD and URD was 12 days (range, 0 to 13 days). The three evaluable patients in the UCB group engrafted neutrophils within a range of 16 and 80 days. Of the two haploidentical transplant recipients, the first patient died early after transplant, and the second patient had neutrophil engraftment by day 19 post-transplant. The pattern was similar for platelet engraftment (defined as a platelet count of >20 × 109 cells/L for seven consecutive days without requiring platelet transfusion). MRD recipients engrafted at a median of 16 days (range 0 to 18 days), URD at 13 days (range, 0 to 18 days), the two evaluable UCB recipients engrafted at 32 and 302 days, and the single, evaluable haploidentical related donor recipient never had a platelet count below 20.
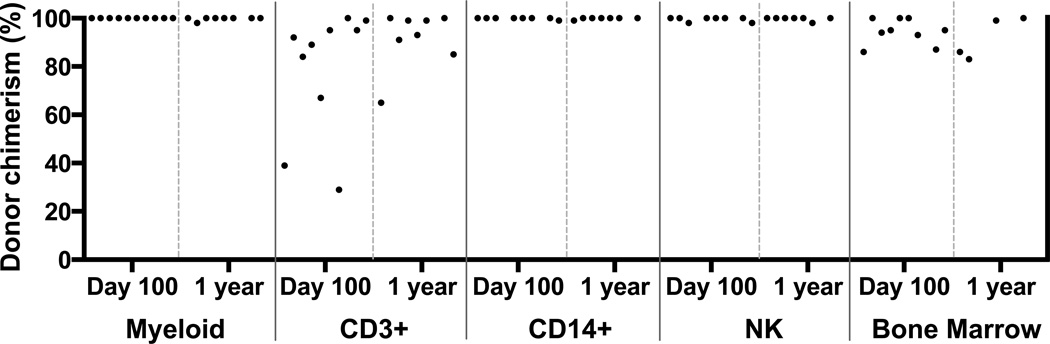
In the evaluable patients who initially engrafted, the percentage of donor chimerism at day 100 and at one year is shown (Figure 2). All evaluable patients achieved 100% donor myeloid cells by day +100 and 98% to 100% at one year. CD14+ monocytes and CD3−/CD56+ NK cells were between 98% and 100% at both day +100 and one year. The number of CD3+ cells at day +100 ranged from 29% to 100% with a median of 91%. At one year, the range was 65% to 100% with a median of 93%. Of the two patients with low CD3 chimerism at day +100, the one with the lowest chimerism (29%) (patient 8) subsequently rejected the unrelated donor graft and required re-transplant. The patient with 39% donor CD3 chimerism at day +100 (patient 1) maintained stable engraftment and ultimately progressed to 100% donor CD3 chimerism at two years post-transplant.
