Abstract
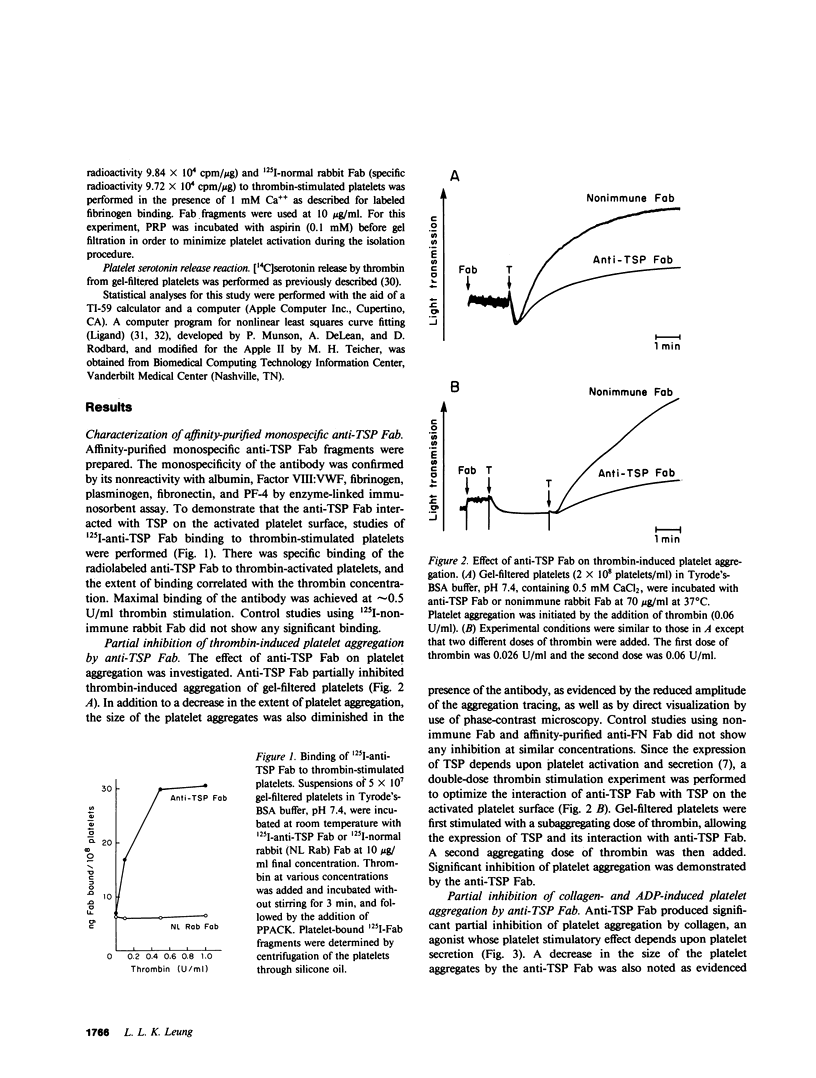
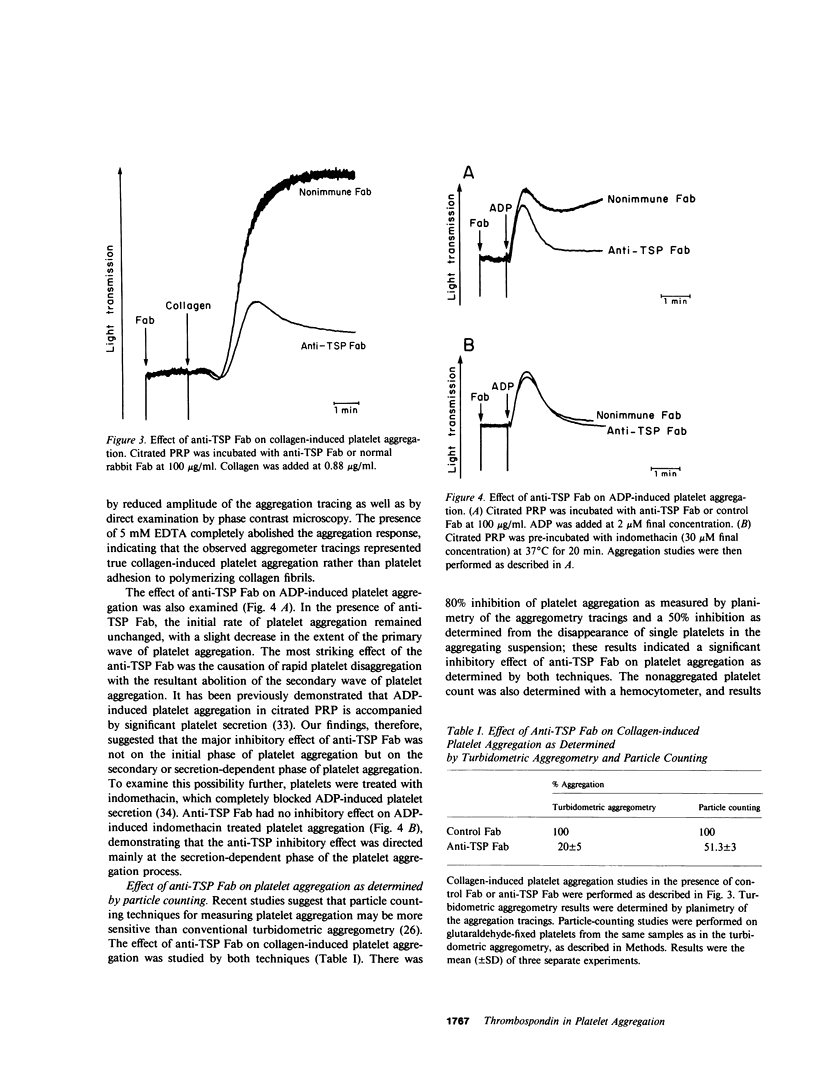
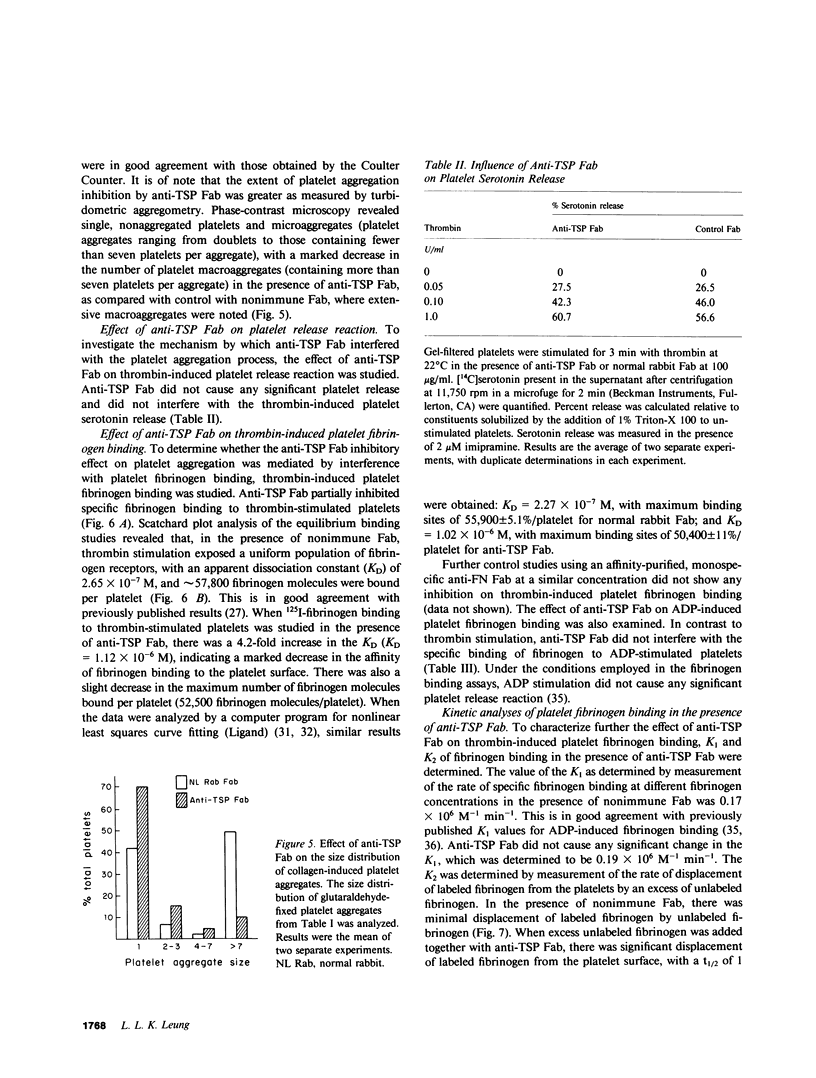
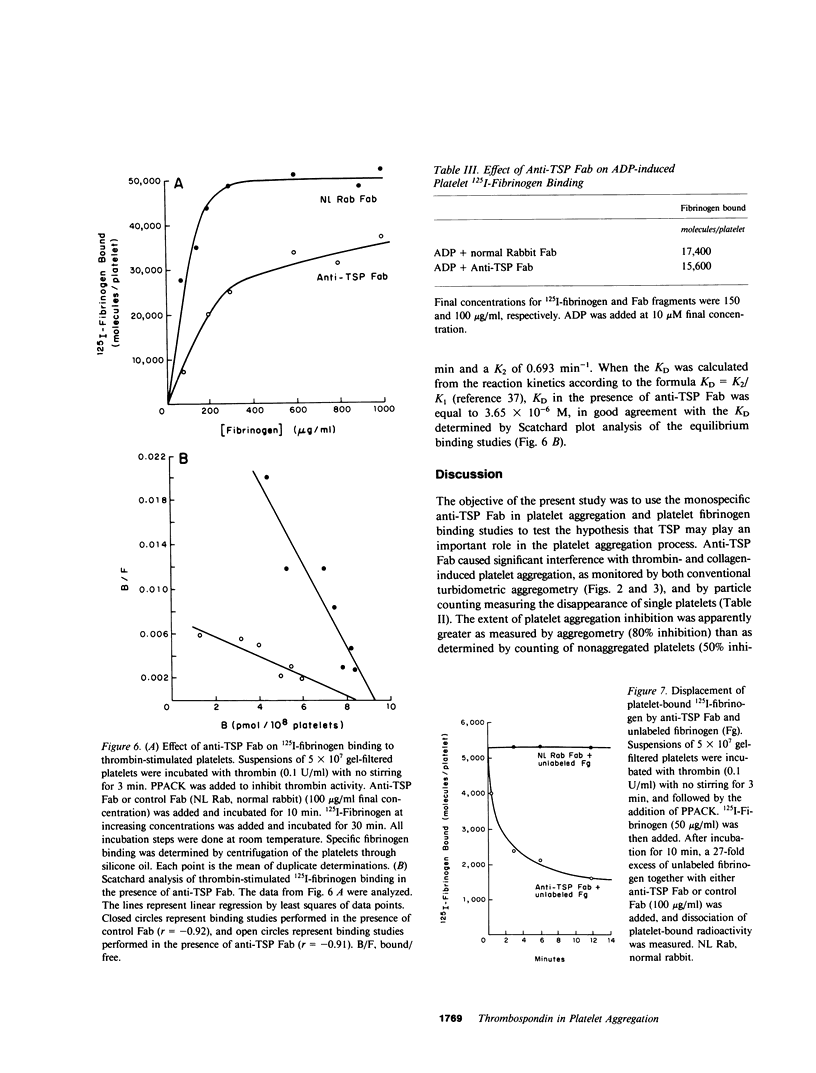
Thrombospondin (TSP), the major alpha-granule protein of human platelets, binds to the activated platelet surface upon platelet stimulation. TSP has hemagglutinating (lectin-like) activity and forms a specific complex with fibrinogen. Based on these observations, it was postulated that the interaction of TSP and fibrinogen on the activated platelet surface may be an important step in the platelet aggregation process. To test this hypothesis, monospecific, affinity-purified anti-TSP Fab fragments were prepared and their effects on platelet aggregation and platelet fibrinogen binding were studied. Anti-TSP Fab caused significant interference with thrombin- and collagen-induced platelet aggregation, as monitored by both turbidometric aggregometry and particle counting measuring the disappearance of single platelets. Phase-contrast microscopy revealed that anti-TSP Fab caused a marked decrease in platelet macroaggregates and an increase in microaggregates and nonaggregated single platelets. Anti-TSP Fab did not affect the initial phase of ADP-induced platelet aggregation but caused rapid platelet disaggregation with the abolition of the secondary phase of aggregation. The effect of anti-TSP Fab was not mediated by a direct inhibition of platelet secretion. The effect of anti-TSP Fab on specific binding of labeled fibrinogen to thrombin-stimulated platelets was also studied. Anti-TSP Fab caused a marked decrease in the affinity of fibrinogen binding to the receptors on the activated platelet surface. Kinetic analyses revealed significant displacement of labeled fibrinogen by unlabeled fibrinogen in the presence of anti-TSP Fab, suggesting that TSP serves to stabilize fibrinogen binding to the activated platelet surface and reinforces the strength of interplatelet interactions. It is proposed that platelet aggregation is a dynamic, multistep process, governed initially by the platelet membrane glycoprotein IIb/IIIa-fibrinogen interaction, with the TSP-fibrinogen interaction playing an important role in determining the size and reversibility of platelet aggregates.