Abstract
Background
Individuals with serious mental illness have high rates of obesity and a need for specialized weight loss intervention programs. This study examines the efficacy of the RENEW weight loss intervention and examines the impact of the intervention setting on outcomes.
Method
136 individuals with serious mental illness from 4 different settings were randomly assigned to receive the RENEW weight loss intervention or a control condition of treatment as usual. The RENEW intervention is a one year program that includes an intensive, maintenance and intermittent supports phase.
Results
The intervention group experienced a modest weight loss of 4.8 lbs at 3 months, 4.1 lbs at 6 months and a slight weight gain of 1.5 lbs at 12 months. The control group gained a total of 6.2 lbs at 12 months. However when settings were examined separately the responder sites had a weight loss of 9.4 lbs at 3 months, 10.9 lbs at 6 months and 7 lbs at 12 months.
Discussion
These results suggest that the settings in which individuals receive services may act as a support or hindrance toward response to weight loss interventions. The concept of the obesogenic environment deserves further examination as a factor in the success of weight loss programs.
Keywords: obesity, schizophrenia, weight loss, intervention
1. Introduction
Individuals with schizophrenia and other serious mental illnesses, particularly women, experience higher rates of obesity than the general population (Allison et al, 2009), and obesity is associated with many negative consequences. For example, obesity in schizophrenia has been correlated with greater severity of psychiatric symptoms, decreased level of functioning, and poorer quality of life (Cerimele & Katon, 2013, Kolotkin et al, 2008). Obesity also increases the risk of many health complications including cardiovascular disease, which is the most common cause of death among individuals with serious mental illness. Of great concern is the effect of obesity on morbidity and mortality, with one study indicating individuals with schizophrenia have a life expectancy reduced by decades (Colton & Manderscheid, 2006).
People with schizophrenia and other serious mental illnesses are at increased risk for obesity for several reasons. Current models of care inadequately address the co-morbid medical conditions of individuals with schizophrenia (Casey et al, 2011). For example one systematic review found that despite high levels of cardiovascular disease, individuals with schizophrenia were less likely than individuals without mental illness to receive procedures or medications typically prescribed to manage common cardiovascular conditions (Mitchell & Lord, 2010). Lifestyle factors also contribute to obesity. Individuals with schizophrenia eat more sugar and fat than the general population and engage in less physical activity (Ratliff et al, 2012). One study found overweight and obese individuals with schizophrenia were sedentary 81% of their waking hours (Janney et al, 2013). Psychotropic medications, particularly the second generation antipsychotics, are associated with weight gain though some antipsychotics present a higher risk than others (Allison et al, 1999; Nasrallah, 2003; Rummel-Kluge et al, 2010). The medications with the greatest risk include clozapine and olanzapine which have both been associated with an increase in appetite and binge eating (Kluge et al, 2007).
The physical health of individuals with schizophrenia and other serious mental illnesses is beginning to receive attention after years of neglect. One response has been the development of behavioral weight loss interventions targeting individuals with serious mental illness. A white paper sponsored by the Substance Abuse and Mental Health Services Administration (Bartels & Desilets, 2012) included six systematic reviews and 24 intervention trials. This review found that 92% of studies examining lifestyle interventions for overweight individuals with serious mental illness resulted in an overall mean weight loss and/or decrease in body mass index (BMI). Unfortunately, the overall amount of weight loss was very modest with a median of 2.5 kg (5.5lbs); however, as this review indicates, mean weight loss for the group can be a poor indicator of individual success. Great variability in the amount of weight loss is typical in weight loss interventions. Identifying factors that contribute to this variability may be useful for identifying strategies that may improve outcomes for a larger number of participants engaged in weight loss programs.
