Abstract
Background & Aims
Mice lacking the receptor toll-like receptor 5 (TLR5-null mice), which recognizes flagellin, have an altered intestinal microbiota composition compared to wild-type mice; they develop low-grade inflammation, metabolic syndrome, and are prone to colitis. The relative roles of intestinal epithelial cell (IEC) vs dendritic cell (DC) TLR5 in mediating these phenotypes is not clear; modification of intestinal microbiota composition has been reported to reflect animal husbandry practices rather than loss of TLR5. We generated mice with specific disruption of Tlr5 in IEC or DC using a breeding scheme that allowed comparison with co-housed siblings, as controls.
Methods
We generated C57BL/6 mice with LoxP sites flanking Tlr5. These mice were crossed with mice expressing Cre recombinase, regulated by the villin or CD11c promoters, to generate mice that lacked expression of TLR5 by IEC (TLR5ΔIEC) or DC (TLR5ΔDC), respectively. Tlr5fl/fl siblings were used as controls. Upon weaning, mice were housed by sex and genotype or by sex only (genotypes cohoused). Mice were examined for basal phenotypes, including microbiota composition; we also analyzed responses to pathobiont challenge, dextran sodium sulfate administration, and high-fat diets.
Results
Similar to previous findings from TLR5-null mice, TLR5ΔIEC mice had low-grade inflammation (mild splenomegaly, shortened colons, and increased fecal levels of lipocalin-2), metabolic syndrome, an inability to clear pathobionts, and were prone to develop colitis compared to their sibling controls, under both housing conditions. Development of this inflammation in the TLR5ΔIEC mice was eliminated by administration of antibiotics, and associated with alterations in localization of microbiota and levels of fecal lipopolysaccharide and flagellin. The composition of the microbiota clustered more closely according to genotype than housing. Loss of TLR5 from DC did not associate with development of inflammation-associated phenotypes or alterations in the composition of the microbiota, but resulted in complete loss of flagellin-induced production of interleukin 22 (IL22).
Conclusion
In mice, flagellin activation of TLR5 on DC leads to IL22 production. Expression of TLR5 on IEC regulates the composition and localization of the intestinal microbiota, preventing diseases associated with intestinal inflammation.
Keywords: LPS, mouse model, IBD, intestine
INTRODUCTION
Several chronic inflammatory diseases affecting the gastro-intestinal tract, as well as a variety of other organ systems, are associated with alterations in gut microbiota composition, suggesting involvement of the microbiota in these disorders 1. Hence, ablation of the microbiota via use of germ-free mice and/or antibiotic treatment prevents development of disease in mouse models of inflammatory diseases 2. Accordingly, use of these tractable models has defined factors that regulate microbiota composition and its influence on inflammation. Such studies indicate that innate immunity maintainins a healthy gut microbiota composition, thus avoiding development of chronic inflammation. For example, loss of the flagellin receptor toll-like receptor 5 (TLR5) results in increased levels of flagellated microbes that promotes low-grade inflammation, metabolic syndrome and colitis 3–5. Somewhat analogous phenotypes, and associated changes in microbiota composition, have also been observed in mice lacking TLR2 and Nod-like receptor P6 6, 7. However, some labs studying these mice have not observed such phenotypes and a recent study that carefully evaluated microbiota composition in several strains of TLR-deficient mice concluded that alterations in microbiota composition in such mice reflect animal housing practices rather than loss of these genes per se 8. Thus, the extent to which loss of select innate immune genes might result in altered microbiota composition and associated phenotypes remains unclear.
An additional ambiguity of mouse microbiota studies is that they have utilized global genetic deletions making it difficult to decipher mechanisms underlying their phenotypes. In the case of TLR5, that several epithelial cell lines respond robustly to flagellin in a TLR5-dependent and polarized manner 9, 10, suggested a major role for intestinal epithelial TLR5 in responding to flagellin released by flagellated intestinal bacteria. Bone marrow chimeras supported a role for TLR5 on non-hemopoietic cells in general 11, but could not distinguish the role of specific cell or tissue type that was important, which is particularly important in the context of disorders such as metabolic syndrome where many organs are affected. Studies with bone marrow chimeric mice have also unequivocally demonstrated a role for TLR5 on bone marrow derived cells in activating a signaling pathway that eventuated in robust induction of IL-22 12, 13 thought to activate protective pathways in epithelial cells, including up-regulation of antimicrobial gene expression. While such IL-22 production required the presence of CD11c+ cells, presumed to be dendritic cells, whether they directly responded to flagellin was not determined. Moreover, the use of bone marrow chimeras have proven difficult in defining cell type contributions to basal phenotypes associated with altered microbiota because the radiation and follow up antibiotics are, themselves, quite disruptive of the gut microbiota.
Hence, we sought to generate mice lacking TLR5 in only intestinal epithelium or CD11c+ cells, and to examine resulting phenotypes relative to their sibling controls that began life with similar microbiota composition. Such studies confirmed the suspected role of dendritic cell TLR5 in being the exclusive driver of flagellin-induced IL-22 production. However, it was loss of intestinal epithelial TLR5 that resulted in low-grade inflammation, metabolic syndrome, and predisposition to chemical- and immune dysregulation-induced colitis. Such phenotypes were associated with altered microbiota composition that resulted in greater levels of fecal bioactive pro-inflammatory flagellin and LPS.
MATERIALS AND METHODS
RESULTS
Generation of mice lacking TLR5 in intestinal epithelial or dendritic cells
Murine TLR5 is located on chromosome 1 in close proximity to a number of genes thought to have key functions in innate immunity. Thus, a caveat of studies performed to date with TLR5 deficient mice is that, since they were generated using ES129 stem cells 14, even extensive backcrossing onto a C57BL6 background does not reliably eliminate the possibility that phenotypes would result from placing some 129 genes onto C57BL6 mice rather than loss of TLR5 per se. Hence, we utilized pure C57BL6 stem cells to generate mice in which exon 1 of the TLR5 gene was flanked by loxP sites (figure 1A and S1). Such mice were then bred to mice engineered to express CRE recombinase under the control of the villin promoter allowing a breeding scheme in which all dams maintained WT TLR5 function while, on average, 50% of each litter would lack TLR5 in villin-expressing cells (TLR5ΔIEC). An analogous scheme was set up to generate mice lacking TLR5 in Cd11c-expressing cells (TLR5ΔDC). Both TLR5ΔIEC and TLR5ΔDC were born at the expected frequencies and appeared healthy. Upon weaning, mice were housed based on sex and genotype.