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger N. L., Brodie G. N., Majerus P. W. A thrombin-sensitive protein of human platelet membranes. Proc Natl Acad Sci U S A. 1971 Jan;68(1):240–243. doi: 10.1073/pnas.68.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. S., Hoxie J. A., Leitman S. F., Vilaire G., Cines D. B. Inhibition of fibrinogen binding to stimulated human platelets by a monoclonal antibody. Proc Natl Acad Sci U S A. 1983 May;80(9):2417–2421. doi: 10.1073/pnas.80.9.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. S., Vilaire G., Cines D. B. Identification of the fibrinogen receptor on human platelets by photoaffinity labeling. J Biol Chem. 1982 Jul 25;257(14):8049–8054. [PubMed] [Google Scholar]
- Bennett J. S., Vilaire G. Exposure of platelet fibrinogen receptors by ADP and epinephrine. J Clin Invest. 1979 Nov;64(5):1393–1401. doi: 10.1172/JCI109597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth W. J., Berndt M. C., Castaldi P. A. An altered platelet granule glycoprotein in patients with essential thrombocythemia. J Clin Invest. 1984 Feb;73(2):291–297. doi: 10.1172/JCI111213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Coller B. S., Peerschke E. I., Scudder L. E., Sullivan C. A. A murine monoclonal antibody that completely blocks the binding of fibrinogen to platelets produces a thrombasthenic-like state in normal platelets and binds to glycoproteins IIb and/or IIIa. J Clin Invest. 1983 Jul;72(1):325–338. doi: 10.1172/JCI110973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lean A., Hancock A. A., Lefkowitz R. J. Validation and statistical analysis of a computer modeling method for quantitative analysis of radioligand binding data for mixtures of pharmacological receptor subtypes. Mol Pharmacol. 1982 Jan;21(1):5–16. [PubMed] [Google Scholar]
- Di Minno G., Thiagarajan P., Perussia B., Martinez J., Shapiro S., Trinchieri G., Murphy S. Exposure of platelet fibrinogen-binding sites by collagen, arachidonic acid, and ADP: inhibition by a monoclonal antibody to the glycoprotein IIb-IIIa complex. Blood. 1983 Jan;61(1):140–148. [PubMed] [Google Scholar]
- Frojmovic M. M., Milton J. G., Duchastel A. Microscopic measurements of platelet aggregation reveal a low ADP-dependent process distinct from turbidometrically measured aggregation. J Lab Clin Med. 1983 Jun;101(6):964–976. [PubMed] [Google Scholar]