In a previous report examining preliminary results (at 6 months) of the Recovering Energy through Nutrition and Exercise for Weight Loss (RENEW) intervention, type of antipsychotic medication was examined as a potential factor affecting response to the intervention (Brown, Goetz & Hamera, 2011). Individuals in the study were stratified by risk of weight gain (high, moderate and low risk) based on the medication they were currently taking. Medication risk was classified as follows: high risk: olanzapine, clozapine and lithium; moderate risk: risperidone, chlorpromazine, quetiapine, and valproate; low risk: molindone, ziprasidone, fluphenazine, haloperidol or aripiprazole. Medication risk was categorized based on the reviews of Casey et al (2004) and Allison et al (1999). Although participants in the RENEW program lost more weight on average than the control participants, medication risk for weight gain was not a factor in response to the intervention.
During the course of the RENEW data analysis an unintended variable was identified that appeared to have an impact on response to the intervention; the site in which participants received mental health services. Community-based programs served as the setting for this study; however, four different sites were used. The primary purpose of this article is to report the final results of the 12 month intervention study. Secondarily this paper examines differential response to the intervention based on site.
2. Experimental/Materials and methods
2.1 Participants
Participants were recruited from four community mental health programs, two in an urban area (sites 1 and 3), and one in a suburban area of greater Kansas City (site 2) and one in an urban location of Las Vegas (site 4).
Inclusion criteria included: 1) a confirmed diagnosis of serious mental illness, 2) age 18 – 65, 3) BMI ≥ 25, and 4) medication stable (no changes in antipsychotic medications or mood stabilizers for the last 3 months). Individuals with intellectual disability or dementia, a diagnosis of an eating disorder or severe cardiovascular or other physical illness that would limit participation in physical activity were excluded. Using a randomized block design, at each setting 46 participants (for a total of 136 participants) were recruited and stratified by antipsychotic medication group (low/no risk, moderate risk, high risk for weight gain) to the intervention or control group. This study was approved by the Institutional Review Boards of the University of Kansas Medical Center and Touro University-Nevada. After complete description of the study to the participants, written informed consent was obtained.
2.2 Intervention
The RENEW program is a manualized intervention combining evidence-based weight loss strategies and psychiatric rehabilitation principles. Participants received education and practice in modifying nutrition and physical activity. This education and practice took the form of psychiatric rehabilitation by incorporating social/instrumental support, goal setting, skills training, transfer training and cognitive compensation.
The RENEW program was divided into three phases: an intensive intervention, maintenance intervention, and intermittent supports. During the intensive phase (weeks 1–12), participants attended weekly 3 hour group-based sessions and had the opportunity to participate in group based exercise sessions on two other days during the week. To facilitate weight loss, participants were given an individual calorie goal (500 kcal caloric restriction) and 2 meal replacements/day (Health Management Resources; Boston MA) to be supplemented with additional foods by the participant. The intensive phase emphasized knowledge and skill acquisition. Participants learned specific information about nutrition and physical activity, and applied the information through skills training. Intensive social support was also provided during this phase. The maintenance phase (weeks 13 – 24), included a 3 hour monthly session, one hour exercise sessions twice a week, weekly phone calls geared towards problem solving and goal setting and a weekly newsletter with tips, reminders and encouragement. During the maintenance phase the emphasis was on transferring behavioral changes into habit patterns. Sessions focused on identifying the eating and activity patterns that were going well and those that were problematic. Participants were also taught how to substitute meal replacements with food available at the grocery store. Social support continued to be an important component of this phase. During the third phase of intermittent supports (weeks 25–52), participants did not receive face-to-face contact, but received feedback, reminders and supports to continue to adhere to the behavioral changes achieved during the earlier phases. This phase included weekly phone calls and monthly mailings with tips, reminders and encouragement.
2.3 Measures
All measures were administered to participants prior to the beginning of the intervention and then after the intensive phase (3 months), maintenance phase (6 months) and intermittent supports phase (12 months). Precise body weight was measured using a digital scale accurate to ± 0.1 kg (Seca Platform Scale, model 707, Seca Corp., Columbia, MD).