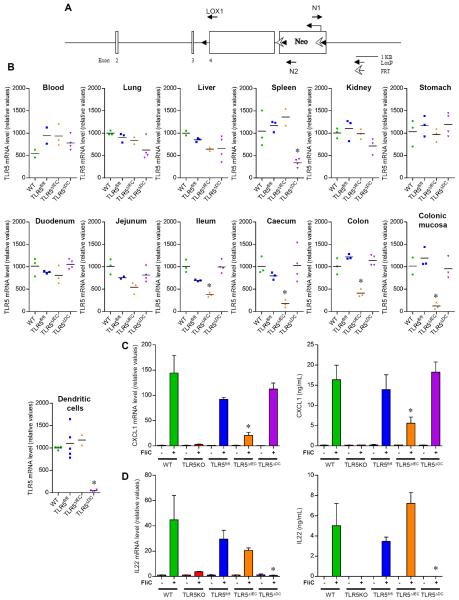
Figure 1. Generation and verification of mice with tissue specific TLR5 deletion.
(A) Schematical representation of the transgene containing TLR5 exon 1 flanked with LoxP sites. (B) Analysis of TLR5 mRNA expression by q-RT-PCR in blood, ling, liver, spleen, kidney, stomach, duodenum, jejunum, ileum, caecum, colon, colonic mucosa and purified CD11c+ splenic cells. (C) Analysis of CXCL1 mRNA expression by q-RT-PCR in the colonic mucosa and CXCL1 protein expression by ELISA in serum. Mice were intra-peritoneally injected with 20μg flagellin (+) or PBS (−). 30 min later, serum and intestinal mRNA were isolated. (D) Analysis of IL22 mRNA expression by q-RT-PCR in the colon and IL22 protein expression by ELISA in the serum of mice treated or not with 20μg of flagellin for 2 hours. Data are the means +/− S.E.M. or points are from individual mice with bar representing the mean. Significance was determined by Student's t-test, * indicates p<0.05 compared to TLR5fl/fl group.
First, we measured extent and specificity of TLR5 deletion based on TLR5 mRNA levels. The littermates of TLR5ΔIEC and TLR5ΔDC – that express flanking LoxP sites but not CRE transgenes – expressed similar levels of TLR5 mRNA as truly WT mice in all the organs tested, indicating that such sites did not significantly affect TLR5 expression levels (figure 1B and S2, table S1). Nor did LoxP sites, by themselves, significantly alter flagellin-induced gene expression as measured in the intestine and in serum (figure 1C). Relative to these TLR5fl/fl, TLR5ΔIEC displayed significant reduction of TLR5 mRNA expression in ileum, cecum and colon, exhibiting a greater than 95% reduction of TLR5 mRNA in colonic mucosal scrapings, which is highly enriched in epithelial cells (figure 1B and S2, table S1). Levels of TLR5 mRNA did not differ significantly from TLR5fl/fl littermates in blood, lung, liver, kidney, stomach, duodenum, spleen, nor purified CD11c+ cells. Thus, TLR5ΔIEC indeed displayed specific deletion of TLR5 expression in IEC, which were a major, but by no means exclusive, contributor to overall intestinal TLR5 expression. TLR5ΔDC displayed a near complete reduction in TLR5 mRNA in CD11c+ cells, demonstrating high efficacy of this CRE driver. Further, these mice displayed a marked reduction in splenic TLR5 mRNA, indicating that DC are the spleen's major cell type expressing TLR5. In contrast, TLR5 mRNA in TLR5ΔDC mice did not differ from that of TLR5fl/fl mice in any other of the above-mentioned organs, consistent with high specificity of this CRE driver and the notion that DC are a relatively rare, albeit very important, cell type in many organs.
Both DC and IEC TLR5 mediate flagellin-induced gene expression in gut
Next, we examined how loss of TLR5 expression affected the innate immune response to purified flagellin. While previous studies indicate that multiple cell types contribute to innate immune recognition of flagellin, rapid production of CXCL1 (mouse homologue of IL-8) has been suggested to largely be mediated by epithelial TLR511. In accord, flagellin-induced CXCL1 mRNA levels in the colonic mucosa was reduced by 75% in TLR5ΔIEC but maintained at WT levels in TLR5ΔDC (figure 1C). Such reductions were nor observed in liver, spleen, or lung tissues, confirming the specificity of the deletion previously observed (figure S4). A similar pattern was seen for induction of flagellin serum CXCL1 protein in response to flagellin, suggesting that the intestine is a major contributor to this response (figure 1C). Similar observations were made for IL-6, indicating that IEC TLR5 also has a major role in mediating expression of this highly-inducible cytokine (figure S3 and S4).
In contrast to the case of CXCL1 and IL-6, TLR5 on bone marrow derived cells mediates expression of IL-23 that results in rapid induction of IL-22 by innate lymphoid cells 12, 13. That depletion of DC ablated this response suggested direct recognition of flagellin might mediate this response but a role by another hemopoietic cell type in recognizing flagellin and then triggering DC activation could not be excluded with this methodology. However, as shown in figure 1D and S3, we observed that absence of TLR5 on DC but not IEC, completely eliminated activation of the IL-23/IL-22 axis, confirming the role of TLR5 on CD11c+ cells in driving this response.