- Gartner T. K., Dockter M. E. Secreted platelet thrombospondin binds monovalently to platelets and erythrocytes in the absence of free Ca2+. Thromb Res. 1984 Jan 1;33(1):19–30. doi: 10.1016/0049-3848(84)90151-8. [DOI] [PubMed] [Google Scholar]
- Gartner T. K., Gerrard J. M., White J. G., Williams D. C. Fibrinogen is the receptor for the endogenous lectin of human platelets. Nature. 1981 Feb 19;289(5799):688–690. doi: 10.1038/289688a0. [DOI] [PubMed] [Google Scholar]
- Gartner T. K., Williams D. C., Minion F. C., Phillips D. R. Thrombin-induced platelet aggregation is mediated by a platelet plasma membrane-bound lectin. Science. 1978 Jun 16;200(4347):1281–1283. doi: 10.1126/science.663608. [DOI] [PubMed] [Google Scholar]
- George J. N., Onofre A. R. Human platelet surface binding of endogenous secreted factor VIII-von Willebrand factor and platelet factor 4. Blood. 1982 Jan;59(1):194–197. [PubMed] [Google Scholar]
- Gerrard J. M., Phillips D. R., Rao G. H., Plow E. F., Walz D. A., Ross R., Harker L. A., White J. G. Biochemical studies of two patients with the gray platelet syndrome. Selective deficiency of platelet alpha granules. J Clin Invest. 1980 Jul;66(1):102–109. doi: 10.1172/JCI109823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogstad G. O., Brosstad F., Krutnes M. B., Hagen I., Solum N. O. Fibrinogen-binding properties of the human platelet glycoprotein IIb-=IIIa complex: a study using crossed-radioimmunoelectrophoresis. Blood. 1982 Sep;60(3):663–671. [PubMed] [Google Scholar]
- Hagen I. Effects of thrombin on washed, human platelets: changes in the subcellular fractions. Biochim Biophys Acta. 1975 Jun 12;392(2):242–254. doi: 10.1016/0304-4165(75)90006-9. [DOI] [PubMed] [Google Scholar]
- Handin R. I., Cohen H. J. Purification and binding properties of human platelet factor four. J Biol Chem. 1976 Jul 25;251(14):4273–4282. [PubMed] [Google Scholar]
- Hawiger J., Parkinson S., Timmons S. Prostacyclin inhibits mobilisation of fibrinogen-binding sites on human ADP- and thrombin-treated platelets. Nature. 1980 Jan 10;283(5743):195–197. doi: 10.1038/283195a0. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Leung L. L., Nachman R. L., Levin R. I., Mosher D. F. Thrombospondin is the endogenous lectin of human platelets. Nature. 1982 Jan 21;295(5846):246–248. doi: 10.1038/295246a0. