Abstract
CD44 is a transmembrane receptor for the glycosaminoglycan hyaluronan, a component of the extracellular matrix. CD44 is expressed by neural stem/progenitor cells, astrocytes, and some neurons but its function in the central nervous system is unknown. To determine the role of CD44 in brain function, we behaviorally analyzed CD44-null (KO) and wild-type (WT) mice. KO mice showed increased activity levels in the light-dark test and a trend towards increased activity in the open field. In addition, KO mice showed impaired hippocampus-dependent spatial memory retention in the probe trial following the first hidden-platform training day in the Morris water maze: WT mice showed spatial memory retention and spent more time in the target quadrant than any other quadrant, while KO mice did not. Although there were no genotype differences in swim speeds during the water maze training sessions with the visible or hidden platform, sensorimotor impairments were seen in other behavioral tests. In the inclined screen and balance beam tests, KO mice moved less than WT mice. In the wire hang test, KO mice also fell off of the wire faster than WT mice. In contrast, there was no genotype difference when emotional learning and memory were assessed in the passive avoidance test. These data support an important role for CD44 in locomotor and sensorimotor functions, and in spatial memory retention.
Keywords: CD44, mouse, locomotor, sensorimotor, memory
1. Introduction
The CD44 transmembrane glycoprotein has been implicated in the regulation of numerous cellular activities including proliferation, differentiation and cell migration [1]. Multiple forms of CD44 are generated by both alternative mRNA splicing and post-translational modifications that include N- and O-linked glycosylation, and the addition of heparin sulfate and chondroitin sulfate side chains. CD44 functions as a receptor for hyaluronan, a glycosaminoglycan found in most extracellular matrices, including perineuronal nets and to a lesser extent in white matter [2, 3]. The contribution of CD44 to nervous system development and how CD44 influences nervous system function is not understood.
In the central nervous system (CNS), CD44 is weakly expressed by astrocytes [4], Müller glial cells and their progenitors in the retina [5], and Bergmann glia in the cerebellum [6]. Some subpopulations of neurons are also at least transiently CD44-positive, including Purkinje and granule neurons in the cerebellum [6, 7]. CD44 is also expressed by a number of progenitor cell populations including oligodendrocyte progenitor cells [6, 8]. Chronically elevated CD44 inhibits oligodednrocyte differentiation and prevents myelination, an effect that likely depends on interactions with hyaluronan [8, 9]. Following a variety of insults including ischemia [10], inflammatory demyelination [11], and traumatic brain and spinal cord injuries [12, 13], CD44 expression is elevated in astrocytes, microglia, oligodendrocyte progenitors and some neurons. Following seizures, CD44 is elevated in the hippocampus and may contribute to mossy fiber sprouting [14]. Hyaluronan accumulates in CNS lesions where CD44 is elevated, especially in areas where there is reactive astrogliosis [8], and has been implicated in blocking oligodendrocyte maturation and remyelination in inflammatory demyelinating disease [8].
CD44 is also expressed by neural stem cells [6, 15]. In the subgranular zone (SGZ) of the hippocampal denate gyrus, most neural stem cells differentiate into dentate granule cells that migrate into the inner granule cell layer then functionally integrate into hippocampal neural circuits. Mice with reduced numbers of adult-born dentate granule cells have cognitive dysfunction characterized by deficiencies in forming hippocampus-dependent long-term spatial memory and impaired performance in contextual fear extinction [16]. Given that CD44 is expressed in numerous CNS cell types including neural stem cells implicated in spatial memory, we postulated that CD44 is required for a wide range of neurological functions. We therefore compared the performance of wild type (WT) and CD44-null (KO) mice in a battery of sensory, motor, and cognitive assays.
2. Methods
2.1. Animals
Six-month old female CD44-null (KO) mice, generated as described [17], and matched C57BL/6J wild-type (WT) mice were used. All mice were bred in the rodent animal care facility at the Oregon National Primate Research Center. The mice were kept on 12:12 hr light-dark schedule (lights on at 6 AM) with chow (PicoLab Rodent Diet 20, #5053; PMI Nutrition International, St. Louis, MO) and water given ad libitum. All the experiments reported here were conducted in accordance with NIH guidelines and approved by the Institutional Animal Care and Use Committee at the Oregon Health & Science University.