Figure 2.
Chimerism after hematopoietic stem cell transplant. Percentage of peripheral blood donor chimerism from myeloid, CD3+, CD14+, NK cell fractions and bone marrow.
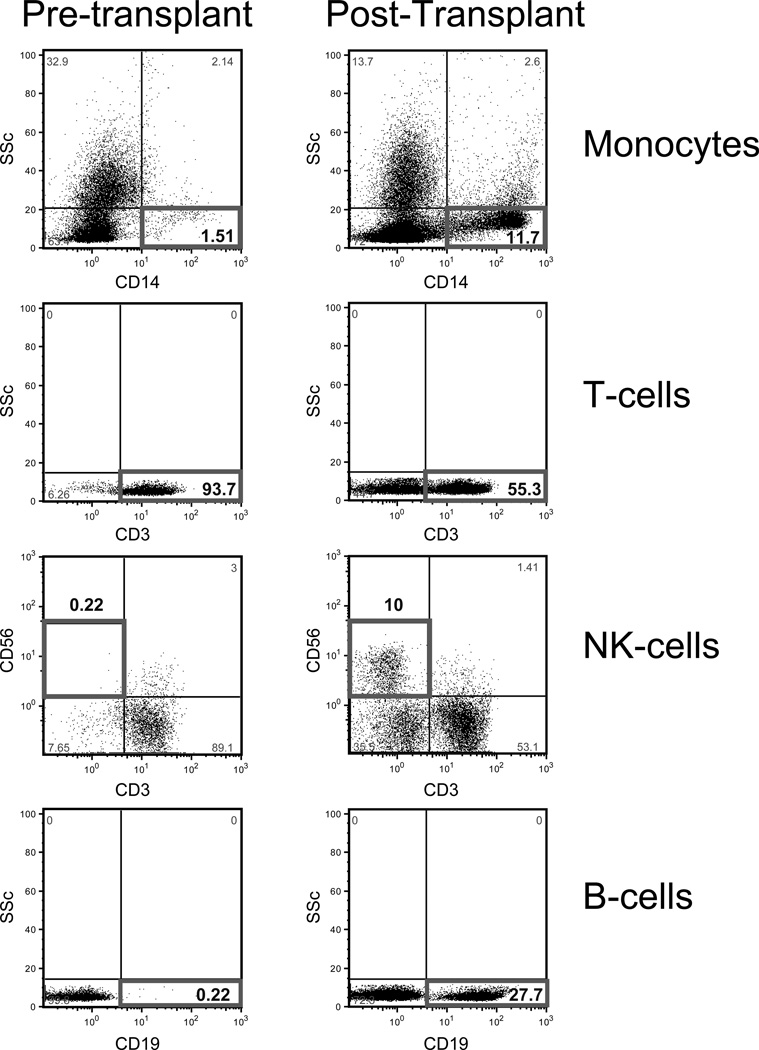
The reconstitution of cell subsets for a single GATA2 patient pre-transplant and at 6 months post-transplant is shown (Figure 3).
Figure 3.
Reconstitution of CD19+ B-cells, NK cells, and monocytes 6 months post-transplant in a GATA2 deficient patient.
Clinical Outcome Following Hematopoietic Stem Cell Transplant
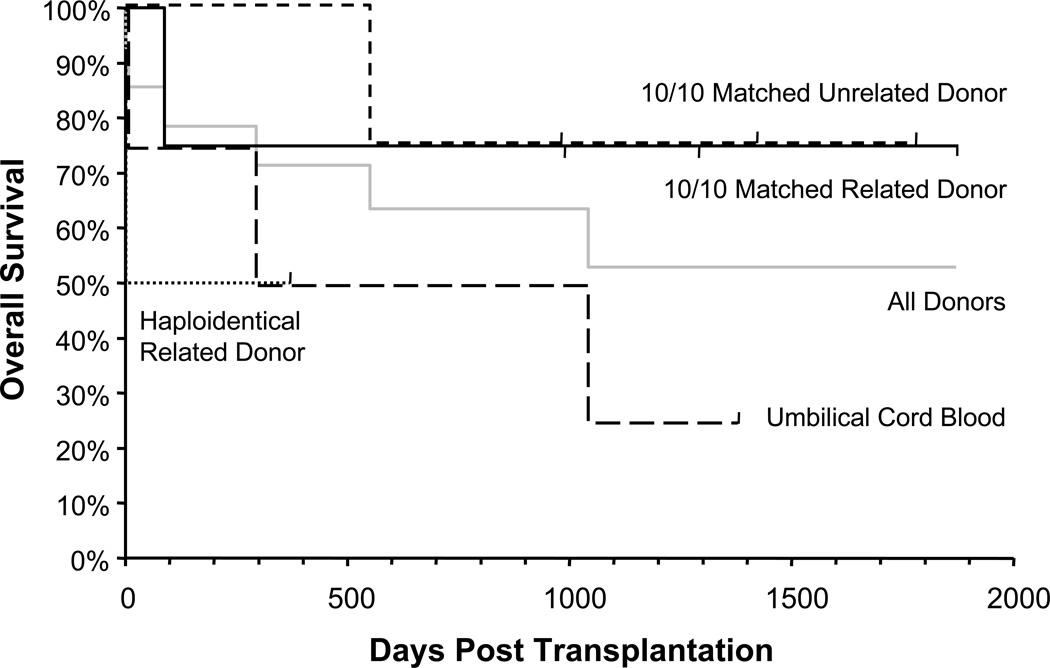
Eight of the 14 (57%) patients in this high-risk cohort of patients are alive at a median follow-up of 3.5 years (range 12 months to 5 years) (Figure 4). The overall survival based on donor type is shown (Figure 4). The mortality was not uniformly distributed in the subgroups: three out of four recipients in both the MRD and URD groups are alive, and one of the two haploidentical related donor recipients is alive. However, only one of the four umbilical cord blood recipients survived (Figure 4). Of note, five of the eight survivors (patients 1, 4, 5, 6, 7) had uneventful hospitalizations and were discharged within one month of transplant.