2.3. Statistical Analyses
Descriptive statistics were used to depict the demographic and baseline characteristics of the two groups. In addition, Chi-square or t-tests were used to compare the intervention and control group at baseline to determine if there were any initial differences between the groups on key demographic and outcome measures.
A mixed model analysis (also called Hierarchical Linear Modeling) was utilized to examine the differences between intervention groups (intervention vs. control) while considering site. The baseline measure (e.g. baseline weight when weight was modeled) was used as a covariate. No other covariates were included in the models. Appropriate interactions were also investigated including time by intervention, intervention by site, and three way time by intervention by site. Compound symmetry correlation structure was specified. Since this study involves measurements over an extended period of time (baseline, three months, six months and 1 year), this method was chosen over a standard repeated measures ANOVA because it produces unbiased estimates of missing data (ignorable missing values assumption). This analysis allows for the inclusion of data from participants that were not present at all time points. Specific outcomes for each individual (Level 1) as nested within the intervention vs. control conditions (level 2 groupings) and blocked by site were performed. The secondary analysis comparing responders and non-responders was conducted using a repeated measures ANOVA.
3. Results
3.1 Participants and Baseline Characteristics
136 individuals with serious mental illness enrolled in the study from four community mental health programs (three in the Kansas City area and one in Las Vegas). Demographic and baseline characteristics are presented in Table 1. There were no differences between the intervention and control group for age, gender, race, diagnosis, medication risk or symptoms as measured by the 24 item Brief Psychiatric Rating Scale (Overall & Gorham, 1962). On baseline measures there were no differences for weight, physical activity as measured by accelerometer, or dietary intake.
Table 1.
Demographic and Clinical Characteristics at Baseline
| Total | Intervention | Control | p | |
|---|---|---|---|---|
| N | 136 | 70 | 66 | |
| Age | 44.4 (11.7) | 44.9 (10.1) | .81 | |
| Gender | ||||
| Male | 25 (36%) | 20 (30%) | .50 | |
| Female | 45 (64%) | 46 (70%) | ||
| Race | ||||
| African American/black | 23 (33%) | 23 (35%) | .99 | |
| Caucasian/white | 42 (60%) | 39 (59%) | ||
| Multiracial | 3 (4.2%) | 2 (3%) | ||
| Other/not reported | 2 (2.8%) | 2 (3%) | ||
| Education | ||||
| Less than high school | 13 (19%) | 9 (14%) | .85 | |
| High School graduate/GED | 20 (29%) | 20 (30%) | ||
| Some college/post HS | 26 (37%) | 27 (41%) | ||
| Bachelor’s degree | 7 (10%) | 8 (12%) | ||
| Beyond bachelor’s degree | 4 (5 %) | 2 (3%) | ||
| Medication Risk (for weight gain) | ||||
| Low | 22 (32%) | 23 (36%) | .76 | |
| Moderate | 34 (49%) | 28 (44%) | ||
| High | 13 (19%) | 13 (20%) | ||
| BPRS Total Score | 39.4 (9.7) | 40.3 (9.1) | .58 | |
| Weight in lbs. | 224.2 (45.4) | 234.1 (55.9) | .26 | |
| Dietary Intake | ||||
| Total Energy | 2098 (866) | 1837 (686) | .08 | |
| Energy from fat | 81.9 (37) | 70 (34) | .08 | |
| Physical activity – minutes of activity | 280.5 (86) | 267.6(77.9) | .55 | |
Ninety-two participants were available for assessment at 12 months; 44 individuals dropped out of the study voluntarily and were thus lost to follow-up (32%). No difference were found between completers and drop outs for age, baseline weight, race or medication risk; however, a larger proportion of women than men dropped out of the study (χ2 = 4.37, p=.037). Of the completers, 47 were randomly assigned to the intervention and 45 to the control group.