2.2 Behavioral Analysis
WT and KO mice were tested in the open field, elevated plus maze, elevated zero maze, and light-dark tests in week 1; the Morris water maze in week 2; then the rotorod, wire hang, inclined screen and balanced beam tests in week 3. Finally, emotional learning and memory were assessed using the passive avoidance test. Performance in the open field, elevated zero maze, elevated plus maze, water maze and passive avoidance tests was analyzed as previously described by us [18]. The rotorod, inclined screen, and wire hanging tests were performed as previously described [19]. In the light-dark test, mice were placed in the open-field enclosure containing black plastic inserts covering from the sides and the top 50% of the open field (Hamilton-Kinder, Poway, CA). A single opening in the wall of the insert adjacent to the open area allowed the mice to enter or exit the more anxiety-provoking light area of the maze. Active times, distance moved and rest time were recorded for a single 10-min session. Breaks in the photo beams were used to calculate path length, active times and rest time in the open and closed compartments of the enclosure. Mice with increased levels of anxiety in this enclosure spend less time in the lighted compartment of the maze.
For the balance beam test, mice were placed in the middle of a horizontal beam (88.5 cm long, 1.8 cm in diameter, 30.5 cm high). Total distance moved and mean velocity of movement in two trials of 2 min each were recorded using a Noldus Instruments’ EthoVision video tracking system.
2.3 Statistical Analyses
The data are expressed as means ± SEM. Differences among means were evaluated by ANOVA, followed by Student’s t-test or Tukey-Kramer posthoc tests, as indicated, using GraphPad Prism (San Diego, CA) and SPSS (Chicago, IL) software. For all analyses, the null hypothesis was rejected at the 0.05 level.
3. Results
Given the cell types that express CD44 in the brain, loss of CD44 might be expected to have significant effects on neurological function. We therefore performed a battery of behavioral and cognitive tests over three weeks to determine if CD44-null mice display cognitive or other neurological disturbances. Mice were tested for exploratory behavior and measures of anxiety in the open field, elevated plus maze, elevated zero maze, and light-dark tests in the first week. Then mice were then tested for spatial learning and memory in the Morris water maze in the second week and for sensorimotor function using the rotorod, wire hang, inclined screen and balanced beam tests in week three. Finally, emotional learning and memory were assessed using the passive avoidance test.
In the open field, there was a trend towards increased activity levels in the KO mice as compared to controls but it did not reach significance (pokes into the periphery: t(14) = 1.990, p = 0.0665; pokes into the center: t(14) = 1.863, p = 0.0836; total pokes: t(14) = 2.121, p = 0.0523; and entries into the center: t(14) = 2.009, p = 0.0642). There were no genotype differences in percent time spent in the more anxiety-provoking center of the open field (t(14) = 1.767, p = 0.0990). In the elevated plus maze and elevated zero maze, there were no genotype differences in activity levels or measures of anxiety (Table 1). However, consistent with the open field data, in the light-dark test, activity levels were higher in KO mice than in controls (t(14) = 4.120, p = 0.001; pokes into the dark compartment: t(14) = 3.473, p = 0.0037; total pokes: t(14) = 2.121, p = 0.0523; and entries into the light compartment: t(14) = 3.941, p = 0.0015).
Table 1.
Elevated plus maze, elevated zero maze, rotorod performance, and passive avoidance learning and memory of CD44 KO and WT female mice
| WT (n = 8) | KO (n = 8) | |
|---|---|---|
| Elevated Plus Maze | ||
| % Time in Open Arms | 20.7 ± 8.9 | 13.2 ± 1.4 |
| Distance moved (cm) | 2,906 ± 362 | 2,970 ± 214 |
| Elevated Zero Maze | ||
| % Time in Open Areas | 7.6 ± 2.8 | 5.6 ± 1.9 |
| Distance moved (cm) | 1,294 ± 220 | 1,463 ± 68 |
| Rotorod Performance | ||
| Mean Fall Latency (sec) | 40.0 ± 3.7 | 46.9 ± 4.0 |
| Passive Avoidance | ||
| Trials to criterion Day 1 | 1.9 ± 0.3 | 2.3 ± 0.3 |
| Latency to Enter Day 2 (s)1 | 300 ± 0 | 300 ± 0 |
None of the mice re-entered the dark compartment.