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Ruggiero J. T., Leung L. K., Doyle M. J., McKeown-Longo P. J., Mosher D. F. Cultured human fibroblasts synthesize and secrete thrombospondin and incorporate it into extracellular matrix. Proc Natl Acad Sci U S A. 1983 Feb;80(4):998–1002. doi: 10.1073/pnas.80.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettner C., Shaw E. D-Phe-Pro-ArgCH2C1-A selective affinity label for thrombin. Thromb Res. 1979;14(6):969–973. doi: 10.1016/0049-3848(79)90014-8. [DOI] [PubMed] [Google Scholar]
- Kloczewiak M., Timmons S., Hawiger J. Recognition site for the platelet receptor is present on the 15-residue carboxy-terminal fragment of the gamma chain of human fibrinogen and is not involved in the fibrin polymerization reaction. Thromb Res. 1983 Jan 15;29(2):249–255. doi: 10.1016/0049-3848(83)90147-0. [DOI] [PubMed] [Google Scholar]
- Lahav J., Schwartz M. A., Hynes R. O. Analysis of platelet adhesion with a radioactive chemical crosslinking reagent: interaction of thrombospondin with fibronectin and collagen. Cell. 1982 Nov;31(1):253–262. doi: 10.1016/0092-8674(82)90425-1. [DOI] [PubMed] [Google Scholar]
- Lawler J. W., Slayter H. S., Coligan J. E. Isolation and characterization of a high molecular weight glycoprotein from human blood platelets. J Biol Chem. 1978 Dec 10;253(23):8609–8616. [PubMed] [Google Scholar]
- Leung L. L., Harpel P. C., Nachman R. L., Rabellino E. M. Histidine-rich glycoprotein is present in human platelets and is released following thrombin stimulation. Blood. 1983 Nov;62(5):1016–1021. [PubMed] [Google Scholar]
- Leung L. L., Nachman R. L. Complex formation of platelet thrombospondin with fibrinogen. J Clin Invest. 1982 Sep;70(3):542–549. doi: 10.1172/JCI110646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung L. L., Nachman R. L., Harpel P. C. Complex formation of platelet thrombospondin with histidine-rich glycoprotein. J Clin Invest. 1984 Jan;73(1):5–12. doi: 10.1172/JCI111206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margossian S. S., Lawler J. W., Slayter H. S. Physical characterization of platelet thrombospondin. J Biol Chem. 1981 Jul 25;256(14):7495–7500. [PubMed] [Google Scholar]
- Marguerie G. A., Ardaillou N., Cherel G., Plow E. F. The binding of fibrinogen to its platelet receptor. J Biol Chem. 1982 Oct 25;257(20):11872–11875. [PubMed] [Google Scholar]