Loss of IEC, but not DC, TLR5 results in low-grade inflammation and metabolic syndrome
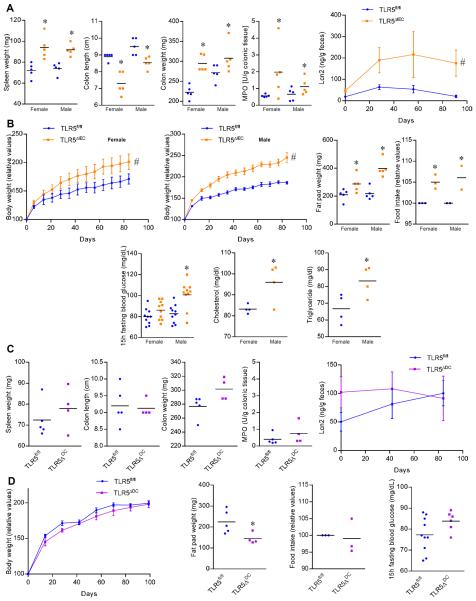
Total TLR5-deficiency makes mice prone to inflammation, including spontaneous colitis, although the extent and severity varies in different vivaria presumably reflecting differences in microbiota composition. Thus, we first examined our colony of tissue-specific TLR5 deletion and TLR5fl/fl control mice for spontaneous colitis but did not observe any incidence of this disorder. Next, we assayed the extent to which loss of TLR5 might result in more subtle inflammatory phenotypes comparing both TLR5ΔIEC and TLR5ΔDC to their age- and sex-matched littermates. We observed that loss of IEC TLR5 resulted in low-grade inflammation (figure 2A). Briefly, both male and female TLR5ΔIEC mice displayed shorter but heavier colons and mild splenomegaly, which was associated with a small increase in MPO and moderately elevated levels of fecal lipocalin-2 (LCN-2), which serves as a broadly dynamic marker of gut inflammation (figure 2A) 15. Such low-grade inflammation in TLR5ΔIEC mice was associated with panoply of characteristics of metabolic syndrome including increased weight and adiposity, hyperphagia, and elevated levels of blood glucose, cholesterol and trigylcerides (figure 2B). Inflammatory cell infiltration was observed in the colon of TLR5ΔIEC animals compare to TLR5fl/fl control mice (figure S5). In contrast, evidence of neither low-grade inflammation nor metabolic syndrome was observed in TLR5ΔDC mice (figure 2C–D and S6). Thus, low-grade inflammation and metabolic syndrome in TLR5KO mice (total knockout) is, at least in large part, a result of loss of intestinal epithelial cell TLR5.
Figure 2. Development of metabolic syndrome and low-grade intestinal inflammation in TLR5ΔIEC but not in TLR5ΔDC mice.
(A) Spleen weight, colon length, colon weight, colonic myeloperoxidase levels and fecal levels of the inflammatory marker Lcn2 over time of 15 weeks old TLR5fl/fl and TLR5ΔIEC mice, both males and females. (B) Body weight over time, fat pad weight, food intake measurement, 15h fasting blood glucose concentration, cholesterol and triglyceride concentration in the serum of 15 weeks old TLR5fl/fl and TLR5ΔIEC mice, both males and females. (C) Spleen weight, colon length, colon weight, colonic myeloperoxidase levels and fecal levels of the inflammatory marker Lcn2 over time of 15 weeks old TLR5fl/fl and TLR5ΔDC mice. (D) Body weight over time, fat pad weight, food intake, and 15h fasting blood glucose concentration of 15 weeks old TLR5fl/fl and TLR5ΔDC mice. Data are the means +/− S.E.M. or points are from individual mice with bar representing the mean. Significance was determined by Student's t-test (* indicates p<0.05) or 2-way group ANOVA (# indicates p<0.05) compared to TLR5fl/fl group.
Loss of IEC and DC TLR5 contribute to microbiota management
Proneness of TLR5KO mice to develop inflammation associates with inability to properly manage commensal microbiota 3. One manifestation is that TLR5KO mice have an approximately 3-fold elevation in levels of fecal bacteria as measured by PCR 4. As shown in figure 3A, such elevation in levels of fecal bacteria was observed in mice lacking IEC, but not DC, TLR5. TLR5ΔIEC also displayed an increase in levels of bacteria adherent to the mucosa (figure 3B). A non-significant trend in this direction was seen with TLR5ΔDC mice. To more directly assess microbiota management in its native state, we examined microbiota localization within the mucosa via confocal microscopy using a non-dehydrating fixative that maintains native mucus structures 16. In WT mice, in accord with previous studies, the closest bacteria were, on average, 22 μM from the epithelium and no bacteria observed within 10 μM of these host cells (figure 3C–D). In contrast, in TLR5ΔIEC, such bacteria were, on average, about 5 μM and many fields examined exhibited bacteria in direct contact with epithelial cells. In TLR5ΔDC, the average closest bacteria was modestly but significantly less than in TLR5fl/fl control mice but, unlike TLR5ΔIEC, bacteria were not observed within a few microns of the epithelium (figure 3C–D).
Figure 3. Loss of IEC and DC TLR5 contributes to microbiota management.
(A) PCR-based quantification of fecal bacterial load. (B) PCR-based quantification of bacterial load adhered to colonic mucosa. (C) Distances of closest bacteria to intestinal epithelial cells (IEC) per condition over 5 high-powered fields per mouse. (D) Confocal microscopy analysis of microbiota localization; Muc2 (green), actin (purple), bacteria (red), and DNA (Blue). (E) AIEC colonization was determinedin WT, TLR5KO, TLR5fl/fl, TLR5ΔIEC and TLR5ΔDC mice by platting on media containing selective antibiotics. Data are the means +/− S.E.M. and points represent data from individual mice. Significance was determined by Student's t-test, * indicates p<0.05 compared to WT or TLR5fl/flgroup.