3.2 Changes in Weight by Group (Intervention group vs. Control group)
The intervention group achieved a weight loss of 4.8lbs during the intensive phase (month 3) and exhibited only a slight regain of weight following the maintenance phase (6 months; 4.1 lbs weight change from baseline). However, intervention participants regained weight during the intermittent supports phase such that the group was 1.5 lbs heavier than at baseline at the 12 month follow-up assessment. Conversely, the control group gained 8.5 lbs at 3 months, but then moderated this weight gain such that participants had gained 7 lbs and 6.2 lbs from baseline at months 6 and 12, respectively. (Table 2). The mixed model analysis indicated a significant difference between the intervention and control group at 3 months (the end of the intensive phase) (F = 6.936, p = .01) but not at 6 months (F = 1.527, p = .22) or 12 months (F = .522, p = .47).
Table 2.
Changes in Weight
| Baseline | 3 Months | 6 Months | 12 Months | |
|---|---|---|---|---|
| Intervention | 224.2 (45.4) | 219.4 (42.9) | 220.1 (43.2) | 225.7 (45.6) |
| −4.8 | −4.1 | +1.5 | ||
| Control | 234.1 (55.9) | 242.6 (57.7) | 241.1 (58.5) | 240.3 (55.8) |
| +8.5 | +7.0 | +6.2 | ||
| Intervention | ||||
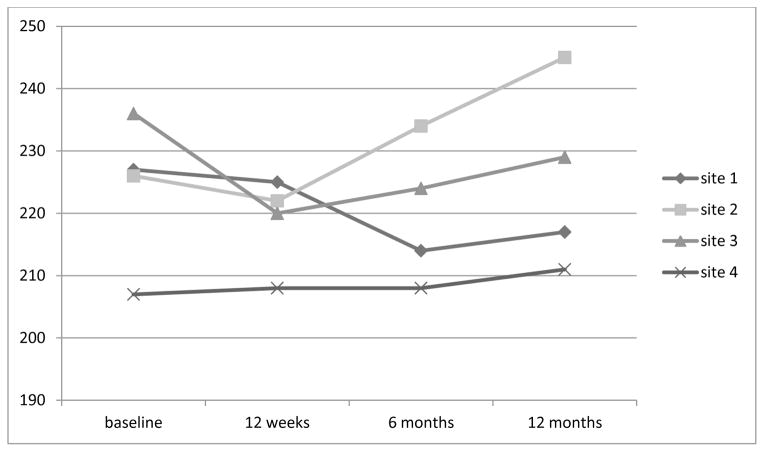
| Site 1 | 227.1 (56.2) | 225.1 (54.4) | 214.3 (49.6) | 217.1 (47.7) |
| −2.0 | −12.8 | −10 | ||
| Site 2 | 226.0 (52.3) | 222.1 (48.7) | 233.7 (47.5) | 244.6 (53.3) |
| −4.9 | +7.7 | +18.6 | ||
| Site 3 | 235.5 (37.3) | 220.2 (38.7) | 224.4 (44.7) | 228.9 (40.9) |
| −15.3 | −11.1 | −6.6 | ||
| Site 4 | 206.7 (24.7) | 208.3 (23.0) | 208.1 (26.9) | 211.3 (35.1) |
| +1.6 | +1.4 | +3.6 | ||
3.3 Differences in Weight Loss by Site
In examining the interaction effect with differences in weight for the intervention and control groups at each of the four sites there was a significant interaction at 3 months (F = 3.36, p = .017) and 6 months (F = 2,831, p = .043), but not at 12 months (F = 2.378, p = .076). The graph (Figure 1) displays the different patterns of weight loss for the two groups at the four different sites.
Figure 1.