- Marguerie G. A., Edgington T. S., Plow E. F. Interaction of fibrinogen with its platelet receptor as part of a multistep reaction in ADP-induced platelet aggregation. J Biol Chem. 1980 Jan 10;255(1):154–161. [PubMed] [Google Scholar]
- Marguerie G. A., Plow E. F. Interaction of fibrinogen with its platelet receptor: kinetics and effect of pH and temperature. Biochemistry. 1981 Mar 3;20(5):1074–1080. doi: 10.1021/bi00508a005. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- McEver R. P., Bennett E. M., Martin M. N. Identification of two structurally and functionally distinct sites on human platelet membrane glycoprotein IIb-IIIa using monoclonal antibodies. J Biol Chem. 1983 Apr 25;258(8):5269–5275. [PubMed] [Google Scholar]
- McPherson J., Sage H., Bornstein P. Isolation and characterization of a glycoprotein secreted by aortic endothelial cells in culture. Apparent identity with platelet thrombospondin. J Biol Chem. 1981 Nov 10;256(21):11330–11336. [PubMed] [Google Scholar]
- Montgomery R. R., Kunicki T. J., Taves C., Pidard D., Corcoran M. Diagnosis of Bernard-Soulier syndrome and Glanzmann's thrombasthenia with a monoclonal assay on whole blood. J Clin Invest. 1983 Feb;71(2):385–389. doi: 10.1172/JCI110780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher D. F., Doyle M. J., Jaffe E. A. Synthesis and secretion of thrombospondin by cultured human endothelial cells. J Cell Biol. 1982 May;93(2):343–348. doi: 10.1083/jcb.93.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby S. M., Raugi G. J., Bornstein P. Interactions of thrombospondin with extracellular matrix proteins: selective binding to type V collagen. J Cell Biol. 1984 Feb;98(2):646–652. doi: 10.1083/jcb.98.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Mustard J. F., Perry D. W., Kinlough-Rathbone R. L., Packham M. A. Factors responsible for ADP-induced release reaction of human platelets. Am J Physiol. 1975 Jun;228(6):1757–1765. doi: 10.1152/ajplegacy.1975.228.6.1757. [DOI] [PubMed] [Google Scholar]
- Nachman R. L., Leung L. L. Complex formation of platelet membrane glycoproteins IIb and IIIa with fibrinogen. J Clin Invest. 1982 Feb;69(2):263–269. doi: 10.1172/JCI110448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurden A. T., Caen J. P. The different glycoprotein abnormalities in thrombasthenic and Bernard-Soulier platelets. Semin Hematol. 1979 Jul;16(3):234–250. [PubMed] [Google Scholar]