Another consequence of complete loss of TLR5 is a delayed clearance of pathobiont bacteria, such as adhesive invasive E. coli (AIEC) 3, 17. Specifically, as shown in figure 3E, WT mice reduce levels of this pathobiont by 2 logs one day post-inoculation whereas levels remain unchanged in TLR5KO mice during this period (figure 3E). Significantly delayed clearance of this E. coli strain was also observed in TLR5ΔIEC and, to a lesser but nonetheless significant degree, TLR5ΔDC mice. Together, these results indicate that both IEC and DC TLR5 contribute to keeping microbiota in check and protecting against potential pathobionts with IEC-TLR5 having a predominant role in these processes.
Effect of high-fat diet (HFD) and TLR4 deficiency on TLR5ΔIEC metabolic syndrome
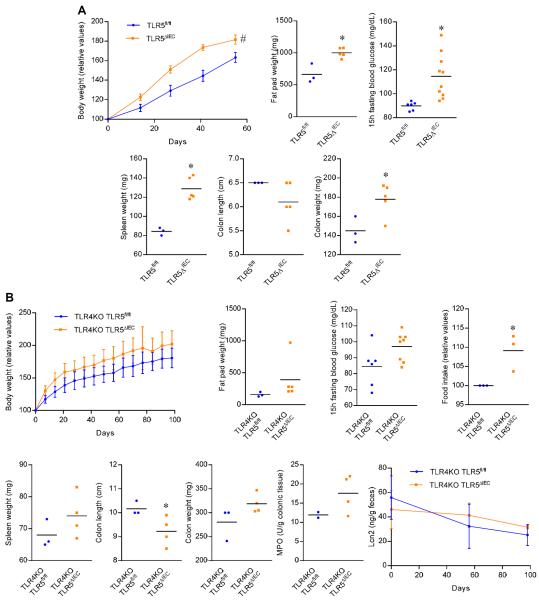
We next examined how well TLR5ΔIEC phenocopied some of our previous findings made with complete TLR5KO. First, we examined if a high-fat diet (FHD) would exacerbate their dysglycemia and/or promote hepatic steatosis. Analogous to findings with mice with complete TLR5 deficiency 4, the HFD exacerbated dysglycemia, particularly in female mice, which are normally more resistant to HFD-induced metabolic syndrome than males (figure 4A). In contrast, TLR5ΔIEC mice were not more prone to developing steatosis than their WT littermates, suggesting a possible direct role for TLR5 in the liver in protecting against this aspect of metabolic syndrome (data not shown). The LPS receptor TLR4 contributes to some aspects of metabolic syndrome in some models. Hence, we next examined the extent to which TLR5ΔIEC low-grade inflammation and metabolic syndrome might require TLR4. We observed that, when bred onto a TLR4-deficient background, loss of IEC TLR5 still displayed evidence of low-grade inflammation and metabolic syndrome relative to their TLR4-deficient TLR5-sufficient littermates, although these phenotypes were less severe than observed in mice with functional TLR4 (figure 4B). Hence, as was the case for mice with complete TLR5 deficiency 4, activation of TLR4 likely contributes to, but is not absolutely necessary for, development of inflammation.
Figure 4. Effect of high-fat diet (HFD) and TLR4 deficiency on TLR5ΔIEC metabolic syndrome.
(A) Body weight over time, fat pad weight, 15h fasting blood glucose concentration, spleen weight, colon length, colon weight, and liver weight of 14 weeks old TLR5fl/fl and TLR5ΔIEC females mice feed with high-fat diet for 8 weeks. (B) Body weight over time, fat pad weight, 15h fasting blood glucose concentration, food intake measurement, spleen weight, colon length, colon weight, colonic myeloperoxidase levels and fecal levels of the inflammatory marker Lcn2 over time of 15 weeks old TLR4KO-TLR5fl/fl and TLR4KO-TLR5ΔIEC males mice. Data are the means +/− S.E.M. or points are from individual mice with bar representing the mean. Significance was determined by Student's t-test (* indicates p<0.05) or 2-way group ANOVA (# indicates p<0.05) compared to TLR5fl/fl group.
TLR5ΔIEC are prone to chemical- and immune dysregulation-induced colitis
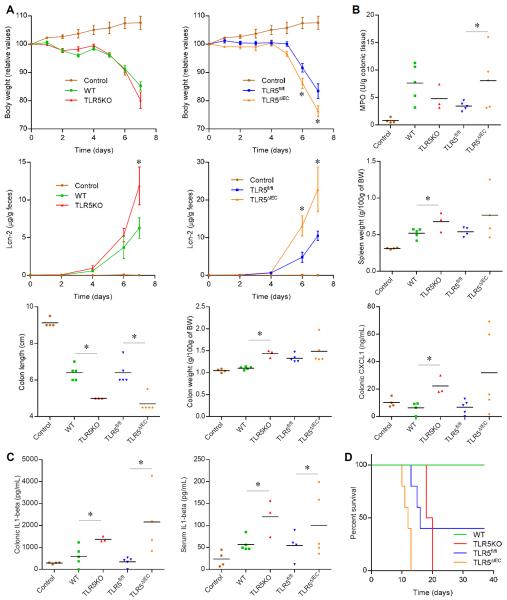
While development of colitis in TLR5KO is sporadic, a more consistent observation is that such mice are prone to develop more severe colitis in response to the chemical colitogen dextran sodium sulfate. Accordingly, relative to WT mice, exposure of TLR5KO mice resulted in more severe gross pathology, particularly colon shortening/thickening, and a greater inflammation as assessed by levels of fecal Lcn-2 and colonic MPO (figure 5A–B). A similar pattern of greater colitis in response to DSS was seen in TLR5ΔIEC relative to their TLR5fl/fl littermates. Such greater colitis severity seen in mice with deficiency in TLR5 correlated with greater levels of colonic interleukin 1β, suggesting increased inflammasome activation had occurred (figure 5C). Lastly, the previously reported greater mortality seen in TLR5KO mice maintained on low-dose DSS exposure was recapitulated by absence of IEC TLR5 (figure 5D).
Figure 5. TLR5ΔIEC are prone to chemical-induced colitis.