Weight change by site for the intervention groups
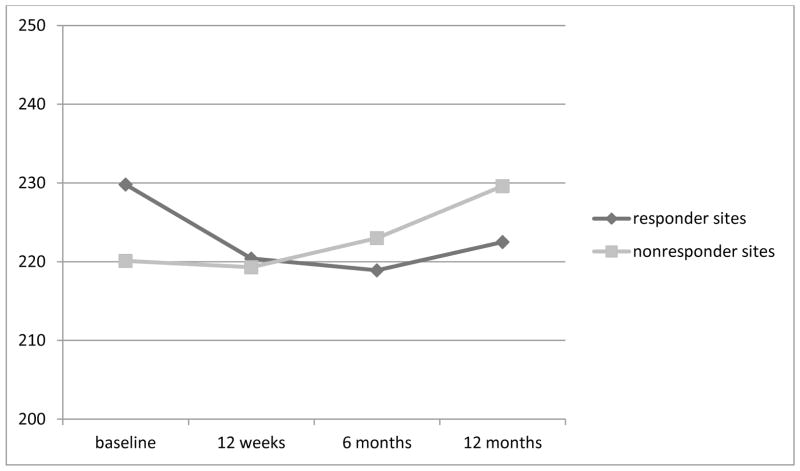
When analyzing the site difference within the intervention group, there appears to be a distinct difference in the pattern of weight change for sites 1 and 3 (both in urban Kansas City) as compared to sites 2 (suburban Kansas City) and 4 (Las Vegas). Sites 1 and 3 lost weight at all three time periods, with a mean weight loss of 10 lbs and 6.6 lbs at 12 months follow-up, respectively. Conversely, sites 2 and 4 gained weight at most time points, with an overall mean weight gain of 18.6 lbs and 3.6 lbs, respectively, at the 12 month follow-up. Because of this perplexing variation in response to the intervention, sites 1 and 3 were combined and labeled “responders”. Sites 2 and 4 were combined and labeled “non- responders”. A repeated measures ANOVA was conducted with time as a within group variable and response (responder vs non-responder) as the between group variable. There was a significant difference for time (F = 7.329, p <.001) and a significant difference for the interaction of time by response (F = 3.36, p = .029). Figure 2 illustrates the different amount of weight loss at the responder and non-responder sites. The responder sites lost 9.4 lbs at 3 months, continued to lose weight for a total loss of 10.9 lbs at 6 months and had an overall weight loss of 7 lbs at 12 months. In contrast, the non-responder sites lost 0.8 lbs at 3 months and gained weight, 2.9 lbs and 9.5 lbs, at 6 months and 12 months, respectively.
Figure 2.
Weight loss responders (Sites 1 and 3) vs non-responders (Sites 2 and 4) in the Intervention group
Differences between the four sites for the intervention group were investigated using independent t-tests and chi-square analyses to determine if these factors may account for site differences. There were no differences detected on the following variables: age, gender, education, BPRS scores, baseline body weight, or medication risk (Table 3). There was a difference in race, with a greater proportion of African Americans at Site 1 and 3, the responder sites. All sites were combined and a one way ANOVA was used to determine if there were differences in the amount of weight lost for the racial groups with no difference found at 3 (p = .23), 6 (p = .38), or 12 months (p = .62)
Table 3.