In the Morris water maze, the visible and hidden platform learning curves were first analyzed using time to reach the platform location as a performance measure (Fig. 1A). CD44-null mice demonstrated no alterations in swim speeds during the visible (WT: 10.95 ± 1.15 cm/s; KO: 12.82 ± 1.04 cm/s) or hidden (WT: 15.00 ± 0.60 cm/s; KO: 13.00 ± 1.31 cm/s) platform training sessions. Both genotypes learned to locate the visible platform location (effect of session: F(3,42) = 22.977, p < 0.0001) but there was a genotype x session interaction (F(3,42) = 3.859, p = 0.016). This interaction was driven by a trend towards a genotype difference in the second visible platform training session (F(1,14) = 4.377, p = 0.055). As the hidden platform data were not normally distributed, they were log transformed first. Both genotypes learned to locate the hidden platform location (effect of session: F(5,70) = 3.994, p = 0.003) with no significant effect of genotype. Learning curves were also analyzed using distance moved as a performance measure (Fig. 1B). Both genotypes learned to locate the visible (effect of session: F(3,42) = 12.464, p < 0.0001) and hidden (effect of session: F(5,70) = 3.994, p = 0.003) platform locations, also with no significant effect of genotype. However, there was a profound genotype difference when spatial memory retention was assessed in the probe trial following the first hidden platform training day. While WT mice showed spatial memory retention and spent more time in the target quadrant than any other quadrant, KO mice did not (Fig. 1C). This impairment could be overcome with additional training. KO mice did show spatial memory retention in the probe trial following the second hidden platform training day (Fig. 1D).
Fig. 1.
CD44 mutant mice have hippocampal memory deficits. A. Water maze learning curves of wild type (WT) and CD44 null mice using time to reach the platform location (latency) as performance measure. B. Water maze learning curves analyzed using distance moved as performance measure. C. Spatial memory retention in the probe trial following the first day of hidden platform training. The mean time spent in each quadrant of the water maze was analyzed and the time spent in the quadrant containing the platform during the hidden platform training (Target quadrant) was compared to that in any other quadrant. *p < 0.04; **p < 0.01. D. Analysis of mean time spent in each quadrant of the Morris water maze by WT as compared to CD44 null mice during the second probe trial. *p < 0.04; **p < 0.02; ***p < 0.01.
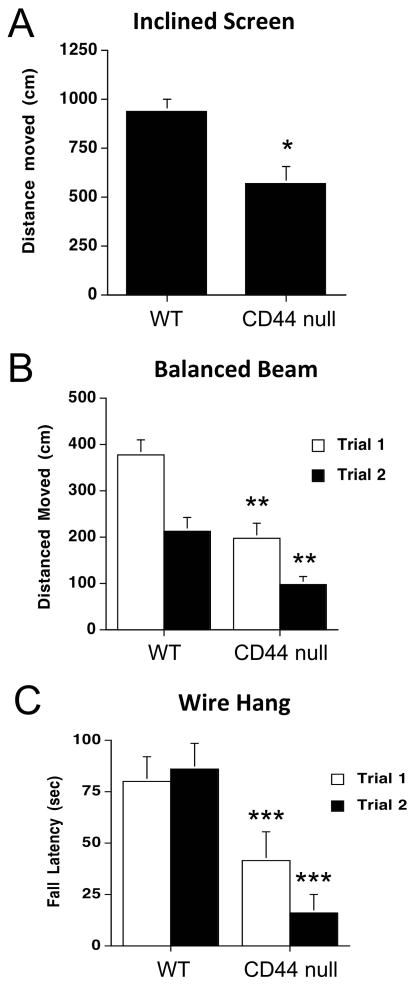
Rotorod performance assesses a combination of balance and muscle strength. There were no significant genotype differences in rotorod performance between WT and KO mice (Table 1). However, in the inclined screen test, KO mice moved less than WT mice (Fig. 2A, t(14) = 3.454, p = 0.0039). In the balance beam test, KO mice also moved less than WT mice (Fig. 2B, t(14) = 4.022, p < 0.0014). In the wire hang test, KO mice fell off the wire faster than WT mice (Fig. 2C, t(14) = 3.818, p < 0.002). These data and our analyses of swimming speeds in the Morris water maze indicate that although motor function is generally intact in the KO mice, they have some sensorimotor deficits.
Fig. 2.
A. CD44 null mice show reduced activity in the inclined screen test compared to wild type (WT) mice. There was a significant effect of genotype, t(14) = 3.454, *p = 0.0039. B. CD44 null mice move less than wild type mice in the balanced beam test, t(14) = 4.022, **p < 0.0014. C. CD44 null mice fall off the wire faster than wild type mice in the wire hang test., t(14) = 3.818, ***p < 0.002).