(A) Body weight over time, (B) colonic myeloperoxidase levels, fecal levels of the inflammatory marker Lcn2 over time, spleen weight, colon length, colon weight and colonic secretion of CXCL1 cytokine, and (C) colonic secretion of IL1β and serum IL1β of WT, TLR5KO, TLR5fl/fl and TLR5ΔIEC males mice treated with DSS 4% in drinking water for 7 days. (D) Survival curves of WT, TLR5KO, TLR5fl/fl and TLR5ΔIEC males mice treated with DSS 1.25% in drinking water for 40 days. Data are the means +/− S.E.M. or points are from individual mice with bar representing the mean. Significance was determined by Student's t-test, * indicates p<0.05 compared to WT or TLR5fl/fl group.
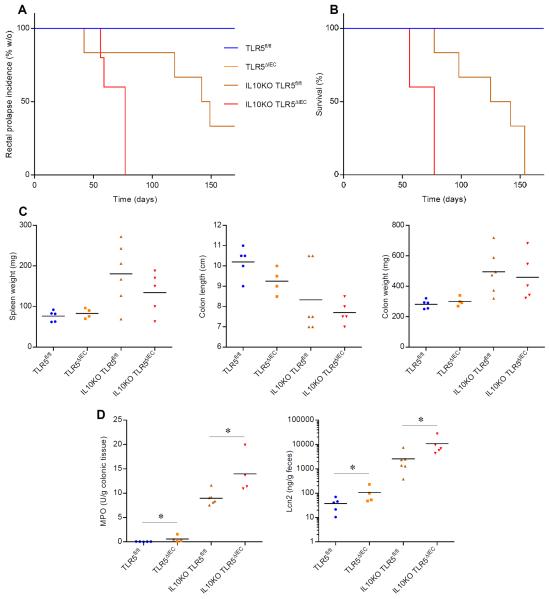
Breeding of TLR5KO onto an IL-10-deficient background resulted in severe uniform colitis in 100% of TLR5/IL-10-DKO mice 5. Hence, we examined if breeding IEC TLR5-deficiency onto this background would recapitulate such severe colitis. While, in our colony/vivarium, absence of IL-10 alone resulted in severe colitis with 100% of mice reaching IACUC endpoints (mostly rectal prolapse) by 5 months of age, their littermates that also had IEC-TLR5 deficiency reached such endpoints significantly faster (figure 6A–B). Further, such mice displayed greater inflammation as assessed by the above-mentioned gross, biochemical, and immunologic markers of colitis (figure 6C–D). Thus, together, these results indicate that the increased susceptibility of TLR5KO mice to develop colitis results, at least in part, from loss of IEC TLR5.
Figure 6. TLR5ΔIEC are prone to immune dysregulation-induced colitis.
(A) Rectal prolapse incidence (% without), (B) survival curves, (C) spleen weight, colon length and colon weight and (D) colonic myeloperoxidase levels and fecal levels of the inflammatory marker Lcn2 over time of TLR5fl/fl, TLR5ΔIEC, IL10KO-TLR5fl/fl and IL10KO-TLR5ΔIEC males mice. Points are from individual mice with bar representing the mean. Significance was determined by Student's t-test, * indicates p<0.05 compared to TLR5fl/fl group.
Loss of IEC TLR5 alters microbiota composition increasing its pro-inflammatory potential
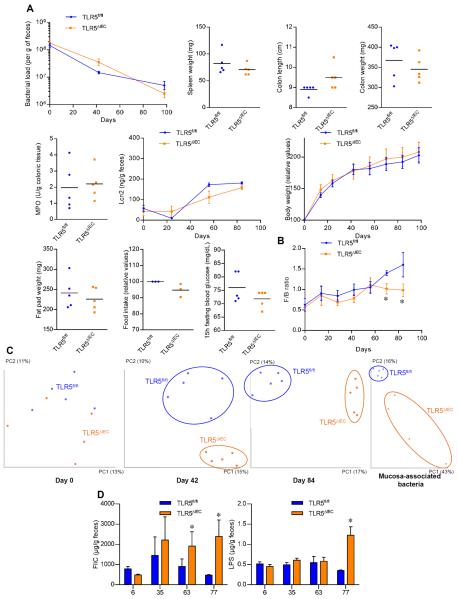
Low-grade inflammation and metabolic syndrome of TLR5KO mice can be prevented by rederiving them in germfree or by antibiotic treatment 4. As shown in figure 7A, this was also true for TLR5ΔIEC indicating basal inflammatory phenotype is a consequence of their inability to manage their gut microbiota. Thus, we examined how loss of IEC TLR5 affected gut microbiota composition. Total loss of TLR5 was reported to alter composition at the species (OTU) but not the family level but such studies compared off-springs of littermates rather than littermates, which would allow more opportunity for composition drift independent of genotype, and, moreover, might have reflected loss of TLR5 from non-IEC 4. We collected fecal samples from groups of TLR5ΔIEC and their TLR5fl/fl littermates (housed according to genotype) upon weaning at 3 weeks of age (day 0), and 42 and 84 days later as well as adherent bacteria at this endpoint. Such samples were subjected to 16S RNA sequencing using the Illumina MiSeq platform. In accord with our previous study, microbiota of TLR5ΔIEC displayed very little difference at the family level relative to their TLR5fl/fl littermates (figure S7). However, by 12-weeks post weaning, a significant increase of the Firmicutes to Bacteroidetes ratio, which has previously been associated with obesity (figure 7B). When using the UniFrac algorithm and principal component analysis at the species (OTU) level, there was no difference upon weaning in accord with the notion that husbandry practices have a great influence over microbiota composition. However, by 6 weeks post weaning, we observed a clear clustering of microbiota composition at the species level in PCoA plots of unweighted UniFrac metric, which was maintained 6 week later (figure 7C). A similar pattern of changes in microbiota composition was also observed for colonic adherent bacteria population (figure 7C).
Figure 7. TLR5ΔIEC have altered microbiota with increased pro-inflammatory potential.