Figure 4.
Kaplan-Meier curves showing overall survival according to type of donor.
In the MRD group where all three survivors have greater than two years of follow-up, all patients engrafted with the only death occurring in the individual on a ventilator at the time of transplant (patient 2). However, one patient (patient 3) relapsed one year post-transplant and required a second transplant using the same sibling donor preceded by a myeloablative regimen with busulfan and fludarabine. This patient remains in complete remission 2 years following retransplant. A second patient (patient 1), who was a lung transplant candidate prior to HSCT due to severe pulmonary hypertension and alveolar proteinosis initially had marked improvement of his lung disease. However, he subsequently developed bronchiolitis obliterans and required lung transplant 4.5 years post-HSCT.
In the URD cohort, three of the four patients are alive with follow-up greater than two years (Figure 4). The only death occurred in a patient (patient 8) who rejected the unrelated donor graft eight months post-transplant. He was retransplanted from a second unrelated donor, developed severe GVHD and died.
The UCB group was the most problematic with only one of four patients surviving long-term (Figure 4 and Table 3). One patient (patient 11) died early from sepsis, another patient (patient 10) rejected the double cord graft and died following a salvage CD34+ selected haploidentical related donor and single cord transplant. The third patient in this group (patient 9) received a single UCB unit, had delayed engraftment and did well for the subsequent two years, but ultimately died 2.5 years post-transplant from a donor cell derived AML. The only long-term survivor in this cohort (patient 6) developed autoimmune nephrotic syndrome and pancytopenia on day 90 which ultimately responded to treatment with rituximab and tacrolimus.
In the haploidentical related donor cohort, one of the two patients (patient 14) is alive nine months post-transplant. The first patient in this group had progressed to a proliferative CMML prior to transplant, required multiple rounds of chemotherapy, and had multiple ongoing refractory infections. She died from respiratory failure on day +1. The second patient had recurrent gastrointestinal bleeding from an EBV+ T-cell lymphoma that involved her gastrointestinal tract, skin and lungs and associated HLH. She received a haploidentical transplant from her sister and had marked resolution of the lymphoma including extensive hydroa vacciniforme-like lesions.
Post-transplant complications
We anticipated major infectious complications in this group of immunocompromised patients. Nine patients (65%) had infections post-transplant (Table 3) and three deaths were attributable to infection. Overall, the most common pathogens were bacteria. Three patients had CMV reactivation (but no invasive disease), and three had invasive fungal infections. Surprisingly, none of the patients had reactivation of their NTM infections following transplant, however all patients with NTM were maintained on anti-mycobacterial therapy throughout the conditioning regimen and for at least 3 months after transplantation.
Eight (57%) of the 14 patients had acute graft versus host disease, most commonly affecting the skin (Table 3). Three patients had Grade III or IV GVHD affecting two or more organs. Acute GVHD was successfully treated with steroids (topical or systemic) in all but Patient 2, whose death at 90 days was attributed in part to severe GVHD, and in Patient 8 who developed severe GVHD after his second transplant. Acute GVHD was most common in the MRD group. Chronic GVHD developed in three patients (1, 4 and 6), but was only severe enough to limit daily activities in one patient.
The baseline cytogenetic abnormalities resolved in all patients except the patient who relapsed and required a second transplant (Table 2). This is particularly relevant in view of the adverse cytogenetics, with monosomy 7 present in five patients.
Although the groups are too small to derive statistically significant conclusions, there is a trend towards worse outcome with umbilical cord grafts relative to the URD and MRD groups where 75% of patients are still alive.
DISCUSSION
We report the outcome in a cohort of patients with GATA2 deficiency who received MRD, URD, UCB or haploidentical related donor hematopoietic stem cells following a nonmyeloablative conditioning regimen. In patients with GATA2 deficiency, nonmyeloablative allogeneic HSCT results in reconstitution of the severely deficient monocyte, B and NK cell populations, correction of the infection susceptibility phenotype, and reversal of the propensity for myeloid progression, including eradication of the abnormal cytogenetic clones. However, the incidence of graft rejection and relapse following nonmyeloablative allogeneic HSCT in GATA2 deficiency indicate that alternative transplant approaches may be required.