Site Differences for Intervention Group on Demographic and Clinical Variables and Baseline Weight
| Site 1 | Site 2 | Site 3 | Site 4 | F/χ2 | p | |
|---|---|---|---|---|---|---|
| N | ||||||
| Age | 41.7 (11.8) | 42.3 (12.5) | 49.6 (9.4) | 43.8 (12.2) | 1.61 | .20 |
| BPRS | 41.6 (9.6) | 38.5 (9.5) | 40.4 (10.4) | 37.0 (9.6) | .728 | .54 |
| Weight | 227.1 (56.2) | 226.0 (52.7) | 235.5 (37.3) | 206.7 (24.7) | 1.19 | .32 |
| Gender | ||||||
| Male | 9 (50%) | 8 (42%) | 4 (26%) | 4 (25%) | 3.84 | .28 |
| Female | 9 (50%) | 11 (58%) | 13 (74%) | 12 (75%) | ||
| Race | ||||||
| African Am | 6 (33%) | 2 (11%) | 10 (59%) | 5 (31%) | 18.27 | .03* |
| White | 11 (61%) | 17 (89%) | 6 (35%) | 8 (50%) | ||
| Multiracial | 0 | 0 | 1 (6%) | 2 (13%) | ||
| Other | 1 (6%) | 0 | 0 | 1 (6%) | ||
| Education | ||||||
| Some HS | 2 | 3 | 5 | 4 | 14.95 | .46 |
| HS degree | 9 | 5 | 4 | 2 | ||
| Post HS | 6 | 6 | 5 | 9 | ||
| Bachelor deg | 0 | 4 | 1 | 2 | ||
| Bachelor + | 1 | 1 | 2 | 0 | ||
| Med Risk | ||||||
| Low | 5 (28%) | 2 (7%) | 8 (47%) | 7 (44%) | 8.84 | .18 |
| Moderate | 10 (56%) | 20 (72%) | 6 (35%) | 8 (50%) | ||
| High | 3 (16%) | 6 (21%) | 3 (18%) | 1 (6%) | ||
4. Discussion
This study found modest amounts of weight loss at 3 and 6 months for the intervention group (4.8 lbs and 4.1 lbs, respectively) and weight gain of 8.5 lbs and 7.0 lbs for the control group during the same time period. At 12 months the intervention group had gained 1.5 lbs over their baseline weight while the control group was 6.2 lbs over their baseline. However more striking differences were identified when site comparisons were made. The mixed model analysis indicated that there was a significant difference in the group by site interaction. When comparing the different sites, 2 intervention sites were identified as responders and two sites as non-responders. The amount of weight lost by the responders (when compared to baseline) was 9.4 lbs at 3 months, 10.9 lbs at 6 months and 7 lbs at 12 months. The weight loss at all time points was greater than the 5.5 lbs. mean weight loss found in the Bartels & Desilets (2012) review of weight loss programs for people with psychiatric disabilities. However, the impressive amount of weight lost in the responder sites is countered by a puzzling weight gain in the non-responder sites. In this study, the control group gained weight with most of their weight gain occurring during the first 3 months. In the non-responder sites, the majority of the weight gain occurred when the intensity of the intervention was reduced. During the last 6 months of the intervention, participants received phone calls and newsletters, but no face to face interventions and no meal replacements. It appears that during this time, the non-responders looked more like the control participants than the intervention participants in the responder sites. Perhaps the non-responders were in greater need of the intensive intervention and were not able to benefit from the lessening of support. On the other hand, not only did the responders have a better response to the intensive intervention (lost more weight), but they continued to fare well during the intermittent supports phase.
Individual differences in weight loss are widely recognized with many intervention studies having participants that both lose and gain weight even when exposed to the same approach (Fogelholm, 2009). A review of predictors of weight loss and maintenance (Stubbs et al, 2011) considered individual factors, process variables such as number of attempts at weight loss, treatment factors such as length of treatment and behavioral change. Overall the review found great heterogeneity among the studies and that most of the variance in weight loss remains unexplained.
In this study, examination of individual factors that might contribute to the difference in response did not yield any useful clues as there were no differences in the sites in terms of age, gender, education, baseline weight or medication risk. There were some differences in race with proportionally more African Americans in the responder sites but race seems a poor explanation. Other studies suggest race is not a major predictor of weight loss (Svetkey et al, 2012) and in this study although there were racial differences by site, the racial groups did not experience differences in amount of weight lost at any time point. Perhaps the difference in response is not a factor associated with the individual but a factor(s) associated with the site. Differential response may be related to environmental as opposed to individual differences. Cook & Mueser (2013) discuss the term obesogenic environment and its relevance for psychiatric rehabilitation. In an ecological model, an obesogenic environment is one that promotes weight gain and inhibits weight loss by encouraging excessive food intake and presenting barriers to physical activity (Hill & Peters, 1998). Cook & Mueser suggest that service delivery programs may unwittingly contribute to the already existing obesogenic environment that the average American experiences. For example, service delivery settings may differ in terms of the types of food served at the program or availability of wellness programming.