(A) PCR-based quantification of fecal bacterial load, spleen weight, colon length and colon weight colonic myeloperoxidase levels, fecal levels of the inflammatory marker Lcn2 over time, body weight over time, fat pad weight, food intake measurement and 15h fasting blood glucose concentration of 15 weeks old TLR5fl/fl and TLR5ΔIEC mice treated with broad spectrum antibiotics for 14 weeks. (B) Firmicutes / Bacteroidetes ratio of TLR5fl/fl and TLR5ΔIEC mice. (C) Principal coordinates analysis (PCoA) of the UniFrac distance matrix of TLR5fl/fl and TLR5ΔIEC mice at days 0, 42 and 84 post-weaning as well as of mucosa-associated bacteria. (D) Bioactive levels of fecal flagellin and LPS assayed with TLR5 and TLR4 reporter cells. Data are the means +/− S.E.M. or points are from individual mice with bar representing the mean. Significance was determined by Student's t-test, * indicates p<0.05 compared to TLR5fl/fl group.
PAM-R analysis revealed that a substantial portion of this strong clustering was driven by an increase in abundance of some OTUs including members of the Prevotellaceae, Alcaligenaceae and Ruminoccoccaceae family and loss of several OTUs including members of the Bifidobacteriaceae family, all of those modifications previously been reported to also occur in intestinal inflammatory diseases 18–21 (figure S8). In addition, the Desulfovibrio genus was enriched in TLR5ΔIEC, analogous to observations in UC patients 22. These bacteria are known to be able to reduce sulfate (SO42−) to the toxic gas hydrogen sulfide (H2S), which may drive acute inflammation in CD and UC, 23 and promote T cell activation associated with chronic inflammation 24.
We next investigated if alteration in the microbiota might alter the inherent ability of its components to activate pro-inflammatory gene expression. Specifically, we generated fecal supernatant extracts and applied them to cells engineered to read out activation of TLR 4 or 5 17. Loss of levels of IEC TLR5 resulted in increased levels of bioactive (i.e. TLR-activating) flagellin and LPS, indicating that alteration of the microbiota composition that results from loss of IEC TLR5 inherently increased the microbiota's pro-inflammatory potential (figure 7D) suggesting a means by which altered microbiota composition contributes to inflammatory phenotypes.
Altered microbiota composition, low-grade inflammation, and metabolic syndrome are maintained in TLR5ΔIEC co-housed with TLR5fl/fl littermates
Lastly, to inquire into potential reasons why some, but not all, investigators have observed loss of select TLRs results in altered gut microbiota composition, we considered the possibility that our practice of caging mice by genotype upon weaning may have resulted in such cages of mice developing distinct microbiota compositions irrespective of genotype. However, that the microbiota composition or its pro-inflammatory potential did not significantly differ between TLR5ΔDC and their TLR5fl/fl littermates that had also been maintained in this manner argued against this possibility (figure S9). Nonetheless, we next examined the extent to which loss of IEC or DC TLR5 might alter microbiota composition and induce low-grade inflammation/metabolic syndrome in mice maintained in the same cages as their TLR5fl/fl siblings. We observed that, similar to TLR5ΔIEC and TLR5fl/fl littermates separated by genotype upon weaning, TLR5ΔIEC mice that were cohoused with their TLR5fl/fl littermates exhibited low-grade inflammation (mild splenomegaly, shortened colons, elevated fecal Lcn-2) and a metabolic syndrome phenotype (elevated body mass, increased adiposity, hyperglycemia) (figure S10). In contrast, these parameters did not significantly differ between TLR5ΔDC and co-housed TLR5fl/fl littermates (figure S10). We next examined microbiota composition in these groups of co-housed mice. Microbiota compositions of TLR5ΔDC and co-housed TLR5fl/fl littermates exhibited clustering based on cages but not genotype (figure S11). This result provides further evidence against a role for DC-TLR5 in determining microbiota composition while supporting the notion that caging is indeed one factor that needs to be controlled for when examining determinants that control microbiota composition. In contrast, when examining the microbiota composition of TLR5ΔIEC and their co-housed TLR5fl/fl littermates, clustering based on genotype was clearly more pronounced than that associated with caging (figure S11). Analogous to mice housed by genotype, loss of IEC, but not DC, TLR5, also resulted in increased microbiota levels of flagellin and LPS. Thus, whether housed by genotype or co-housed with WT mice, loss of IEC, but not DC, TLR5 results in altered microbiota composition and associated inflammatory phenotypes, particularly metabolic syndrome, indicating that IEC TLR5 plays an important role in protecting the gut against the microbiota.
DISCUSSION
Motility, or lack of it, determines a bacterium's modus vivendi impacting upon nutrient/niche acquisition, metabolism, and pathogenesis/commensalism. Accordingly, the mucosal immune system utilizes at least 2 means of innate immune recognition, namely TLR5 and NLRC4 to detect flagellin, which mediates bacterial locomotion 25. While both TLR5 and NLRC4 play a role in host defense against flagellated pathogens, TLR5 has also been observed to help manage the commensal microbiota, including efficiently clearing pathobionts that can promote disease if left un-checked 3, 17. Accordingly, absence of TLR5 has been reported to result in dysregulated microbiota composition, low-grade inflammation, metabolic syndrome and proneness to colitis 4, 5. Yet, the underlying mechanisms are not well defined, in part because TLR5 is expressed by multiple cell types and tissues. Herein, we report that loss of TLR5 on intestinal epithelial cells (IEC) is sufficient to result in alteration of gut microbiota composition, low-grade inflammation, metabolic syndrome and predisposition to development of colitis thus advancing mechanistic understanding of TLR5 function, and providing a tractable focused model by which an innate immune deficiency promote chronic inflammatory disease.