Recently, deficits in the expression of GATA2, a “master regulator” of hematopoiesis, have been shown to lead to opportunistic infections, myeloid malignancies, and pulmonary alveolar proteinosis [2,3,4,5]. Although HSCT represents the only definitive therapy for this disease, the indications for transplant, optimal conditioning regimen, timing of transplant, and donor source of stem cells remain unclear. In addition, the decision to undertake allogeneic transplant has to be weighed against the complications inherent to HSCT, including regimen related toxicity, GVHD, infection, and death.
The development of progressive bone marrow dysplasia and cytogenetic abnormalities represents one of the primary indications for allogeneic HSCT in GATA2 deficiency. GATA2 is crucial for the maintenance of hematopoietic stem cells in that both mice and humans with GATA2 deficiency lack multi-lineage progenitors and have a severe depletion of granulocyte-macrophage progenitors in the bone marrow [15, 16, 17]. This defect in cell production typically evolves into a hypocellular MDS, and ultimately progresses to AML or a proliferative CMML.
The largest series published on patients with mutations in GATA2 found that 84% of patients with GATA2 deficiency met criteria for MDS on bone marrow biopsy [6]. Similarly, a high frequency of GATA2 mutations was reported in 14 patients in the French Severe Chronic Neutropenia Registry [15]. In this cohort, the risk of malignant transformation was high with six of the fourteen patients developing RCMD and four developing AML. The median age at diagnosis of MDS or AML was 15 years (range 7 to 35). Of note, none of the patients treated with chemotherapy alone survived (median survival of 1.5 years after diagnosis of AML).
The majority of patients with GATA2 deficiency have low or intermediate-1 International Prognostic Scoring System (IPSS) scores at the time of diagnosis [16,17]. All 14 of the patients in this study had low or intermediate −1 IPSS scores when diagnosed. According to the IPSS, the time for 25% of low risk MDS patients to transform to AML is 9.4 years [16]. However, in our cohort two out of four patients with low risk IPSS scores had a rapid transformation from a hypocellular MDS to a hypercellular MDS/refractory anemia with excess blasts. Thus, once the diagnosis of GATA2 deficiency is established, close follow-up of these patients is warranted, and patients should be considered for transplant even prior to the appearance of blasts in their bone marrow.
The development of recurrent severe infections is a second indication to proceed to allogeneic HSCT. A subset of patients with mutations in GATA2 present in their second or third decade of life with recurrent life-threatening opportunistic infections (frequently NTM), but lack features of a malignant hematologic disease [6]. The mortality associated with these infections is enough to warrant replacement of the dysfunctional and deficient immune system. This is exemplified in two 20-year-old patients in our study who died awaiting transplant within the preceding year: one patient died from sepsis and one died from CMV pneumonia (D. Hickstein, unpublished).
Progressive lung injury from infection and PAP represents a third reason for allogeneic HSCT in patients with GATA2 deficiency. Both frequent pneumonias, as well as PAP, result in gradual deterioration of lung function. The first two patients transplanted on this protocol had severe PAP requiring pulmonary lavage prior to transplant, and both required supplemental oxygen at the time of transplant. As mentioned previously, one patient has gone on to require bilateral lung transplant, and the other died early after transplant. Therefore, initiating allogeneic HSCT before PAP develops would be anticipated to result in lower post transplant morbidity.
The ideal pre-transplant conditioning regimen for GATA2 deficiency is unclear. Moreover, it is likely that the stage of the disease at the time of transplant may influence the type of conditioning. This study was initiated prior to the identification of the GATA2 gene as the cause of the MonoMAC syndrome, and all of the initial patients had considerable co-morbidities necessitating a nonmyeloablative approach to allogeneic HSCT in this cohort. However, relapse in one of the four MRD recipients and graft rejection in one of the four recipients from each of the URD and UBC groups support the use of a more intensive conditioning regimen. Also, three patients required pre-transplant chemotherapy, which may have been unnecessary if a myeloablative regimen was used.