The passive avoidance test has been used to study learning and memory in the context of a stressful experience. In contrast to the spatial memory deficits we observed in the Morris water maze, there were no genotype differences in trials to criterion in passive avoidance learning or memory retention 24 hrs later (Table 1). These data suggest that the cognitive phenotypes of KO mice are limited to spatial memory retention deficits.
4. Discussion
We are the first group to identify sensorimotor and memory retention phenotypes in CD44 null animals. Mice lacking CD44 are viable, fertile, and do not exhibit gross physical or behavioral abnormalities [17]. A small number of previous studies examined CD44 null mice in some sensory and motor assays, including in the context of brain injury studies [e.g. 20, 21], but failed to find any deficits. We find that CD44 null animals have deficits in one behavioral test and one cognitive test: the light-dark test, which measures anxiety based on the innate aversion of rodents to brightly illuminated areas and on the spontaneous exploratory behaviors of rodents in response to mild stressors, and the Morris water maze, which measures spatial learning and memory. Interestingly, there were no differences between genotypes in the passive avoidance test, a test of emotional learning and memory, or in the elevated or plus maze tests. Similarly, while we found that the KO mice had deficits in some sensorimotor tests (the inclined screen, balanced beam, and wire hang), they did not have deficits in swimming speed or in rotorod performance. These findings suggest that the behavioral, cognitive and sensorimotor deficits we observed in the KO mice are not due to widespread CNS abnormalities but rather subtle changes in brain development and function.
The cognitive phenotype of the CD44-null mice is consistent with disturbances in hippocampal function. Combined with the fact that CD44 is expressed by neural stem cells [6, 15] and that neural stem cells undergoing neurogenesis are implicated in hippocampal learning and memory [16], our data raise the possibility that CD44 may influence adult hippocampal neurogenesis. Some components of contextual fear conditioning involve hippocampal neurogenesis [22] and spatial memory in the Morris water maze is also sensitive to alterations in adult neurogenesis [23]. Given the contributions of CD44 and hyaluronan to regulating cell proliferation and differentiation, it will be interesting to test how their disruption influences cells within neural stem cell niches.
CD44 null mice exhibited memory retention deficits in the Morris water maze task
CD44 null mice exhibited sensorimotor deficits in some tests
CD44 is necessary for normal nervous system development and function
Acknowledgments
We thank Dr. Steven Matsumoto for helpful comments. This work was supported by National Institutes of Health grants NS056234 (to LSS), core grant RR00163 supporting the Oregon National Primate Research Center, NIDA training grant T32 DA07262, and the Portland Saturday Academy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 2.Galtrey CM, Kwok JC, Carulli D, Rhodes KE, Fawcett JW. Distribution and synthesis of extracellular matrix proteoglycans, hyaluronan, link proteins and tenascin-R in the rat spinal cord. Eur J Neurosci. 2008;27:1373–90. doi: 10.1111/j.1460-9568.2008.06108.x. [DOI] [PubMed] [Google Scholar]
- 3.Asher R, Perides G, Vanderhaeghen JJ, Bignami A. Extracellular matrix of central nervous system white matter: demonstration of an hyaluronate-protein complex. J Neurosci Res. 1991;28:410–21. doi: 10.1002/jnr.490280314. [DOI] [PubMed] [Google Scholar]
- 4.Girgrah N, Letarte M, Becker LE, Cruz TF, Theriault E, Moscarello MA. Localization of the CD44 glycoprotein to fibrous astrocytes in normal white matter and to reactive astrocytes in active lesions in multiple sclerosis. J Neuropathol Exp Neurol. 1991;50:779–92. doi: 10.1097/00005072-199111000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Shinoe T, Kuribayashi H, Saya H, Seiki M, Aburatani H, Watanabe S. Identification of CD44 as a cell surface marker for Müller glia precursor cells. J Neurochem. 2010;115:1633–42. doi: 10.1111/j.1471-4159.2010.07072.x. [DOI] [PubMed] [Google Scholar]