The notion that TLR5 on IEC plays a role in keeping microbiota in-check is consistent with in vitro observations that several epithelial cell lines are highly responsive to flagellin despite being hyporesponsive to some other TLR agonists. However, as intestinal CD11c+ phagocytes (herein referred to as DC but may well include some intestinal macrophages) are also highly responsive to flagellin while being relatively unresponsive to LPS 14, suggesting a potentially important role for DC TLR5 in host defense. TLR5ΔDC mice exhibited mild impairment in maintaining proper IEC-microbiota distance and clearing AIEC. Moreover, we confirmed the suspected essential role of DC-TLR5 for flagellin-induced activation of the IL-23/23 axis, which has increasingly-appreciated importance in intestinal host defense. Considering these results and the lack of basal clinical-type phenotype of TLR5ΔDC mice suggests that DC-TLR5 plays a role in diseases states associated with marked epithelial damage and acute colitis. Conversely, our results that TLR5ΔIEC mice developed low-grade inflammation and metabolic syndrome are in accord with the notion that innate immune signaling in IEC plays an essential role in managing the microbiota to maintain healthy homeostasis in the gut.
While our approach of generating and studying tissue-specific TLR5 deficient mice (technically difficult due to TLR5 having a single large coding exon) was initiated to distinguish the role of TLR5 on IEC vs. that of other cells, this model also serves to address discordant results between ourselves and others. Potential explanations to such discordance included potential anomalies in the generation of TLR5KO mice used in our previous studies to their having acquired an unusual microbiota while being bred in select vivariums. To avoid the former concern, targeting of TLR5 herein was performed on a pure C57BL/6 stem cell. Re the latter concern, our floxed mice were sent to Jackson Labs for sperm-based rederivation and, thereafter, bred only to CRE-expressing mice purchased from this supplier using a breeding scheme in which female breeders were effectively WT and litters would yield equal numbers of tissue-specific KO and effectively WT littermates. Hence, all mice studied herein likely began life with a microbiota similar to that of WT C57BL6 mice purchased from Jackson labs. Moreover, differences in phenotype and microbiota composition resulting from loss of IEC TLR5 were maintained when cohoused with effectively littermate WT mice. Thus, our observations argue that the propensity of TLR5-deficient mice to develop an altered microbiota composition, low-grade inflammation and metabolic syndrome is indeed a consequence of loss of TLR5 function rather than a caging artifact as recently suggested 8.
While the precise mechanism by which loss of IEC TLR5 promotes inflammatory diseases remains under investigation, our results to date are consistent with the notion that a small but not insignificant numbers of bacteria frequently breech the mucus barrier to contact or surmount the epithelium. Flagellated bacteria would likely have increased ability to transit through the mucus layer and would thus likely be enriched in such perturbing bacteria. Activation of TLR5 on IEC by bacteria approaching, adhering to, or breaching the epithelium can be envisaged to result in efficient recruitment of immune cells. In the absence of IEC-TLR5 signaling, clearance of commensal and/or pathobionts bacteria that might occasionally breech the mucosa would be delayed, as we observed herein was the case of adherent-invasive E. coli. The modest delay of AIEC clearance by TLR5ΔDC suggests DC TLR5 also contributes to recognition of this pathobiont. The quick response by the WT mucosal immune system to perturbing bacteria might quickly restore proper IEC-microbiota distance without inducing harmful inflammation that might also change in microbiota composition. Indeed, delayed clearance of this exogenously added pathobiont promotes chronic colitis when occurring in the context of microbiota acquisition and mucosal immune development 3, 17. Another consequence of reduced TLR5 activation might be reduced epithelial production of anti-microbial peptides that can result from direct activation of IEC TLR5 or via activation of the IL22/23 axis via activation of DC-TLR5. The reduced basal bacterial epithelial distance observed in both TLR5ΔIEC and TLR5ΔDC support a role for both pathways in this process, but that a much greater reduction in microbiota-epithelial distance and associated phenotype of low-grade inflammation/metabolic syndrome suggest a more prominent role for IEC-TLR5 in keeping the inner mucus layer clear of bacteria.
Another important means by which the mucosal immune system protects against microbiota-induced inflammation may be targeting of flagellin by the adaptive immune system. Specifically, TLR5 promotes generation of flagellin-specific IgA that serves to suppress flagellin gene expression 26. Analogously, humans carrying a dominant-negative TLR5 allele displayed reduce levels of naturally acquired flagellin specific antibodies 27. These results suggest the possibility that the mechanism by which loss of TLR5 results in low-grade inflammation may involve loss of antibody-mediated containment of motile bacteria that might activate TLR4, the NLRC4 inflammasome, and/or other pathways of innate immune recognition to promote pro-inflammatory gene expression 28, 29. That loss of TLR5 still results in low-grade inflammation upon a Rag-1KO background 4 (i.e. complete loss of adaptive immunity) argues against this being the sole mechanism, it may nonetheless be an important contributor. While bone marrow chimera-based studies suggest that flagellin recognition by hemopoietic and non-hemopoietic cells promote adaptive immunity to flagellin 11, the better controlled and more tractable model described here should prove useful to deciphering the underlying mechanisms. Further understanding of how activation of IEC-TLR5 occurs and how it helps maintain microbial order in the mucus layer may allow development of means to prevent diseases driven by a disturbances in the host-microbiota relationship.
Supplementary Material
Acknowledgments
Author Contributions: BC, REL and ATG study concept and design; BC acquired the data; BC, REL and ATG analyzed the data; BC and ATG draft the manuscript; REL critically revised the manuscript; BC, REL and ATG obtained funding.
Grant support: This work was supported by NIH grant DK099071 and DK083890. BC is a recipient of the Research Fellowship award from the Crohn's and Colitis Foundation of America (CCFA).
Abbreviations used in this paper
- TLR5
Toll Like Receptor 5
- IEC
intestinal epithelial cells
- DC
dendritic cells
- LPS
lipopolysaccharide.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors disclose no conflicts.