The timing of HSCT is well defined for many malignancies; however, the timing of allotransplant is less clear for many immune deficiencies. GATA2 deficiency is particularly difficult given its variable natural history with some patients developing symptoms only after many decades. However, once symptoms develop, survival declines [6]. The uninterrupted progression of GATA2 deficiency that we have seen suggests that the ideal time for transplant would be after the onset of pathologic abnormalities, but prior to the development of organ damage or malignancy. Identifying more precise indicators of disease progression is urgently needed.
In this regard, we are currently investigating markers that portend myeloid progression. For example, it is well known that acquired mutations lead to poor risk MDS/AML. To identify acquired somatic mutations associated with myeloid transformation in patients with GATA2 deficiency, we sequenced the region of the ASXL1 gene previously shown to be associated with transformation from MDS to AML [8,17]. Heterogeneous somatic ASXL1 mutations were identified in nearly 30% of patients, including four out of five patients who developed a proliferative CMML. Patients with GATA2 and ASXL1 mutations were considerably younger and almost exclusively female compared to patients with MDS and ASXL1 alone [8,17].
Donor source remains a critical variable in the outcome of HSCT and this is evident in our study. MRD and URD transplants were associated with the best outcomes with 75% survival in both groups. The poor outcome with UCB supports the use of alternative donor sources, such as haploidentical related donors in cases where a 10/10 matched donor is not available. The rapid reconstitution of the deficient compartments is essential to clear the ongoing infections that affect many of the patients at the time of transplant. As such, the prolonged time to engraftment with UCB transplant is especially detrimental in this population.
Previously, patients with GATA2 deficiency were transplanted without prior knowledge of the genetic defect. Bigley et al. described two patients aged 12 and 21 who received URD transplants for GATA2 deficiency and both were alive two years post-transplant [17]. In a report of patients with Emberger’s syndrome, four young patients (ages 9, 11, 12, and 16) received HSCT, primarily from URD, and only one survived [18]. In contrast, all six of the 14 patients in the French registry study who developed MDS or AML and underwent allotransplant were alive at a median of two years post transplant [15]. Our pilot study in 2011 described encouraging results using a nonmyeloablative regimen in six patients with GATA2 deficiency [9]. However, with further follow-up of the original cohort, and the inclusion of 8 additional patients, it now appears that more intensive conditioning is indicated in patients with GATA2 deficiency. Lastly, our overall mortality (42%) in this high-risk group of patients, though heavily skewed by the poor outcome of cord blood recipients, is comparable to recently reported transplant outcomes [19].
In summary, although nonmyeloablative HSCT results in reversal of the hematologic, immunologic, and clinical manifestations of GATA2 deficiency, more uniform engraftment and a reduced risk of relapse would be anticipated with a more intensive conditioning regimen. To this end, we are now using a myeloablative regimen with busulfan and fludarabine for patients with GATA2 deficiency [20, 21] The optimal time to transplant appears to be during the hypocellular MDS phase and before significant organ dysfunction develops. We anticipate that with the increasing frequency of genetic testing for GATA2 mutations, patients will be transplanted earlier in the course of the disease, before significant organ damage or clonal evolution of MDS to AML or CMML occurs, and that the outcome of allogeneic HSCT in these patients will continue to improve.
Highlights.
Mutations in the GATA2 gene result cause immune deficiency as well as bone marrow dysplasia and hematologic malignancy.
We report outcomes on a cohort of 14 patients who were treated with hematopoietic stem cell transplantation using a non-myeloablative conditioning regimen.
MRD and URD groups had the best survival with 3/4 patients in each group alive at 2 years.
The ideal timing of transplant and conditioning regimen have yet to be determined. Given the high incidence of relapse and rejection, a myeloablative regimen may be optimal.
Acknowledgments
This research was supported in part by the Intramural Research program of the NIH including the National Cancer Institute, National Institutes of Health, in part by the Division of Intramural Research, and in part under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
This research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
This research was supported [in part] by the National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no conflict of interest.
Authorship
Contribution: J.G. drafted the manuscript. D.D.H., S.M.H., and J.C.R designed the research, supervised the study and drafted the manuscript; J.G.B., C.Z., T.H., K.C., K.R.C., A.F., and M.P. provided critical revision of the manuscript for important intellectual content; K.R.C. reviewed the histopathology.