- Nurden A. T., Kunicki T. J., Dupuis D., Soria C., Caen J. P. Specific protein and glycoprotein deficiencies in platelets isolated from two patients with the gray platelet syndrome. Blood. 1982 Apr;59(4):709–718. [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. R., Jennings L. K., Prasanna H. R. Ca2+-mediated association of glycoprotein G (thrombinsensitive protein, thrombospondin) with human platelets. J Biol Chem. 1980 Dec 25;255(24):11629–11632. [PubMed] [Google Scholar]
- Plow E. F., Ginsberg M. H. Specific and saturable binding of plasma fibronectin to thrombin-stimulated human platelets. J Biol Chem. 1981 Sep 25;256(18):9477–9482. [PubMed] [Google Scholar]
- Plow E. F., Marguerie G. A. Participation of ADP in the binding of fibrinogen to thrombin-stimulated platelets. Blood. 1980 Sep;56(3):553–555. [PubMed] [Google Scholar]
- Raugi G. J., Mumby S. M., Abbott-Brown D., Bornstein P. Thrombospondin: synthesis and secretion by cells in culture. J Cell Biol. 1982 Oct;95(1):351–354. doi: 10.1083/jcb.95.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbard D., Feldman H. A. Theory of protein-ligand interaction. Methods Enzymol. 1975;36:3–16. doi: 10.1016/s0076-6879(75)36003-5. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E. G., Pierschbacher M., Engvall E. Fibronectin: purification, immunochemical properties, and biological activities. Methods Enzymol. 1982;82(Pt A):803–831. doi: 10.1016/0076-6879(82)82103-4. [DOI] [PubMed] [Google Scholar]
- Sage H., Farin F. M., Striker G. E., Fisher A. B. Granular pneumocytes in primary culture secrete several major components of the extracellular matrix. Biochemistry. 1983 Apr 26;22(9):2148–2155. doi: 10.1021/bi00278a015. [DOI] [PubMed] [Google Scholar]
- Silverstein R. L., Leung L. L., Harpel P. C., Nachman R. L. Complex formation of platelet thrombospondin with plasminogen. Modulation of activation by tissue activator. J Clin Invest. 1984 Nov;74(5):1625–1633. doi: 10.1172/JCI111578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. B., Ingerman C., Kocsis J. J., Silver M. J. Formation of prostaglandins during the aggregation of human blood platelets. J Clin Invest. 1973 Apr;52(4):965–969. doi: 10.1172/JCI107262. [DOI] [PMC free article] [PubMed] [Google Scholar]