- 6.Naruse M, Shibasaki K, Yokoyama S, Kurachi M, Ishizaki Y. Dynamic changes of CD44 expression from progenitors to subpopulations of astrocytes and neurons in developing cerebellum. PLoS One. 2013;8:e53109. doi: 10.1371/journal.pone.0053109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sretavan DW, Feng L, Puré E, Reichardt LF. Embryonic neurons of the developing optic chiasm express L1 and CD44, cell surface molecules with opposing effects on retinal axon growth. Neuron. 1994;12:957–75. doi: 10.1016/0896-6273(94)90307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Back SA, Tuohy TM, Chen H, Wallingford N, Craig A, Struve J, Luo NL, Banine F, Liu Y, Chang A, Trapp BD, Bebo BF, Jr, Rao MS, Sherman LS. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005 Sep;11(9):966–72. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- 9.Tuohy TM, Wallingford N, Liu Y, Chan FH, Rizvi T, Xing R, Bebo B, Rao MS, Sherman LS. CD44 overexpression by oligodendrocytes: a novel mouse model of inflammation-independent demyelination and dysmyelination. Glia. 2004 Sep;47(4):335–45. doi: 10.1002/glia.20042. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Zhan Y, Xu L, Feuerstein GZ, Wang X. Use of suppression subtractive hybridization for differential gene expression in stroke: discovery of CD44 gene expression and localization in permanent focal stroke in rats. Stroke. 2001;32:1020–7. doi: 10.1161/01.str.32.4.1020. [DOI] [PubMed] [Google Scholar]
- 11.Girgrah N, Letarte M, Becker LE, Cruz TF, Theriault E, Moscarello MA. Localization of the CD44 glycoprotein to fibrous astrocytes in normal white matter and to reactive astrocytes in active lesions in multiple sclerosis. J Neuropathol Exp Neurol. 1991;50:779–92. doi: 10.1097/00005072-199111000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Jones LL, Liu Z, Shen J, Werner A, Kreutzberg GW, Raivich G. Regulation of the cell adhesion molecule CD44 after nerve transection and direct trauma to the mouse brain. J Comp Neurol. 2000;426:468–92. doi: 10.1002/1096-9861(20001023)426:3<468::aid-cne9>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 13.Moon C, Heo S, Sim KB, Shin T. Upregulation of CD44 expression in the spinal cords of rats with clip compression injury. Neurosci Lett. 2004;367:133–6. doi: 10.1016/j.neulet.2004.05.101. [DOI] [PubMed] [Google Scholar]
- 14.Bausch SB. Potential roles for hyaluronan and CD44 in kainic acid-induced mossy fiber sprouting in organotypic hippocampal slice cultures. Neuroscience. 2006;143:339–50. doi: 10.1016/j.neuroscience.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 15.Oishi K, Ito-Dufros Y. Angiogenic potential of CD44+ CD90+ multipotent CNS stem cells in vitro. Biochem Biophys Res Commun. 2006;349:1065–72. doi: 10.1016/j.bbrc.2006.08.135. [DOI] [PubMed] [Google Scholar]
- 16.Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–42. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Protin U, Schweighoffer T, Jochum W, Hilberg F. CD44-deficient mice develop normally with changes in subpopulations and recirculation of lymphocyte subsets. J Immunol. 1999;163:4917–23. [PubMed] [Google Scholar]
- 18.Siegel JA, Haley GE, Raber J. Apolipoprotein E isoform-dependent effects on anxiety and cognition in female TR mice. Neurobiol Aging. 2012;33:345–58. doi: 10.1016/j.neurobiolaging.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matagne V, Budden S, Ojeda SR, Raber J. Correcting deregulated Fxyd1 expression ameliorates a behavioral impairment in a mouse model of Rett syndrome. Brain Res. 2013;1496:104–14. doi: 10.1016/j.brainres.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Xu L, Wang H, Zhan Y, Puré E, Feuerstein GZ. CD44 deficiency in mice protects brain from cerebral ischemia injury. J Neurochem. 2002;83:1172–9. doi: 10.1046/j.1471-4159.2002.01225.x. [DOI] [PubMed] [Google Scholar]
- 21.Hertzano R, Puligilla C, Chan SL, Timothy C, Depireux DA, Ahmed Z, Wolf J, Eisenman DJ, Friedman TB, Riazuddin S, Kelley MW, Strome SE. CD44 is a marker for the outer pillar cells in the early postnatal mouse inner ear. J Assoc Res Otolaryngol. 2010;11:407–18. doi: 10.1007/s10162-010-0211-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan YW, Chan GC, Kuo CT, Storm DR, Xia Z. Inhibition of adult neurogenesis by inducible and targeted deletion of ERK5 mitogen-activated protein kinase specifically in adult neurogenic regions impairs contextual fear extinction and remote fear memory. J Neurosci. 2012;32:6444–55. doi: 10.1523/JNEUROSCI.6076-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–54. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]