Unfortunately, we did not anticipate the discrepancy in response across the sites, and did not collect data that could identify environmental factors associated with weight loss. Although some variability in intervention leaders existed across the four sites, the primary facilitators provided an intervention at both a responder and non-responder site. Thus, discrepancy in response across sites is likely not due to intervention leaders. Anecdotal evidence from the intervention leaders suggests differences in group cohesion along with a more established culture of wellness may have played a role in response to the intervention. The leaders reported that the responder sites appeared to have greater peer support both within and outside of the group intervention. In addition, the responder sites seemed to value and model wellness to a greater extent and were more likely to have existing wellness programs such as walking groups or Wellness Recovery Action Planning. Therefore, as the intervention intensity waned, it is possible that the individuals at the responder sites then moved to using existing programs. At least one of the non-responder sites seemed to have greater challenges related to transportation. A major limitation of this study is the lack of objective data to verify these hypotheses. Future research would benefit from data collection on these factors to determine if significant differences existed. Unfortunately valid and reliable measures of obesogenic environments are limited for the general public (Giskes et al, 2010) and are unexplored in relation to mental health service settings. However, a greater recognition of the role of the environment in weight loss should lead to the development of these much needed measures.
Individual differences in response to weight loss interventions are not well understood. The results of this study suggest that the setting in which the intervention is administered may have an impact on the response and should be considered when administering weight loss interventions. Environmental factors that contribute to and prevent weight loss deserve additional attention.
Acknowledgments
Funding
This research was supported by grant # R34 MH077282 from the National Institutes of Mental Health. In addition, meal replacements were partially subsidized by Health Management Resources.
Footnotes
Contributors
This study was designed by C. Brown, E. Hamera and J. Goetz who were also primarily responsible for collection of the data. B. Gajewski and C. Brown are responsible for analysis of the results. C. Brown wrote the first draft and all authors contributed to and have approved the final article.
Conflict of interest
There were no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Catana Brown, Midwestern University - Glendale – College of Health Sciences.
Jeannine Goetz, University of Kansas – School of Allied Health.
Edna Hamera, University of Kansas – School of Nursing.
Byron Gajewski, University of Kansas – School of Allied Health.
References
- Allison DB, Mentore JL, Heo M. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156(11):1686–1696. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- Allison DB, Newcomer JW, Dunn AL, Blumenthal JA, Fabricatore AN, Daumit GL, Cope MB, Riley WT, Vreeland B, Hibbeln JR, Alpert JE. Obesity among those with mental disorders: a National Institute of Mental Health meeting report. Am J Prev Med. 2009;36(4):341–350. doi: 10.1016/j.amepre.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Bartels S, Desilets R. Health promotion programs for people with serious mental illness (Prepared by the Dartmouth Health Promotion Research Team) SAMHSA-HRSA Center for Integrated Health Solutions; Washington, D.C: 2012. [Google Scholar]
- Brown C, Goetz J, Hamera E. Weight loss intervention for people with serious mental illness: A randomized controlled trial of the RENEW program. Psychiatr Serv. 2011;62(7):800–803. doi: 10.1176/appi.ps.62.7.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DE, Haupt DW, Newcomer JW, Henderson DC, Sernyak MJ, Davidson M, Lindenmayer JP, Manoukian SV, Banerji MA, Lebovitz HE, Hennekens CH. Antipsychotic-induced weight gain and metabolic abnormalities: implications for increased mortality in patients with schizophrenia. J Clin Psychiatry. 2004;65(Suppl 7):4–18. [PubMed] [Google Scholar]