REFERENCES
- 1.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chassaing B, Darfeuille-Michaud A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1720–28. doi: 10.1053/j.gastro.2011.01.054. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho FA, Koren O, Goodrich JK, et al. Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell Host & Microbe. 2012;12:139–52. doi: 10.1016/j.chom.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vijay-Kumar M, Aitken JD, Carvalho FA, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–31. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vijay-Kumar M, Sanders CJ, Taylor RT, et al. Deletion of TLR5 results in spontaneous colitis in mice. The Journal of clinical investigation. 2007;117:3909–21. doi: 10.1172/JCI33084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caricilli AM, Picardi PK, de Abreu LL, et al. Gut microbiota is a key modulator of insulin resistance in TLR 2 knockout mice. PLoS Biol. 2011;9:e1001212. doi: 10.1371/journal.pbio.1001212. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Elinav E, Strowig T, Kau AL, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–57. doi: 10.1016/j.cell.2011.04.022. Author names in bold designate shared co-first authors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ubeda C, Lipuma L, Gobourne A, et al. Familial transmission rather than defective innate immunity shapes the distinct intestinal microbiota of TLR-deficient mice. J Exp Med. 2012;209:1445–56. doi: 10.1084/jem.20120504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gewirtz AT, Navas TA, Lyons S, et al. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. Journal of immunology. 2001;167:1882–5. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 10.Hobert ME, Sands KA, Mrsny RJ, et al. Cdc42 and Rac1 regulate late events in Salmonella typhimurium-induced interleukin-8 secretion from polarized epithelial cells. J Biol Chem. 2002;277:51025–32. doi: 10.1074/jbc.M210466200. [DOI] [PubMed] [Google Scholar]
- 11.Sanders CJ, Moore DA, 3rd, Williams IR, et al. Both radioresistant and hemopoietic cells promote innate and adaptive immune responses to flagellin. J Immunol. 2008;180:7184–92. doi: 10.4049/jimmunol.180.11.7184. [DOI] [PubMed] [Google Scholar]
- 12.Kinnebrew MA, Buffie CG, Diehl GE, et al. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012;36:276–87. doi: 10.1016/j.immuni.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Maele L, Carnoy C, Cayet D, et al. TLR5 signaling stimulates the innate production of IL-17 and IL-22 by CD3(neg)CD127+ immune cells in spleen and mucosa. J Immunol. 2010;185:1177–85. doi: 10.4049/jimmunol.1000115. Author names in bold designate shared co-first authors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uematsu S, Jang MH, Chevrier N, et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol. 2006;7:868–74. doi: 10.1038/ni1362. Author names in bold designate shared co-first authors. [DOI] [PubMed] [Google Scholar]
- 15.Chassaing B, Srinivasan G, Delgado MA, et al. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS One. 2012;7:e44328. doi: 10.1371/journal.pone.0044328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson ME, Phillipson M, Petersson J, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008;105:15064–9. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chassaing B, Koren O, Carvalho FA, et al. AIEC pathobiont instigates chronic colitis in susceptible hosts by altering microbiota composition. Gut. 2013 doi: 10.1136/gutjnl-2013-304909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Png CW, Linden SK, Gilshenan KS, et al. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol. 2010;105:2420–8. doi: 10.1038/ajg.2010.281. [DOI] [PubMed] [Google Scholar]
- 19.Joossens M, Huys G, Cnockaert M, et al. Dysbiosis of the faecal microbiota in patients with Crohn's disease and their unaffected relatives. Gut. 2011;60:631–7. doi: 10.1136/gut.2010.223263. [DOI] [PubMed] [Google Scholar]
- 20.Lucke K, Miehlke S, Jacobs E, et al. Prevalence of Bacteroides and Prevotella spp. in ulcerative colitis. J Med Microbiol. 2006;55:617–24. doi: 10.1099/jmm.0.46198-0. [DOI] [PubMed] [Google Scholar]
- 21.Kim N, Kunisawa J, Kweon MN, et al. Oral feeding of Bifidobacterium bifidum (BGN4) prevents CD4(+) CD45RB(high) T cell-mediated inflammatory bowel disease by inhibition of disordered T cell activation. Clin Immunol. 2007;123:30–9. doi: 10.1016/j.clim.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Rowan F, Docherty NG, Murphy M, et al. Desulfovibrio bacterial species are increased in ulcerative colitis. Dis Colon Rectum. 2010;53:1530–6. doi: 10.1007/DCR.0b013e3181f1e620. [DOI] [PubMed] [Google Scholar]
- 23.Bhatia M. Role of hydrogen sulfide in the pathology of inflammation. Scientifica (Cairo) 2012;2012:159680. doi: 10.6064/2012/159680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller TW, Wang EA, Gould S, et al. Hydrogen sulfide is an endogenous potentiator of T cell activation. J Biol Chem. 2012;287:4211–21. doi: 10.1074/jbc.M111.307819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carvalho FA, Aitken JD, Vijay-Kumar M, et al. Toll-like receptor-gut microbiota interactions: perturb at your own risk! Annual review of physiology. 2012;74:177–98. doi: 10.1146/annurev-physiol-020911-153330. Author names in bold designate shared co-first authors. [DOI] [PubMed] [Google Scholar]
- 26.Cullender TC, Chassaing B, Janzon A, et al. Innate and adaptive immunity interact to quench microbiome flagellar motility in the gut. Cell Host Microbe. 2013;14:571–81. doi: 10.1016/j.chom.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gewirtz AT, Vijay-Kumar M, Brant SR, et al. Dominant-negative TLR5 polymorphism reduces adaptive immune response to flagellin and negatively associates with Crohn's disease. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1157–63. doi: 10.1152/ajpgi.00544.2005. [DOI] [PubMed] [Google Scholar]
- 28.Carvalho FA, Nalbantoglu I, Aitken JD, et al. Cytosolic flagellin receptor NLRC4 protects mice against mucosal and systemic challenges. Mucosal immunology. 2012 doi: 10.1038/mi.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carvalho FA, Nalbantoglu I, Ortega-Fernandez S, et al. Interleukin-1beta (IL-1beta) promotes susceptibility of Toll-like receptor 5 (TLR5) deficient mice to colitis. Gut. 2012;61:373–84. doi: 10.1136/gut.2011.240556. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.