REFERENCES
- 1.Vicente C, Vazquez I, Conchillo A, Garcia-Sanchez MA, Marcotegui N, Fuster O, et al. Overexpression of GATA2 predicts an adverse prognosis for patients with acute myeloid leukemia and it is associated with distinct molecular abnormalities. Leukemia. 2012;26:550–554. doi: 10.1038/leu.2011.235. [DOI] [PubMed] [Google Scholar]
- 2.Hahn CN, Chong CE, Carmichael CL, Wilkins EJ, Brautigan PJ, Li XC, et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat Genet. 2011;43:1012–1017. doi: 10.1038/ng.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu AP, Sampaio EP, Khan J, Calvo KR, Lemieux JE, Patel SY, et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood. 2011;118:2653–2655. doi: 10.1182/blood-2011-05-356352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickinson RE, Griffin H, Bigley V, Reynard LN, Hussain R, Haniffa M, et al. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood. 2011;118:2656–2658. doi: 10.1182/blood-2011-06-360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostergaard P, Simpson MA, Connell FC, Steward CG, Brice G, Woollard WJ, et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome) Nat Genet. 2011;43:929–931. doi: 10.1038/ng.923. [DOI] [PubMed] [Google Scholar]
- 6.Spinner MA, Sanchez LA, Hsu AP, Shaw PA, Zerbe CS, Calvo KR, et al. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123:809–821. doi: 10.1182/blood-2013-07-515528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickinson RE, Milne P, Jardine L, Zandi S, Swierczek SI, McGovern N, et al. The evolution of cellular deficiency in GATA2 mutation. Blood. 2014;123:863–874. doi: 10.1182/blood-2013-07-517151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.West RR, Hsu AP, Holland SM, Cuellar-Rodriguez J, Hickstein DD. Acquired ASXL1 mutations are common in patients with inherited GATA2 mutations and correlate with myeloid transformation. Haematologica. 2013 doi: 10.3324/haematol.2013.090217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuellar-Rodriguez J, Gea-Banacloche J, Freeman AF, Hsu AP, Zerbe CS, Calvo KR, et al. Successful allogeneic hematopoietic stem cell transplantation for GATA2 deficiency. Blood. 2011;118:3715–3720. doi: 10.1182/blood-2011-06-365049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vinh DC, Patel SY, Uzel G, Anderson VL, Freeman AF, Olivier KN, et al. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood. 2010;115:1519–1529. doi: 10.1182/blood-2009-03-208629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maris MB, Niederwieser D, Sandmaier BM, Storer B, Stuart M, Maloney D, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102:2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 12.Luznik L, O'Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 15.Pasquet M, Bellanne-Chantelot C, Tavitian S, Prade N, Beaupain B, Larochelle O, et al. High frequency of GATA2 mutations in patients with mild chronic neutropenia evolving to MonoMac syndrome, myelodysplasia, and acute myeloid leukemia. Blood. 2013;121:822–829. doi: 10.1182/blood-2012-08-447367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 17.Bigley V, Haniffa M, Doulatov S, Wang XN, Dickinson R, McGovern N, et al. The human syndrome of dendritic cell, monocyte, B and NK lymphoid deficiency. J Exp Med. 2011;208:227–234. doi: 10.1084/jem.20101459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mansour S, Connell F, Steward C, Ostergaard P, Brice G, Smithson S, et al. Emberger syndrome-primary lymphedema with myelodysplasia: report of seven new cases. Am J Med Genet A. 2010;152A:2287–2296. doi: 10.1002/ajmg.a.33445. [DOI] [PubMed] [Google Scholar]
- 19.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell JA, Tran HT, Quinlan D, Chaudhry A, Duggan P, Brown C, et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant. 2002;8:468–476. doi: 10.1053/bbmt.2002.v8.pm12374451. [DOI] [PubMed] [Google Scholar]
- 21.Andersson BS, de Lima M, Thall PF, Wang X, Couriel D, Korbling M, et al. Once daily i.v. busulfan and fludarabine (i.v. Bu-Flu) compares favorably with i.v. busulfan and cyclophosphamide (i.v. BuCy2) as pretransplant conditioning therapy in AML/MDS. Biol Blood Marrow Transplant. 2008;14:672–684. doi: 10.1016/j.bbmt.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]