- Casey DE, Rodriguez M, Northcott C, Vickar G, Shihabuddin L. Schizophrenia: medical illness, mortality, and aging. Int J Psychiatry Med. 2011;41:245–251. doi: 10.2190/PM.41.3.c. [DOI] [PubMed] [Google Scholar]
- Cerimele JM, Katon WJ. Associations between health risk behaviors and symptoms of schizophrenia and bipolar disorder: A systematic review. Gen Hosp Psychiatry. 2013;35(1):16–22. doi: 10.1016/j.genhosppsych.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):1–14. [PMC free article] [PubMed] [Google Scholar]
- Cook JA, Mueser KT. The challenge of obesity. Psychiatr Rehabil J. 2013;36:129–132. doi: 10.1037/prj0000021. [DOI] [PubMed] [Google Scholar]
- Fogelholm M. Individual differences in weight change – scientific challenges or personal frustrations. Obes Rev. 2009;10(6):587–588. doi: 10.1111/j.1467-789X.2009.00640.x. [DOI] [PubMed] [Google Scholar]
- Giskes K, van Lenthe F, Avendano-Pabon M, Brug J. A systematic review of environmental factors and obesogenic dietary intakes among adults: Are we getting closer to understanding obesogenic environments? Obes Reviews. 2011;12(5):e95–e106. doi: 10.1111/j.1467-789X.2010.00769.x. [DOI] [PubMed] [Google Scholar]
- Janney CA, Ganguli R, Richardson CR, Holleman RG, Tang G, Cauley JA, Kriska AM. Sedentary behavior and psychiatric symptoms in overweight and obese adults with schizophrenia and schizoaffective disorders (WAIST Study) Schizophr Res. 2013;145(1–3):63–68. doi: 10.1016/j.schres.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280(5368):1371–1374. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]
- Kluge M, Schuld A, Himmerich H. Clozapine and olanzapine are associated with food craving and binge eating: results from a randomized double-blind study. J Clin Psychopharmacol. 2007;27(6):662–666. doi: 10.1097/jcp.0b013e31815a8872. [DOI] [PubMed] [Google Scholar]
- Kolotkin RL, Corey-Lisle PK, Crosby RD, Swanson JM, Tuomari AV, L’italien GJ, Mitchell JE. Impact of obesity on health-related quality of life in schizophrenia and bipolar disorder. Obesity. 2008;16(4):749–754. doi: 10.1038/oby.2007.133. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, Lord O. Do deficits in cardiac care influence high mortality rates in schizophrenia? A systematic review and pooled analysis. J Psychopharmacol. 2010;24(4 Suppl):69–80. doi: 10.1177/1359786810382056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah H. A review of the effect of atypical antipsychotics on weight. Psychoendocrinol. 2003;28(Suppl 1):83–96. doi: 10.1016/s0306-4530(02)00114-2. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:790–812. [Google Scholar]
- Ratliff JC, Palmese LB, Reutenaeur EL, Liskove E, Grilo CM, Tek C. The effect of dietary and physical activity pattern on metabolic profile in individuals with schizophrenia: A cross sectional study. Comp Psychiatry. 2012;53(7):1028–1033. doi: 10.1016/j.comppsych.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummel-Kluge C, Komossa K, Schwarz S, Hunger H, Schmid f, Lobos CA, Kissling W, Davis JM, Leucht S. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: A systematic review and meta-analysis. Schizophr Res. 2010;123(2):225–233. doi: 10.1016/j.schres.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs J, Whybrow S, Teixeira P, Blu dell J, Lawton C, Westenhoefer J, Engel D, Shepherd R, Mcconnon A, Gilbert P, Raats M. Problems in identifying predictors and correlates of weight loss and maintenance: Implications for weight control therapies based on behaviour change. Obes Rev. 2011;12(9):688–708. doi: 10.1111/j.1467-789X.2011.00883.x. [DOI] [PubMed] [Google Scholar]
- Svetkey LP, Ard JD, Steven VJ, Loria CM, Young DY, Hollis JF, Appel LJ, Brantley PJ, Kennedy BM, Kumanyika SK, Batch BC, Corsino L, Lien LF, Vollmer WM Weight Loss Maintenance Collaborative Research Group. Predictors of long-term weight loss in adults with modest initial weight loss, by sex and race. Obesity. 2012;20(9):1820–1828. doi: 10.1038/oby.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]